Abstract
Galleria mellonella larvae are widely used for assessing the virulence of microbial pathogens and for measuring the in vivo activity of antimicrobial agents and produce results comparable to those that can be obtained using mammals. The aim of the work described here was to ascertain the effect of pre-incubation at 15°C for 1, 3, 6 or 10 weeks on the susceptibility of larvae to infection with Candida albicans and Staphylococcus aureus. Larvae infected with C. albicans after 1 week pre-incubation at 15°C showed 73.3 ± 3.3% survival at 24 hours post-infection while those infected after 10 weeks pre-incubation showed 30 ± 3.3% survival (P < 0.01). Larvae infected with S. aureus after 1 week pre-incubation showed 65.5 ± 3.3% survival after 24 hours while those infected after 10 weeks pre-incubation showed 13.3 ± 3.3% (P < 0.001). Analysis of the haemocyte density in larvae pre-incubated for 3–10 weeks showed a reduction in haemocytes over time but a proportionate increase in the density of granular haemocytes in the population as determined by FACS analysis. Proteomic analysis revealed decreased abundance of proteins associated with metabolic pathways (e.g. malate dehydrogenase, fructose-1,6-bisphosphatase, glyceraldehyde-3-phosphate dehydrogenase) and prophenoloxidase. G. mellonella larvae are a useful in vivo model system but the duration of the pre-incubation stage significantly affects their susceptibility to microbial pathogens possibly as a result of altered metabolism.
Introduction
Due to the structural and functional similarities between the immune system of insects and the innate immune system of mammalsCitation1-3 insects have become popular choices for evaluating the virulence of microbial pathogens and for measuring the potency and/or toxicity of novel antimicrobial agents and produce results comparable to those that may be obtained using mammals.Citation4-6 The use of insects in these applications has many advantages including the ease of inoculation, lack of legal or ethical constraints and the fact that results may often be obtained within a relatively short space of time (e.g., 24 – 48 hours). A variety of insects are now used as mini-models and these include Galleria mellonella (Greater Wax moth), Drosophila melanogatser (Fruit fly), Manduca sexta (Tobacco horn worm) and Bombyx mori (Silk worm).Citation1,2,7
Larvae of G. mellonella have been employed to assess the virulence of fungalCitation8,9 and bacterialCitation10,11 pathogens and a strong correlation between the virulence of a number of pathogens in larvae and mice has been demonstrated.Citation4,5 G. mellonella larvae have also been employed to assess the potency and efficacy of novel antimicrobial agents.Citation12,13
While insects are now popular and effective models, no standardized procedures for their use have been developed.Citation14 Larvae of G. mellonella are commonly stored at 15°C or room temperature and may be maintained under these conditions for 1 to 3 weeks in advance of inoculation.Citation14 We have previously established that variations in incubation temperatureCitation15 physical stressCitation16 and the access to nutrientsCitation17 significantly altered the response of G. mellonella larvae to infection. It was also established that prior exposure of larvae to yeast cellsCitation18 or β-glucanCitation19 stimulated their immune response and lead to reduced sensitivity to infection. This priming effect was mediated by an elevated density of circulating haemocytes (immune cells) and increased abundance of immune related and antimicrobial proteins in the haemolymph.
The aim of the work presented here was to establish whether incubating G. mellonella larvae for extended periods at 15°C in advance of infection affected their susceptibility to infection and if so to determine how this was mediated. Identification of the role of pre-incubation in altering the larval immune response would be useful for those using larvae as an in vivo model system.
Results
Effect of pre-incubation at 15°C on susceptibility of G. mellonella larvae to infection
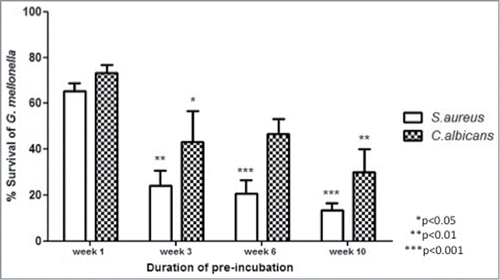
Larvae were stored in the dark at 15°C for 1, 3, 6 or 10 weeks prior to being inoculated through the last left pro-leg with C. albicans or S. aureus as described.Citation8 Subsequent to infection larvae were placed at 30°C and survival was monitored over 24h. The results demonstrated that those larvae that were incubated at 15°C for 3, 6 or 10 weeks were the most sensitive to infection with C. albicans with survival at 24h post-infection being 43.3 ± 13.3% (P < 0.01), 46.7 ± 6.6% and 30.0 ± 10.0% (P < 0.05) respectively, while larvae infected after 1 week pre-incubation showed 73.3 ± 3.3% survival at the same time point (). Similarly, larvae pre-incubated for 3, 6 or 10 weeks and infected with S. aureus showed significantly reduced survivals 24h after inoculation, i.e. 24.1 ± 6.6% (P < 0.01), 20.7 ± 5.7% (P < 0.001) or 13.3 ± 3.3% (P < 0.001) respectively, compared to larvae incubated for 1 week at 15°C in advance of infection which showed 65.5 ± 3.3% survival at the same time point post-infection ().
