Abstract
Cholesterol catabolism is thought to be a key factor contributing to the pathogenesis of Mycobacterium tuberculosis. Previous epistasis and mutant screening studies predicted that the P55 efflux pump (Rv1410c) positively interacts with the Mce4 transporter, a major cholesterol import system of M. tuberculosis and is needed for optimal growth in vitro, in macrophages, and in vivo. Using a combination of cell growth kinetic techniques, cholesterol consumption, and [4–14C]cholesterol uptake studies, we demonstrated that the Mycobacterium bovis BCG rv1410c gene indeed is needed for optimal in vitro growth on cholesterol and other carbon sources. Our data, together with previous predictions, support hypotheses that the P55 efflux pump functions in maintaining general metabolism or as a subunit of the Mce4 transport apparatus (catalyzing its assembly or providing cell wall integrity) to allow more efficient cholesterol uptake.
Importance
Tuberculosis (TB) remains one of the most devastating infectious diseases in the world killing 1.3 million people in 2012. Current TB treatment takes 6 months with cure rates of 87% under optimal conditions. The emergence of drug resistant strains makes successful treatment even more challenging. Alternative strategies are urgently needed to treat TB, including multidrug resistant infections.
Cholesterol catabolism by Mycobacterium tuberculosis, the causative agent of TB, is essential for virulence. Our observations show that the mycobacterial P55 efflux pump, which provides resistance to the first-line anti-TB drug rifampin, is needed for optimal growth of M. tuberculosis on cholesterol. This helps explain the increasing body of evidence showing its role in virulence, and suggests an alternative drug development strategy. P55 inhibitors would improve the activity of currently used antibiotics and assist the immune system to clear the infection.
Introduction
The ability of M. tuberculosis to use cholesterol as a carbon source is a key element contributing to its pathogenesis.Citation1 Analyses of cholesterol-grown Rhodococcus sp. strain RHA1, a soil bacterium related to M. tuberculosis, revealed that 51 rhodococcal genes specifically expressed during growth on cholesterol were conserved within an 82-gene cluster in M. tuberculosis H37Rv and Mycobacterium bovis bacillus Calmette–Guerin (BCG).Citation2 These studies provided the first indication of a cholesterol catabolic pathway critical for intracellular survival, and led to the identification of the Mce4 system, a multi-subunit ABC-like transporter functioning in cholesterol uptake by Rhodococcus,Citation3 Mycobacterium smegmatis Citation4 and M. tuberculosis.Citation5 The Mce4 transporter is essential for survival during the chronic stages of infection. In fact, M. tuberculosis with mutations in the mce4 operon had a marked growth defect in media containing cholesterol as the sole carbon sourceCitation5 and reduced survival during the chronic stage of murine in vivo infection.Citation6 Joshi et al. Citation7 defined functional genetic interactions with mce4 by identifying mutations that not only share the same phenotypes in vivo but also had much reduced effects in mce mutant backgrounds (positive interactions). Positive genetic interactions are often found among genes that lie within the same pathway. This genetic interaction mapping approach predicted mycobacterial cholesterol catabolic virulence genes that affect the activities of genes in the mce locus such as mceG (rv0655) or the igr locus (rv3545c to rv3540c). The igr genes are required for intracellular growth and igr-deficient strains are unable to grow on cholesterol even in the presence of alternative carbon sources, suggesting the accumulation of toxic cholesterol metabolic intermediates.Citation8 Joshi et al.Citation7 also reported that the gene encoding the P55 efflux pump (rv1410c) positively interacted with the mce1 and mce4 gene clusters in vivo, suggesting that they depend on P55 to provide a shared function.
