Abstract
The aim of this study was to investigate if the alternative in vivo model Galleria mellonella can be used (i) to determine differences in pathogenicity of amphotericin B (AMB) resistant and susceptible A. terreus isolates, (ii) to evaluate AMB efficacy in vivo (iii) and to correlate outcome to in vitro susceptibility data. Larvae were infected with 2 A. terreus AMB resistant (ATR) and 3 AMB susceptible (ATS) isolates and survival rates were correlated to physiological attributes and killing ability of larval haemocytes. Additionally, infected larvae were treated with different concentrations of L-AMB. Haemocyte density were ascertained to evaluate the influence of L-AMB on the larval immune cells. Larvae were sensitive to A. terreus infection in an inoculum-size and temperature dependent manner. In vitro susceptibility to L-AMB correlated with in vivo outcome of antifungal treatment, defining an AMB susceptible strain cluster of A. terreus. Susceptibility to L-AMB increased virulence potential in the larval model, but this increase was also in accordance with faster growth and less damage caused by larval haemocytes. L-AMB treatment primed the larval immune response by increasing haemocyte density. G. mellonella provides a convenient model for the in vivo screening of A. terreus virulence and treatment options, contributing to the generation of a hypothesis that can be further tested in refined experiments in mammalian models.
Introduction
Invasive aspergillosis (IA) is associated with considerable morbidity and mortality in patients with compromised immunity due to cancer chemotherapy, haematopoietic stem-cell transplantation and solid organ transplantation.Citation1-3 While A. fumigatus is the most commonly cause of IA worldwide, A. terreus appears to be frequently isolated in some institutions; e.g. the Medical University Hospital of Innsbruck, Austria, where approximately 15% of all Aspergillus infections are caused by this pathogen, and the M.D. Anderson Cancer Center in Houston, Texas, USA.Citation4,5 In general, mold infections remain difficult to manage because of late diagnosis and complication during treatment due to drug toxicities.Citation6,7 Due to the high side effects caused by AMB such as nephrotoxicity and infusional toxicity, lipid formulations of AMB (L-AMB) are the reference formulation in the treatment of invasive fungal infections. Citation8,9 In vivo and in vitro data indicate that A. terreus isolates are intrinsically resistant to amphotericin B (AMB) (MIC ≥ 2 µg/mL).Citation10,11 The mechanisms involved in AMB resistance are only partly elucidated and special focus has been put on comparing cellular and metabolic differences in AMB resistant isolates (ATR) to one susceptible isolate (ATS) from the University of Milano (MIC of 0.5 µg/mL).Citation12,13 Currently, 2 further A. terreus isolates from the USA have been found to be susceptible against AMB.
The larvae of the greater wax moth, Galleria mellonella, have been widely used as alternative models to evaluate the virulence of microbial pathogens, including fungal pathogens, and to evaluate the efficacy of antimicrobial drugs.Citation14,15 Of particular importance is the strong correlation to data obtained in murine studies.Citation16 Insects possess a cellular and humoral innate immune system which bears similarities to innate immune response of vertebrates.Citation17 The cellular immune response is mediated by haemocytes, which show strong structural and functional similarities with mammalian phagocytes.Citation18,19 Six different types of haemocytes are defined in Galleria which are involved in phagocytosis, encapsulation and melanization.Citation20 Because their density is not stable, examining changes in haemocyte populations can be used to estimate immune responses to fungal infections or drug treatment.Citation14,21
The aim of this study was to establish and characterize a simple alternative in vivo model to study infections due to A. terreus. Two ATR isolates and 3 ATS isolates were used to determine differences in virulence potential and survival rates were correlated to physiological attributes and to in vitro AMB susceptibility profile. The wax moth larvae were further used to evaluate L-AMB efficacy in vivo and to compare outcome to in vitro susceptibility data. Additionally, the influence of L-AMB on haemocyte density was determined.
