Abstract
The metropolitan region of Rio de Janeiro is hyperendemic for cat-associated sporotrichosis. This study aimed to assess the virulence of serial Sporothrix isolates from a 61-year-old male patient with chronic, destructive disseminated sporotrichosis. Five Sporothrix isolates were cultured from skin exudates and bone samples over a 5-year period, and all were molecularly identified as Sporothrix brasiliensis. The final isolate was significantly more virulent in Galleria mellonella larvae compared to earlier isolates. We conclude that S. brasiliensis has the capacity to increase in virulence in vivo. This finding is significant to clinicians caring for individuals with S. brasiliensis disease and it suggests that further studies are needed to identify the mechanisms underlying pathogenicity enhancement during chronic disease.
Introduction
Sporotrichosis is a subcutaneous mycosis caused by a complex of Sporothrix species.Citation1 Molecular studies have identified Sporothrix brasiliensis, Sporothrix globosa, Sporothrix luriei, Sporothrix mexicana, Sporothrix pallida and Sporothrix schenckii, the most common species globally, as responsible to sporotrichosis in different regions.Citation1-9
The metropolitan region of Rio de Janeiro is hyperendemic for cat-associated sporotrichosis,Citation10,11 primarily due to S. brasiliensis.Citation1,5 S. brasiliensis patients have presented with acute, chronic, fixed, lymphangitic, mucosal, disseminated and relapsing sporotrichosis, as well as manifestations of hypersensitivity.Citation10,12 Disseminated cases in non-immunosuppressed patients have been more common than previously reported for other regions and other species, which likely is associated with the high inoculums into tissue due to the multiple and deep scratches from the infected catsCitation13 as well as a greater virulence of S. brasiliensis relative to the other Sporothrix species.Citation14
In addition to inoculum size, candidate factors that facilitate S. brasiliensis’ capacity to cause frequent and severe disease are thermotolerance, production of enzymes and other molecules, and resistance to oxidative stress and to antifungals. At present, there is only limited information about the virulence of S. brasiliensis.Citation14-16 The aim of this study is to describe the evolution of increased virulence of sequential Sporothrix isolates from a patient with chronic, destructive disseminated sporotrichosis.
Case Report
A 61-year-old retired bricklayer with diabetes and hypertension was referred to the Laboratory of Infectious Dermatology at the Instituto Nacional de Infectologia Evandro Chagas (INI), in Rio de Janeiro, Brazil due to a 6-year history of cutaneous lesions. After his initial diagnosis, he had received 5 months of potassium iodide, which resulted in the healing of his cutaneous lesions. However, he complained of progressive joint pains in the knees and left wrist that worsened to the point that he required a wheelchair 2 years prior to presenting to INI. Six months prior to our evaluation at INI, he noted recurrence of skin lesions.
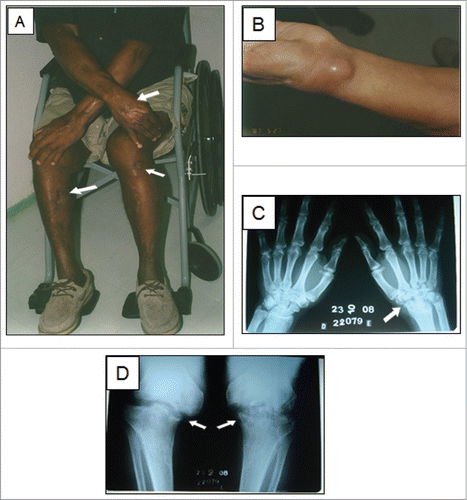
On our initial exam, the patient had multiple scars on the limbs, an exudative small ulcerated lesion and a cystic lesion on the dorsum of the left wrist (). There was ankylosis of both knees and the left wrist. Radiographs showed lytic lesions and sclerosis at these joints (). Tests for human immunodeficiency virus and human T-lymphotropic virus were negative. The total lymphocyte count was 1,470 /μL and the T CD4+ count was 554 /μL, both normal. The number of naïve T cells was considerably low (24 /μL). Sporothrix spp. was cultured from a skin lesion on the left wrist and antibody testing for sporotrichosis by an enzyme-linked immunosorbent assay methodCitation17 was positive (OD: 2.548 / Cut-Off: 0.605). Due to potential harmful interactions of his regular medications (captopril, hydrochlorothiazide and glibenclamide) with itraconazole, he was treated with terbinafine 500 mg/day for 32 months, until his skin lesions healed and the radiographic patterns stabilized. However, due to the subsequent development of recurrent skin lesions 11 months later, terbinafine was restarted and he currently continues to receive this antifungal due to the recalcitrant, relapsing nature of his disease. He remains wheelchair-bound due to severe, permanent deformities of both knees and the left wrist (confirmed by magnetic resonance). Over the course of his disease, skin samples were collected from the left forearm and the left knee, as well as a biopsy sample from the left femur.
Figure 1. A sixty-one-year-old patient with disseminated sporotrichosis. (A) Multiple scars are visible on the limbs (arrows), where cutaneous lesions were previously present. (B) Inflammatory cyst on the left wrist. The first 3 isolates were collected from the wrist lesion, by puncture with fine needle at different time points over a 6-month period. C-D) Radiographs showed severe lytic lesions and sclerosis at the left wrist and knees (arrows).