P55 is a mycobacterial efflux pump belonging to the Major Facilitator Superfamily (MFS) of membrane transporters.Citation9 We previously demonstrated that P55 has a role in at least 3 important processes: (i) it exports and thus provides resistance to several drugs (including the first-line anti-tuberculosis drug rifampin), (ii) it is involved in the maintenance of cellular redox balance, and (iii) it is needed to maintain normal growth characteristics both on solid and liquid media.Citation10 The rv1410c gene encoding the P55 efflux pump forms an operon with rv1411c, encoding the LprG lipoprotein. LprG is essential for normal expression of surface lipoarabino-mannans (LAM). Surface-exposed LAMs allow interaction of M. tuberculosis with mannose receptors on macrophages, providing a specific adsorption process, inhibition of phagosome-lysosome fusion, and intracellular survival that may explain their critical role in virulence.Citation11 The rv1411c-rv1410c operon is required for survival in the presence of toxic compounds and for maintenance of a normal cell surface composition in M. smegmatis,Citation12 in M. bovis BCG Citation10 and in M. tuberculosis.Citation13 Its deletion also results in strong attenuation of M. tuberculosis in vivo.Citation14 This observation is reinforced by high throughput screening studies indicating that transposon mutants of rv1410c gene are attenuated in vivo Citation6 and in macrophages.Citation15 From a total of 194 genes that were specifically required for mycobacterial growth in vivo, the rv1410c transposon mutant was among the most attenuated, ranking 7th, 3rd, 2nd and 2nd after 1, 2, 4 and 8 weeks of infection.Citation6 Similarly, from a total of 126 genes specifically required for mycobacterial growth in macrophages in any of the experimental conditions tested, i.e., unactivated, pre-activated or post-activated macrophages with interferon gamma, the P55 mutant ranked 6th, 4th and 2nd, respectively.Citation15 These 2 studies strongly suggest a role for P55 in the late stages of infection and, together with the strong positive genetic interaction of the rv1410c gene with the mce genes reported by Joshi et al.Citation7 led us to hypothesize a potential role of the P55 efflux pump in cholesterol catabolism.
Results
To validate these observations, we analyzed the contribution of the P55 efflux pump to support in vitro growth on cholesterol. Studies were performed using M. bovis BCG, a strain often used as a model for M. tuberculosis research. We used a previously described M. bovis BCG P55 mutant and its genetic derivatives Citation10 and two cholesterol concentrations of 0.5 and 0.05 mM in polystyrene rolling bottles. Other studies have used optical density (OD) values as the readout for bacterial growth on cholesterol.Citation5,8,16 However, these measurements can be confounded by cholesterol precipitation. After a few days of incubation, cholesterol precipitation can variably increase OD up to 0.6 units compromising data reproducibility (data not shown). We thus used protein content determinations to obtain a more reliable measurement of growth. Our previous studies with the M. bovis BCG P55 efflux pump mutant described a growth defect in standard 7H9 broth supplemented with either dextrose or glycerol as carbon sources.Citation10 Here we show that the P55 mutant displayed a similar growth defect using acetate (with or without cholesterol) as carbon source, suggesting that general metabolism of the P55 mutant is compromised (). Importantly, this growth defect was markedly enhanced when cholesterol was the sole carbon source (); under these conditions the P55 mutant strain exhibited an extended lag phase and its growth rate was lower (). These phenotypes could be fully complemented by the introduction of the p55 efflux pump gene (rv1410c) in trans.
Figure 1. Growth kinetics of M. bovis BCG wild-type, P55 mutant (KOP55) and complemented (KOP55c) strains on cholesterol. Ten mM acetate and/or 0.5 (full lines) and 0.05 mM (dotted lines) cholesterol were used as carbon sources. (A) Growth kinetics were monitored by total protein measurements. Values are the mean, and error bars indicate standard deviations of 3 technical replicates from one typical biological experiment; (B) the mean of values displayed in A are plotted as logarithmic ratios (cholesterol or cholesterol plus acetate vs. acetate) to show KOP55 cholesterol specific growth deficiencies; (C) the cholesterol concentrations (µM) were measured in culture supernatant and standardized to the number of cells (Log cfu/mL). Values represent single time points and are representative of 2 independent experiments.
