?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Aspergillus westerdijkiae is a major producer of ochratoxin A (OTA), a highly toxic and carcinogenic mycotoxin found in various food and feed products. A. westerdijkiae produces excessive amount of OTA under various water activity (aw) conditions that occur during food and feed storage. The biosynthetic gene clusters associated with OTA production include OTAbZIP, which plays a key role in controlling mycotoxin production in response to environmental conditions. This study explored the regulation of OTA biosynthesis in A. westerdijkiae fc-1, focusing on the OTAbZIP gene’s influence under aw stress. The mycelium growth of A. westerdijkiae fc-1 wild type and OTAbZIP mutant strains increased by 40.7% and 50.5% under high water activity (0.96 aw) respectively, at 6 days post-inoculation (dpi), indicating a stress on A. westerdijkiae fc-1. While OTAbZIP mutant did not produce OTA under both high and moderate aW conditions. The wild type produced OTA and OTA biosynthetic gene expression levels were downregulated under high (0.96 aw) and moderate (0.91 aw) water activity. The expression level of hog1 gene in OTAbZIP mutant was significantly lower than in the wild type. Pathogenicity tests revealed that deletion of OTAbZIP did not significantly affect disease infection. This study shows that deleting OTAbZIP gene greatly reduces OTA production, affecting the strain’s adaptability to water activity stress.
1. Introduction
Ochratoxin A (OTA) producing fungi widely contaminate various foods and their byproducts resulting in the substantial production of OTA. OTA causes high levels of toxicity including carcinogenicity, hepatotoxicity, genotoxicity, cytotoxicity, and immunotoxicity. OTA is listed as a 2-B carcinogen by the International Agency for Research on Cancer, which seriously threatens food safety and endangers human health (Gonzalez et al. Citation2020; Kumar et al. Citation2020). The National Cancer Institute/National Toxicology Program (NCI/NTP) demonstrated that OTA is the most potent renal carcinogen ever studied in rodents (National Toxicology Program Citation1989). Several species of Aspergillus and Penicillium produce OTA, which has been detected in a variety of common cereals such as rice, wheat, corn, barley, and oats, as well as in grapes, spices, coffee, grape juice, and other agricultural products (Zhihong et al. Citation2015). In addition, OTA has also been found in herbal medicines (Özden and Özden Citation2018; Zhu et al. Citation2022), food additives (Solfrizzo et al. Citation2015), and water (Mata et al. Citation2015).
The mycelium growth and mycotoxin production of Aspergillus species are influenced by various environmental factors, including water activity, pH, light, and temperature. Among these factors, water activity (aw) stands out as a key determinant affecting the production of mycotoxins during the storage of food and feed ingredients (Cervini et al. Citation2020; Mutlu-İNgök et al. Citation2020; Priesterjahn et al. Citation2020). Water activity serves as a crucial indicator for assessing the moisture content in food. In general, foods with high aw are more susceptible to fungal infection and spoilage compared to those with low aw. OTA-producing fungi Aspergillus and Penicillium species have the potential to contaminate foods both in high and low aw conditions. The influence of aw extends to the growth and secondary metabolism of fungi, consequently regulating the production of mycotoxins (Kapetanakou et al. Citation2009; Wang et al. Citation2018; Zhang et al. Citation2021). Fungal species possess a signalling pathway known as the HOG-MAPK (high osmolarity glycerol mitogen-activated protein kinase) pathway, which plays a critical role in responding to a diverse range of environmental stresses. These stresses include osmotic, oxidative, aw, cold, heat, and light (Fernandes et al. Citation2015; Bersching and Jacob Citation2021; Mo et al. Citation2021). The hog1 gene in the HOG-MAPK pathway plays a vital regulatory role under aw. Knockout of the afsakA (hog1) gene increases the sensitivity of Aspergillus flavus to aw stress and induces toxin production, indicating that hog1 is involved in the aw stress regulation of growth, sporulation and toxin production in A. flavus (Zhang et al. Citation2014, Citation2015). Understanding the regulatory mechanism of aw on mycotoxins is beneficial during the storage of grain and feed materials, aiding in the prevention and control of mycotoxin contamination in these substances. Currently, several studies have investigated the biosynthesis pathway of OTA including the biosynthesis mechanism of OTA such as polyketide synthase gene otaA, the nonribosomal polypeptide synthase gene otaB, the P450 oxidase gene otaC, the halogenase gene otaD, and the bZIP transcription factor gene otaR1 which are directly implicated in the process of OTA biosynthesis (Wang et al. Citation2015, Citation2016). Functioning as a bZIP regulator, otaR1 governs the expression of four biosynthetic genes: otaA, otaB, otaC, and otaD. The bZIP protein is a conserved, specific pathway-transcription factor found in eukaryotes, and it regulates a congregation of genes associated with environmental stress adaptation (Hurst Citation1995; Wang et al. Citation2018). The bZIP transcription factor atfA in Penicillium marneffei is partially involved in the regulation of oxidative stress but not in the regulation of osmotic and UV light stress (Nimmanee et al. Citation2014). Knock-out mutants of the bZIP transcription factor fvatfA in Fusarium verticillioides are more sensitive to oxidative stress factors (Szabó et al. Citation2020). The overexpression of the OTA biosynthesis gene cluster was observed in A. carbonarius under conditions of high water activity (aw = 0.99). Notably, the expression level of the bZIP gene was found to be the highest, suggesting that bZIP plays a crucial role in the regulation of aw stress in Aspergillus species (Cervini et al. Citation2020). A novel bZIP transcription factor gene, atfC, has been identified in A. flavus, demonstrating its advantageous role in enhancing A. flavus tolerance under drought stress conditions (Fountain et al. Citation2020). The mechanism of the bZIP response to aw stress in A. westerdijkiae remains elusive. To unveil this mechanism, we introduced glycerol into the culture medium to simulate aw stress. This approach aims to elucidate how the OTAbZIP gene regulates the mycelium growth and OTA production of A. westerdijkiae fc-1 under aw stress.
