Abstract
Mucormycoses are fungal infections caused by the ancient Mucorales. They are rare, but increasingly reported. Predisposing conditions supporting and favoring mucormycoses in humans and animals include diabetic ketoacidosis, immunosuppression and haematological malignancies. However, comprehensive surveys to elucidate fungal virulence in ancient fungi are limited and so far focused on Lichtheimia and Mucor. The presented study focused on one of the most important causative agent of mucormycoses, the genus Rhizopus (Rhizopodaceae). All known clinically-relevant species are thermotolerant and are monophyletic. They are more virulent compared to non-clinically, mesophilic species. Although adaptation to elevated temperatures correlated with the virulence of the species, mesophilic strains showed also lower virulence in Galleria mellonella incubated at permissive temperatures indicating the existence of additional factors involved in the pathogenesis of clinical Rhizopus species. However, neither specific adaptation to nutritional requirements nor stress resistance correlated with virulence, supporting the idea that Mucorales are predominantly saprotrophs without a specific adaptation to warm blooded hosts.
Introduction
Zygomycetes belong to one of the oldest fungal groups on earth, with known fossils from the Middle Triassic of AntarcticaCitation1 and a diverging time calculated for their origin of around 600 mya years.Citation2 Contemporary descendants of these early ancestors can be found all over the world colonizing a wide range of ecological habitats, and are currently classified in several subphyla, namely Mucoromycotina, Kickxellomycotina, Zoopagomycotina, Mortierellomycotina,Citation3,4 and the phylum Entomophthoromycota.Citation5 Within the Mucoromycotina, the largest order Mucorales comprises predominantly saprotrophic inhabitants of soil and organic decaying matter. Some species are also able to parasitize on plants, insects and fungi, or they can be found as opportunistic pathogens of man and animals.
The mucoralean family Rhizopodaceae K. Schum. today encompasses 3 genera, namely Sporodiniella, Syzygites and Rhizopus. Although the family comprises only 11 species, saprotrophic, parasitic and pathogenic life-styles are represented within the Rhizopodaceae in a species-specific manner.
While Sporodiniella umbellata, sole species of its genus, is a facultative parasite of insect larvae,Citation6,7 Syzygites megalocarpus, also sole species of its genus, is parasitic on members of the Dikarya.Citation8 In contrast, Rhizopus species display a high variability of lifestyles and habitats. Being primarily saprotrophic fungi, several species are important plant-pathogens or spoilage agents of fresh and manufactured food e.g. soft rot caused by R. stolonifer, R. arrhizus (syn. R. oryzae) or R. microsporus.Citation9-12 Yet, Rhizopus plays also an important role in industrial biotransformations or food processing through fermentation, especially in Asia and Africa.Citation13-15
However, Rhizopus species are also the most common cause of life-threatening mucormycoses. Citation16-19 These infections often develop rapidly, predominately as rhinocerebral and pulmonary manifestations; and are often associated with dissemination and high mortality rates.Citation16-20 Although mucormycoses are uncommon fungal infections compared to aspergillosis or candidiasis, their incidence is increasing in clinical settings.Citation17,21 Major risk factors for mucormycoses are diabetic ketoacidosis, immunosuppression and malignancies.Citation17,18 In addition, infections have been found to be associated with administration of certain antifungals such as voriconazole or iron chelators like deferoxamine.Citation22,23
In addition to human predispositions, fungal prerequisites are also required for infection. Such virulence factors include pathways that facilitate adaptation to the host environment, e.g., to elevated temperatures, unfavoured pH, unbalanced osmotic conditions and nutrient limitation.Citation24 Furthermore, some morphological features are linked to virulence: e.g. fungal spore size is known to be related to fungal pathogenesis in Mucor circinelloides.Citation25,26 Finally, the relative burden of asexual spores in the environment might contribute to the establishment of mucormycoses. In Rhizopus the amount of spores produced differs between species and is known to be reduced for R. schipperae, a rare causative agent of mucormycosis.Citation16
Although mucormycoses are seen as emerging serious fungal infections, with a large number of case reports and studies concentrating on susceptibility to antifungal drugs,Citation27 comprehensive evaluations of the pathogenic potential at genus- or family level so far only exist for the Lichtheimiaceae.Citation28 In addition to evaluating fungal traits potentially involved in virulence we investigated the pathogenic potential of the Rhizopodaceae applying the embryonated chicken egg model, a model with proven suitability to assess the virulence potential of fungi, and Galleria mellonella as a second alternative infection model.Citation28-31
Results
Phylogeny, clinical relevance and infection model
The family Rhizopodaceae comprises the genera Rhizopus, Syzygites and Sporodiniella with few, closely related species (). Only species of the genus Rhizopus have clinical relevance, with R. arrhizus and R. microsporus predominantly described as potential agents of severe mucormycoses.Citation16,17 The species R. schipperae, R. caespitosus and R. homothallicus are less frequently observed in human infections.Citation32-34
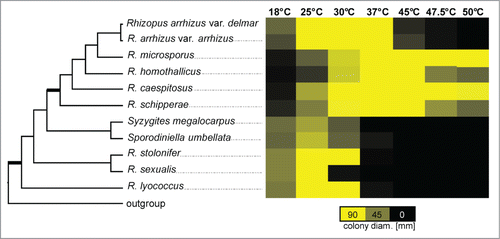
Figure 1. Schematic cladogram of the Rhizopodaceae, modified after Walther et al. 2013.Citation39 Bold branches show considerable bootstrap support (100 %) in the original phylogram which based on ITS sequences. The second part displays growth at different temperatures after 48 h. Maximum diameter possible for a colony is 90.00 mm, equal to the size of a petridish. The mean diameter was determined by 3 biological replicates, each with 3 technical replicates.