Results
Molecular identification
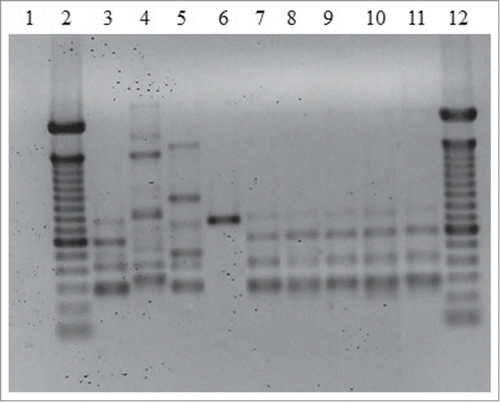
T3B PCR fingerprinting of the 5 isolates showed DNA fragments from 300 to 800 bp that allowed a clear identification by similarity with the profile obtained using the S. brasiliensis type strain (). Moreover, the profiles generated from all of the patient isolates presented 100% similarity.
Figure 2. Representative T3B PCR fingerprinting profiles of the 5 Sporothrix isolates. (1) Negative control. (2 and 12) Molecular marker DNA ladder, 100 bp (Invitrogen). (3) S. brasiliensis (CBS120339). (4) S. mexicana (MUM11.02). (5) S. schenckii (ATCC32286). (6) S. globosa (IPEC27135). (7) IPEC32742. (8) IPEC33070. (9) IPEC33718. (10) IPEC33946. (11) IPEC43174.
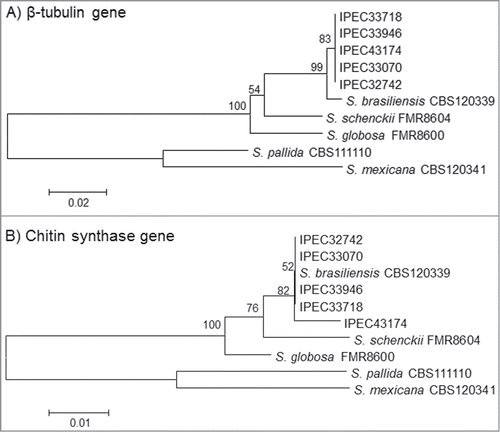
The Basic Local Alignment Search Tool (BLAST) analysis comparing the β-tub and CHS sequences from isolates in the NCBI GenBank database (AM116954, AM116950, AM116956, AM116946, AM114898, AM117414, AM114889, and AM117417) with the patient isolates identified these isolates as S. brasiliensis with 99-100% similarity. Moreover, comparison and phylogenetic analysis of the partial β-tub and CHS sequences obtained from all the isolates with the reference strains of the Sporothrix complex available in GenBank database presented similarity with S. brasiliensis and showed high bootstrap support values for both genes (). The partial CHS sequence of isolate 5 had a 2-bp polymorphism (positions 109 A/C and 151 G/C) compared to the first 4 isolates, suggesting a variability of this isolate in the phylogenetic analysis. The sequences obtained for β-tub and CHS-encoding genes were deposited in the GenBank database under the accession numbers from KM222420 to KM222429.
Figure 3. Neighbor-joining phylogram of the partial β-tubulin (A) and Chitin synthase (B) genes obtained for the 5 isolates (IPEC32742, IPEC33070, IPEC33718, IPEC33946, IPEC43174) and S. brasiliensis (CBS120339), S. globosa (FMR8600), S. mexicana (CBS120341), S. pallida (CBS111110) and S. schenckii (FMR8604), reference strains in NCBI public GenBank sequences constructed with MEGA version 4.0.2. Bootstrap values after 1,000 replicates are presented in the branch node.
In vitro assays
To characterize their virulence, in vitro assays using J774 macrophages were performed, including phagocytosis, fungal killing and macrophage viability assays. Interestingly, no differences among the isolates were noted (p > 0.05), and similar results were obtained using the S. brasiliensis type strain. The phagocytosis index after 2 h of yeasts-macrophages interaction ranged from 89% for isolate 5 to 95% for isolate 4, and the macrophages were unable to inhibit the S. brasiliensis growth, with a fungal survival rate varying from 96 to 123% after 18 h. Macrophages viability was ∼90% (range from 89% for isolate 5 to 92% for isolate 3) after this time period, for all conditions.
Galleria mellonella infection model
In vivo assays with Galleria mellonella larvae were performed by challenging larva with 107 yeast cells. Enhanced pigmentation of G. mellonella occurred in larvae inoculated with yeasts compared to controls (). Survival curves were obtained for 3 separate experiments. All the isolates reduced the survival of the larvae, compared to the controls (P < 0.0001), but this reduction was consistently significantly higher for the larvae injected with the isolate 5 compared to the other groups. shows the survival curve for the combined data of the 3 experiments, in which the median survival for isolate 5 was 7 days compared to 9–11 days for the other isolates (P < 0.0001). The median survival for uninfected controls was 14 days. We also performed experiments with 104 and 106 yeasts, but at these inoculums, the survival of the larvae was similar to that of uninfected controls (data not shown).
Figure 4. Galleria mellonella infection model. (A) Photographs of 2 groups of larvae (PBS [left] and isolate 5 [right]) 2 days after injection; the five dark brown / black larvae were dead. (B) Survival curves of the larvae. Lines represent the percentage of live individuals at each day. Control: uninfected larvae; PBS: larvae inoculated with 10 μL sterile PBS; numeric codes: larvae inoculated with 107 yeasts of each isolate, or with the reference CBS strain of S. brasiliensis in 10 μL sterile PBS. The larvae were maintained at 37°C. N = 60 larvae per group.
