Abstract
Candida biofilms play an important role in infections associated with medical devices and are resistant to antifungals. We hypothesized that the echinocandin micafungin (MICA) exerts an enhanced antifungal activity against caspofungin (CAS)-susceptible (CAS-S) and CAS–non-susceptible (CAS-NS) Candida albicans and Candida parapsilosis which is at least in part through apoptosis, even in the biofilm environment. Apoptosis was characterized by detecting reactive oxygen species (ROS) accumulation, depolarization of mitochondrial membrane potential (MMP), DNA fragmentation, lack of plasma membrane integrity, and metacaspase activation following exposure of Candida biofilm to MICA for 3h at 37°C in RPMI 1640 medium. The minimum inhibitory concentration was higher for CAS (2.0–16.0 μg/mL) than for MICA (1.0–8.0 μg/mL) for Candida biofilms. Elevated intracellular ROS levels and depolarization of MMP was evident in CAS-S C. albicans (3.0–4.2 fold) and C. parapsilosis (4.8–5.4 fold) biofilms compared with CAS-NS (1.2 fold) after exposure to MICA (0.25x-1xMIC). Elevated intracellular ROS levels and depolarization of MMP was evident in CAS-S C. albicans (3.0–4.2 fold) and C. parapsilosis (4.8–5.4 fold) biofilms compared with CAS-NS (1.2 fold) after exposure to MICA (0.25x-1xMIC). Finally higher ß-1, 3 glucan levels were seen in sessile cells compared to planktonic cells, especially in CAS-NS strains. MICA treatment might induce a metacaspase-dependent apoptotic process in biofilms of both CAS-S C. albicans and C. parapsilosis, and to some degree in CAS-NS strains.
Introduction
Candida spp are an increasingly common cause of bloodstream infections in hospitalized patients.Citation1,2 These infections are commonly related to organism's ability to produce biofilms on medical devices.Citation3 Candida biofilms are heterogeneous sessile communities of yeast and hyphal cells in the extracellular matrix. Biofilm-associated organisms are phenotypically different from planktonic cells and, characteristically, are significantly less susceptible to antimicrobial agents, display resistance to antifungals and are protected from host immune cells, making the management of infections with these organisms difficult.Citation4-6
Recent evidence suggests that echinocandins, in contrast to amphotericin B or azoles, exert fungicidal activity against Candida biofilms.Citation4,7-9 The mechanism of this activity and whether it is influenced by the specific Candida spp. treated or its degree of susceptibility to caspofungin (CAS) are not known. The existence of a small number of drug-tolerant (persister cells) Candida cells that neither grow nor die in the presence of antimicrobial agents has gained considerable attention in recent years.Citation10-12 Persisters can withstand drug concentrations substantially above the minimum inhibitory concentration and represent specialized survivor cells that are phenotypic variants of the wild type rather than mutants. It has been suggested that Candida cells with no persister cells exposed to antimicrobial agents undergo apoptosis, whereas this process has been disabled in persisters, however not all strains of Candida produce persisters.Citation10,13
Apoptotic responses have been described in variety of fungi after exposure to the wide range of conditions, such as weak acid stress, oxidative stress, or ultraviolet irradiation.Citation14 Fungal cells dying in this way display several characteristics of apoptosis, including the exposure of phosphatidylserine at the plasma membrane and DNA degradation.Citation14 Recent studies have demonstrated that planktonic and sessile C. albicans cells undergo apoptosis when exposed to amphotericin B and caspofungin, via activation of cysteine-dependent, aspartate-specific proteases (caspases).Citation13,15,16 However, to our knowledge, no study has assessed in a biofilm environment, the mechanisms of cell death in Candida albicans and Candida parapsilosis induced by the echinocandin micafungin (MICA), an echinocandin that inhibits (1, 3)-β-D glucan synthesis in the cell wall. Elucidation of novel targets or mechanisms for enhancing the activity of current antifungals against Candida biofilms is critical for improving outcomes of biofilm associated Candida infection. Herein we extend further observations that the fungicidal activity of the echinocandins (inhibitors of ß-1,3 glucan synthase) is mediated, at least in part via apoptosis in biofilm environment. If correct, using pro-apoptotic agonists with or without echinocandins could improve outcomes in biofilm associated Candida infections. Specifically, the administration of echinocandins in combination with pro-apoptotic agents as lock therapy could turn out to be an important catheter retention strategy in catheter-associated candidemias. Understanding of apoptotic responses in the Candida spp biofilms may offer the opportunity for exploiting the fungal death machinery to control biofilms infections as an adjunct, catheter-retention strategy.