Figure 1. Susceptibility of G. mellonella larvae to infections with S. aureus or C. albicans. Larvae were incubated at 15°C for 1, 3, 6 or 10 weeks in advance of infection with S. aureus or C. albicans. Larvae were subsequently incubated for 24 h at 30°C at which time the survival was assessed. All values are the mean ± SE of 3 independent determinations. (p refers to susceptibility compared to week 1 response).
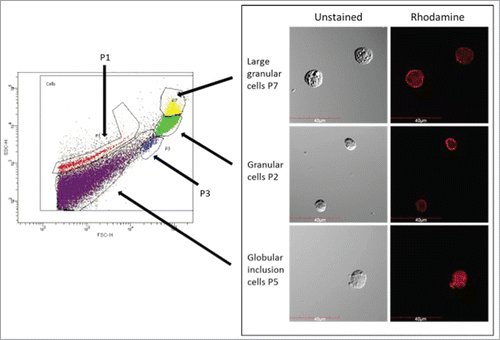
Pre-incubation leads to alteration in the haemocyte population of G. mellonella larvae
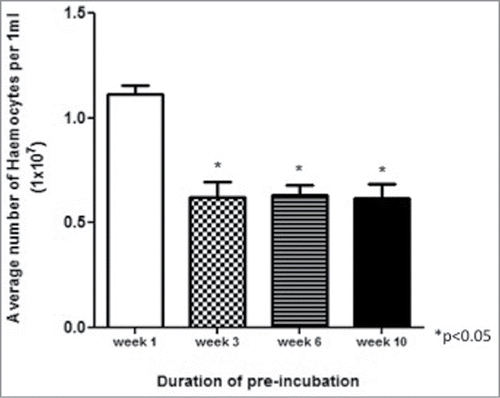
Haemocytes were extracted from larvae incubated at 15°C for up to 10 weeks and enumerated. The results demonstrated a decline (P < 0.05) in the haemocyte density of larvae incubated for 3, 6 or 10 weeks compared to the density in larvae incubated at 15°C for 1 week ().
Figure 2. Haemocyte density in larvae incubated at 15°C for up to 10 weeks. Haemocytes were extracted from larvae incubated at 15°C for 1, 3, 6 and 10 weeks and enumerated.
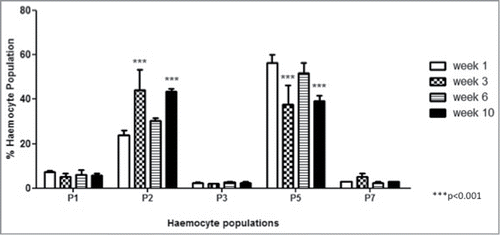
FACS analysis was employed to establish if there was a change in the relative proportion of each haemocyte sub-population in larvae pre-incubated for up to 10 weeks. Haemocyte populations were differentiated on the basis of size and granularity and at least 5 distinct sub-populations, labeled P1, P2, P3, P5 and P7, were visible (). The results () demonstrated an increase in the relative abundance of P2 haemocytes (granular cells) in the total haemocyte population over time i.e., week 1; 23.8 ± 2.1%, week 3; 44.0 ± 9.4 % (P < 0.001), week 6; 30.3 ± 1.3 % and week 10; 43.55 ± 1.25 % (P < 0.001) while the proportion of P5 haemocytes (cells with globular inclusions) in the population decreased over time, i.e. week 1; 56.4 ± 3.8 %, week 3; 37.65 ± 8.75 (P < 0.001), week 6; 51.7 ± 4.8 % and week 10; 43.55 ± 1.25 % (P < 0.001) (). The relative abundance of the other haemocyte populations (P1, P3 and P7) remained relatively constant in larvae incubated for 1, 3, 6 and 10 weeks ().
Analysis of changes in proteome of larvae incubated for up to 10 weeks at 15°C
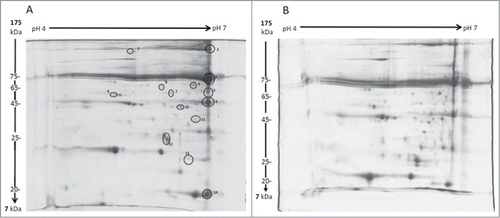
The proteome of larvae incubated at 15°C for 1 or 10 weeks was resolved by 2D SDS-PAGE. In total, 14 peptide spots were shown to be altered in abundance in larvae (). Proteins showing alteration in abundance were excised, digested and identified by LC/MS as described (). The results demonstrated that proteins associated with the prophenoloxidase (PPO) pathway (e.g. masquerade-like serine proteinase, protease serine 1 precursor and PPO subunit-2) showed a decrease in abundance at week 10 (). Proteins with functions in metabolic pathways (e.g., malate dehydrogenase, fructose-1,6-bisphosphatase and aliphatic nitrilase) also showed a decrease in abundance in those larvae incubated at 15°C for 10 weeks. In contrast, the relative abundance of selected immune proteins (e.g. apolipophorin 3 and β-1, 3-glucan recognition protein precursor) increased in abundance over the course of the incubation period. It was also observed that the abundance of transferrin precursor remained relatively constant while ferritin 1 heavy chain and ferritin 2 light chain demonstrated an increase in abundance in larvae incubated at 15°C for 10 weeks. In contrast, the abundance of arylphorin declined during the incubation period.