2. Materials and methods
2.1. Fungal strain and culture mediums
The fungus studied in the current research is OTA producing A. westerdijkiae fc-1 (accession number PRJNA264608). Potato dextrose agar (PDA) medium and potato dextrose broth (PDB) medium supplemented with glycerol were utilised. The aw of the PDA/PDB medium was maintained at 0.98 aw, 0.96 aw, 0.95 aw, 0.93 aw, 0.91 aw, and 0.88 aw by adding different amounts (v/v) of glycerol (Nakagawa and Oyama Citation2019). Both PDA and PDB media were subjected to autoclaving at 121 °C for 20 min to facilitate the morphological characterisation and determination of OTA production in A. westerdijkiae fc-1.
2.2. Identification of the OTAbZIP gene
The characteristics of the OTAbZIP gene were analysed by conducting a BLASTp alignment, comparing the OTAbZIP gene with the bZIP genes found in currently known fungi. This analysis was carried out on the NCBI website (https://www.ncbi.nlm.nih.gov/).
2.3. Knockout of the OTAbZIP gene in A. westerdijkiae fc-1
Primer design was executed using Primer Five-Software. The promoter and terminator regions (−1.5 kb) were subsequently amplified from the A. westerdijkiae fc-1 genome utilising Top-Taq DNA polymerase from Bioron GmbH, Ludwigshafen, Germany.
The PCR programme is performed according to . The PCR-amplified DNA, along with 5 μL of sodium heparin, was introduced into A. westerdijkiae fc-1 protoplasts. Positive transformants were then screened through three passages on PDA supplemented with 100 μg/mL hygromycin B, and homology-adjusted PCR patterns corresponding to T-DNA at the target site were assessed.
Table 1. Knockout PCR programme of the OTAbZIP gene in Aspergillus westerdijkiae fc-1.
The A. westerdijkiaefc-1 protoplasts were solubilised and subjected to centrifugation at 4 °C and 5,000 r/min for 5 min. The resultant filter residue was collected, and 800 μL of STC solution (comprising 0.8 mol/L sorbitol, 50 mmol/L Tris-HCl, and 50 mmol/L calcium chloride) was added to re-suspend the protoplasts. This suspension was then gradually added dropwise to 200 μL of SPTC solution (containing 0.8 mol/L sorbitol, 40% polyethylene glycol, 50 mmol/L Tris-HCl, and 50 mmol/L calcium chloride), thoroughly mixed, and kept on ice for subsequent use.
The PCR cloned DNA solution was transformed, and 5 μL of sodium heparin (0.9 U/L) was mixed gently with the A. westerdijkiae fc-1 protoplasts and allowed to stand on ice for 30 min, with periodic mixing to prevent the formation of protoplasts. Precipitation affects transformation efficiency. Then, one-third volume of SPTC solution was added dropwise, mixed gently, and allowed to stand at room temperature for 20 min. The protoplasts were then plated on a medium containing hygromycin for selection. After incubating at 28 °C for 3 d, the fungi exhibiting normal growth were transferred to PDB medium without hygromycin and shaken at 28 °C and 180 r/min for 3 d to obtain the transformed cells of A. westerdijkiae fc-1.
2.4. Phenotypic characterization
The ΔOTAbZIP mutant and wild-type strains were inoculated on a PDA medium with varying water activity (0.98 aw, 0.96 aw, 0.95 aw, 0.93 aw, 0.91 aw, and 0.88 aw). The inoculated plates were then kept in the dark at 28 °C, and the colony diameter was observed and measured on the 3 and 6 dpi. The inhibition was calculated using the formula.
2.5. Pathogenicity test
The ripe pear (Sydney var.) fruit was detached from the stem, and its surface was subjected to sterilisation three times using 75% alcohol, followed by allowing it to evaporate in ultra clean laminar flow chamber. Subsequently, 10 μL of conidial suspension (107 spores/mL) from both the wild-type strain and mutant fc-1 strain was injected into the pear fruit using a needle syringe. for control, the spore suspension was replaced with an equivalent volume of sterile water. The inoculated fruits were incubated at 28 °C for 3, 6, and 9 dpi. The growing colony diameter of the infection site on the fruit was measured at 3, 6 and 9 dpi.