In this study, we hypothesized that MICA exerts pro-apoptotic activity against not only CAS-susceptible (CAS-S) but also CAS–non-susceptible (CAS-NS) C. albicans and C. parapsilosis embedded biofilms. We therefore investigated apoptotic features including increased reactive oxygen species (ROS), decreased mitochondrial membrane potential, DNA damage, and activation of metacaspases in biofilms of CAS-S and CAS-NS C. albicans and C. parapsilosis exposed to a range of concentrations of MICA.
Results
Effects of CAS and MICA on CAS-S and CAS-NS C. albicans and C. parapsilosis isolates in susceptibility assays
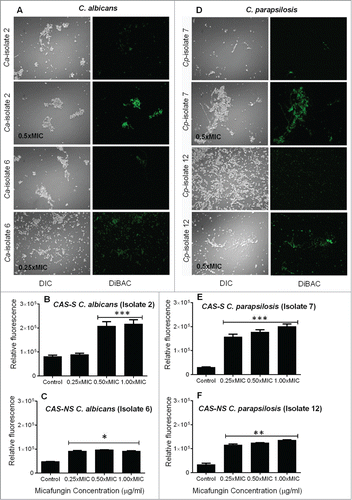
MICA was more active than CAS against sessile C. albicans and C. parapsilosis cells. However, Candida biofilms were 4 to 33 times less susceptible to both drugs () than the planktonic cells (data not shown). To further elucidate the mechanisms of the antifungal action of MICA, we stained C. albicans and C. parapsilosis biofilms with the morbidity dye bis-[1,3-dibutylbarbituric acid] trimethine oxonol (DiBAC). We observed increased DiBAC uptake for cells treated with MICA compared with untreated controls at all concentrations tested, indicating enhanced membrane damage due to MICA (0.25x-1xMIC) (). However, this effect was more prominent in CAS-S C. albicans and C. parapsilosis (2.7 to 6.4-fold, isolate 2 and 7; P = 0.0056) biofilms treated with MICA (0.25x-1xMIC) than in CAS-NS C. albicans and C. parapsilosis biofilms (2 to 3.9-fold, isolate 6 and 12) treated with MICA (0.25x-1xMIC) ().
Table 1. In vitro antimicrobial activity of CAS and MICA against C. albicans and C. parapsilosis strains biofilms
Figure 1. Fungicidal action of MICA against C. albicans (A–C) and C. parapsilosis (D–F) biofilms as shown by the morbidity stain DiBAC. (A) Fluorescence images of C. albicans stained with DiBAC. Relative fluorescence of DiBAC staining of CAS-S C. albicans (isolate 2) (B) and CAS-NS C. albicans (isolate 6) (C) treated with MICA. (D) Fluorescence images of C. parapsilosis stained with DiBAC. Relative fluorescence of DiBAC staining of CAS-S C. parapsilosis (isolate 7) (E) and CAS-NS C. parapsilosis (isolate 12) (F) treated with MICA. DIC: differential interference contrast; Ca: C. albicans; Cp: C. parapsilosis; C: untreated control. *P < 0.05; **P < 0.001; ***P < 0.0001 (compared with untreated control). The experiments were performed in triplicate and repeated 3 times.
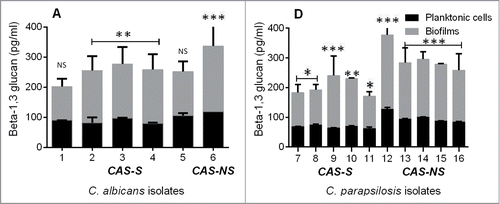
Comparison of extracellular β-1,3 glucan levels from planktonic and sessile CAS-S and CAS-NS C. albicans and C. parapsilosis
Reports of a link between matrix glucan levels and drug resistance led us to evaluate the impact of the glucan matrix on CAS-S and CAS-NS cells in a biofilm environment.Citation6 Given the high concentration of β-1,3 glucan observed in the cell wall of CAS-S Candida biofilms compared with planktonic cells and its even greater concentration in CAS-NS biofilms, we reasoned that the higher matrix glucan levels may decrease the susceptibility of Candida spp. to echinocandins in CAS-NS biofilms compared with planktonic cells. To test this hypothesis, we compared β glucan levels of C. albicans and C. parapsilosis (CAS-S and CAS-NS) biofilms with those of planktonic cells. However, the biofilm contained significantly higher concentrations of β-1,3 glucan than the planktonic cells (). Moreover, higher glucan levels were observed in CAS-NS strains than in CAS-S strains in biofilms and in planktonic cells.