Table 1. Protein identities from excised and trypsin digested spots 1–14 () identified by LC/MS. The relative fold changes in proteins abundance was determined from 1 and 10 week old larvae using Progenesis SameSpot Software.
Figure 5. 2D SDS PAGE gels of G. mellonella haemolymph from larvae pre-incubated for 1 or 10 weeks. Haemolymph was extracted from larvae incubated for 1 (A) or 10 (B) weeks and proteins were resolved on a 12.5% acrylamide gels as described. Proteins showing alterations in abundance over the course of the incubation period were identified (1 – 14), excised, digested and analyzed by LC/MS.
Label free quantitative analysis of week 1 and 10 haemolymph
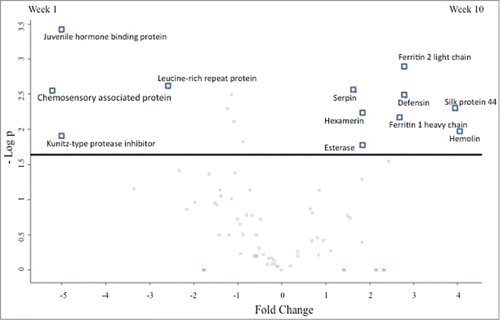
In order to analyze changes in the total proteome of larvae, label free shotgun quantitative proteomics was performed. In total 1060 peptides were identified representing 140 proteins with 2 or more unique peptides from Galleria haemolymph. Fourteen statistically significant differentially abundant proteins were identified (ANOVA P < 0.05) of which 12 had a fold change >1.5 between week 1 and 10 haemolymph samples (). Four proteins had higher abundance in week 1 larvae and include a leucine-rich repeat-containing protein (2.6 fold change), kunitz-type protease inhibitor (5 fold change), juvenile hormone binding protein (5 fold change) and a chemosensory associated protein (5.3 fold change). Eight proteins were more abundant in week 10 haemolymph and include hemolin (4 fold change), silk protein 44 (2.4 fold change), defensin (2.8 fold change), ferritin 2 light chain (2.8 fold change), ferritin 1 heavy chain (2.6 fold change), Esterase (1.9 fold change), hexamerin (1.8 fold change) and serpin (1.7 fold change). Nine proteins were identified that were absent in all 4 replicates of one group (). Elongation factor 1, isocitrate dehydrogenase, glyceraldehyde-3-phosphate dehydrogenase and hypothetical protein KGM_16590 were detected in week 1 haemolymph only. Odorant degrading enzyme cxe5, 23 kda glycoprotein, an alcohol dehydrogenase, protease inhibitor 3 and immune-related protein 2 were detected in week 10 haemolymph only.
Table 2. Protein LFQ intensities from individual haemolymph samples from G. mellonella larvae incubated for 1 and 10 weeks. Zero values indicate proteins that were apparently absent or non detected in a given sample and only proteins that were absent in all 4 replicates of one group but present in 3 of the 4 replicates of the other were consider for further discussion
Figure 6. Volcano plot representing differential abundances in proteins between haemolymph extracted from week 1 and week 10 incubated G. mellonella larvae. Fold changes in protein abundance are plotted against the negative logarithmic p value of a t-test. Proteins above the line are statistically significant (P < 0.05) and positive and negative fold changes indicate higher abundance in weeks 10 and 1 larvae respectively.
Discussion
Insects have become popular and useful alternatives to the use of mammals for assessing the virulence of microbial pathogens and for determining the in vivo efficacy of antimicrobial agents.Citation1,2,6,20 Despite their widespread use standardized procedures for their incubation and infection have not yet been developed or adopted.Citation14 G. mellonella larvae are commonly incubated at 15°C or room temperature for various periods of time prior to infection. In the results presented here evidence is provided that incubation of larvae at 15°C for up to 10 weeks prior to infection leads to a decrease in the ability of G. mellonella larvae to withstand bacterial and fungal infection. Larvae incubated at 15°C for 3 weeks or more showed a lower density of haemocytes and the composition of the haemocyte population was altered compared to that in larvae incubated for 1 week. Previous work demonstrated a reduction of 31.8% in the number of circulating haemocytes in 4 week old Drosophila relative to that in one week old flies. The same study also observed that the phagocytosing capacity of cells reduced from 24.3% ± 1.15% in one week old flies to 16.7% ± 0.99% in 4 week old flies.Citation21
While the overall density of haemocytes decreased there was an increase in the relative proportion of P2 haemocytes but a corresponding decrease in the proportion of P5 haemocytes in larvae incubated for up to 10 weeks. P5 haemocytes demonstrated globular inclusions and resembled adipohaemocytesCitation1,22 which store energy in the form of lipids and glycogen.Citation22 The reduction in P5 cells in the haemocyte population of larvae incubated for up to 10 weeks may be an indication that energy reserves in the form of lipids are being utilized by the larvae during the prolonged incubation stage. The P2 haemocytes are granular cells which function in phagocytosing pathogens.Citation1,22 The increase in the proportion of P2 haemocytes in the population occurs as the overall density of haemocytes declines by approximately 40% ().