![Figure 4. Galleria mellonella infection model. (A) Photographs of 2 groups of larvae (PBS [left] and isolate 5 [right]) 2 days after injection; the five dark brown / black larvae were dead. (B) Survival curves of the larvae. Lines represent the percentage of live individuals at each day. Control: uninfected larvae; PBS: larvae inoculated with 10 μL sterile PBS; numeric codes: larvae inoculated with 107 yeasts of each isolate, or with the reference CBS strain of S. brasiliensis in 10 μL sterile PBS. The larvae were maintained at 37°C. N = 60 larvae per group.](/cms/asset/5f5f90fc-dc3f-4825-a39a-04ed8c883140/kvir_a_1014274_f0004_oc.gif)
There were no significant differences in fungal burden both at days 3 and 6 after G. mellonella infection. The CFUs obtained at day 6 post-infection ranged from 1.4 × 107 for isolate 4 to 8.2 × 107 for isolate 1.
Putative virulence factors
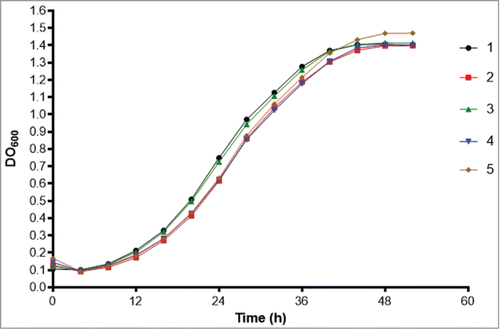
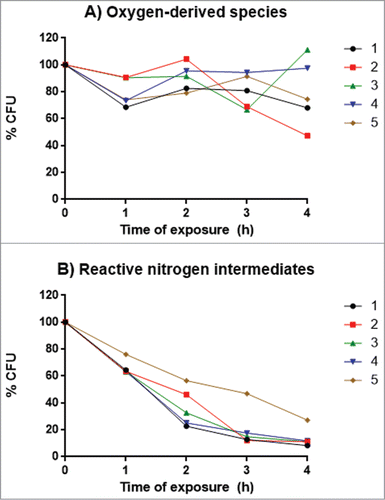
Since we identified a difference in virulence of isolate 5 in G. mellonella compared to the other isolates, we assessed whether factors associated with virulence could explain the cause of this change. Urease production using Christensen's urea broth at 37°C was positive for all 5 isolates, after 4 and 7 days, with no difference in the mean absorbance at 559 nm. The proteinase activity, determined by the measurement of the azoalbumin degradation halo on agar, ranged from 0.6 to 0.76, with a higher activity achieved by isolate 1 (p/z = 0.6). This activity differed significantly from isolates 2 (p = 0.016) and 4 (p = 0.0005). The proportion of DHN-melanin produced by the isolates, obtained by the ratio of dry DHN-melanin / dry mass of yeasts, showed no statistical differences. All the isolates grew at 35 and 37°C, and none grew at 39 °C. Moreover, growth curves of the isolates in BHI medium were similar, with no statistical difference among them (P > 0.05; ). For the exposure to oxygen-derived species, isolate 2 had the highest inhibition of growth at 4 h of exposure, and this was significantly different (P < 0.05) from isolates 3 and 4 (). The differences at other time intervals were not significant. Exposure to reactive nitrogen intermediates reduced the survival of the isolates, but the survival of isolate 5 was consistently higher than the other isolates over the examined times. Nevertheless, these differences were not significant ().
Figure 6. Percentage of viable yeasts after 4 h of exposure to different oxidative stress conditions. (A) Exposure to oxygen-derived species. (B) Exposure to reactive nitrogen intermediates. The 5 isolates (107 yeasts / mL) were in the specific media at 37°C and after each time point samples of 100 μL were plated onto PCA for CFU determinations. Points represent medians of 2 experiments performed in triplicate.
Susceptibility testing
The susceptibility to antifungals was similar for all the isolates, with MICs of 0.06 μg/mL for terbinafine, 1.0 μg/mL for itraconazole, 2.0 μg/mL for ketoconazole and amphotericin, and ≥ 8.0 μg/mL for voriconazole.
Discussion
Our study focused on the characterization of sequential isolates from this patient given the clinical aggressiveness and therapeutic difficulties he faced with sporotrichosis over an 11 year period, with dissemination to skin and bones. We must note that the lack of prior treatment is unfortunately all too common in certain populations in Brazil, due to poor access to care based on the distance from rural locations to medical clinics, the cost of even inexpensive medications such as potassium iodide, and reluctance to engage the healthcare system. Despite having diabetes and hypertension, the patient had previously been healthy, as demonstrated by his capacity to actively work under the physically stressful conditions as a bricklayer, and tests performed in a search for a possible underlying immunosuppressed condition did not show abnormalities. The reduction of naïve T cells may be related to the constant stimulation of the lymphocytes due to the chronic infection, inducing their differentiation.
Given the chronicity and relapsing nature of the disease in this patient, we considered the possibility that he may have been 1) re-infected with different species or strains or 2) that there was a change in the virulence of the infecting strain over time. However, we confirmed that each isolate was S. brasiliensis. Although not definitive, the finding that the isolates did not have differences in their patterns of bands in the fingerprinting PCR () suggests that they were the same; however, this does not rule out re-infection with the same strain over time instead of relapses of chronic disease.