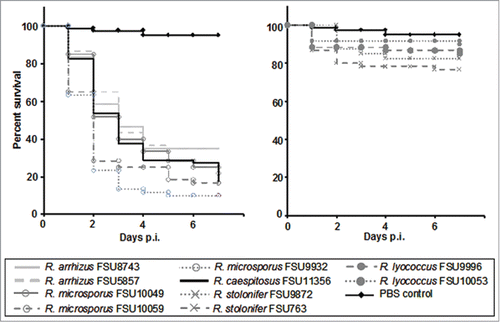
The virulence potential of the different Rhizopus species was determined in chicken embryos. The two most-common pathogenic species R. arrhizus and R. microsporus resulted in high mortality with survival rates of 35 % and 10–22 %, what is comparable to R. caespitosus with 17.5 % survival (). The less common clinical species R. homothallicus and R. schipperae produced only small amounts of spores. Therefore infection experiments were performed with a lower infection dose. Both species showed high mortality comparable to or even higher than R. microsporus (Fig. S1). Rhizopus schipperae is the most virulent strain tested in this study with 98 % mortality as early as 3 d after infection (Fig. S1). Mesophilic, non-clinical species were found less virulent than the thermotolerant, clinically relevant species with survival rates between 76–90 % at day 7 post infection ().
Figure 2. Virulence of different Rhizopus species in embryonated chicken eggs. Eggs were infected via the chorio-allantoic membrane at developmental day 10 using 106 spores (n = 20) from the thermotolerant species (left) and mesophilic species (right). Spore-depleted PBS was used as negative control. Survival was assessed daily over a period of 7 d post infection. Experiments were performed 3 times (except FSU 9872 which was performed twice). Kaplan-Meier-curves represent average survival rates.
To assess the variability of the virulence potential within a species, 17 additional strains of R. microsporus isolated from the environment, food and human patients () were tested in chicken embryos. No significant difference was found regarding their origin and their potential to cause lethal infections (Fig. S2). While some clinical isolates showed higher virulence than food isolates (e.g., CBS124669 [human] vs. CBS228.95 [tempeh] P= 0.5721), there were also isolates from tempeh with higher virulence compared to clinical isolates (e.g., CBS339.62 [tempeh] vs. CBS124669 [human] P = 0 .0428). Overall mortality ranged between 60 to 100 % (average 80 %) for all strains (Fig. S2).
Table 1. List of isolates of R. microsporus used for extension of the virulence test in chicken eggs to survey isolate specificity
Role of temperature adaptation
Growth at elevated temperatures is known to be an important virulence factor in several fungal pathogens. To investigate if thermotolerance of the different species correlated with virulence in the embryonated egg model, growth at different temperatures was determined. A clear shift in the temperature profiles between the virulent and attenuated species was found (). While the attenuated species grew well between 25°C and 30°C, the growth optimum for the virulent clade including R. microsporus, R. arrhizus, R. caespitosus, R. homothallicus and R. schipperae was 37°C or higher. The mesophilic R. stolonifer and R. lyococcus were able to germinate at 37°C, but did not grow well ().
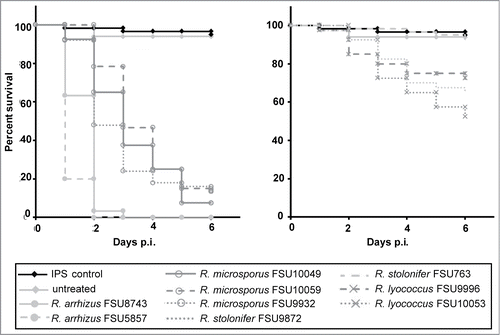
While thermotolerance is a prerequisite for a pathogen to cause infections in warm-blooded animals, additional virulence factors have been found to be involved in the infection process of fungal pathogens. To investigate whether the observed reduced virulence of the mesophilic Rhizopus species was caused only by their reduced thermotolerance, infection experiments were carried out using wax moth larvae. In contrast to the chicken embryos the larvae could be incubated at 30°C, a temperature at which the growth rate of the mesophilic species was comparable to or even higher than for the thermotolerant species (). Rhizopus arrhizus and R. microsporus as representatives of the thermotolerant species induced high mortality rates in Galleria (86–100 %) with R. arrhizus being significantly more virulent than R. microsporus (P < 0.0001; ). Yet, R. arrhizus was faster growing at this temperature than R. microsporus (), eventually supporting faster spreading within the larvae. The tested isolates of R. stolonifer and R. lyococcus were significantly less virulent than R. arrhizus and R. microsporus (P<0.0001; ). Despite the lower incubation temperature, the results from the Galleria experiments () resemble those from the chicken model (), indicating additional adaptations supporting virulence of the thermotolerant Rhizopus species.
Figure 3. Virulence of different Rhizopus species in Galleria mellonella. Twenty sixth-instar larvae per group were infected each via injection in the hemocoel with 106 spores of thermotolerant species (left panel) and mesophilic species (right panel). Spore-depleted IPS was used as negative control. Experiments were performed 3 times; curves represent average survival rates over a period of 6 d post infection.
Stress resistance and metabolic flexibility
In addition to adaptation to temperature, coping with arising stress conditions in the changing host environment is an important feature affecting virulence in fungal pathogens. Therefore, resistance toward osmotic stress and cell wall stress was tested. Thermotolerant and mesophilic species showed comparable susceptibility to the different stressors and no correlation was found between stress resistance and virulence of the species (Table S1).