Analysis of the changes in the proteome of larvae incubated at 15°C for 10 weeks indicated decreased abundance of a number of proteins involved in metabolism Malate dehydrogenase, an enzyme associated with the TCA cycle, aliphatic nitrilase and fructose-1,6-bisphosphatase demonstrated a decrease in abundance. Aconitase, an enzyme in the TCA cycle, and adenine nucleotide translocator which regulates the intra-mitochondrial ADP/ATP ratio, were also shown to decline by approximately 50% in abundance during aging in insects.Citation23,24 The abundance of proteins with homology to prophenoloxidase subunit-2, masquerade-like serine proteinase and protease serine 1 precursor declined in larvae pre-incubated for 10 weeks relative to the control (). Serine type proteases were also down-regulated at the gene transcript level in aged Drosophila.Citation25 Masquerade-like serine proteinase has been demonstrated to have PRR capability toward bacterial and fungal cell wall components and functions in phenoloxidase activity.Citation26,27 The reduced levels of PPO and serine proteinases from long term storage of larvae at 15°C highlights their limited melanisation capacity. The label free analysis also demonstrated the absence of protease inhibitor 3 and a lower abundance of serpin but a significant fold increase in kunitz-type protease inhibitor in one week pre-incubated larvae which may contribute to inhibition of PPO activation, in the absence of a microbial challenge. In contrast the abundance of ferritin 1 heavy chain and ferritin 2 light chain increased over time particularly in the 10 week pre-incubated larvae when analyzed using 2D SDS PAGE and label free proteomic analyses. Ferritins function in iron storageCitation28 and transport,Citation29 and have antioxidant properties.Citation30 Increased expression of ferritin may improve resistance to oxidative stress.Citation31
The relative abundance of apolipophorin-3 increased in 10 week pre-incubated larvae. The abundance of hemolin was observed to decline in the 2D SDS PAGE analysis () but increased through the label free proteomic analysis (). Hemolin is upregulated in response to infectionCitation32 and prior to metamorphosis into pupa,Citation33 therefore a lower abundance would support the observation of enhanced susceptibility to infection in 10 week pre-incubated larvae. In contrast β-1, 3-glucan recognition protein precursor showed an increase of 81% in abundance in larvae pre-incubated for 10 weeks relative to those incubated for one week. The abundance of apolipophorin and arylphorin was reduced in 10 week pre-incubated larvae when compared to that in one week pre-incubated larvae. Apolipophorin is known to enhance clotting and cellular and humoral defenses.Citation34 The up-regulation of some PRRs and immune peptides in 10 week pre-incubated larvae may be as a result of decreased phagocytosis ability and melanisation potential which has been observed in Drosophila.Citation35
The increased susceptibility of larvae to infection observed here may arise as a result of a change in the metabolism of larvae due to the prolonged incubation at 15°C. The alteration in the relative populations of haemocytes and the decreased abundance of selected metabolic proteins (e.g., malate dehydrogenase, aliphatic nitrilase, fructose-1,6-bisphosphatase () and isocitrate dehydrogenase, glyceraldehyde-3-phosphate dehydrogenase ()) may indicate a change in the physiology of larvae to adapt to the prolonged incubation state. These findings have implications for those utilizing G. mellonella larvae and this effect may contribute to some of the inter-experimental variability that can be encountered when using larvae as in vivo models.Citation16 Using larvae that have been incubated for different periods of time prior to infection with a pathogen of interest will give variable results. As a consequence steps should be taken to ensure that larvae of the equivalent age are used and preferably those that have been stored for less than 3 weeks. However, this finding may be exploited if studying pathogens of relatively low virulenceCitation36 where larvae pre-incubated for extended periods (3 – 10 weeks) and with a weaker immune response may be more susceptible to infection compared to those pre-incubated for only a short period of time.
While G. mellonella larvae are now widely used for assessing the virulence of microbial pathogensCitation4,5,8,10,11 and for measuring the efficacy of antimicrobial agentsCitation12,13,20 their potential usefulness for studying Listeria induced neural pathologies has been described.Citation37 The continued exploitation of insects as models of infection will depend upon the standardization of parameters for their use. This work shows that storage conditions need to be optimized to ensure reproducibility of results.