The polymorphisms in the encoding gene CHS of isolate 5 suggest an alteration in the chitin synthase gene, which could increase virulence by affecting chitin synthesis and cell wall structure. Chitin is an essential component of the fungal cell wall and septa that is present in all known pathogenic fungi, but not in humans.Citation18,19 Virulence determinants include factors required for recognition and invasion of the host and for protection against host defense systems.Citation20 Since the cell wall is involved in these processes, it protects the fungal cell from external injury, regulates adherence of host cells to components of the cell-wall componentsCitation21-23 and, therefore, mediates immune responses.Citation24-27 Therefore, any changes in the cell-wall structure as well as in its composition may affect fungal virulence. To date, chitin has been correlated to virulence in other fungi, as Paracoccidioides brasiliensis,Citation28 Histoplasma capsulatumCitation29 and Blastomyces dermatitidis.Citation30 Chitin deposition may impact melanization,Citation31,32 which is another important factor associated with the cell wall and virulence in pathogenic fungi,Citation33-36 including Sporothrix.Citation15,37 Hence, alterations in chitin can lead to alterations in virulence.
Our in vitro macrophage assays did not show virulence difference among the patient's isolates: the macrophages effectively phagocytosed the yeasts, but were unable to kill them once ingested. However, our in vivo assays using G. mellonella larvae resulted in a consistent higher mortality among larvae inoculated with isolate 5, which was obtained after 5 years of follow-up at INI (after a total of 11 years of disease), which strongly suggests a change in the virulence of this isolate. It should be noted that the G. mellonella model has been successfully used in other fungi and bacteria,Citation38-40 and this study represents its first use in S. brasiliensis. The G. mellonella model has advantages over rodent systems, such as low cost, ease of storage and handling, and more favorable ethical aspects.
Assays to measure urease and DHN-melanin as well as our assessments of thermotolerance showed no difference among the isolates. Proteinase activity was not uniform, but the higher activity measured for isolate 1 was not consistent in comparison with all the other isolates and it does not seem to have implied in an increase in virulence of this isolate, as evidenced by the virulence assays. The antifungal susceptibilities were similar for all the isolates. Comparing the MICs found in the strains of our patient with the previously published susceptibility profile for Sporothrix spp. from Rio de JaneiroCitation41 and S. brasiliensis,Citation42 terbinafine produced the best in vitro efficacy against the isolates, while the azoles and amphotericin B had poor and intermediate MIC results, respectively. It is notable that despite the low MICs for terbinafine, this was the drug used for the treatment of this patient over several years with only moderate success.
Finally, tests to assess the susceptibility to oxidative stress revealed that the isolates were more susceptible to reactive nitrogen intermediates than to oxygen-derived species. Notably, isolate 5 was the most resistant to reactive nitrogen intermediates. The advantage of resisting adverse conditions similar to those found within lysosomes in macrophages could explain the higher virulence of this isolate; however, this cannot be fully concluded by our results. Nevertheless, this finding might in part contribute to the higher virulence of this isolate.
Since the patient was retired since the onset of the disease, away from the environment where he likely acquired the infection, our data suggests that it is reasonable to conclude that he has been chronically infected. Also, the identification of this patient's isolates as S. brasiliensis was somewhat surprising, as we did not elicit any epidemiological link with exposure to cats and the patient lived in a municipality outside of the “belt” of sporotrichosis in Rio de Janeiro.Citation11
Our data supports the conclusion that this patient had the same fungus over an 11-year period. Despite its in vitro susceptibility to terbinafine, the S. brasiliensis strain effectively remained in the patient by evading his immune system responses. Moreover, the G. mellonella studies demonstrate that virulence evolved over the course of disease in this patient. We suspect that there are additional aspects in the parasite-host relationship, not studied here, that contributed to this increased virulence, and further studies are needed to elucidate these factors. We consider this study, in vivo and in vitro, singular, since it demonstrates the evolution of virulence of S. brasiliensis in a host with chronic and disseminated sporotrichosis.
Materials and Methods
Ethics statement
The Research Ethics Committee of the INI/Fiocruz, RJ, Brazil, approved this study under the protocol number 0024.0.009.000-10.
Clinical specimens
Five biological specimens were collected from the lesions of the patient over a 5-year period. The first 3 samples (IPEC32742, IPEC33070 and IPEC33718, hereafter identified as “1,” “2” and “3,” respectively) were collected by sterile puncture with a fine needle from a cystic lesion on the left wrist between September 2007 and March 2008; the fourth sample (IPEC33946, subsequently referred to as “4”) was from a left femur biopsy in April 2008; and the fifth sample (IPEC43174, hereafter identified as “5”) was from an ulcerated nodule on the skin overlying the left knee in February 2012. The isolates were stored in cryotubes with 10% skim milk in a −20 °C freezer.
Sporotrichosis diagnosis
All clinical specimens underwent routine mycological examination, which involved direct microscopy of KOH 10% wet mounts and culture on Sabouraud dextrose agar 2% and Mycobiotic agar (Difco). Cultures were incubated at 25 °C and were observed over 4 weeks for fungal growth. Dimorphism was demonstrated by conversion to the yeast-like form on Brain heart infusion (BHI) agar (Difco) at 37 °C, for 7 days.
Molecular identification
Genomic DNA (DNA) was extracted from the yeast phase of the isolates by the chloroform/isoamyl alcohol methodCitation5 and used for sequencing of the partial β-tubulin (β-tub) and chitin synthase (CHS) encoding genesCitation1, as well as for T3B Polymerase Chain Reaction (PCR) fingerprintingCitation43 as described. In the T3B PCR fingerprinting, the control strains were S. brasiliensis (CBS120339), S. mexicana (MUM11.02), S. schenckii (ATCC32286) and S. globosa (IPEC27135). In the sequencing analysis, S. brasiliensis (CBS120339), S. globosa (FMR8600), S. mexicana (CBS120341), S. pallida (CBS111110) and S. schenckii (FMR8604) were used as reference strains from the National Center for Biotechnology Information (NCBI) public Genbank.