MICA increases intracellular ROS accumulation in CAS-S and to a lesser extent in CAS-NS sessile C. albicans and C. parapsilosis
ROS play an important role as early initiators of apoptosis in yeasts and other filamentous fungi. Using a fluorimetric assay with dihydrorhodamine (DHR)-123 staining, we found increased intracellular ROS levels in MICA-treated (0.25x-1xMIC) CAS-S C. albicans and C. parapsilosis (isolates 2 and 7, respectively) and CAS-NS C. albicans and C. parapsilosis (isolates 6 and 12, respectively) compared with untreated controls (). Specifically, in CAS-S C. albicans (isolate 2) and C. parapsilosis (isolate 7) biofilms treated with MICA (0.25x-1xMIC), the relative fluorescence increased 4.8-fold compared with untreated controls, whereas in CAS-NS C. albicans (isolate 6) and C. parapsilosis (isolate 12) treated with MICA (0.25x-1xMIC) only 1.2 to 2.0-fold changes in fluorescence were observed (P = 0.0150; ).
Figure 3. Intracellular ROS accumulation detected using fluorescence microscopy and fluorescence spectrophotometry in C. albicans (CAS-S isolate 2 and CAS-NS isolate 6) and C. parapsilosis (CAS-S isolate 7 and CAS-NS isolate 12) cells treated with MICA. Fluorescence images and relative fluorescence of CAS-S and CAS-NS strains of C. albicans (A, B) stained with DHR-123. Fluorescence images and relative fluorescence of CAS-S and CAS-NS strains of C. parapsilosis (C, D) stained with DHR-123. DIC: differential interference contrast; Ca: C. albicans; Cp: C. parapsilosis; C: untreated control. *P < 0.05; ***P < 0.0001 (compared with untreated controls).
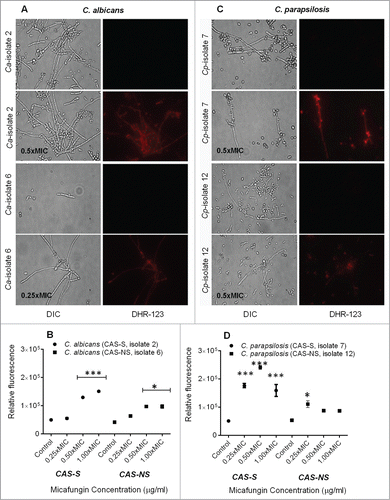
MICA decreases mitochondrial membrane potential in CAS-S and to a lesser extent in CAS-NS sessile C. albicans and C. parapsilosis
Mitochondrial membrane depolarization was assessed by staining with rhodamine (Rh)-123, a fluorescent dye that is distributed in the mitochondrial matrix, as previously described.Citation17 MICA-treated (0.25x-1xMIC) CAS-S C. albicans (isolate 2) and C. parapsilosis (isolate 7) sessile cells showed sharp decrease in mitochondrial potential (5.4-fold compared with controls), as indicated by an increase in fluorescence, whereas the mitochondrial potential in the MICA-treated (0.25x-1xMIC) CAS-NS strains decreased only by 2.3-fold (P = 0.0023, compared to CAS-S) ().
Figure 4. Measurement of depolarization of mitochondrial membrane potential by Rh-123 in C. albicans and C. parapsilosis sessile cells treated with MICA. Fluorescence images and relative fluorescence of CAS-S and CAS-NS strains of C. albicans (A, B) stained with Rh-123. Fluorescence images and relative fluorescence of CAS-S and CAS-NS strains of C. parapsilosis (C, D) stained with Rh -123. DIC: differential interference contrast; Ca: C. albicans; Cp: C. parapsilosis; C: untreated control. *P < 0.05; **P < 0.001; ***P < 0.0001 (compared with untreated control).