2.6. OTA detection
The ΔOTAbZIP mutant and the wild-type A. westerdijkiae fc-1 strains were inoculated on PDB medium containing various concentrations of glycerol to maintain varying aw conditions and incubated at 28 °C and 180 r/min. The OTA content was analysed at 6 and 9 dpi. For HPLC sample preparation, 5 mL of culture was mixed with 10 mL of methanol (Sigma-Aldrich Inc., St. Louis, USA) in a 50 mL centrifuge tube. The tube was vigorously shaken using a shaker (Crystal Technology & Industries, Inc. ISRDD3, Suzhou, China) for 3 min and sonicated for 30 min. The samples were then centrifuged at 5,000 r/min for 10 min using a centrifuge (Eppendorf Inc., Hamburg, Germany). The supernatant (1.5 mL) in the centrifuge tube was extracted and filtered through a 0.22 μm organic microporous filter (Bkmam Biotechnology Inc., Hunan, China). The concentration of OTA was detected using HPLC-FLD (Agilent Inc., California, USA) detector. Agilent 1260 analytical liquid chromatography coupled with an FLD-fluorescence detector was used. The excitation and emission wavelength were set at 336 nm and 440 nm respectively. Agilent ZorbaxSB-C18 column (4.6 mm × 250 mm, 5 μm) was used for the detection of OTA and mobile phase run as acetonitrile: 2% acetic acid water (60/40, v/v) with the flow rate of 1.0 mL/min. The sample injected volume was 20 μL and the detection time was set to 10 minutes for each sample.
2.7. Detection of gene expression by RT-qPCR
The ΔOTAbZIP mutant and the wild type of strain were inoculated in a PDB medium containing different concentrations of glycerol and placed at 28 °C and 180 r/min in the dark. The relative expression levels of the OTA biosynthesis genes otaA, otaB, otaC, otaD, and hog1 were determined at 3 dpi. Primers were designed according to the target gene to be detected. After extracting RNA from the sample, the integrity was checked by electrophoresis, and the purity and concentration were checked by measuring the OD value. One microgram of RNA was synthesised into cDNA using M-MLV reverse transcriptase (Life Technologies, Milan, Italy) and primers for the bZIP gene. The expression levels of OTA biosynthetic gene cluster genes (otaA, otaB, otaC, and otaD) and hog1 were assessed using a real-time PCR detection system. The amplification conditions comprised 45 cycles of 95 °C for 3 min, followed by 95 °C for 10 s, and 60 °C for 45 s. The relative gene expression was calculated utilising the CFX Manager software from Bio-Rad Laboratories and the 2−ΔΔCT method, with triplicate measurements for every sample.
3. Results
3.1. Identification of the OTAbZIP gene
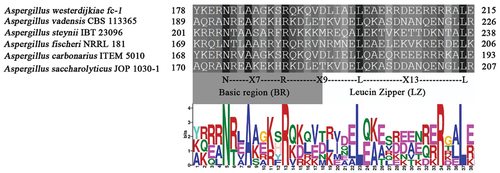
The OTAbZIP gene of A. westerdijkiae fc-1 is located in Scaffold_Scf_8 of the A. westerdijkiae fc-1 genome, with a length of 767 bp, and it encodes a protein of 263 aa. In A. fischeri, A. vadensis, A. carbonarius, A. saccharolyticus, and A. steynii, according to the calibration and prediction of the fungal BRLZ domain, the conserved canonical N-X7-R region BR domain, R-X9-L region, distinguishable BR domain and LZ domain and at least four leucine residues of the LZ domain were identified (), indicating that the OTAbZIP gene is a bZIP-regulated gene.
3.2. Knockout of the OTAbZIP gene in A. westerdijkiae fc-1
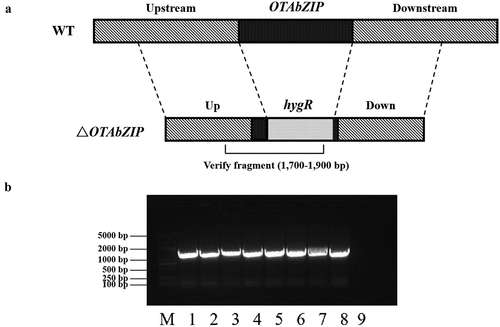
To investigate the role of OTAbZIP in OTA biosynthesis, this gene was substituted with a hygromycin resistance gene in the A. westerdijkiae fc-1 strain. ΔOTAbZIP mutants were chosen for examination, and the number of T-DNA copies in the genome was determined through qPCR, using the wild-type strain as a control. In the OTAbZIP mutant A. westerdijkiae fc-1 strain, the amplified band was 1,700–1,900 bp, representing a part of the replaced hygromycin gene, indicating that the ∆OTAbZIP gene was successfully knocked out, and the mutant strain showed a visible band after amplification (). On the contrary, there were no target bands in the wild-type A. westerdijkiae fc-1 strain.
Figure 2. (a) Identification of the hygromycin gene in the ∆OTAbZIP mutant Aspergillus westerdijkiae. (b) Strategy of gene replacement. Agarose gel electrophoresis showing PCR amplified products for wild type and ∆OTAbZIP mutant A. westerdijkiae fc-1. M: DL 5000 DNA Marker; 1–8: ∆OTAbZIP mutant A. westerdijkiae fc-1; 9: Wild-type A. westerdijkiae fc-1.