Macrophage assays
In vitro virulence assays with J774 macrophages were performed to determine the ability of the macrophages to phagocytose and kill S. brasiliensis yeasts, as well as the macrophages viability after this interaction.Citation44 Macrophages of the J774 lineage were grown in monolayer in culture plates, in a medium containing 10% fetal bovine serum, 10% NCTC-109, 1% non-essential amino acids and 1% penicillin/streptomycin diluted in Dulbecco's Modified Eagle's medium (DMEM), at 37ºC, 10% CO2, and split onto 96-well plates. Yeast cells from the 5 S. brasiliensis isolates, previously opsonized for 30 min by incubation with Guinea pig serum complement (MP Biomedicals), were added to monolayers of the macrophages, in a 5:1 ratio.
In vivo experiments
To assess virulence in vivo, larvae of the lepidoptera Galleria mellonella (Vanderhorst Wholesale, Inc..) were inoculated using 25 μL Hamilton® syringes with 104, 106 or 107 yeasts of one of the 5 S. brasiliensis isolates diluted in 10 μL PBS and the larval survival rate was analyzed.Citation40 As a control, a group of larvae was inoculated with the reference strain of S. brasiliensis (CBS120339). Negative control groups included uninoculated larvae (“Control”) and larvae injected with 10 μL sterile PBS (“PBS”). These experiments were performed in triplicate on different days. Each group was comprised by 20 larvae maintained in 90 mm Petri dishes at 37°C, and the Galleria were assessed daily. The groups were photographed (Canon EOS DIGITAL REBEL) at days 0 (day of inoculation) and 2 (48 h after inoculation). Dead larvae (recognized by absence of active or touch-induced movement and usually dark-colored) were removed from the group and discarded. Survival curves were obtained in order to compare the effect of the isolates on the larvae.
Parallel groups of 10 larvae each under the same conditions were followed for CFU determinations. Three larvae from each group were sacrificed after 3 or 6 days of inoculation. The internal content was macerated and homogenized in 2 mL PBS, and then filtered through 40 μm-cell strainers. An aliquot of 100 μL of this homogenate was plated onto BHI agar with 0.4 g/L cycloheximide and 1% penicillin / streptomycin to prevent contamination, and incubated at 37°C. After five days, the CFUs were determined.
In vitro production of putative virulence factors
a. Urease
To verify the urease production, 0.5 mL of a suspension equivalent to a 2.0 McFarland scale of yeast cells of each isolate was inoculated in 4.5 mL of Christensen urea broth,Citation45,46 and incubated at 37°C. After four and 7 days, the tubes were centrifuged at 1,575 g (Centrifuge 5804R, Eppendorf) and 100 μL of the supernatant were transferred in triplicate to a 96-well polystyrene flat bottom-plate (Corning, Tewksbury, USA). Cryptococcus neoformans (ATCC32045) and Candida parapsilosis (ATCC22019) were used as positive and negative controls, respectively. The samples O.D. were obtained using a Biotek spectrophotometer (Epoch model), at a 559 nm wavelength.
b. Proteinase
Proteinase activity was measured as described.Citation47 with the following mixture: 2% agar, 15 mM glucose, 13 mM glycine, 29.4 mM KH2PO4, 10 mM MgSO4, 3 mM thiamine, 0.1% azoalbumin (pH 4.5). For protein agar clearance, yeast cells of each isolate were plated through punctures on this mixture and incubated at 37°C for 21 days. After incubation, plates were inspected for the production of a degradation halo of azoalbumin around the colonies. When proteolytic activity was observed, the diameter of the colony (p) and the diameter of the halo of azoalbumin degradation (z) were measured with a millimeter ruler, and the p/z value was calculated as the ratio between the 2 diameters.
c. Melanin
Melanin ghosts were obtained according to the method described by Almeida-Paes et al.Citation48 The proportion of dihydroxynaphtalene (DHN)-melanin per isolate was then calculated by simple ratio DHN-melanin/yeasts (w/w).
Thermotolerance
Yeasts of each of the 5 isolates were grown at 37°C in BHI broth and, after 3 days, 2 mL of the solution of yeasts were centrifuged, suspended in PBS and 10-fold diluted to obtain 3 different dilutions. Drops of 2.5 μL were inoculated on BHI Agar plates and incubated at 35, 37 or 39 °C on a Type 37900 Culture Incubator. After three and 6 days, the plates were verified to determine the presence or absence of colony growth, in a qualitative manner.
Growth curves
S. brasiliensis yeast cells of the 5 isolates were grown in BHI broth (Difco) at 37°C for 3 days. With minor modifications from the described method by Medina et al.,Citation49 a Bioscreen C Microbiological Growth Curves Analysis System (Labsystems, Helsinki, Finland) was used to serially assess turbidity as a measurement of growth. Data were recorded using the software Easy Bioscreen Experiment (EZExperiment) provided by the manufacturer.
Susceptibility to oxidants
Fungal cells (1 × 107 yeasts) of each isolate were harvested after 5 days of growth on BHI agar slants at 37°C, washed 3 times with PBS, and submitted to chemically generated nitric oxide, reactive nitrogen intermediates and oxygen-derived oxidants, as previously described.Citation15 After 1, 2, 3, and 4 h of incubation at 37°C with the oxidants, the yeasts were plated onto plate count agar (Bio-Rad) to determine viability by CFUs counting. Aliquots of untreated cells were also plated as controls. The survival for each isolate at a time-point was the ratio of the CFU count at that time / the CFU count for the control, expressed in percentage.