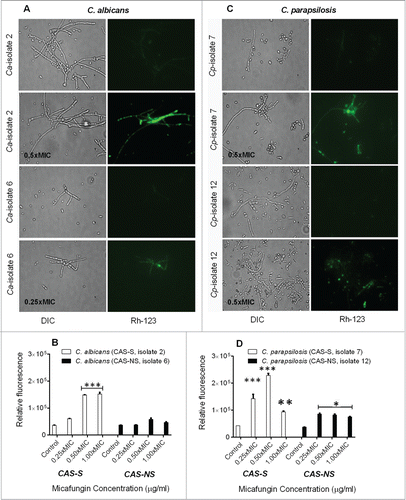
MICA induces more pronounced apoptosis in CAS-S and to a lesser extent in CAS-NS sessile C. albicans and C. parapsilosis
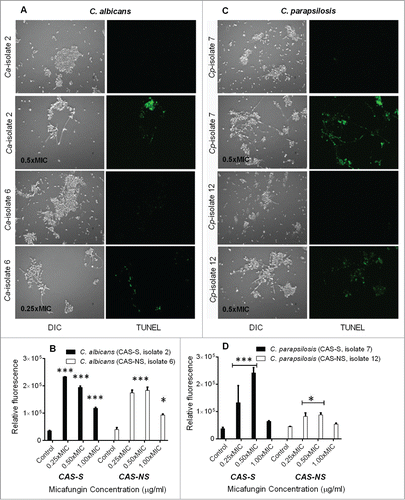
To assess whether cell death due to CAS and MICA occurs through induction of apoptosis, we performed terminal deoxynucelotidyltransferase-mediated dUTP nick end-labeling (TUNEL) assay to detect apoptosis as shown by DNA fragmentation ().Citation17-19 Compared with no treatment group, pre-exposure to MICA at 0.25x-0.5xMIC resulted in a 5.5 to 6.6-fold increase in fluorescence in CAS-S C. albicans (isolate 2), and 0.25x-0.5xMIC of MICA led to a 5.0 to 6.5-fold increase in fluorescence in CAS-S C. parapsilosis (isolate 7) (). However, in CAS-NS strains, 0.25x-1xMIC of MICA led to a 2.3-fold increase in fluorescence in C. albicans biofilms and a 1.2-fold increase in fluorescence in C. parapsilosis biofilms ().
Figure 5. Detection of DNA fragmentation by TUNEL assay in CAS-S and CAS-NS C. albicans (isolates 2 and 6, respectively) and C. parapsilosis (isolates 7 and 12, respectively) sessile cells treated with MICA. Fluorescence images and relative fluorescence of CAS-S and CAS-NS strains of C. albicans (A, B) as detected by TUNEL assay. Fluorescence images and relative fluorescence of CAS-S and CAS-NS strains of C. parapsilosis (C, D) as detected by TUNEL. DIC: differential interference contrast; Ca: C. albicans; Cp: C. parapsilosis; C: untreated control. *P < 0.05; ***P < 0.0001 (compared with untreated control).
Micafungin activates metacaspases (caspase- like) activity in CAS-S and CAS-NS sessile C. albicans and C. parapsilosis
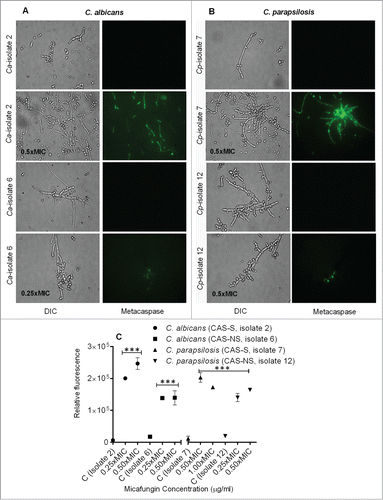
Caspases are activated in the early stages of apoptosis and play a central role in the apoptotic cascade.Citation20,21 Although caspases are not present in fungi, researchers have identified orthologs of mammalian caspases in fungi and plants called metacaspases. Activation of metacaspases was detected with the CaspACE FITC-VAD-FMK In Situ Marker (Promega), which fluoresces green when it binds to active metacaspases.Citation17 To detect metacaspase activation, CAS-S and CAS-NS strains of sessile C. albicans and C. parapsilosis that were pretreated with 0.25x-0.5xMIC MICA were incubated with CaspACE FITC-VAD-FMK In Situ Marker (). Indeed, cells of CAS-S and CAS-NS C. albicans and C. parapsilosis treated with MICA showed green fluorescence, which is consistent with activation of the marker, and suggesting that apoptosis in these strains might occur via metacaspase activation ().
Figure 6. Caspase-like activity in CAS-S and CAS-NS C. albicans and C. parapsilosis strains treated with MICA. Fluorescence images of CAS-S and CAS-NS strains of C. albicans (A) and C. parapsilosis (B) treated with MICA and stained with CaspACE FITC-VAD-FMK. (C) Relative fluorescence of CAS-S and CAS-NS C. albicans and C. parapsilosis sessile cells stained with CaspACE FITC-VAD-FMK. DIC: differential interference contrast; Ca: C. albicans; Cp: C. parapsilosis; C: untreated control. ***P < 0.0001 (compared with untreated control).