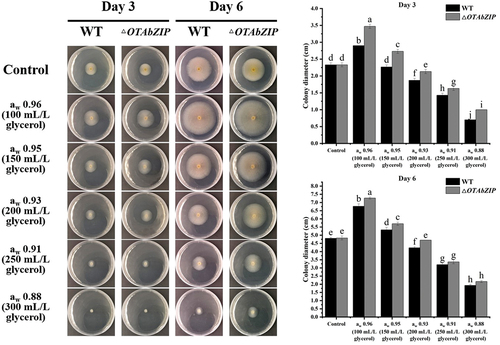
3.3. Phenotypic characterization
The wild-type strain exhibited enhanced growth at 0.96 aw, with increases of 24.4%. Conversely, growth inhibition was observed at 0.95 aw, 0.93 aw, 0.91 aw, and 0.88 aw, with inhibition rates of 2.7%, 19.7, 38.6%, and 59.9%, respectively, at 3dpi. In comparison, the ΔOTAbZIP mutant demonstrated improved growth at 0.96 aw and 0.95 aw, with increases of 48.9% and 7.2%, respectively. Growth inhibition for the ΔOTAbZIP mutant commenced at 0.93 aw, 0.91 aw, and 0.88 aw, with inhibition rates of 8.6%, 30.0%, and 57.1%, respectively. These results suggest that the mutant strains have a higher tolerance to water action. On 6 dpi, the wild-type strain exhibited enhanced growth at 0.96 aw and 0.95 aw, with increases of 40.7% and 10.8%, respectively. Conversely, growth inhibition was observed at 0.93 aw, 0.91 aw, and 0.88 aw, with inhibition rates of 12.1%, 33.5%, and 59.9%, respectively. In comparison, the ΔOTAbZIP mutant demonstrated improved growth at 0.96 aw and 0.95 aw, with increases of 50.5% and 18.4%, respectively. Growth inhibition for the ΔOTAbZIP mutant commenced at 0.91 aw and 0.88 aw, with inhibition rates of 30.2% and 55.1%, respectively. Importantly, the inhibition rates were lower than those observed in the wild type (). These findings indicate increased tolerance to water activity in the ΔOTAbZIP mutant strains.
Figure 3. Mycelium growth of wild type and ∆OTAbZIP mutant Aspergillus westerdijkiae fc-1 strains in different aw culture medium. WT: Wild-type A. westerdijkiae fc-1; ∆OTAbZIP: ∆OTAbZIP mutant A. westerdijkiae fc-1; aw: Water activity. Different lowercase letters represent significant differences, p < 0.05.
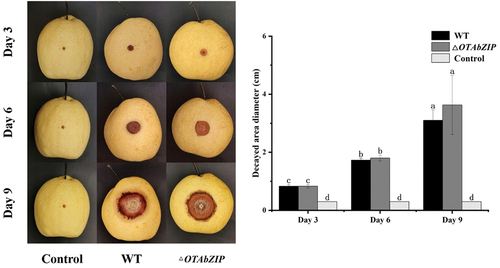
3.4. Pathogenicity test
The disease infection of pear fruit showed scabs caused by both wild-type and mutant A. westerdijkiae fc-1 at 3, 6, and 9 dpi (). The size of the decayed area was not significantly different in either wild type or mutant strains, indicating that the pathogenicity of the mutant strain with the OTAbZIP gene was not significantly different from that of the wild type. The deletion of OTAbZIP does not affect the disease infection.
3.5. OTA detection
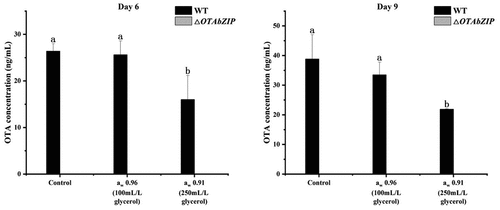
The wild-type A. westerdijkiae fc-1 produces OTA as it is growing. On the 9 days of growth under high water activity conditions (0.96 aw), the toxigenicity of the wild-type strain was inhibited by 13.7% compared to the control. Similarly, under conditions of moderate water activity (0.91 aw), the toxigenicity of the wild-type strain was inhibited, with a decrease of 43.6%. These results suggest that both high aw and moderate aw are not conducive to the toxigenic production of A. westerdijkiae fc-1. Additionally, the toxigenic production of A. westerdijkiae is more sensitive to the addition of glycerol and changes in aw. However, OTA was not detected in the ∆OTAbZIP mutant strain on the 9 days of growth (), indicating that the strain lost its toxigenic ability following the deletion of OTAbZIP.
Figure 5. OTA production of the wild type and ∆OTAbZIP mutant Aspergillus westerdijkiae fc-1 strains under different aw conditions. WT: Wild-type A. westerdijkiae fc-1; ∆OTAbZIP: ∆OTAbZIP mutant A. westerdijkiae fc-1; aw: Water activity. Different lowercase letters represent significant differences, p < 0.05.