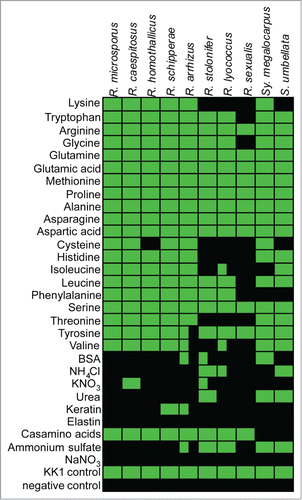
In order to survive in the host, pathogens have to be able to acquire nutrients from the resources within the host. Thus, metabolic flexibility might influence virulence. Therefore, we analyzed the utilization of different carbon- and nitrogen sources by Rhizopus species. As primary soil inhabiting fungi, all species tested were able to utilize carbon sources originating from living or decaying plant material like xylose, xylitol, pectin, cellobiose and common sugars or sugar alcohols like glucose, fructose, galactose, mannose, mannitol and sorbitol. Maltose and starch could not be utilized by R. stolonifer, R. sexualis and Syzygites. Sporodiniella was unable to use soluble starch. None of the tested species could use the complex polysaccharids xylan or cellulose (Table S2). Rhizopus caespitosus and Syzygites megalocarpus are the only fungal species tested capable to utilize citric acid, a common organic acid in mushrooms.Citation35
Within animal hosts fermentable sugars like glucose, fructose or galactose have often limited availability. All of them can be assimilated by all Rhizopus species. For alternative carbon sources only the amino acids arginine, tyrosine and partially phenylalanine were exclusively metabolized by the virulent species. Most of the other amino acids could not be metabolized by any Rhizopus species (Table S2).
A similar effect was observed when amino acids were used as sole nitrogen source (, Table S3). Thermotolerant species were generally able to utilize all 20 tested amino acids while mesophilic Rhizopus species lacked the ability to grow on several amino acids, including lysine, cysteine, histidine, isoleucine, threonine and valine. All other nitrogen sources tested revealed no obvious differences.
Infection-related morphological features
Since infections with Rhizopus species occur mainly in the respiratory tract, the small size of fungal spores may contribute to the success of fungal infections. In addition, fungal spore size is known to be related to fungal pathogenesis in Mucor circinelloides with larger spores being more virulent.Citation26 For the genus Rhizopus, spore size differs largely between species ranging from average volume of 28 μm3 to 555 μm3. Spores from thermotolerant species were in general smaller compared to spores from mesophilic species (). However, there was no correlation between spore size and virulence in the thermotolerant species.
Table 2. List of species used in this study. Strain numbers, origin, mating type, spore size and sequences generated for identification are given
In addition to spore size, the burden of fungal spores in the environment can be important for the development of mucormycoses as a high spore burden increases the likelihood that spores are inhaled in sufficient numbers to establish infection. In our artificial setting the relative amount of spores produced in a specific period of time differed considerably between Rhizopus species (Fig. S3). Within the thermotolerant species R. schipperae and the homothallic R. homothallicus produced the lowest number of spores. Generally, homothallic species (also R. sexualis) produced less asexual spores than heterothallic species.
Discussion
All recent phylogenetic analyses strongly support separation of mesophilic and thermotolerant species of the Rhizopodaceae (), although the relationship between the species in each supported group is not finally solved.Citation36-39 The mesophilic group contains species able to grow around 25°C to 30°C but displaying reduced growth rates at higher temperatures. However, the ability to grow at elevated temperatures of 37°C or above, as seen for the thermotolerant Rhizopus species (), is a prerequisite for colonization warm blooded hosts.
The genus Rhizopus exhibits the largest impact on mankind, being important in agriculture and industry and is furthermore the main causing agent of mucormycoses, followed by Lichtheimia and Mucor. All three genera being responsible for 70 to 80 % of the reported infections, predominantly as rhinocerebral, pulmonary or disseminated manifestations; associated with high mortality rates.Citation18-20 From the mesophilic species of the genus Rhizopus, only R. stolonifer can be found in clinical settings, but is seen rarely; mostly as agents of allergic alveolitis or superficial infections but being predominantly non-invasive.Citation16,40,41 For the thermotolerant species R. arrhizus and R. microsporus are reported more frequently in severe infections than any other species from the Rhizopodaceae.Citation19,42 In our study, R. schipperae, R. caespitosus and R. homothallicus displays a virulence potential comparable to R. arrhizus and R. microsporus (, Fig. S1), yet they were only isolated rarely from human infections.Citation32-34 This suggests that additional factors might be required for infections in humans. One of those aspects could be the abundance of species and the burden of fungal spores in human environment. Although all species of Rhizopus are distributed worldwide, and the natural habitats are similar for nearly all species, like soil, or on decaying organic matter including wood, and especially sugar-rich fruits,Citation16 they are isolated from environmental samples with different frequencies. Rhizopus arrhizus is found most frequently, followed by R. stolonifer and less frequently by R. microsporus.Citation16 While R. arrhizus and R. stolonifer are found to similar extends, the majority of infections is caused by R. arrhizus (50%) and R. microsporus (15–25%).Citation16,17,43 This could be explained by the lower virulence potential of R. stolonifer observed in this study. In contrast, R. schipperae, R. caespitosus and R. homothallicus appear to be less abundant in the environment, if judging from the few available specimens from public culture collections, or the fact, that R. schipperae is only known from 2 reported cases with no obvious natural substrate presented.Citation34 Furthermore, R. schipperae fails to sporulate on most artificial media,Citation16 which was confirmed in this study. If sporulation is also low in natural habitats, this could explain the few known isolates. Furthermore, low numbers of spores in the environment would likely result in very limited exposure of humans to R. schipperae, thereby explaining the limited number of reported human infections despite the significant virulence potential.