Discussion
In this study we showed that apoptosis accounts for MICA-induced cell death in CAS-S Candida isolates and to some degree in CAS-NS Candida biofilms. Our findings demonstrate that MICA triggers a mitochondria-mediated apoptotic process in C. albicans and C. parapsilosis biofilms that requires the proteolytic activity of metacaspases. Not surprisingly, a higher percentage of CAS-S sessile C. albicans and C. parapsilosis cells underwent apoptosis compared with CAS-NS isolates in the presence of echinocandins. Apoptosis was also found to trigger in CAS-NS strains at sub MICs at higher concentration of echinocandins compared to CAS-S strains. This phenomenon might be attributed to the more complex glucan matrix in the cell walls of CAS-NS strains, which may account for these strains' resistance to antifungal drugs, as described previously.Citation6,22 In fact, we found that sessile cells had significantly higher concentrations of β-1, 3 glucan than planktonic cells. This difference in glucan levels between planktonic cells and biofilms was prominent in CAS-S Candida cells and even more so in CAS-NS isolates ().
Apoptosis is a cellular process that leads to cell death in multicellular organisms and is crucial for normal cell growth, development, and maintenance. The features of apoptosis include externalization of phosphatidylserine, chromatin condensation, accumulation of ROS, DNA degradation, and caspase activation.Citation15,23,24 Candida spp undergoing apoptosis exhibit biochemical hallmarks similar to those of mammalian apoptosis in response to diverse stimuli,Citation15,25 in which mitochondria play a pivotal role in controlling both caspase-dependent and caspase-independent cell death pathways. ROS accumulation and mitochondrial dysfunction signal the onset of apoptosis.Citation26 Moreover, it has been shown that activation of C. albicans metacaspase is associated with elevation of intracellular ROS levels.Citation23
To detect these mechanisms of apoptosis in Candida spp., we evaluated the effect of MICA on ROS accumulation and mitochondrial homeostasis in C. albicans and C. parapsilosis biofilms. As expected, MICA elicited a greater concentration-dependent depolarization of mitochondrial membrane potential in CAS-S C. albicans and C. parapsilosis biofilms than in CAS-NS isolates. Similarly, ROS accumulation after treatment with MICA was greater in CAS-S strains than in CAS-NS strains, which supports a previous report ROS accumulation induced apoptosis in the presence of miconazole and purpurin in C. albicans and C. dubliniensis.Citation26
The collapse of mitochondrial potential triggers a series of downstream cellular events that lead to DNA fragmentation and thus to cell death.Citation14,18 Our assessment of the effect of MICA on DNA integrity and metacaspase activity in C. albicans and C. parapsilosis biofilms showed that apoptosis in CAS-S Candida spp might be metacaspase-dependent. Metacaspase activation, a distinct biochemical marker of apoptosis, has been previously characterized in Saccharomyces cerevisiae and C. albicans.Citation16,19,23 Recently, other research groups have demonstrated metacaspase-dependent apoptosis in planktonic and sessile cells of C. albicans.Citation23-25
In conclusion, we provide evidence that MICA triggers apoptotic features in C. albicans and C. parapsilosis biofilms including in CAS-NS strains. Strategies that work by switching on endogenous cell death mechanisms in the C. albicans and C. parapsilosis biofilms would be a novel antifungal approach against biofilm-associated Candida infections. Although the in vitro data presented here are an important “proof of concept,” in vivo studies in murine model of catheter-related candidiasis in the setting of lock therapy comparing different echinocandins would be needed.
Materials and Methods
Echinocandins
Pharmacy-grade CAS (5.0 mg/mL; Merck & Co., Inc.) and pure MICA powder (5.0 mg/mL; Astellas Pharma US, Inc.), dilutions were prepared in distilled water and stored at −80°C until use.
Isolates and growth conditions
We used strains of the Candida species C. albicans (five CAS-S isolates and one CAS-NS isolate) and C. parapsilosis (five CAS-S isolates and 5 CAS-NS isolates) that had been isolated from the blood cultures of cancer patients with candidemia at The University of Texas MD Anderson Cancer Center, Houston, TX. The isolates were grown on yeast peptone dextrose (YPD) agar plates overnight at 37°C. The cells were then collected, washed twice in phosphate-buffered saline (PBS) and counted using a hemocytometer (Hausser Scientific). Next, 106 cells/mL of each isolate were resuspended in liquid YPD and incubated at 37°C for 12 h until reaching mid-log phase, and then recounted with a hemocytometer to achieve final testing inocula.