Materials and Methods
Inoculation of Galleria mellonella larvae
Sixth instar larvae of G. mellonella (Lepidoptera: Pyralidae, the Greater Wax Moth) (Mealworm Company, Sheffield, England) were stored in the dark at 15°C in wood shavingsCitation19 for 1, 3, 6 or 10 weeks prior to use. Larvae were stored immediately upon receipt from the supplier. Larvae weighing 0.27 ± 0.005g were inoculated with 20 μl of PBS containing either 1 × 106 C. albicans cells or 4 × 107 S. aureus cells through the last pro-leg using a Myjector U100 insulin syringe (Terumo Europe, Leuven, Belgium) as described previously.Citation8
Microbial strains
C. albicans MEN (serotype B, wild-type originally isolated from an eye infection (a gift from Dr. D. Kerridge, Cambridge, UK) was cultured to the stationary phase (approx. 1 × 108/ml) overnight in YEPD broth (2% w/v glucose (Sigma-Aldrich), 2% w/v bacteriological peptone (Difco), 1% w/v yeast extract (Oxoid)) at 30°C and 200rpm. S. aureus (clinical isolate) was cultured to the stationary phase (OD600nm = 2) overnight in nutrient broth (Oxoid) at 37°C and 200 rpm.
Determination of haemocyte density
The density of circulating haemocytes in larvae was assessed as described previously.Citation36 Experiments were performed on 3 independent occasions and the means ± standard errors (SE) were determined.
Extraction of Haemocytes for Fluorescence-activated cell sorting (FACS) Analysis
Haemolymph (150 μl) was extracted from larvae as describedCitation36 and diluted in ice cold PBS (800 μl). Haemocytes were enumerated and the density was adjusted to 1 × 106 cells/ml. Cells were fixed in 4% formaldehyde (Sigma-Aldrich) in PBS for 10 mins at 4°C. Haemocytes were washed in 1% BSA/PBS, 1500 x g for 5min at 4°C and re-suspended in BSA/PBS at a density of 1 × 106 cells/ml. Haemocyte populations were characterized using a FACSAria (Becton Dickinson) flow cytometer and cells were differentiated based on side and forward scatter with a total of 10,000 events measured per sample. Cells were subsequently separated using a cell sorter and images of haemocytes were captured using an Olympus IX81 confocal immunofluorescence microscope.
2 D SDS-PAGE analysis of protein expression in larvae
Haemolymph (100 μl) was collected into a pre-chilled microcentrifuge tube from larvae pre-incubated at 15°C for 1 or 10 weeks and haemocytes were removed by centrifugation (1500g, 5min at 4°C). The protein concentration was determined by the Bradford assay before adjusting to 200 μg per sample in isoelectric focusing buffer (IEF). Isoelectric focusing of protein samples on a pH 4-7 strip (GE Healthcare) and subsequent protein separation by mini-2D electrophoresis was performed as described.Citation18 Each 2D gel was scanned on a Hewlett Packard scanjet 5100c scanner and the images were analyzed using Progenesis SameSpot Software (Nonlinear Dynamics, Newcastle, UK). Progenesis software enabled the analysis of protein expression changes between gel replicates with significance determined using ANOVA. A table of protein spots was built and every protein was linked to the matching proteins between the gels creating a list of proteins that can be cross referenced as a final check to ensure correct alignment.
LC/MS analysis of peptides
In-gel digestion was performed on spots resolved by 2D-SDS-PAGE on a reference gel. The gel pieces were excised, trypsin digestedCitation38 and fragmented protein samples were eluted through an LC/MS (Aglient 6340 Ion Trap) which determines the relative charge to mass ratio from detected ionized particles. The data were searched against the NCBI non redundant database using the mascot search engine to identify proteins (www.matrixscience.com). MASCOT scores above 67 were deemed to have a significant match (P < 0.05). The mass error tolerance was 1 Da allowing for a maximum of no more than 2 missed cleavages. Verification of protein sequences was confirmed by blasting the protein sequence on the Uniprot (www.uniprot.org) and NCBI (www.ncbi.nlm.nih.gov) websites. Progenesis was used to determine the protein fold changes between 2-Dimensional gels.
Label free quantitative proteomics of larval haemolymph
Label free shotgun quantitative proteomics was conducted on haemocyte-free haemolymph from 1 week and 10 week pre-incubated larvae. Protein (75 μg) was reduced with dithiotreitol (DTT; 200 mM) (Sigma-Aldrich), alkylated with iodoacetamide (IAA; 1 M) (Sigma-Aldrich) and digested with sequence grade trypsin (Promega, Ireland) at a trypsin:protein ratio of 1:40, overnight at 37°C. Tryptic peptides were purified for mass spectrometry using C18 spin filters (Medical Supply Company, Ireland) and 1 μg of peptide mix was eluted onto a QExactive (ThermoFisher Scientific, USA) high resolution accurate mass spectrometer connected to a Dionex Ultimate 3000 (RSLCnano) chromatography system. Peptides were separated by an increasing acetonitrile gradient on a Biobasic C18 PicofritTM column (100 mm length, 75 mm ID), using a 120 mins reverse phase gradient at a flow rate of 250 nL /min. All data were acquired with the mass spectrometer operating in automatic data dependent switching mode. A high resolution MS scan (300-2000 Dalton) was performed using the Orbitrap to select the 15 most intense ions prior to MS/MS.