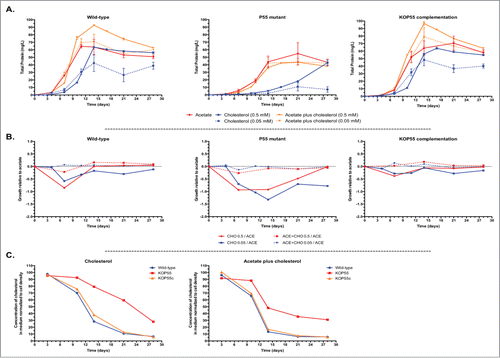
To further confirm that the mutant strain had a decreased ability to use cholesterol, we analyzed the cholesterol concentration in the supernatants at different times during the mycobacterial growth curve. After 14 days, a high proportion of the initial cholesterol was still present in the P55 mutant supernatant while both wild-type and complemented strains had consumed more than half of the cholesterol in the medium. After 21 days, when wild-type and complementation cells had already reached stationary phase, only a small fraction of the initial cholesterol was found in their supernatants; in contrast, more than half of the initial cholesterol was still found in the supernatant of the mutant (). To test whether the mutant's growth delay on cholesterol was linked to reduced uptake, we performed cholesterol accumulation assays as described previously.Citation3 Uptake experiments demonstrated that after 20 minutes the P55 mutant cells accumulated ∼25% less [4–14C]cholesterol than the wild-type cells (), consistent with its growth defect. In summary, growth kinetics, uptake assays and cholesterol consumption (either with or without acetate) confirmed that the P55 mutant had defects when grown on a variety of carbon sources and that this general growth defect was increased when growing solely on cholesterol.
Discussion
Although our growth kinetics studies demonstrated that the P55 efflux pump was needed for optimal growth on cholesterol in vitro (), the gene encoding the P55 efflux pump (rv1410c) was not identified in a previous screen for genes essential for growth on cholesterol.Citation17 This is consistent with our growth kinetic analyses of the P55 mutant demonstrating that it had a dramatic fitness reduction on cholesterol but was able to grow after prolonged incubation. The fact that P55 is able to export toxic compounds such as antibiotics raised the possibility that it might export toxic intermediates of cholesterol metabolism observed in other cholesterol catabolic mutants.Citation8 If so, growth inhibition caused by toxic cholesterol metabolites would also be observed in cultures co-metabolizing acetate, a carbon source that supports more rapid growth. However, the mutant was able to grow normally when both acetate and cholesterol were present in the medium (), and cholesterol was being consumed. This suggested that accumulation of bactericidal toxic cholesterol catabolites was not the main cause of the phenotype observed for the P55 mutant. Interestingly, the presence of acetate in the medium enhanced cholesterol consumption and growth not only in the mutant but also in the wild-type strain (). Unlike other genera that display diauxic growth kinetics when cultured in the presence of more than one carbon source, mycobacteria are able to catabolize multiple carbon sources simultaneously to achieve enhanced monophasic growth;Citation18 thus, the observed effect could be due to a more efficient cholesterol catabolism in the presence of acetate.
The requirement for P55 to support optimal growth on cholesterol, a primary carbon source in vivo, correlates with previous studies showing a potential virulence role of P55 in chronic in vivoCitation6 mouse modelsCitation6 and during macrophage infection.Citation15 In fact, the rv1410c gene has a positive genetic interaction with the mce4 locus, suggesting a role in the same pathway and function dependencyCitation7 and an M. tuberculosis mce4 mutant grows more slowly on cholesterol,Citation5 suggesting alternative cholesterol importers. Efflux pumps such as P55 are known to confer drug resistance. It is also evident that the primary role of MFS proteins is related to physiological cell homeostasis (metabolite import or export) or cell envelope assembly; for pathogenic bacteria these also affects virulence.Citation19 MFS transporters include proton-drug antiporters that export a wide range of compounds. Since the P55 mutation affects growth on many carbon sources, its primary role is not likely to be uptake of these carbon sources, and slow cholesterol uptake in the mutant would be an indirect effect. Integrity of the rv1411c-rv1410c operon is essential for normal cell surface structure in M. smegmatis,Citation12 in M. bovis BCG Citation10 and in M. tuberculosis.Citation20 Studies have also shown that accumulation of cholesterol by the tubercle bacilli affects cell wall permeability.Citation16 Thus, P55 could be a subunit of the larger Mce4 transport apparatus or provide cell wall integrity by lipid transport in conjunction with LprG. In fact, catabolism of cholesterol has important effects on the structure and abundance of bacterial components critical for virulence, such as the cell wall lipid phthiocerol dimycocerosate (PDIM);Citation21 this could also help explain the proposed avirulent phenotype of the P55 efflux pump mutant.Citation6,15
Conclusions
Our results show that mutations in the p55 gene preferentially affect growth on cholesterol, an important carbon source for virulence of M. tuberculosis. Further in depth biochemical and growth kinetic studies using a wide variety of carbon sources are needed to fully decipher the mechanisms underlying this phenotype. The development of new therapies for bacterial infections has traditionally focused on empirical screening for single compounds that inhibit growth in vitro. Our study suggests an alternative strategy in which multiple cellular functions might be inactivated by a single drug, a P55 inhibitor. Our data demonstrate the role of P55 in growth on cholesterol and other carbon sources.Citation10 In addition, there is an increasing body of evidence suggesting a role of the P55 efflux pump in virulence, especially during chronic infection.Citation6,15 The P55 efflux pump also provides resistance to the first-line anti-TB drug rifampin and other antibiotics.Citation10 Therefore, the development of P55 inhibitors would provide a dual strategy for drug discovery and development. It could not only increase the activities of currently used antibiotics, but also would compromise cholesterol metabolism and perhaps LprG lipoprotein function,Citation11 thus inhibiting in vivo bacterial growth and assisting the immune system to clear the infection faster. This is especially important for treatment of chronic infection, the stage of the disease most refractory to therapy.