3.6. Detection of gene expression by RT-qPCR
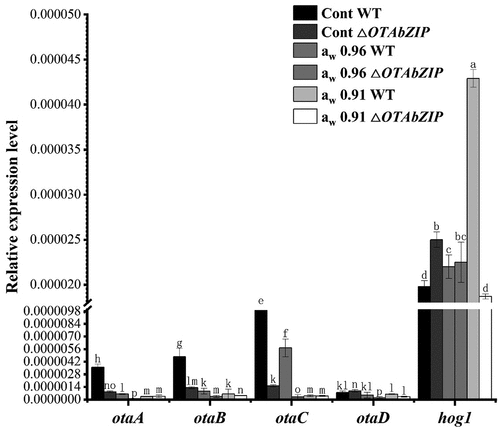
To investigate the regulatory function of the transcription factor OTAbZIP on the expression of OTA biosynthesis genes, the expression levels of OTA biosynthesis genes (otaA, otaB, otaC, and otaD) were analysed in both wild-type and mutant strains under different water activity conditions (0.96 aw and 0.91 aw). Expression levels were analysed () and under high water activity (0.96 aw) conditions, the wild type of OTA biosynthesis genes was downregulated compared with the control group (decrease in otaA, 82.9%; otaB, 80.9%; otaC, 41.8%; and otaD, 36.4%), and the OTA biosynthesis genes of the mutant strain were downregulated (decrease in otaA, 88.8%; otaB, 73.8%; otaC, 80.1%; and otaD, 77.9%). Under moderate water activity, (0.91 aw), the wild type of OTA biosynthesis genes was basically downregulated (decrease in otaA, 91.4%; otaB, 87.2%; otaC, 95.9%; and otaD, 22.0%), and the OTA biosynthesis genes of the mutant strain were downregulated (decrease in otaA, 57.6%; otaB, 66.2%; otaC, 74.8%; and otaD, 68.4%). The results show that the expression of OTA biosynthesis genes in wild-type and mutant A. westerdijkiae can be inhibited under both high-water activity and moderate water activity conditions. The lower the water activity was the greater the decrease in the expression of OTA biosynthesis genes in the wild type of strain (the opposite occurred for the mutant strain). For the core gene hog1 in the HOG-MAPK pathway, under high water activity (0.96 aw), the wild-type hog1 gene was upregulated by 10.5%, and the mutant hog1 gene was downregulated by 9.6% (no significant difference); under moderate water activity (0.91 aw), the wild-type hog1 gene was upregulated by 121.1%, and the mutant hog1 gene was downregulated by 25.3%. The findings indicate that the expression of the hog1 gene in wild-type A. westerdijkiae was upregulated under both high and moderate water activity conditions, with a more pronounced upregulation as water activity decreased. On the other hand, in the mutant A. westerdijkiae, hog1 gene expression was downregulated under both high and moderate water activity scenarios, with a more significant downregulation becoming more pronounced as water activity dropped.
Figure 6. OTA biosynthesis gene (otaA, otaB, otaC, and otaD) and hog1 gene expression of the wild type (WT) and ∆OTAbZIP mutant Aspergillus westerdijkiae fc-1 strains under different aw treatment. Cont: Control group, 0 mL/L glycerine; aw 0.96: 100 mL/L glycerine; aw 0.91: 250 mL/L glycerine. Different lowercase letters represent significant differences, p < 0.05.
4. Discussion
In majority of Aspergillus species A. fischeri NRRL 181, 66.67% homology (Mead et al. Citation2019); A. vadensis CBS 113,365, 80.90% homology (de Vries et al. Citation2005); A. carbonarius ITEM 5010, 71.15% homology (Gerin et al. Citation2021); A. saccharolyticus JOP 1030-1, 66.18% homology (Yang et al. Citation2016); and A. steynii IBT 23096, 71.07% homology (Gil-Serna et al. Citation2015) the bZIP gene is located in the OTA biosynthetic gene cluster, with a length of 767 bp, encoding a 263 aa protein. bZIP is a class of transcription factors ubiquitous in plants, animals and microorganisms and consists of two parts: the basic region (BR) and the leucine zipper (LZ) region. Based on homologous sequence comparison analysis (), the conserved structural domains of bZIP in A. westerdijkiae were identified and the BR and LZ domains could be distinguished, suggesting that OTAbZIP is a bZIP transcriptional regulator.
In the glycerol-conditioned system, higher water activity favoured A. westerdijkiae fc-1 growth, while as glycerol continued to increase, water activity decreased, leading to inhibition of A. westerdijkiae fc-1 growth.