Biofilm formation and susceptibility testing of Candida cells
C. albicans and C. parapsilosis cells were grown overnight, and then the cells were harvested and washed in PBS. Next, cells were counted with a hemocytometer, and suspensions of 1 × 106 cells/mL were prepared in Roswell Park Memorial Institute (RPMI) 1640 medium. One hundred micro liters (μL) of cell suspension were placed in a 96-well plate and allowed to incubate for 48 h to form biofilms as described.Citation27,28 After biofilm formation, plates were washed 3 times with sterile PBS. CAS or MICA dissolved in RPMI 1640 medium were added to each well, and the plates were incubated at 37°C for 24 h. After incubation, plates were washed with PBS, and hyphal damage with the use of the 2,3-bis[2-methyloxy-4-nitro-5-[(sulfenylamino) carbonyl]-2H-tetrazolium-5-carboxanilide] (XTT) colorimetric formazan reduction assay.100 μL of XTT (1 mg) /menadione (0.17 mg) solution was added to each well containing a pre-washed biofilms. Plates were covered in aluminum foil and incubated in the dark for 2 h at 37°C. After incubation, 80 μL of the resulting colored supernatant from each well was removed and transferred into the corresponding wells of a new microtiter plate. Absorbance was recorded at 490 nm. From the resulting colorimetric readings and after subtracting the corresponding values for negative controls (XTT only), we calculated the minimum inhibitory concentration for each isolate at which 80% decrease in absorbance was detected in comparison to control biofilms in the absence of antifungal drug.
Matrix collection and matrix ß-1,3 glucan measurements in Candida biofilms
The matrix ß-1,3 glucan content was measured using a Glucatell (1,3)-ß-D-glucan detection kit (Associates of Cape Cod, Inc.), as previously described.Citation6 Matrix was collected from C. albicans and C. parapsilosis biofilms grown in 24-well polystyrene plates for 48 h and planktonic cells were grown for 12h under shaking condition at 37°C. Biofilms were dislodged using a sterile spatula, sonicated for 10 min, and centrifuged 3 times at 4,500 × g for 20 min to separate cells from soluble matrix material. Samples were stored at –20°C, and glucan concentrations were determined per the manufacturer's directions.
Viability assay of Candida cells
C. albicans and C. parapsilosis cells pretreated with MICA (0.25x-1xMIC) for 3 h at 37°C were stained with the morbidity dye bis-[1,3-dibutylbarbituric acid] trimethine oxonol (DiBAC; Molecular Probes), which enters depolarized cells, as described previously.Citation17,29
Detection of intracellular ROS in Candida biofilms
Intracellular ROS levels in C. albicans and C. parapsilosis biofilms were measured as previously described.Citation17 The C. albicans and C. parapsilosis biofilms were treated with MICA (0.25x-1xMIC) for 3 h at 37°C and then spiked with dihydrorhodamine (DHR)-123 (5.0 μg/mL). After 2 h at room temperature, the cells were harvested after centrifugation at 13,000 × g for 5 min and then observed with a Nikon Microphot SA fluorescence microscope (excitation, 488 nm; emission, 520 nm). For quantitative assays, fluorescence intensity values were recorded by using a POLARstar Galaxy microplate reader (excitation, 490 nm; emission, 590 nm; BMG LABTECH).
Mitochondrial membrane potential measurements in Candida biofilms
Mitochondrial membrane depolarization was assessed by staining with rhodamine (Rh)-123, a fluorescent dye that is distributed in the mitochondrial matrix, as previously described.Citation17 Briefly, cells exposed to MICA (0.25x-1xMIC) for 3 h at 37°C were harvested via centrifugation, washed twice, and resuspended in PBS. Rh-123 was added to the final concentration of 10 μM, and the dyed cells were incubated for 30 min in the dark at room temperature. Fluorescence intensity was recorded as described above (excitation, 488 nm; emission, 520 nm).
Measurement of DNA damage in Candida biofilms
DNA fragmentation, a characteristic change in apoptosis, was detected by subjecting the biofilms to a terminal deoxynucelotidyltransferase-mediated dUTP nick end-labeling (TUNEL) assay.Citation17 Biofilms pretreated with MICA (0.25–4.0 μg/mL) for 3 h at 37°C were fixed with 3.7% formaldehyde for 30 min on ice and were digested with a lysing enzyme mixture (0.25 mg/mL of chitinase, 15 U of lyticase, and 20 mg/mL lysing enzyme; Sigma-Aldrich) for 3 h at 30°C. The enzyme-digested cells were used to detect DNA fragmentation by means of the TUNEL assay as previously described.Citation16 The cells were observed for fluorescence as described above, with excitation and emission wavelengths of 488 nm and 520 nm, respectively.
Detection of metacaspase activity in Candida biofilms
Activation of metacaspases was detected with the CaspACE FITC-VAD-FMK In Situ Marker (Promega), which fluoresces green when it binds to active metacaspases.Citation17 Biofilms pretreated with MICA (0.25x-1xMIC) for 3 h at 37°C were harvested, washed in PBS, and then re-suspended in 10 μM CaspACE FITC-VAD-FMK solution. After 2 h of incubation at room temperature, cells were washed twice and re-suspended in PBS. Samples were mounted and viewed in a fluorescence microscope as described above (emission, 488 nm; excitation, 520 nm).