Materials & Methods
Strains and general growth conditions
M. bovis BCG strains used in this study (wild-type, P55 mutant and complementation) are described elsewhere.Citation10 Strains were routinely propagated at 37°C in Middlebrook 7H9 broth (Difco) supplemented with 10% Middlebrook albumin-dextrose-catalase (Difco), 0.2% glycerol and 0.05% (vol/vol) Tyloxapol or on Middlebrook 7H10 agar plates (Difco) supplemented with 10% (vol/vol) oleic acid albumin-dextrose- catalase (Difco).
Growth on cholesterol
Cells were incubated for up to 35 d in either polycarbonate or polystyrene (490 cm2, Corning®) roller bottles in 7H9 broth (Difco) supplemented with 10 mM acetate and/or 0.5 mM or 0.05 mM cholesterol as sole carbon sources plus 0.5% (vol/vol) Tyloxapol. Cholesterol is a highly hydrophobic molecule that precipitates and generates turbidity in culture medium. This can limit the ability to measure cell growth by optical density (OD600). Several approaches were used to overcome this issue: (i) Total protein content (Thermo Scientific, Micro BCA assay) was measured in the pelleted cell fraction after alkaline cell lysis using BSA as internal standard; (ii) polystyrene rolling bottles, instead of the typically used polycarbonate rolling bottles, allowed reliable OD600 readings of cultures containing 0.5 mM cholesterol; (iii) while at the concentration used in this study (0.5 mM) cholesterol precipitates out of solution after 3 to 5 d of incubation at 37˚C (even in polystyrene rolling bottles), this precipitation was not observed at a cholesterol concentration fold10- lower (0.05 mM) that equally supported growth even after 28 d of incubation; however, it did not allow for accurate cholesterol determination in the medium supernatant.
Gas Chromatography–Mass Spectrometry (GC-MS) analysis
A cholesterol concentration of 0.5 mM in polystyrene rolling bottles was used for GC-MS analysis. Concentrations of cholesterol at different time points were quantified in the supernatant fraction by GC-MS. Briefly, 1 mL culture supernatants were acidified, extracted twice with ethyl acetate and derivatized with 1:1 pyridine: Sylon BFT (Sigma) at 30°C for 30 min. Cholestane was used as an internal standard. Samples (1.0 µL) were applied to an Agilent 6890N GC with an HP5ms column (30 m × 0.25 mm, 0.25 µm film thickness) and detected by an Agilent 5973 N mass selective detector in electron ionization mode. Analytical standards of cholesterol were used for quantitation.
[4–14C]cholesterol accumulation
Uptake experiments were performed as previously described.Citation3 M. bovis BCG cultures were grown to mid-log phase on 7H9 broth, harvested at room temperature and concentrated to an OD600 = 15.0 in 7H9 broth supplemented with 0.1% (vol/vol) Tyloxapol. Cholesterol uptake was then measured at a final cell density of OD600 = 7.5. Cells were pre-incubated for 15 min at 37°C and the assay was started by the addition of 5800 dpm of [4–14C] cholesterol (53 mCi/mmol, PerkinElmer Life Sciences) from an ethanol stock solution. At different time intervals, cholesterol uptake was stopped by diluting the suspensions with 1.0 ml of ice-cold buffer, collecting the cells on a 0.22-µm Millipore nitrocellulose filter (Fisher Scientific, Mississauga, Ontario), washing the cells with 10 ml of 5% Tween 20 (Fisher), then with 10 ml of 50% ethanol and, finally, with 10 ml of 5% Tween 20. The background signal of this washing procedure was less that 1% of total dpm (a light wash protocol i.e., only 10 mL of 5% Tween 20, gave background signals of up to 10% of total dpm). Filters were placed in Beckman Ready-Safe scintillation mixture (Beckman Coulter) and counted in a Beckman LS-600IC scintillation counter to determine the amount of cholesterol taken up by the cells.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Author Contributions
S.R-G. conceived the project, designed research, performed experiments (growth kinetics and [4–14C]cholesterol accumulation), analyzed data, and drafted and wrote the manuscript; G.R.S. designed research, performed experiments (GC-MS cholesterol analysis and [4–14C]cholesterol accumulation) and analyzed data; Z.K.H. performed experiments (growth kinetics); W.W.M. designed research and critically reviewed the manuscript; C.J.T. designed research, and wrote the manuscript.