The results of the growth experiment of A. westerdijkiae fc-1 showed that high water activity (0.96 aw) promoted the growth of A. westerdijkiae fc-1 strains, indicating that glycerol was necessary for the growth of A. westerdijkiae fc-1, and this glycerol concentration was beneficial to the growth of A. westerdijkiae fc-1; moderate water activity conditions of 0.91 aw inhibited the growth of A. westerdijkiae fc-1 strains, indicating that although glycerol is necessary for the growth of A. westerdijkiae fc-1, when the concentration is too high, it has a stress effect on A. westerdijkiae fc-1, which is not conducive to the growth of A. westerdijkiae fc-1. Compared with the wild type, the mutant strain grew better under the condition of high-water activity (0.96 aw) and moderate water activity (0.91 aw), the growth of the mutant strain was still better than that of the wild type, indicating that the mutant strain was more tolerant to water activity than the wild type, and the bZIP gene increased the strain’s sensitivity to water activity. Among other toxin-producing fungi, there are cases where their growth is regulated by water activity, the optimal growing conditions of A. flavus are 0.99 aw and 30 °C, and its growth rate is regulated by the water activity of the growth medium and decreases with decreasing water activity (Romero Donato et al. Citation2022). At 0.99 aw, A. westerdijkiae fc-1 grows at the highest rate, moreover, the bZIP gene expression in its OTA synthesis gene cluster is the highest at this water activity (Cervini et al. Citation2020). The above results indicate that water activity has a significant regulatory effect on the growth of toxin-producing fungi, with high water activity (0.96 aw) usually promoting growth and medium water activity (0.91 aw) beginning to inhibit growth.
Generally, bZIP can regulate the pathogenic fungi’s virulence by affecting its mycotoxins production and growth (Leiter et al. Citation2021). The bZIP-type transcription factor of F. verticillioides, FvAtfA, its knockout mutant strain is less virulent in tomatoes than in WT (Szabó et al. Citation2020). But the results of the pathogenicity test of A. westerdijkiae fc-1 showed that the wounds infected with Sydney pears treated with the spore suspensions of the wild type and mutant strains had scabs and spores, and the size of the rotten area was basically the same between the wild type and mutant strains, indicating that the OTAbZIP gene had no obvious influence on the pathogenicity of A. westerdijkiae fc-1.
The results from the toxicity experiment of A. westerdijkiae fc-1 revealed a decrease in toxin production for the wild-type strain under high water activity (0.96 aw) and a further decreased under moderate water activity (0.91 aw). This suggests that the toxin production of A. westerdijkiae fc-1 is influenced by the degree of water activity. Notably, the mutant strains did not produce any toxicity under any water activity conditions. Upon combining the results of the biosynthetic gene expression analysis, it was observed that the expression levels of the OTA biosynthetic genes (otaA, otaB, otaC, and otaD) were inhibited under conditions of high-water activity (0.96 aw) in the wild-type A. westerdijkiae fc-1. Under moderate water activity (0.91 aw) conditions, the expression levels of these biosynthetic genes in the mutant strain were inhibited to extremely low levels. Additionally, the expression levels of the OTA biosynthetic genes in the wild-type strain were also inhibited, with otaD showing an opposite trend.
This result is consistent with the results of the toxin production experiment. At 0.95 aw, F. graminearum produced the largest and fastest amount of deoxynivalenol (DON) (Ramírez Albuquerque et al. Citation2022). Alternaria arborescens produces the most toxins at 0.995 aw and decreases as aw decreases (Vaquera et al. Citation2014). Likewise, the deletion of the OTAbZIP gene greatly reduced the expression level of the OTA biosynthesis genes, resulting in the loss of the toxigenic ability of A. westerdijkiae fc-1, indicating that OTAbZIP controls the production of OTA by regulating the expression of OTA biosynthesis genes.
Hog1 is a component of the HOG-MAPK pathway that is closely related to the water activity stress signal (Fernandes et al. Citation2015). The experimental results showed that the reduction in water activity in the wild-type of strain increased the expression level of the hog1 gene, while the mutant strain showed the opposite effect. Decreased water activity decreased the expression level of the hog1 gene. This indicates that the OTAbZIP gene is indeed involved in the expression of OTA biosynthesis genes and that OTA biosynthesis is mediated by water activity. In wild-type strains, with the increase in glycerol concentration, water activity decreased, resulting in drought stress and activation of the HOG-MAPK pathway, promoting the expression of the hog1 gene in the wild type, which greatly alleviated the damage of the stress to the strain. After knocking out OTAbZIP, the water activity that stimulated the HOG-MAPK pathway was cut off, the HOG-MAPK pathway was not activated, and the water activity was reduced (Zhang et al. Citation2015; Mo et al. Citation2021). The reduction damaged the strain, and then the growth of the strain and the expression of hog1 were inhibited, so the deletion of the OTAbZIP gene greatly reduced the adaptability of the strain to water activity stress.
Supplemental Material
Download MS Word (11.5 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/21501203.2024.2355333
Additional information
Funding
References
- Bersching K, Jacob S. 2021. The molecular mechanism of fludioxonil action is different to osmotic stress sensing. J Fungi (Basel). 7(5):393. doi: 10.3390/jof7050393.
- Cervini C, Gallo A, Piemontese L, Magistà D, Logrieco AF, Ferrara M, Solfrizzo M, Perrone G. 2020. Effects of temperature and water activity change on ecophysiology of ochratoxigenic Aspergillus carbonarius in field-simulating conditions. Int J Food Microbiol. 315:108420. doi: 10.1016/j.ijfoodmicro.2019.108420.
- de Vries RP, Frisvad JC, van de Vondervoort PJ, Burgers K, Kuijpers AF, Samson RA, Visser J. 2005. Aspergillus vadensis, a new species of the group of black Aspergilli. Antonie Van Leeuwenhoek. 87:195–203.