Antifungal susceptibility tests
Conidia were used for susceptibility testing according to the Clinical and Laboratory Standards Institute (CLSI) protocol. The susceptibility of the 5 isolates to amphotericin B, ketoconazole, itraconazole, voriconazole and terbinafine was studied using the microdilution method (0.016-8 μg/mL) for filamentous fungi (including S. schenckii) M38-A2, described by the CLSI.Citation50 The cells were diluted in RPMI 1640 resulting in a final concentration of 2 × 104 to 1 × 105 cells/mL. For amphotericin B, itraconazole and voriconazole, the minimum inhibitory concentration (MIC) endpoint was the lowest concentration that produced complete inhibition of growth. For ketoconazole, the MIC was the lowest concentration producing a 50% reduction in growth, and for terbinafine it was the lowest concentration producing at least 80% of reduction in growth.
Statistical tests
Non-parametric tests (e.g., Kruskal-Wallis test to compare the growth curves and CFUs, Kaplan-Meier estimator for the survival of G. mellonella and Log-rank (Mantel-Cox) test for the comparison of the survival curves) were used in the analyses to compare the 5 isolates and a P-value < 0.05 was considered significant. GraphPad Prism 6 for Windows, GraphPad Software, Inc.., was used for the graphs construction and for the statistics. Microsoft Excel 2010/2013 (Microsoft Corporation, Redmond, Washington, USA) was used for percentage calculations and analyses.
Acknowledgments
Thanks to David Alejandro Sanchez for the help with the virulence experiments, to Dr. Otávio Espíndola for the flow cytometry analysis and to Dr. Nelson Lima for the S. mexicana strain.
funding
DFSF received financial support from CNPq, CAPES and Fogarty International Center (NIH D43-TW007129). SSS received financial support from FAPESP/São Paulo, Brazil (grant proc. 2010/50439-0). RMZ-O was supported in part by CNPq 350338/2000-0 and FAPERJ E-26/103.157/2011. JDN is supported in part by NIH AI52733. Financial support provided by FAPERJ/Rio de Janeiro, Brazil (grant proc. E-26/110.619/2012) and PAPES VI – CNPq/Fiocruz (grant proc. 407693/2012-2).
References
- Marimon R, Cano J, Gené J, Sutton DA, Kawasaki M, Guarro J. Sporothrix brasiliensis, S. globosa, and S. mexicana, three new Sporothrix species of clinical interest. J Clin Microbiol 2007; 45:3198-206; PMID:17687013; http://dx.doi.org/10.1128/JCM.00808-07
- Marimon R, Gené J, Cano J, Guarro J. Sporothrix luriei: a rare fungus from clinical origin. Med Mycol 2008a; 46:621-5; http://dx.doi.org/10.1080/13693780801992837
- Madrid H, Cano J, Gené J, Bonifaz A, Toriello C, Guarro J. Sporothrix globosa, a pathogenic fungus with widespread geographical distribution. Rev Iberoam Micol 2009; 26:218-22; PMID:19635441; http://dx.doi.org/10.1016/j.riam.2009.02.005
- Oliveira MME, Almeida-Paes R, Muniz MM, Barros MBL, Gutierrez-Galhardo MC, Zancopé-Oliveira RM. Sporotrichosis caused by Sporothrix globosa in Rio de Janeiro, Brazil: case report. Mycopathologia 2010; 169:359-63; PMID:20131099; http://dx.doi.org/10.1007/s11046-010-9276-7
- Oliveira MM, Almeida-Paes R, Muniz MM, Gutierrez-Galhardo MC, Zancope-Oliveira RM. Phenotypic and molecular identification of Sporothrix isolates from an epidemic area of sporotrichosis in Brazil. Mycopathologia 2011; 172:257-67; PMID:21701792; http://dx.doi.org/10.1007/s11046-011-9437-3
- Rodrigues AM, de Hoog S, de Camargo ZP. Emergence of pathogenicity in the Sporothrix schenckii complex. Med Mycol 2013a; 51:405-12; http://dx.doi.org/10.3109/13693786.2012.719648
- Rodrigues AM, Teixeira MdM, de Hoog GS, Schubach TMP, Pereira SA, Fernandes GF, Bezerra LM, Felipe MS, de Camargo ZP. Phylogenetic Analysis Reveals a High Prevalence of Sporothrix brasiliensis in Feline Sporotrichosis Outbreaks. PLoS Negl Trop Dis 2013b; 7(6):e2281; http://dx.doi.org/10.1371/journal.pntd.0002281
- Yu X, Wan Z, Zhang Z, Li F, Li R, Liu X. Phenotypic and molecular identification of Sporothrix isolates of clinical origin in Northeast China. Mycopathologia 2013; 176:67-74; PMID:23771481; http://dx.doi.org/10.1007/s11046-013-9668-6
- Morrison AS, Lockhart SR, Bromley JG, Kim JY, Burd EM. An environmental Sporothrix as a cause of corneal ulcer. Med Mycol Case Rep 2013; 2:88-90; PMID:24432225; http://dx.doi.org/10.1016/j.mmcr.2013.03.002
- Freitas DFS, Valle ACF, Almeida-Paes R, Bastos FI, Galhardo MCG. Zoonotic sporotrichosis in Rio de Janeiro, Brazil: a protracted epidemic yet to be curbed. Clin Infect Dis 2010; 50:453; PMID:20064034; http://dx.doi.org/10.1086/649891
- Silva MB, Costa MM, Torres CC, Galhardo MC, Valle AC, Magalhães Mde A, Sabroza PC, Oliveira RM. Urban sporotrichosis: a neglected epidemic in Rio de Janeiro, Brazil. Cad Saude Publica 2012; 28:1867-80; PMID:23090167