Protein identification from the MS/MS data was performed using the Andromeda search engineCitation39 in MaxQuant (version 1.2.2.5; http://maxquant.org/) to correlate the data against a 6-frame translation of the EST contigs for G. mellonella.Citation40 The following search parameters were used: first search peptide tolerance of 20 ppm, second search peptide tolerance 4.5ppm with cysteine carbamidomethylation as a fixed modification and N-acetylation of protein and oxidation of methionine as variable modifications and a maximum of 2 missed cleavage sites allowed. False Discovery Rates (FDR) were set to 1% for both peptides and proteins and the FDR was estimated following searches against a target-decoy database. Peptides with minimum length of 7 amino acid length were considered for identification and proteins were only considered indentified when more than one unique peptide for each protein was observed.
Results processing, statistical analyses and graphics generation were conducted using Persues v. 1.5.0.31. LFQ intensities were log2-transformed and ANOVA of significance and t-tests between the haemolymph proteomes of week 1 and week 10 larvae was performed using a p-value of 0.05 and significance was determined using FDR correction (Benjamini-Hochberg). Proteins that had non-existent values (indicative of absence or very low abundance in a sample) were included in the study only when they were completely absent from one group and present in at least 3 of the 4 replicates in the second group (referred to as qualitatively differentially abundant proteins). The Blast2GO suite of software tools was utilised to assign gene ontology terms (GO terms) relating to biological processes, molecular function and cellular component. Enzyme commission (EC) numbers and Kyoto Encyclopaedia of Genes and Genomes (KEGG) pathway mapping was performed as part of the Blast2GO annotation pipeline.Citation41
Statistical analysis
All experiments were performed on 3 independent occasions and results are expressed as the mean ± SE. Changes in larval survival were analyzed with the log rank (Mantel-Cox) method. Analysis of changes in haemocyte density and protein abundance were performed by One-way ANOVA. FACS results were analyzed using Two-way ANOVA with all statistical analysis listed performed using GraphPad Prism version 5.00 for Windows 8, GraphPad Software, San Diego California USA, (www.graphpad.com). Differences were considered significant at P ≤ 0.05.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
N. Browne is the recipient of a Hume Postgraduate Scholarship from NUI Maynooth. C. Surlis is the recipient of funding from the Irish Research Council. The assistance of Dr. Ilona Dix with confocal microscopy is acknowledged. The Q-Exactive quantitative mass spectrometer was funded under the SFI Research Infrastructure Call 2012; Grant Number: 12/RI/2346 (3) to Prof S. Doyle.
References
- Kavanagh K, Reeves EP. Exploiting the potential of insects for in vivo pathogenicity testing of microbial pathogens. FEMS Microbiol Rev 2004; 28:101-12; PMID:14975532; http://dx.doi.org/10.1016/j.femsre.2003.09.002
- Fuchs BB, Mylonakis E. Using non-mammalian hosts to study fungal virulence and host defense. Curr Opin Microbiol 2006; 9:346-51; PMID:16814595; http://dx.doi.org/10.1016/j.mib.2006.06.004
- Browne N, Heelan M, Kavanagh K. An analysis of the structural and functional similarities of insect hemocytes and mammalian phagocytes. Virulence 2013; 7:1-7; PMID:23921374
- Brennan M, Thomas DY, Whiteway M, Kavanagh K. Correlation between virulence of Candida albicans mutants in mice and Galleria mellonella larvae. FEMS Immunol Med Microbiol 2002; 34:153-7; PMID:12381467; http://dx.doi.org/10.1111/j.1574-695X.2002.tb00617.x
- Jander G, Rahme LG, Ausubel FM. Positive correlation between virulence of pseudomonas aeruginosa mutants in mice and insects. J Bacteriol 2000; 182:3843-5; PMID:10851003; http://dx.doi.org/10.1128/JB.182.13.3843-3845.2000
- Lionakis MS, Lewis RE, May GS, Wiederhold NP, Albert ND, Halder G, Kontoyiannis DP. Toll-deficient Drosophila flies as a fast, high-throughput model for the study of antifungal drug efficacy against invasive aspergillosis and Aspergillus virulence. J Infect Dis 2005; 191:1188-95; PMID:15747256; http://dx.doi.org/10.1086/428587
- Kemp M, Massey RC. The use of insect models to study human pathogens. Drug Discov Today 2007; 4:105-10.