- Fernandes ÉKK, Rangel DEN, Braga GUL, Roberts DW. 2015. Tolerance of entomopathogenic fungi to ultraviolet radiation: a review on screening of strains and their formulation. Curr Genet. 61(3):427–440. doi: 10.1007/s00294-015-0492-z.
- Fountain JC, Clevenger JP, Nadon B, Youngblood RC, Korani W, Chang PK, Starr D, Wang H, Isett B, Johnston HR, et al. 2020. Two new Aspergillus flavus reference genomes reveal a large insertion potentially contributing to isolate stress tolerance and aflatoxin production. G3: Genes | Genomes | Genetics (G3) (TSI). 10(10):3515–3531. doi: 10.1534/g3.120.401405.
- Gerin D, Garrapa F, Ballester AR, González-Candelas L, De Miccolis Angelini RM, Faretra F, Pollastro S. 2021. Functional role of Aspergillus carbonarius AcOTAbZIP gene, a bZIP transcription factor within the OTA gene cluster. Toxins. 13(2):111. doi: 10.3390/toxins13020111.
- Gil-Serna J, Vázquez C, González-Jaén MT, Patiño B. 2015. Clustered array of ochratoxin A biosynthetic genes in Aspergillus steynii and their expression patterns in permissive conditions. Int J Food Microbiol. 214:102–108. doi: 10.1016/j.ijfoodmicro.2015.07.020.
- Gonzalez AL, Lozano VA, Escandar GM, Bravo MA. 2020. Determination of ochratoxin A in coffee and tea samples by coupling second-order multivariate calibration and fluorescence spectroscopy. Talanta. 219:121288. doi: 10.1016/j.talanta.2020.121288.
- Hurst HC. 1995. Transcription factors 1: bZIP proteins. Protein Profile. 2(2):101–168.
- Kapetanakou AE, Panagou EZ, Gialitaki M, Drosinos EH, Skandamis PN. 2009. Evaluating the combined effect of water activity, pH and temperature on ochratoxin A production by Aspergillus ochraceus and Aspergillus carbonarius οn culture medium and Corinth raisins. Food Control. 20(8):725–732. doi: 10.1016/j.foodcont.2008.09.008.
- Kumar P, Mahato DK, Sharma B, Borah R, Haque S, Mahmud MMC, Shah AK, Rawal D, Bora H, Bui S. 2020. Ochratoxins in food and feed: Occurrence and its impact on human health and management strategies. Toxicon. 187:151–162. doi: 10.1016/j.toxicon.2020.08.031.
- Leiter É, Emri T, Pákozdi K, Hornok L, Pócsi I. 2021. The impact of bZIP Atf1ortholog global regulators in fungi. Appl Microbiol Biotechnol. 105(14–15):5769–5783. doi: 10.1007/s00253-021-11431-7.
- Mata AT, Ferreira J, Oliveira BR, Batoreu C, Crespo MT, Pereira V, Bronze M. 2015. Bottled water: analysis of mycotoxins by LC-MS/MS. Food Chem. 176:455–464. doi: 10.1016/j.foodchem.2014.12.088.
- Mead ME, Knowles SL, Raja HA, Beattie SR, Kowalski CH, Steenwyk JL, Silva LP, Chiaratto J, Ries LNA, Goldman GH, et al. 2019. Characterizing the pathogenic, genomic, and chemical traits of Aspergillus fischeri, a close relative of the major human fungal pathogen Aspergillus fumigatus. mSphere. 4(1):e00018–19. doi: 10.1128/mSphere.00018-19.
- Mo S, Qian Y, Zhang W, Qian L, Wang Y, Cailin G, Ding H. 2021. Mitogen-activated protein kinase action in plant response to high-temperature stress: a mini review. Protoplasma. 258(3):477–482. doi: 10.1007/s00709-020-01603-z.
- Mutlu-İNgök A, EliKoğlu C, TemiR HN, Karbancioğlu-Güler F. 2020. Growth and ochratoxin A production by Aspergillus carbonarius isolated from dried figs in aegean region of Turkey affected by temperature and water activity. Sakarya Univ J Sci. 24(1):140–150. doi: 10.16984/saufenbilder.494882.
- Nakagawa H, Oyama T. 2019. Molecular basis of water activity in glycerol-water mixtures. Front Chem. 7:731. doi: 10.3389/fchem.2019.00731.
- National Toxicology Program. 1989. Toxicology and carcinogenesis studies of ochratoxin A (CAS No. 303-47-9) in F344/N rats (gavage studies). Natl Toxicol Program Tech Rep Ser. 358:1–142.
- Nimmanee P, Woo PCY, Vanittanakom P, Youngchim S, Vanittanakom N, Yu JH. 2014. Functional analysis of atfA gene to stress response in pathogenic thermal dimorphic fungus Penicillium marneffei. PLoS One. 9(11):e111200. doi: 10.1371/journal.pone.0111200.
- Özden H, Özden S. 2018. Levels of heavy metals and ochratoxin A in medicinal plants commercialized in Turkey. Turk J Pharm Sci. 15(3):376–381. doi: 10.4274/tjps.74936.