Acknowledgments
We are grateful to Dr. José A. Aínsa for providing M. bovis BCG strain and its P55-genetic derivatives.
Funding
This work was supported by grants from The Canadian Institute of Health Research (MOP-82855) and the British Columbia Lung Association to Charles J. Thompson, and an NSERC Discovery Grant (RGPIN 155184–13) to William W. Mohn. Zhao Kun Hui was supported by a Natural Sciences and Engineering Research Council of Canada - Undergraduate Student Research Award (NSERC-USRA).
References
- Gatfield J, Pieters J. Essential role for cholesterol in entry of mycobacteria into macrophages. Science (2000); 288:1647-50; PMID:10834844
- Van der Geize R, Yam K, Heuser T, Wilbrink MH, Hara H, Anderton MC, Sim E, Dijkhuizen L, Davies JE, Mohn WW, et al. A gene cluster encoding cholesterol catabolism in a soil actinomycete provides insight into Mycobacterium tuberculosis survival in macrophages. Proc Natl Acad Sci U S A (2007); 104:1947-52; PMID:17264217; http://dx.doi.org/10.1073/pnas.0605728104
- Mohn WW, van der Geize R, Stewart GR, Okamoto S, Liu J, Dijkhuizen L, Eltis LD. The actinobacterial mce4 locus encodes a steroid transporter. J Biol Chem (2008); 283:35368-74; PMID:18955493; http://dx.doi.org/10.1074/jbc.M805496200
- Klepp LI, Forrellad MA, Osella AV, Blanco FC, Stella EJ, Bianco MV, Santangelo Mde L, Sassetti C, Jackson M, Cataldi AA, et al. Impact of the deletion of the six mce operons in Mycobacterium smegmatis. Microbes Infect (2012); 14:590-9; PMID:22353253; http://dx.doi.org/10.1016/j.micinf.2012.01.007
- Pandey AK, Sassetti CM. Mycobacterial persistence requires the utilization of host cholesterol. Proc Natl Acad Sci U S A (2008); 105:4376-80.
- Sassetti CM, Rubin EJ. Genetic requirements for mycobacterial survival during infection. Proc Natl Acad Sci U S A (2003); 100:12989-94; PMID:14569030; http://dx.doi.org/10.1073/pnas.2134250100
- Joshi SM, Pandey AK, Capite N, Fortune SM, Rubin EJ, Sassetti CM. Characterization of mycobacterial virulence genes through genetic interaction mapping. Proc Natl Acad Sci U S A (2006); 103:11760-5; PMID:16868085; http://dx.doi.org/10.1073/pnas.0603179103
- Chang JC, Miner MD, Pandey AK, Gill WP, Harik NS, Sassetti CM, Sherman DR. igr genes and Mycobacterium tuberculosis cholesterol metabolism. J Bacteriol (2009); 191:5232-9; PMID:19542286; http://dx.doi.org/10.1128/JB.00452-09
- De Rossi E, Arrigo P, Bellinzoni M, Silva PA, Martín C, Aínsa JA, Guglierame P, Riccardi G. The multidrug transporters belonging to major facilitator superfamily in Mycobacterium tuberculosis. Mol Med (2002); 8:714-24; PMID:12520088
- Ramon-Garcia S, Martin C, Thompson CJ, Ainsa JA. Role of the Mycobacterium tuberculosis P55 efflux pump in intrinsic drug resistance, oxidative stress responses, and growth. Antimicrob Agents Chemother (2009); 53:3675-82; PMID:19564371; http://dx.doi.org/10.1128/AAC.00550-09
- Gaur RL, Ren K, Blumenthal A, Bhamidi S, Gibbs S, Jackson M, Zare RN, Ehrt S, Ernst JD, Banaei N. LprG-mediated surface expression of lipoarabinomannan is essential for virulence of Mycobacterium tuberculosis. PLoS Pathog (2014); 10:e1004376; PMID:25232742; http://dx.doi.org/10.1371/journal.ppat.1004376