No clear correlation between virulence and special nutritional requirements or differences in the ability to cope with stress was observed in our study. Whether the observed small differences in the profiles of C- and N-sources contribute to virulence remains to be determined. A recent study of pathogenic Lichtheimia species likewise identified only few differences in nutritional requirements between strains.Citation28 For Lichtheimia and Rhizopus the carbon utilization profiles differ for raffinose, lactose, melibiose, inosine, glycine and pyruvate which could be utilized by Lichtheimia spp. but not by any Rhizopus spp. On the other hand, Rhizopus spp. are able to use glycerol and ethanol, whereas Lichtheimia spp do not (Table S2 and ref 28). Yet there are few amino acids which were exclusively used by thermotolerant Rhizopus species, a feature which could contribute to the survival within the host, but needs further studies.
Beside adaption to temperature or available nutrients, coping with arising stress conditions in the changing host environment affects virulence, as demonstrated for e.g., Candida albicans and Aspergillus fumigatus.Citation44–46 In our study, mesophilic species showed a trend toward higher tolerance to osmotic stress due to excess of sodium chloride, but without species-specific differences (Table S1). A similar concordance was observed between virulent and attenuated species of Lichtheimia.Citation28 No obvious differences could be observed for cell wall stresses, although the applied stress conditions generally led to reduced growth compared to normal conditions in Rhizopus. Yet, this is less pronounced than in other mucoralean pathogens (Table S1 and ref.Citation28). Nevertheless, no correlation between tolerance to stress and the observed virulence could be made, in contrast to virulence of evolutionary derived fungi like Candida.Citation44-46 Yet, pathogens of the derived fungi are often adapted to their hosts, whereas mucoralean fungi seem to be not, which could explain why there is not obvious difference in stress tolerance between potential pathogenic and non-pathogenic species.
An additional factor that could affect virulence of fungi is the ability to produce hydrolytic enzymes aiding in the degradation of host tissue, such as glycosidases, lipases and proteases. Previous comprehensive tests for the presence of gelatinase, urease, lipase, amylase, cellulase, laccase and tyrosinase within different isolates of R. microsporus sampled from various substrates (environment, food, clinical) revealed no differences in hydrolytic activity. In contrast, significant difference was observed in the production of the iron chelating compounds, the siderophores, by strains of food and clinical origin.Citation47 As siderophores are important for iron acquisition of some pathogens within the host,Citation48 this observation suggests a link between siderophore production and clinical relevance of strains. However, no correlation between the presence of siderophores or the origin of the isolateCitation47 and the observed virulence of R. microsporus strains was observed in this study.
Another interesting observation in recent studies on the virulence of Mucorales is the relation between differences in spore size and virulence, where larger spores produced by the minus mating type of Mucor circinelloides are more virulent than smaller spores produced by the plus mating type.Citation25,26 A second observation is that hyphal-stage of a fungus is more virulent than yeast-stage.Citation25 This study by Lee et al.Citation25 demonstrated for the first time, that morphogenesis is also linked to virulence in zygomycetes. Comparing spore size with the genus Rhizopus, no correlation to virulence could be made. Further studies on species level will reveal if spore size in mucoralean fungi is related to virulence as demonstrated for Mucor circinelloides. Recent studies revealed also the iron permease FTR1 and the surface protein cotH as known factors contributing to the virulence of these species.Citation23,53 However, both factors are present in a variety of mucoralean fungi and are not sufficient to explain the clinical importance of certain species. Comparative analyses of closely related virulent and non-virulent species may improve the understanding of the evolution of the pathogenicity mechanisms. Avian infection model-mediated virulence analysis yields objective results that overcome the disadvantages of mammalian infection models being time consuming, laborious and conflicting with ethic aspects. Therefore, it can be expected that the assessment of virulence of Rhizopus spp. applied to the embryonated hen egg infection model will play a crucial role in future investigations of host-pathogen interactions by the utilization of knock-out mutant-based identification of virulence factors. Future experiments should also include various, distinctly related zygomycetes to elucidate comparability of virulence factors with the background of long time speciation of microorganisms not specifically adapted to warm blooded hosts.
Material and Methods
Ethics statement
All experiments were performed in compliance with the European and German animal protection law. According to this, no specific approval is needed for work performed in avian embryos before the time of hatching. The experimental protocols were reviewed and approved in regard to ethical and welfare issues by the responsible animal welfare officer. Experiments were terminated latest on developmental day 18, 3 d before hatching, by chilling the eggs on ice for 30–60 min.
Fungal isolates
A total of 34 isolates of the family Rhizopodaceae were included in this study (). Strains were obtained from the Jena Microbial Resource Collection and from the Centraalbureau voor Schimmelcultures (CBS). Isolates were identified by standard microbiological procedures and by sequencing of 18S rDNA, 28S rDNA, and ITS regions. For DNA isolation strains were grown for 5–10 d on medium KK1, especially composed for Mucorales (1 % glucose, 0.44 % NaCl, 0.3 % KH2PO4, 0.125% K2HPO4, 0.2 % yeast extract, 0.1 % KNO3, 0.05 % MgSO4*7H2O, 0.05 % KCl (all Carl Roth)) at room temperature. DNA isolation, PCR and sequencing were conducted as described previously.Citation38 Primers used for amplification were: NL1 and NL4 (for 28S rDNA),Citation49 NS1 and NS4 (for 18S rDNA)Citation50 and ITS1 and ITS4 (for ITS).Citation50 Sequences generated in this study are given in .