- Priesterjahn EM, Geisen R, Schmidt-Heydt M. 2020. Influence of light and water activity on growth and mycotoxin formation of selected isolates of Aspergillus flavus and Aspergillus parasiticus. Microorganisms. 8(12):E2000. doi: 10.3390/microorganisms8122000.
- Ramírez Albuquerque D, Patriarca A, Fernández Pinto V. 2022. Water activity influence on the simultaneous production of DON, 3-ADON and 15-ADON by a strain of Fusarium graminearum ss of 15-ADON genotype. Int J Food Microbiol. 373:109721. doi: 10.1016/j.ijfoodmicro.2022.109721.
- Romero Donato CJ, Cendoya E, Demonte LD, Repetti MR, Chulze SN, Ramirez ML. 2022. Influence of abiotic factors (water activity and temperature) on growth and aflatoxin production by Aspergillus flavus in a chickpea-based medium. Int J Food Microbiol. 379:109841. doi: 10.1016/j.ijfoodmicro.2022.109841.
- Solfrizzo M, Piemontese L, Gambacorta L, Zivoli R, Longobardi F. 2015. Food coloring agents and plant food supplements derived from Vitis vinifera: a new source of human exposure to ochratoxin A. J Agric Food Chem. 63(13):3609–3614. doi: 10.1021/acs.jafc.5b00326.
- Szabó Z, Pákozdi K, Murvai K, Pusztahelyi T, Kecskeméti Á, Gáspár A, Logrieco AF, Emri T, Ádám AL, Leiter É, et al. 2020. FvatfA regulates growth, stress tolerance as well as mycotoxin and pigment productions in Fusarium verticillioides. Appl Microbiol Biotechnol. 104(18):7879–7899. doi: 10.1007/s00253-020-10717-6.
- Vaquera S, Patriarca A, Fernández Pinto V. 2014. Water activity and temperature effects on growth of Alternaria arborescens on tomato medium. Int J Food Microbiol. 185:136–139. doi: 10.1016/j.ijfoodmicro.2014.06.007.
- Wang X, Wu F, Liu L, Liu X, Che Y, Keller NP, Guo L, Yin WB. 2015. The bZIP transcription factor PfZipA regulates secondary metabolism and oxidative stress response in the plant endophytic fungus Pestalotiopsis fici. Fungal Genet Biol. 81:221–228. doi: 10.1016/j.fgb.2015.03.010.
- Wang X, Wu Q, Wan D, Liu Q, Chen D, Liu Z, Martínez-Larrañaga MR, Martínez MA, Anadón A, Yuan Z. 2016. Fumonisins: oxidative stress-mediated toxicity and metabolism in vivo and in vitro. Arch Toxicol. 90(1):81–101. doi: 10.1007/s00204-015-1604-8.
- Wang Y, Wang L, Wu F, Liu F, Wang Q, Zhang X, Selvaraj JN, Zhao Y, Xing F, Yin WB, et al. 2018. A consensus ochratoxin a biosynthetic pathway: insights from the genome sequence of Aspergillus ochraceus and a comparative genomic analysis. Appl Environ Microbiol. 84(19):e01009-18. doi: 10.1128/AEM.01009-18.
- Yang L, Lübeck M, Ahring BK, Lübeck PS. 2016. Enhanced succinic acid production in Aspergillus saccharolyticus by heterologous expression of fumarate reductase from Trypanosoma brucei. Appl Microbiol Biotechnol. 100(4):1799–1809. doi: 10.1007/s00253-015-7086-z.
- Zhang F, Guo Z, Zhong H, Wang S, Yang W, Liu Y, Wang SH. 2014. RNA-Seq-based transcriptome analysis of aflatoxigenic Aspergillus flavus in response to water activity. Toxins. 6(11):3187–3207. doi: 10.3390/toxins6113187.
- Zhang F, Zhong H, Han X, Guo Z, Yang W, Liu Y, Yang K, Zhuang Z, Wang S. 2015. Proteomic profile of Aspergillus flavus in response to water activity. Fungal Biol. 119(2):114–124. doi: 10.1016/j.funbio.2014.11.005.
- Zhang H, Yan A, Liu X, Ma Y, Zhao F, Wang M, Loor JJ, Wang H. 2021. Melatonin ameliorates ochratoxin A induced liver inflammation, oxidative stress and mitophagy in mice involving in intestinal microbiota and restoring the intestinal barrier function. J Hazard Mater. 407:124489. doi: 10.1016/j.jhazmat.2020.124489.
- Zhihong L, Kunlun H, Yunbo L. 2015. Ochratoxin A and ochratoxin-producing fungi on cereal grain in China: a review. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 32(4):461–470. doi: 10.1080/19440049.2014.996787.
- Zhu Y, Wang S, Xu X, Wang L, Zhou H, Ying X, Hu Q, Wang X, Ji S, Cai Q. 2022. Exposure assessment and risk-based limit levels evaluation of ochratoxin a in Astragali Radix in China. Ecotox Environ Safe. 237:113517. doi: 10.1016/j.ecoenv.2022.113517.