- Cotter G, Doyle S, Kavanagh K. Development of an insect model for the in vivo pathogenicity testing of yeasts. FEMS Immunol Med Microbiol 2000; 27:163-9; PMID:10640612; http://dx.doi.org/10.1111/j.1574-695X.2000.tb01427.x
- Mylonakis E, Moreno R, El Khoury JB, Idnurm A, Heitman J, Calderwood SB, Ausubel FM, Diener A. Galleria mellonella as a model system to study Cryptococcus neoformans pathogenesis. Infect immun 2005; 73:3842-50; PMID:15972469; http://dx.doi.org/10.1128/IAI.73.7.3842-3850.2005
- Senior NJ, Bagnall MC, Champion OL, Reynolds SE, La Ragione RM, Woodward MJ, Salguero FJ, Titball RW. Galleria mellonella as an infection model for Campylobacter jejuni virulence. J Med Microbiol 2011; 60:661-9; PMID:21233296; http://dx.doi.org/10.1099/jmm.0.026658-0
- Mukherjee K, Altincicek B, Hain T, Domann E, Vilcinskas A, Chakraborty T. Galleria mellonella as a model system for studying Listeria pathogenesis. Appl Environ Microbiol 2010; 76:310-7; PMID:19897755; http://dx.doi.org/10.1128/AEM.01301-09
- Rowan R, Moran C, McCann M, Kavanagh K. Use of Galleria mellonella larvae to evaluate the in vivo anti-fungal activity of [Ag2 (mal)(phen) 3]. Biometals 2009; 22:461-7; PMID:19082779; http://dx.doi.org/10.1007/s10534-008-9182-3
- Desbois AP, Coote PJ. 2 Utility of greater wax moth larva (Galleria mellonella) for evaluating the toxicity and efficacy of new antimicrobial agents. Adv Appl Microbiol 2012; 78:25; PMID:22305092; http://dx.doi.org/10.1016/B978-0-12-394805-2.00002-6
- Cook SM, McArthur JD. Developing Galleria mellonella as a model host for human pathogens. Virul 2013; 4:350-3; PMID:23799664; http://dx.doi.org/10.4161/viru.25240
- Mowlds P, Kavanagh K. Effect of pre-incubation temperature on susceptibility of Galleria mellonella larvae to infection by Candida albicans. Mycopathol 2008; 165:5-12; PMID:17922218; http://dx.doi.org/10.1007/s11046-007-9069-9
- Mowlds P, Barron A, Kavanagh K. Physical stress primes the immune response of Galleria mellonella larvae to infection by Candida albicans. Microb Infect 2008; 10:628-34; PMID:18457977; http://dx.doi.org/10.1016/j.micinf.2008.02.011
- Banville N, Browne N, Kavanagh K. Effect of nutrient deprivation on the susceptibility of Galleria mellonella larvae to infection. Virulence 2012; 3:497-503; PMID:23076277; http://dx.doi.org/10.4161/viru.21972
- Bergin D, Murphy L, Keenan J, Clynes M, Kavanagh K. Pre-exposure to yeast protects larvae of Galleria mellonella from a subsequent lethal infection by Candida albicans and is mediated by the increased expression of antimicrobial peptides. Microb Infect 2006; 8:2105-12; PMID:16782387; http://dx.doi.org/10.1016/j.micinf.2006.03.005
- Mowlds P, Coates C, Renwick J, Kavanagh K. Dose-dependent cellular and humoral responses in Galleria mellonella larvae following β-glucan inoculation. Microb Infect 2010; 12:146-53; PMID:19925881; http://dx.doi.org/10.1016/j.micinf.2009.11.004
- Fallon J, Kelly J, Kavanagh K. Galleria mellonella as a model for fungal pathogenicity testing. Methods Mol Biol 2012; 845:469-85; PMID:22328396
- Mackenzie DK, Bussiere LF, Tinsley MC. Senescence of the cellular immune response in Drosophila melanogaster. Exp Gerontol 2011; 11:853-9; PMID:21798332; http://dx.doi.org/10.1016/j.exger.2011.07.004
- Araújo H, Cavalcanti M, Santos S, Alves L, Brayner F. Hemocytes ultrastructure of Aedes aegypti (Diptera: Culicidae). Micron 2008; 39:184-9; PMID:17329111; http://dx.doi.org/10.1016/j.micron.2007.01.003
- Yan L-J, Sohal RS. Mitochondrial adenine nucleotide translocase is modified oxidatively during aging. Proc Natl Acad Sci U S A 1998; 95:12896-901; PMID:9789011; http://dx.doi.org/10.1073/pnas.95.22.12896
- DAS N, Levine R, Orr W, Sohal R. Selectivity of protein oxidative damage during aging in Drosophila melanogaster. Biochem J 2001; 360:209-16; PMID:11696009; http://dx.doi.org/10.1042/0264-6021:3600209