Embryonated chicken egg model
Infections at developmental day 10 was done via the chorio-allantoic membrane as described previouslyCitation29,30 with 105 and 106 spores/egg. Twenty eggs were used for each strain. Experiments were performed 3 times, except for R. homothallicus FSU 2530 and R. schipperae FSU 10234 for which spore concentrations of 106 could not be reached and R. stolonifer FSU 9872 which was tested only twice. Rhizopus homothallicus and R. schipperae were tested for 3 times with 105 spores/egg in comparison to R. microsporus. Spore solution was prepared in PBS, which was used as negative control. Eggs were incubated at 37 °C; survival was assessed daily by candling. Pooled data was analyzed with GraphPad Prism v5.03 and displayed in and Fig. S1. To assess strain dependent differences, 17 additional isolates of R. microsporus were checked once (Fig. S2). Syzygites and Sporodiniella were not tested because they do not grow at elevated temperatures and did not produce the necessary amount of spores.
Galleria mellonella infection model
In order to test whether the virulence data observed in the chicken eggs correlate to the elevated temperature used for incubation and the different abilities of the strains to grow at this temperature; a second, widely accepted, infection model was applied. Rhizopus lyococcus and R. stolonifer were chosen as representatives of the mesophilic group, R. arrhizus and R. microsporus for the thermotolerant.
Sixth-instar larvae of Galleria mellonella (Kurt Pechmann, Langenzersdorf, Austria) were stored in the dark at 18°C prior to use. Larvae weighing between 0.3 and 0.4 g were used, each (n = 20) infected with 1 × 106 spores. Inocula were diluted in insect physiological saline (IPS) and a volume of 20 μl was injected into the hemocoel via the hind pro-leg. Untouched larvae and larvae injected with 20 μl of IPS served as control. Larvae were incubated at 30°C, respectively, in the dark and monitored daily up to 6 d Significance of mortality rates was evaluated by using Kaplan-Meier survival curves with the PRISM statistics software (Mantel-Cox log rank test) using pooled data. All experiments were performed 3 times, each time with duplicates. Survival rates are displayed in . Syzygites and Sporodiniella were not tested because they did not produce enough spores.
Relation of growth and temperature
Petridishes with medium KK1 were inoculated with 10 μl spore suspension containing 1000 spores. In cases of growth, the initial colony was 6 mm in diameter. Sporodiniella umbellata and Syzygites megalocarpus were inoculated as agar slants with 6 × 6 mm. Plates were incubated at different temperatures. The diameter was measured 2 times a day across 3 defined lines at the bottom of the petridishCitation51 for 3 technical replicates. The mean diameters of 3 biological replicates at 48 h are given in . Maximum diameter possible is 90.00 mm, equal to the size of the petridish.
Relation of growth and stress conditions
Petridishes with medium KK1 were supplemented with 1 M NaCl, 1.5 M NaCl, 30 μg/ml SDS, 7.5 mM caffeine, 100 μg/ml CongoRed (all Carl Roth). Petridishes were inoculated with 1000 spores in 10 μl. Plates were incubated at 30°C (25°C for Sporodiniella umbellata, Syzygites megalocarpus, Rhizopus sexualis). The relative growth [%] compared to medium without stress inducers of 3 replicates at 24 h (48 h for Sporodiniella umbellata, Syzygites megalocarpus) is given in Table S1.
Carbon and nitrogen assimilation profiles
Agar plates with medium MM (0.5 % (NH4)2SO4, 0.05 % MgSO4, 0.1 % KH2PO4, 2 % agar), supplemented with 0.2 % carbon source were inoculated with 2 × 105 spores in 20 μl and incubated at 30°C (25°C for Sporodiniella umbellata, Syzygites megalocarpus, Rhizopus sexualis) for 3–4 d Evaluation of growth was performed visually and categorized in: inhibition (−), growth arrest after germination (0/−), no growth (0, but this includes ´background` growth due to carbon traces contained in the agar), slight or no growth (0/+, difficult to distinguish from the ´background` growth), weak growth (+), normal growth (++), strong growth (+++, similar to the glucose containing media), stronger growth (++++) (Table S6). Since Syzygites and Sporodiniella did not grow in appropriate time on this medium, a different medium (10 mM KH2PO4/K2HPO4 (pH6.6), 1.25 mM MgSO4*7H2O, 0.3 mM ZnSO4*7H2O, 0.09 mM FeCl3*6H2O, 0.03 mM CuSO4*5H2O (all Carl Roth), modified after ref 52) was used to analyze a reduced second set of carbon sources. This time, liquid media was used in 96-well plates. Each well was supplemented with a carbon source and 500 spores. Plates were incubated for up to 6 d at optimal temperatures (37 °C, 30 °C, room temperature). Experiment was done up to 3 times, each time with triplicates; except for species where no differences between isolates were observed. In those cases only 2 repetitions were performed. After incubation the plates were analyzed visually for growth (p) or lack of growth (0). Weak growth was considered negative because of the difficulty in differentiation from background growth. Additionally chitin, pectin, citric acid and cellulose were tested as carbon sources. Results are supplemented in Table S2.