- Barros MB, de Almeida-Paes R, Schubach AO. Sporothrix schenckii and Sporotrichosis. Clin Microbiol Rev 2011; 24:633-54; PMID:21976602; http://dx.doi.org/10.1128/CMR.00007-11
- Freitas DF, de Siqueira Hoagland B, do Valle AC, Fraga BB, de Barros MB, de Oliveira Schubach A, de Almeida-Paes R, Cuzzi T, Rosalino CM, Zancopé-Oliveira RM, et al. Sporotrichosis in HIV-Infected patients: report of 21 cases of endemic sporotrichosis in Rio de Janeiro, Brazil. Med Mycol 2012; 50:170-8; PMID:21859385; http://dx.doi.org/10.3109/13693786.2011.596288
- Arrillaga-Moncrieff I, Capilla J, Mayayo E, Marimon R, Mariné M, Gené J, Cano J, Guarro J. Different virulence levels of the species of Sporothrix in a murine model. Clin Microbiol Infect 2009; 15:651-5; PMID:19624508; http://dx.doi.org/10.1111/j.1469-0691.2009.02824.x
- Almeida-Paes R, Frases S, Araújo Gde S, de Oliveira MM, Gerfen GJ, Nosanchuk JD, Zancopé-Oliveira RM. Biosynthesis and functions of a melanoid pigment produced by species of the Sporothrix complex in the presence of L-tyrosine. Appl Environ Microbiol 2012; 78:8623-30; PMID:23042177; http://dx.doi.org/10.1128/AEM.02414-12
- Fernandes GF, dos Santos PO, Rodrigues AM, Sasaki AA, Burger E, de Camargo ZP. Characterization of virulence profile, protein secretion and immunogenicity of different Sporothrix schenckii sensu stricto isolates compared with S. globosa and S. brasiliensis species. Virulence 2013; 4:241-9; PMID:23324498; http://dx.doi.org/10.4161/viru.23112
- Almeida-Paes R, Pimenta MA, Pizzini CV, Monteiro PC, Peralta JM, Nosanchuk JD, Zancopé-Oliveira RM. Use of mycelial-phase Sporothrix schenckii exoantigens in an enzyme-linked immunosorbent assay for diagnosis of sporotrichosis by antibody detection. Clin Vaccine Immunol 2007; 14:244-9; PMID:17215334; http://dx.doi.org/10.1128/CVI.00430-06
- Lenardon MD, Munro CA, Gow NA. Chitin synthesis and fungal pathogenesis. Curr Opin Microbiol 2010; 13:416-23; PMID:20561815; http://dx.doi.org/10.1016/j.mib.2010.05.002
- Munro CA, Gow NA. Chitin biosynthesis as a target for antifungals. In: Dixon GK, Copping LG, Hollomon DW, editors. Antifungal Agents: Discovery and Mode of Action. Oxford: Bios Scientific Publishers 1995; p. 161-71.
- Bulawa CE, Miller DW, Henry LK, Becker JM. Attenuated virulence of chitin-deficient mutants of Candida albicans. Proc Natl Acad Sci USA 1995; 92:10570-4; PMID:7479842
- Calderone RA, Braun PC. Adherence and Receptor Relationships of Candida albicans. Microbiol Rev 1991; 55:1-20; PMID:2030668
- Barki M, Koltin Y, van Wetter M, Rosenberg M. A Candida albicans surface antigen mediating adhesion and autoaggregation in Saccharomyces cerevisiae. Infect Immun 1994; 62:4107-11; PMID:7927663
- Klotz SA, Hein RC, Smith RL, Rouse JB. The fibronectin adhesin of Candida albicans. Infect Immun 1994; 62:4679-81; PMID:7927741
- Cassone A. Cell wall of Candida albicans: its functions and its impact on the host. Curr Top Med Mycol 1989; 3:248-314; PMID:2688918
- Domer JE. Candida cell wall mannan: a polysaccharide with diverse immunologic properties. Crit Rev Microbiol 1989; 17:33-51; PMID:2669830
- Nelson RD, Shibata N, Podzorski RP, Herron MJ. Candida mannan: chemistry, suppression of cell-mediated immunity, and possible mechanisms of action. Clin Microbiol Rev 1991; 4:1-19; PMID:2004345
- Abel G, Czop JK. Stimulation of human monocyte beta-glucan receptors by glucan particles induces production of TNF-alpha and IL-1 beta. Int J Immunopharmacol 1992; 14:1363-73; PMID:1334474
- San-Blas G, San-Blas F. Paracoccidioides brasiliensis: cell wall structure and virulence. A review. Mycopathologia 1977; 62:77-86; PMID:340954
- Klimpel KR, Goldman WE. Cell walls from avirulent variants of Histoplasma capsulatum lack alpha-(1,3)-glucan. Infect Immun 1988; 56:2997-3000; PMID:3169995
- Hogan LH, Klein BS. Altered Expression of Surface α-1,3-Glucan in Genetically Related Strains of Blastomyces dermatitidis That Differ in Virulence. Infect Immun 1994; 62:3543-6; PMID:8039925
- Walton FJ, Idnurm A, Heitman J. Novel gene functions required for melanization of the human pathogen Cryptococcus neoformans. Mol Microbiol 2005; 57:1381-96; PMID:16102007
- Gerik KJ, Bhimireddy SR, Ryerse JS, Specht CA, Lodge JK. PKC1 is essential for protection against both oxidative and nitrosative stresses, cell integrity, and normal manifestation of virulence factors in the pathogenic fungus Cryptococcus neoformans. Eukaryot Cell 2008; 7:1685-98; PMID:18689526; http://dx.doi.org/10.1128/EC.00146-08
- Gómez BL, Nosanchuk JD. Melanin and fungi. Curr Opin Infect Dis 2003; 16:91-6.