Statistical analysis
For all assays, 3 independent experiments on different days were performed in triplicate. Multiple treatment groups were compared using Kruskall-Wallis tests and post-hoc paired comparisons were performedusing Dunnett's tests. Calculations were made with InStat (GraphPad Software). All results are expressed as means ± standard deviations. Two-tailed P values of less than 0.05 were considered statistically significant.
Disclosure of Potential Conflicts of Interest
DPK has received research support and honoraria from Pfizer, Astellas Pharma US, and Merck and Co., Inc.; FS has no conflicts of interest.
Funding
DPK. acknowledges the Frances King Black Endowed Professorship for Cancer Research. This research was supported in part by grant from Astellas Pharma US, Inc.
References
- Pfaller MA, Diekema DJ. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev 2007; 20:133-63; PMID:17223626; http://dx.doi.org/10.1128/CMR.00029-06
- Pappas PG, Kauffman CA, Andes D, Benjamin DK Jr, Calandra TF, Edwards JE Jr, Filler SG, Fisher JF, Kullberg BJ, Ostrosky-Zeichner L, et al. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis 2009; 48:503-35; PMID:19191635; http://dx.doi.org/10.1086/596757
- Kojic EM, Darouiche RO. Candida infections of medical devices. Clin Microbiol Rev 2004; 17:255-67; PMID:15084500; http://dx.doi.org/10.1128/CMR.17.2.255-267.2004
- Douglas LJ. Candida biofilms and their role in infection. Trends Microbiol 2003; 11:30-36; PMID:12526852; http://dx.doi.org/10.1016/S0966-842X(02)00002-1
- ten Cate JM, Klis FM, Pereira-Cenci T, Crielaard W, de Groot PW. Molecular and cellular mechanisms that lead to Candida biofilm formation. J Dent Res 2009; 88:105-15; PMID:19278980; http://dx.doi.org/10.1177/0022034508329273
- Taff HT, Nett JE, Zarnowski R, Ross KM, Sanchez H, Cain MT, Hamaker J, Mitchell AP, Andes DR. A Candida biofilm-induced pathway for matrix glucan delivery: implications for drug resistance. PLOS Pathog 2012; 8:e1002848; http://dx.doi.org/10.1371/journal.ppat.1002848
- Choi HW, Shin JH, Jung SI, Park KH, Cho D, Kee SJ, Shin MG, Suh SP, Ryang DW. Species-specific differences in the susceptibilities of biofilms formed by Candida bloodstream isolates to echinocandin antifungals. Antimicrob Agents Chemother 2007; 51:1520-23; PMID:17283191; http://dx.doi.org/10.1128/AAC.01141-06
- Katragkou A, Chatzimoschou A, Simitsopoulou M, Dalakiouridou M, Diza-Mataftsi E, Tsantali C, Roilides E. Differential activities of newer antifungal agents against Candida albicans and Candida parapsilosis biofilms. Antimicrob Agents Chemother 2008; 52:357-60; PMID:17938192; http://dx.doi.org/10.1128/AAC.00856-07
- Ramage G, Saville SP, Thomas DP, Lopez-Ribot JL. Candida biofilms: an update. Eukaryot Cell 2005; 4:633-38; PMID:15821123; http://dx.doi.org/10.1128/EC.4.4.633-638.2005
- Lewis K. Programmed cell death in bacteria. Microbiol Mol Biol Rev 2000; 64:503-14; PMID:10974124; http://dx.doi.org/10.1128/MMBR.64.3.503-514.2000
- Lewis K. Riddle of biofilm resistance. Antimicrob Agents Chemother 2001; 45:999-1007; PMID:11257008; http://dx.doi.org/10.1128/AAC.45.4.999-1007.2001
- Lewis K. Persister cells, dormancy and infectious disease. Nat Rev Microbiol 2007; 5:48-56; PMID:17143318; http://dx.doi.org/10.1038/nrmicro1557
- Al-Dhaheri RS, Douglas LJ. Absence of amphotericin B tolerant persister cells in biofilms of some Candida species. Antimicrob Agents Chemother 2008; 52:1884-87; PMID:18285487; http://dx.doi.org/10.1128/AAC.01473-07
- Ramsdale M. Programmed cell death in pathogenic fungi. Biochim Biophys Acta 2008; 1783:1369-80; PMID:18294459; http://dx.doi.org/10.1016/j.bbamcr.2008.01.021