The liquid medium was also used to analyze the nitrogen utilization profile. Growth was evaluated after 70–88 h at appropriate growth temperatures. Results are shown in Table S3.
Size of sporangiospores and amount of spores
The size of the spores for each species was determined according to standard rules after harvest from KK1 medium cultivated for 5 d under optimal growth conditions ().
R. schipperae is known to produce fewer spores on artificial media. To assess the relative amount of spores produced in a specific period of time R. schipperae was cultivated on KK1 medium (petridish with 5.5 cm diam.) for 3 days, at appropriate temperatures (room temperature, 30°C or 37°C). Spores were harvested by extensive washing with PBS and counted in a haemocytometer. Mean spore burden out of 3 replicates is given in Figure S3.
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
1029219_Suplemental_files.zip
Download Zip (275.8 KB)Acknowledgment
We thank Birgit Weber (HKI, Jena) for excellent technical assistance in the performance of the virulence tests in the embryonated hen egg model and Caroline Hörtnagl (Medical University Innsbruck) for valuable help with the Galleria infection model. We thank Domenica Schnabelrauch (MPI Chemical Ecology, Jena) for technical support in DNA sequencing.
Funding
Research of INy and TP was supported by the grants OTKA PD101613 and OTKA NN106394, respectively.
References
- Krings M, Taylor TN, Dotzler N, Persichini G. Fossil fungi with suggested affinities to the Endogonaceae from the Middle Triassic of Antarctia. Mycologia 2012; 104:835-44; PMID:22453117; http://dx.doi.org/10.3852/11-384
- Berbee ML, Taylor JW. Fungal molecular evolution: gene trees and geologic time. In: McLaughlin DJ, McLaughlin EG, Lemke PA, editors. The Mycota. Vol. VII. Part B. Systematics and Evolution. Berlin: Springer Verlag; 2001. page 229-45
- Hibbett DS, Binder M, Bischoff JF, Blackwell M, Cannon PF, Eriksson OE, Huhndorf S, James T, Kirk PM, Lücking R, et al. A higher-level phylogenetic classification of the Fungi. Mycol Res 2007; 111:509-47; PMID:17572334; http://dx.doi.org/10.1016/j.mycres.2007.03.004
- Hoffmann K, Voigt K, Kirk PM. Mortierellomycotina subphyl. nov., based on multi-gene genealogies. Mycotaxon 2011; 115:353-63; http://www.mycotaxon.com
- Humber RA. Entomophthoromycota: a new phylum and reclassification of entomophthoroid fungi. Mycotaxon 2012; 120:477-92; http://www.mycotaxon.com
- Evans HC, Samson RA. Sporodiniella umbellata, an entomogenous fungus of the Mucorales from cocoa farms in Ecuador. Can J Bot 1977; 55:2981-84; http://dx.doi.org/10.1139/b77-334
- Chien C-Y, Huang B-C. First record of the occurance of Sporodiniella umbellata Mucorales in Taiwan. Mycoscience 1997; 38:343-46; http://link.springer.com/article/10.1007%2FBF02464094
- Kovacs RL, Sundberg WJ. Syzygites megalocarpus (Mucorales, Zygomycetes) in Illinois. T Illinois Acad Sci 1999; 92:181-90; http://ilacadofsci.com/archives/2432
- Fajola AO. The post-harvest fruit rots of tomato (Lycopersicum esculentum) in Nigeria. Nahrung 1979; 23:105-9; PMID:471028
- Shtienberg D. Rhizopus head rot of confectionery sunflower: effects on yield quantity and quality and implications for disease management. Phytopathology 1997; 87:1226-32; PMID:18945022; http://dx.doi.org/10.1094/PHYTO.1997.87.12.1226
- Lackner G, Partida-Martinez LP, Hertweck C. Endofungal bacteria as producers of mycotoxins. Trends Microbiol 2009; 17:570-76; PMID:19800796; http://dx.doi.org/10.1016/j.tim.2009.09.003
- Kwon JH, Kim J, Kim WI. First Report of Rhizopus oryzae as a postharvest pathogen of apple in Korea. Mycobiology 2011; 39:140-42; PMID:22783094; http://dx.doi.org/10.4489/MYCO.2011.39.2.140
- Nout MJR, Kiers JL. Tempe fermentation, innovation and functionality: update into the third millennium. J Appl Microbiol 2005; 98:789-805; PMID:15752324; http://dx.doi.org/10.1111/j.1365-2672.2004.02471.x
- Nout MJ. Rich nutrition from the poorest - cereal fermentations in Africa and Asia. Food Microbiol 2009; 26:685-92; PMID:19747601; http://dx.doi.org/10.1016/j.fm.2009.07.002
- Iqbal Choudhary M, Mohammad MY, Musharraf SG, Onajobi I, Mohammad A, Anis I, Shah MR, Atta-Ur-Rahman. Biotransformation of clerodane diterpenoids by Rhizopus stolonifer and antibacterial activity of resulting metabolites. Phytochemistry 2013; 90:56-61; PMID:23535269; http://dx.doi.org/10.1016/j.phytochem.2013.02.007