- Farrow MF, Rubin EJ. Function of a mycobacterial major facilitator superfamily pump requires a membrane-associated lipoprotein. J Bacteriol (2008); 190:1783-91; PMID:18156250; http://dx.doi.org/10.1128/JB.01046-07
- Bianco MV, Blanco FC, Imperiale B, Forrellad MA, Rocha RV, Klepp LI, Cataldi AA, Morcillo N, Bigi F. Role of P27 -P55 operon from Mycobacterium tuberculosis in the resistance to toxic compounds. BMC Infect Dis (2011); 11:195; PMID:21762531; http://dx.doi.org/10.1186/1471-2334-11-195
- Bigi F, Gioffré A, Klepp L, Santangelo MP, Alito A, Caimi K, Meikle V, Zumárraga M, Taboga O, Romano MI, et al. The knockout of the lprG-Rv1410 operon produces strong attenuation of Mycobacterium tuberculosis. Microbes Infect (2004); 6:182-7; PMID:14998516; http://dx.doi.org/10.1016/j.micinf.2003.10.010
- Rengarajan J, Bloom BR, Rubin EJ. Genome-wide requirements for Mycobacterium tuberculosis adaptation and survival in macrophages. Proc Natl Acad Sci U S A (2005); 102:8327-32; PMID:15928073; http://dx.doi.org/10.1073/pnas.0503272102
- Brzostek A, Pawelczyk J, Rumijowska-Galewicz A, Dziadek B, Dziadek J. Mycobacterium tuberculosis is able to accumulate and utilize cholesterol. J Bacteriol (2009); 191:6584-91; PMID:19717592; http://dx.doi.org/10.1128/JB.00488-09
- Griffin JE, Gawronski JD, Dejesus MA, Ioerger TR, Akerley BJ, Sassetti CM. High-resolution phenotypic profiling defines genes essential for mycobacterial growth and cholesterol catabolism. PLoS Pathog (2011); 7:e1002251; PMID:21980284; http://dx.doi.org/10.1371/journal.ppat.1002251
- de Carvalho LP, Fischer SM, Marrero J, Nathan C, Ehrt S, Rhee KY. Metabolomics of Mycobacterium tuberculosis reveals compartmentalized co-catabolism of carbon substrates. Chem Biol (2010); 17:1122-31; PMID:21035735; http://dx.doi.org/10.1016/j.chembiol.2010.08.009
- Piddock LJ. Multidrug-resistance efflux pumps - not just for resistance. Nat Rev Microbiol (2006); 4:629-36; PMID:16845433; http://dx.doi.org/10.1038/nrmicro1464
- Shukla S, Richardson ET, Athman JJ, Shi L, Wearsch PA, McDonald D, Banaei N, Boom WH, Jackson M, Harding CV. Mycobacterium tuberculosis lipoprotein LprG binds lipoarabinomannan and determines its cell envelope localization to control phagolysosomal fusion. PLoS Pathog (2014); 10:e1004471; PMID:25356793; http://dx.doi.org/10.1371/journal.ppat.1004471
- Yang X, Nesbitt NM, Dubnau E, Smith I, Sampson NS. Cholesterol metabolism increases the metabolic pool of propionate in Mycobacterium tuberculosis. Biochemistry (2009); 48:3819-21; PMID:19364125; http://dx.doi.org/10.1021/bi9005418

![Figure 2. Cholesterol uptake in M. bovis BCG. [4–14C]cholesterol uptake by wild-type and KOP55 mutant cells. Values are the mean and error bars indicate standard deviation of 3 replicates. Pair wise comparisons indicate statistically significant differences (**, P <0.005; ***, P<0.0001).](/cms/asset/63b953b2-0423-4b72-adb8-dacc903bf426/kvir_a_1044195_f0002_c.gif)