- Pletcher SD, Macdonald SJ, Marguerie R, Certa U, Stearns SC, Goldstein DB, Partridge L. Genome-wide transcript profiles in aging and calorically restricted Drosophila melanogaster. Curr Biol 2002; 12:712-23; PMID:12007414; http://dx.doi.org/10.1016/S0960-9822(02)00808-4
- Lee SY, Söderhäll K. Characterization of a pattern recognition protein, a masquerade-like protein, in the freshwater crayfish Pacifastacus leniusculus. J Immunol 2001; 166:7319-26; PMID:11390482; http://dx.doi.org/10.4049/jimmunol.166.12.7319
- Kwon TH, Kim MS, Choi HW, Joo CH, Cho MY, Lee BL. A masquerade-like serine proteinase homologue is necessary for phenoloxidase activity in the coleopteran insect, Holotrichia diomphalia larvae. Eur J Biochem 2000; 267:6188-96; PMID:11012672; http://dx.doi.org/10.1046/j.1432-1327.2000.01695.x
- Arosio P, Ingrassia R, Cavadini P. Ferritins: a family of molecules for iron storage, antioxidation and more. Biochim Biophys Acta 2009; 1790:589-99; PMID:18929623; http://dx.doi.org/10.1016/j.bbagen.2008.09.004
- Zhou G, Kohlhepp P, Geiser D, Frasquillo MDC, Vazquez-Moreno L, Winzerling JJ. Fate of blood meal iron in mosquitoes. J Insect Physiol 2007; 53:1169-78; PMID:17689557; http://dx.doi.org/10.1016/j.jinsphys.2007.06.009
- Strickler-Dinglasan PM, Guz N, Attardo G, Aksoy S. Molecular characterization of iron binding proteins from Glossina morsitans morsitans (Diptera: Glossinidae). Insect Biochem Mol Biol 2006; 36:921-33; PMID:17098167; http://dx.doi.org/10.1016/j.ibmb.2006.09.003
- Missirlis F, Holmberg S, Georgieva T, Dunkov BC, Rouault TA, Law JH. Characterization of mitochondrial ferritin in Drosophila. Proc Natl Acad Sci U S A 2006; 15:5893-8; PMID:16571656; http://dx.doi.org/10.1073/pnas.0601471103
- Daffre S, Faye I. Lipopolysaccharide interaction with hemolin, an insect member of the Ig-superfamily. FEBS Lett 1997; 408:127-30; PMID:9187352; http://dx.doi.org/10.1016/S0014-5793(97)00397-9
- Yu XQ, Kanost MR. Developmental expression of Manduca sexta hemolin. Arch Insect Biochem Physiol 1999; 42:198-212; PMID:10536048; http://dx.doi.org/10.1002/(SICI)1520-6327(199911)42:3%3c198::AID-ARCH4%3e3.0.CO;2-G
- Altincicek B, Stötzel S, Wygrecka M, Preissner KT, Vilcinskas A: Host-derived extracellular nucleic acids enhance innate immune responses, induce coagulation, and prolong survival upon infection in insects. J Immunol 2008; 181(4):2705-12; PMID:18684961; http://dx.doi.org/10.4049/jimmunol.181.4.2705
- Zerofsky M, Harel E, Silverman N, Tatar M. Aging of the innate immune response in Drosophila melanogaster. Aging cell 2005; 4:103-8; PMID:15771614; http://dx.doi.org/10.1111/j.1474-9728.2005.00147.x
- Bergin D, Brennan M, Kavanagh K. Fluctuations in haemocyte density and microbial load may be used as indicators of fungal pathogenicity in larvae of Galleria mellonella. Microb Infect 2003; 5:1389-95; PMID:14670452; http://dx.doi.org/10.1016/j.micinf.2003.09.019
- Mukherjee K, Hain T, Fischer R, Chakraborty T, Vilcinskas A. Brain infection and activation of neuronal repair mechanisms by the human pathogen Listeria monocytogenes in the lepidopteran model host Galleria mellonella. Virulence 2013; 4:324-32; PMID:23348912; http://dx.doi.org/10.4161/viru.23629
- Shevchenko A, Tomas H, Havli J, Olsen JV, Mann M. In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat protoc 2007; 1:2856-60; PMID:17406544; http://dx.doi.org/10.1038/nprot.2006.468
- Cox J, Neuhauser N, Michalski A, Scheltema RA, Olsen JV, Mann M. Andromeda: a peptide search engine integrated into the MaxQuant environment. J Proteome Res 2011; 10:1794-805; PMID:21254760; http://dx.doi.org/10.1021/pr101065j
- Vogel H, Altincicek B, Glöckner G, Vilcinskas A. A comprehensive transcriptome and immune-gene repertoire of the lepidopteran model host Galleria mellonella. BMC Genomics 2011; 12:308; PMID:21663692; http://dx.doi.org/10.1186/1471-2164-12-308
- Conesa A, Gotz S. Blast2GO: a comprehensive suite for functional analysis in plant genomics. Int J Plant Genomics 2008; 2008:619832; PMID:18483572; http://dx.doi.org/10.1155/2008/619832