- Ribes JA, Vanover-Sams CL, Baker DJ. Zygoymcetes in human disease. Clin Microbiol Rev 2000; 13:236-301; PMID:10756000; http://dx.doi.org/10.1128/CMR.13.2.236-301.2000
- Roden MM, Zaoutis TE, Buchanan WL, Knudsen TA, Sarkisova TA, Schaufele RL, Sein M, Sein T, Chiou CC, Chu JH, et al. Epidemiology and outcome of zygomycosis: A review of 929 reported cases. Clin Infect Dis 2005; 41:634-53; PMID:16080086; http://dx.doi.org/10.1086/432579
- Skiada A, Pagano L, Groll A, Zimmerli S, Dupont B, Lagrou K, Lass-Florl C, Bouza E, Klimko N, Gaustad P, et al. Zygomycosis in Europe: analysis of 230 cases accrued by the registry of the European Confederation of Medical Mycology (ECMM) Working Group on Zygomycosis between 2005 and 2007. Clin Microbiol Infect 2011; 17:1859-67; PMID:21199154; http://dx.doi.org/10.1111/j.1469-0691.2010.03456.x
- Lanternier F, Dannaoui E, Morizot G, Elie C, Garcia-Hermoso D, Huerre M, Bitar D, Dromer F, Lortholary O, French Mycosis Study Group. A global analysis of mucormycosis in France: The RetroZygo Study (2005–2007). Clin Infect Dis 2012; 54 (Suppl 1): S35-43; PMID:22247443; http://dx.doi.org/10.1093/cid/cir880
- Gomes MZ, Lewis RE, Kontoyiannis DP. Mucormycosis caused by unusual mucormycetes, non-Rhizopus, -Mucor, and -Lichtheimia species. Clin Microbiol Rev 2011; 24:411-45; PMID:21482731; http://dx.doi.org/10.1128/CMR.00056-10
- Greenberg RN, Scott LJ, Vaughn HH, Ribes JA. Zygomycosis (mucormycosis): emerging clinical importance and new treatments. Curr Opin Infect Dis 2004; 17:517-25; PMID:15640705
- Lamaris GA, Ben-Ami R, Lewis RE, Chamilos G, Samonis G, Kontoyiannis DP. Increased virulence of zygomycetes organisms following exposure to voriconazole: a study involving fly and murine models of zygomycosis. J Infect Dis 2009; 199:1399-1406; PMID:19358672; http://dx.doi.org/10.1086/597615
- Ibrahim AS, Spellberg B, Edwards J Jr. Iron acquisition: a novel perspective on mucormycosis pathogenesis and treatment. Curr Opin Infect Dis 2008; 21:620-25; PMID:18978530; http://dx.doi.org/10.1097/QCO.0b013e3283165fd1
- Cooney NM, Klein BS. Fungal adaptation to the mammalian host: it's a new world, after all. Curr Opin Microbiol 2008; 11:511-16; PMID:18955154; http://dx.doi.org/10.1016/j.mib.2008.09.018
- Lee SC, Li A, Calo S, Heitman J. Calcineurin plays key roles in the dimorphic transition and virulence of the human pathogenic zygomycete Mucor circinelloides. PLoS Pathogens 2013; 9:e1003625; PMID:24039585; http://dx.doi.org/10.1371/journal.ppat.1003625
- Li CH, Cervantes M, Springer DJ, Boekhout T, Ruiz-Vázquez RM, Torres-Martínez SR, Heitman J, Lee SC. Sporangiospore size dimorphism is linked to virulence of Mucor circinelloides. PLoS Pathogens 2011; 7:e1002086; PMID:21698218; http://dx.doi.org/10.1371/journal.ppat.1002086
- Alastruey-Izquierdo A, Castelli MV, Cuesta I, Monzon A, Cuenca-Estrella M, Rodriguez-Tudela JL. Activity of posaconazole and other antifungal agents against Mucorales strains identified by sequencing of internal transcribed spacers. Antimicrob Agents Chemother 2009; 53:1686-89; PMID:19171801; http://dx.doi.org/10.1128/AAC.01467-08
- Schwartze VU, Hoffmann K, Nyilasi I, Papp T, Vágvölgyi C, de Hoog S, Voigt K, Jacobsen ID. Lichtheimia species exhibit differences in virulence potential. PLoS One 2012; 7:e40908; PMID:22911715; http://dx.doi.org/10.1371/journal.pone.0040908
- Jacobsen ID, Grosse K, Slesiona S, Hube B, Berndt A, Brock M. Embryonated eggs as an alternative infection model to investigate Aspergillus fumigatus virulence. Infect Immun 2010; 78:2995-3006; PMID:20421382; http://dx.doi.org/10.1128/IAI.00268-10
- Olias P, Gruber AD, Hafez HM, Lierz M, Slesiona S, Brock M, Jacobsen ID. Molecular epidemiology and virulence assessment of Aspergillus fumigatus isolates from white stork chicks and their environment. Vet Microbiol 2011; 148:348-55; PMID:20880638; http://dx.doi.org/10.1016/j.vetmic.2010.08
- Chamilos G, Lionakis MS, Lewis RE, Kontoyiannis DP. Role of mini-host models in the study of medically important fungi. Lancet Infect Dis 2007; 7:42-55; PMID:17182343; http://dx.doi.org/10.1016/S1473-3099(06)70686-7
- Anstead GM, Sutton DA, Thompson EH, Weitzman I, Otto RA, Ahuja SK. Disseminated zygomycosis due to Rhizopus schipperae after heatstroke. J Clin Microbiol 1999; 37:2656-62; PMID:10405417
- Chakrabarti A, Marak RSK, Shivaprakash MR, Gupta S, Garg R, Sakhuja V, Singhal S, Baghela A, Dixit A, Garg MK, Padhye AA. Cavitary pulmonary zygomycosis caused by Rhizopus homothallicus. J Clin Microbiol 2010; 48:1965-69; PMID:20200286; http://dx.doi.org/10.1128/JCM.01272-09