- Morris-Jones R, Youngchim S, Gomez BL, Aisen P, Hay RJ, Nosanchuk JD, Casadevall A, Hamilton AJ. Synthesis of melanin-like pigments by Sporothrix schenckii in vitro and during mammalian infection. Infect Immun 2003; 71:4026-33; PMID:12819091
- Nosanchuk JD, Casadevall A. Impact of Melanin on Microbial Virulence and Clinical Resistance to Antimicrobial Compounds. Antimicrob Agents Chemother 2006; 50:3519-28; PMID:17065617; http://dx.doi.org/10.1128/AAC.00545-06
- Taborda CP, da Silva MB, Nosanchuk JD, Travassos LR. Melanin as a virulence factor of Paracoccidioides brasiliensis and other dimorphic pathogenic fungi: a minireview. Mycopathologia 2008; 165:331-9; PMID:18777637
- Romero-Martinez R, Wheeler M, Guerrero-Plata A, Rico G, Torres-Guerrero H. Biosynthesis and functions of melanin in Sporothrix schenckii. Infect Immun 2000; 68:3696-703; PMID:10816530
- Mylonakis E. Galleria mellonella and the Study of Fungal Pathogenesis: Making the Case for Another Genetically Tractable Model Host. Mycopathologia 2008; 165:1-3; PMID:18060516
- García-Rodas R, Casadevall A, Rodríguez-Tudela JL, Cuenca-Estrella M, Zaragoza O. Cryptococcus neoformans capsular enlargement and cellular gigantism during Galleria mellonella infection. PLoS ONE 2011; 6:e24485; http://dx.doi.org/10.1371/journal.pone.0024485
- Thomaz L, García-Rodas R, Guimarães AJ, Taborda CP, Zaragoza O, Nosanchuk JD. Galleria mellonella as a model host to study Paracoccidioides lutzii and Histoplasma capsulatum. Virulence 2013; 4:139-46; PMID:23302787; http://dx.doi.org/10.4161/viru.23047
- Gutierrez-Galhardo MC, Oliveira RMZ, Valle ACF, Paes RA, Silvatavares PM, Monzon A, Mellado E, Rodriguez-Tudela JL, Cuenca-Estrella M. Molecular epidemiology and antifungal susceptibility patterns of Sporothrix schenckii isolates from a cat-transmitted epidemic of sporotrichosis in Rio de Janeiro, Brazil. Medical Mycology 2008; 46:141-51; PMID:18324493; http://dx.doi.org/10.1080/13693780701742399
- Marimon R, Serena C, Gené J, Cano J, Guarro J. In vitro antifungal susceptibilities of five species of Sporothrix. Antimicrob Agents Chemother 2008b; 52:732-4; http://dx.doi.org/10.1128/AAC.01012-07
- Oliveira MME, Sampaio P, Almeida-Paes R, Pais C, Gutierrez-Galhardo MC, Zancopé-Oliveira RM. Rapid Identification of Sporothrix Species by T3B Fingerprinting. J Clin Microbiol 2012; 50:2159-62; PMID:22403427; http://dx.doi.org/10.1128/JCM.00450-12
- Nicola AM, Casadevall A. In Vitro Measurement of Phagocytosis and Killing of Cryptococcus neoformans by Macrophages. Methods Mol Biol 2012; 844:189-97; PMID:22262444; http://dx.doi.org/10.1007/978-1-61779-527-5_14
- Christensen WB. Urea decomposition as a means of differentiating Proteus and paracolon cultures from each other and from Salmonella and Shigella. J Bacteriol 1946; 52:461-6; PMID:16561200
- Kane J, Fischer JB. The differentiation of T. rubrum and T. mentagrophytes by use of Christensen's urea broth. Can J Microbiol 1971; 17:911-3; PMID:5094601
- Chen LC, Blank ES, Casadevall A. Extracellular proteinase activity of Cryptococcus neoformans. Clin Diagn Lab Immunol 1996; 3:570-4; PMID:8877137
- Almeida-Paes R, Frases S, Monteiro PCF, Gutierrez-Galhardo MC, Zancopé-Oliveira RM, Nosanchuk JD. Growth conditions influence melanization of Brazilian clinical Sporothrix schenckii isolates. Microbes Infect 2009; 11:554-62; PMID:19328867; http://dx.doi.org/10.1016/j.micinf.2009.03.002
- Medina A, Lambert RJ, Magan N. Rapid throughput analysis of filamentous fungal growth using turbidimetric measurements with the Bioscreen C: a tool for screening antifungal compounds. Fungal Biol 2012; 116:161-9; PMID:22208611; http://dx.doi.org/10.1016/j.funbio.2011.11.001
- CLSI. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi; approved standard – second edition. CLSI document M38-A2. Wayne, PA: Clinical and Laboratory Standards Institute 2008.