- Phillips AJ, Sudbery I, Ramsdale M. Apoptosis induced by environmental stresses and amphotericin B in Candida albicans. Proc Natl Acad Sci USA 2003; 100:14327-32; PMID:14623979; http://dx.doi.org/10.1073/pnas.2332326100
- Hao B, Cheng S, Clancy CJ, Nguyen MH. Caspofungin kills Candida albicans by causing both cellular apoptosis and necrosis. Antimicrob Agents Chemother 2013; 57:326-32; PMID:23114781; http://dx.doi.org/10.1128/AAC.01366-12
- Shirazi F, Kontoyiannis DP. Mitochondrial respiratory pathways inhibition in Rhizopus oryzae potentiates activity of posaconazole and itraconazole via apoptosis. PLoS One 2013; 8:e63393; PMID:23696824; http://dx.doi.org/10.1371/journal.pone.0063393
- Madeo F, Herker E, Wissing S, Jungwirth H, Eisenberg T, Fröhlich KU. Apoptosis in yeast. Curr Opin Microbiol 2004; 7:655-60; PMID:15556039; http://dx.doi.org/10.1016/j.mib.2004.10.012
- Madeo F, Herker E, Maldener C, Wissing S, Lächelt S, Herlan M, Fehr M, Lauber K, Sigrist SJ, Wesselborg S, Fröhlich KU. A caspase-related protease regulates apoptosis in yeast. Mol Cell 2002; 9:911-17; PMID:11983181; http://dx.doi.org/10.1016/S1097-2765(02)00501-4
- Cho J, Lee DG. The antimicrobial peptide arenicin-1 promotes generation of reactive oxygen species and induction of apoptosis. Biochim Biophys Acta 2011; 1810: 1246-51; PMID:21875650; http://dx.doi.org/10.1016/j.bbagen.2011.08.011
- Hwang In-S, Lee J, Hwang JH, Kim KJ, Lee DG. Silver nanoparticles induce apoptotic cell death in Candida albicans through the increase of hydroxyl radicals. FEBS J 2012; 279:1327-38; PMID:22324978; http://dx.doi.org/10.1111/j.1742-4658.2012.08527.x
- Nett JE, Crawford K, Marchillo K, Andes DR. Role of Fks1p and matrix glucan in Candida albicans biofilm resistance to an echinocandin, pyrimidine, and polyene. Antimicrob Agents Chemother 2012; 54:3505-08; http://dx.doi.org/10.1128/AAC.00227-10
- Cao YY, Huang S, Dai B, Zhu Z, Lu H, Dong L, Cao Y, Wang Y, Gao P, Chai Y. Candida albicans cells lacking CaMCA1-encoded metacaspase show resistance to oxidative stress-induced death and change in energy metabolism. Fungal Genet Biol 2009; 46;183-89; PMID:19049890; http://dx.doi.org/10.1016/j.fgb.2008.11.001
- Shirtliff ME, Krom BP, Meijering RA, Peters BM, Zhu J, Scheper MA, Harris ML, Jabra-Rizk MA. Farnesol-induced apoptosis in Candida albicans. Antimicrob Agents Chemother 2009; 53:2392-401; PMID:19364863; http://dx.doi.org/10.1128/AAC.01551-08
- Al-Dhaheri RS, Douglas LJ. Apoptosis in Candida biofilms exposed to amphotericin B. J Med Microbiol 2010; 59:149-57; PMID:19892857; http://dx.doi.org/10.1099/jmm.0.015784-0
- Tsang P W-K, Wong A P-K, Yang H-P, Li N-F. Purpurin triggers caspase dependent-independent apoptosis in Candida dubliniensis biofilms. PLoS One 2013; 8: e86032; PMID:24376900; http://dx.doi.org/10.1371/journal.pone.0086032
- Pierce CG, Uppuluri P, Tristan AR, Wormley FL Jr, Mowat E, Ramage G, Lopez-Ribot JL. A simple and reproducible 96-well plate based method for the formation of fungal biofilms and its application to antifungal susceptibility testing. Nature protocols 2008; 3:1494-1500; PMID:18772877; http://dx.doi.org/10.1038/nprot.2008.141
- Pierce CG, Uppuluri P, Tummala S, Lopez-Ribot JL. A 96 well microtiter plate based method for monitoring formation and antifungal susceptibility testing of Candida albicans biofilms. J Vis Exp 2010; 44:e2287; PMID:21048668
- Ben-Ami R, Lewis RE, Tarrand J, Leventakos K, Kontoyiannis DP. Antifungal activity of colistin against Mucorales species in vitro and in a murine model of Rhizopus oryzae pulmonary infection. Antimicrob Agents Chemother 2010; 54:484-90; PMID:19858263; http://dx.doi.org/10.1128/AAC.00956-09