- Weitzman I, McGough DA, Rinaldi MG, Della-Latta P. Rhizopus schipperae spp nov., a new agent of zygomycosis. Mycotaxon 1996; 59:217-25; http://www.mycotaxon.com
- Valentão P, Lopes G, Valente M, Barbosa P, Andrade PB, Silva BM, Baptista P, Seabra RM. Quantitation of nine organic acids in wild mushrooms. J Agric Food Chem 2005; 53:3626-30; PMID:15853411; http://dx.doi.org/10.1021/jf040465z
- Abe A, Asano K, Sone T. A molecular phylogeny-based taxonomy of the genus Rhizopus. Biosci Biotechnol Biochem 2010; 74:1325-31; PMID:20622457; http://dx.doi.org/10.1271/bbb.90718
- Abe A, Oda Y, Asano K, Sone T. The molecular phylogeny of the genus Rhizopus based on rDNA sequences. Biosci Biotechnol Biochem 2006; 70:2387-93; PMID:17031057; http://dx.doi.org/10.1271/bbb.60101
- Hoffmann K, Pawłowska J, Walther G, Wrzosek M, de Hoog GS, Benny GL, Kirk PM, Voigt K. The family structure of the Mucorales: a synoptic revision based on comprehensive multigene-genealogies. Persoonia 2013; 30:57-76; PMID:24027347; http://dx.doi.org/10.3767/003158513´666259
- Walther G, Pawłowska J, Alastruey-Izquierdo A, Wrzosek M, Rodriguez-Tudela JL, Dolatabadi S, Chakrabarti A, de Hoog GS. DNA barcoding in Mucorales: an inventory of biodiversity. Persoonia 2013; 30:11-47; PMID:24027345; http://dx.doi.org/10.3767/003158513´665070
- O'Connell MA, Pluss JL, Schkade P, Henry AR, Goodman DL. Rhizopus-induced hypersensitivity pneumonitis in a tractor driver. J Allergy Clin Immunol 1995; 95:779-81; PMID:7897166
- Wimander K, Belin L. Recognition of allergic alveolitis in the trimming department of a Swedish sawmill. Eur J Respir Dis Suppl 1980; 61:163-69; PMID:6934078
- Scholer HJ, Müller E, Schipper MAA. Mucorales. In Howard DH, editior. Fungi pathogenic for humans and animals. Part A. Biology. New York, NY: Marcel Dekker; 1983. page 9-59
- Alvarez E, Sutton DA, Cano J, Fothergill AW, Stchigel A, Rinaldi MG, Guarro J. Spectrum of zygomycete species identified from clinically significant specimens in the United States. J Clin Microbiol 2009; 47:1650-56; PMID:19386856; http://dx.doi.org/10.1128/JCM.00036-09
- Bates S, Hughes HB, Munro CA, Thomas WPH, MacCallum DM, Bertram G, Atrih A, Ferguson MAJ, Brown AJP, Odds FC, Gow NAR. Outer chain N-glycans are required for cell wall integrity and virulence of Candida albicans. J Biol Chem 2005; 281:90-8; PMID:16263704; http://dx.doi.org/10.1074/jbc.M510360200
- Duran R, Cary JW, Calvo AM. Role of the osmotic stress regulatory pathway in morphogenesis and secondary metabolism in filamentous fungi. Toxins 2010; 2:367-81; PMID:22069590; http://dx.doi.org/10.3390/toxins2040367
- Nakagawa Y, Kanbe T, Mizuguchi I. Disruption of the human pathogenic yeast Candida albicans catalase gene decreases survival in mouse-model infection and elevates susceptibility to higher temperature and to detergents. Microbiol Immunol 2003; 47:395-403; PMID:12906099; http://dx.doi.org/10.1111/j.1348-0421.2003.tb03376.x
- Dolatabadi S, Walther G, Gerrits van den Ende AHG, de Hoog GS. Diversity and delimitation of Rhizopus microsporus. Fungal Diversity 2014; 64:145-63; http://dx.doi.org/10.1007/s13225-013-0229-6
- Symeonidis AS. The role of iron and iron chelators in zygoymcosis. Clin Microbiol Infect 2009; S5:26-32; PMID:19754753; http://dx.doi.org/10.1111/j.1469-0691.2009.02976.x
- O'Donnell K. Fusarium and its near relatives. In: Reynolds DR, Taylor JW, editiors. The Fungal Holomorph: Mitotic, meiotic and pleomorphic speciation in fungal systematics. Wallingford, UK: CAB International; 1993. page 225-33
- White TJ, Bruns T, Lee S, Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR protocols: a guide to methods and applications. San Diego: Academic Press; 1990. page 315-22
- Rosenberg SL. Temperature and pH optima for 21 species of thermophilic and thermotolerant fungi. Can J Microbiol 1975; 21:1535-40; PMID:138; http://dx.doi.org/10.1139/m75-225
- Sorenson WG, Hesseltine CW. Carbon and nitrogen utilization by Rhizopus oligosporus. Mycologia 1966; 58:681-89; PMID:5976683; http://www.jstor.org/stable/3756843
- Gebremariam T, Liu M, Luo G, Bruno V, Phan QT, Waring AJ, Edwards JE Jr, Filler SG, Yeaman MR, Ibrahim AS. CotH3 mediates fungal invasion of host cells during mucormycosis. J Clin Invest 2014; 124:237-50; PMID:24355926; http://dx.doi.org/10.1172/JCI71349