Results
Killing of Galleria mellonella larvae by A. terreus isolates is dose- and temperature dependent
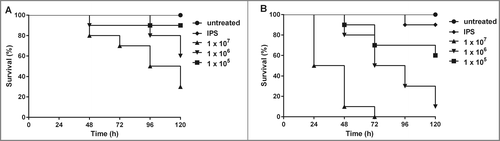
To establish G. mellonella larvae as a model for A. terreus infections, first, the optimal conidial dosage for virulence studies was determined. Therefore, larvae were infected with 1 × 105 – 1 × 107 conidia per larva of the ATR isolate (T90) and survival was monitored in parallel at 30°C or 37°C. As expected, killing of the larvae depended on the number of conidia injected and the incubation temperature (). Larvae were killed significantly faster (p ≤ 0.05) when incubated at 37°C compared to 30°C at each inoculum density tested. Significant difference between all 3 inocula was observed at both temperatures. At 37°C, all larvae infected with 1 × 107 conidia died within 72 h, while survival still reached 70% in those incubated at 30°C and it took 6 days before all larvae died. Infection with 1 × 106 conidia at 37°C resulted in 100% mortality at day 6, while almost 50% survival was monitored at 30°C. Lower inoculum density (1 × 105) resulted in 50% mortality at day 6 at 37°C, whereas larvae kept at 30°C survived the infection (less than 10% mortality). Heat-inactivated conidia of T90 were not able to cause mortality and survival was more than 80% after 120 h incubation at either 30°C or 37°C (data not shown). The dose and temperature dependency of this killing indicates that G. mellonella larvae have the potential to serve as a non-vertebrate animal host model for this group of Aspergilli.
Figure 1. Dose- and temperature dependent mortality of G. mellonella larvae infected with A. terreus. Ten larvae each were infected with different conidial concentrations of A. terreus (T90) and incubated at 30°C (A) or 37°C (B), respectively. Experiments were performed at least 3 times. Kaplan-Meyer curves represent one out of 3 experiments. Significant difference (p ≤ 0.05) was observed within different inocula tested at each temperature.
A. terreus isolates exhibit different virulence potential
Distinct morphological variations were observed between the ATR and ATS isolates. In order to see if the various isolates exhibit differences in their virulence potential and to check if this could be linked to morphological characteristics or AMB susceptibility, the virulence potential of the 5 isolates was compared. Significant differences in survival rates of larvae were determined, when larvae were infected with 1 × 107 conidia of each strain and incubated at 30°C (). In summary, the ATR strains (T9 and T90) showed lower virulence potential compared to the ATS strains (T77, 09-164 and 09-175), indicated by significant differences in the median survival of each strain, which was 60–72 h for the ATS strains and 96–108 h for ATR isolates. T77, the isolate exhibiting fastest killing of larvae, showed significantly higher virulence potential compared to T9 (p = 0.0310) and T90 (p = 0.0157), and isolate 09-175 displayed significantly higher virulence compared to T9 (p = 0.0444) and T90 (p = 0.0214) (). No significant difference compared to ATR isolates was detected for the ATS isolate 09-164 (p > 0.05). As total mortality is reached earlier at 37°C, we performed virulence studies with 1 × 106 conidia of each strain and incubated larvae at 37°C (, solid lines). Difference in virulence potential was less prominent at 37°C than at 30°C. Still, the difference is reflected in the median survival of each strain. While larvae infected with any of the ATS strains demonstrated a median survival of 72 h, for groups infected with ATR strains median survival was 84 h. The correlation between virulence potential and physiological attributes of the infectious agent, or the size of the infecting agent was already discussed before.Citation22 Here too, higher virulence potential correlated with higher germination rates and increased growth rates in vitro (Figs. S1 and S2). For further support, tissue sections of infected larvae embedded in paraffin were performed and revealed that A. terreus is able to proliferate within the host (). Additionally, faster growth ability, connected with higher virulence potential, was obvious in vivo too, as for the ATS strain T77 faster development of fungal hyphae within the host tissue was observerd.
Figure 2. Survival of G. mellonella larvae following inoculation with different A. terreus isolates. Larvae were infected with 20 µL IPS containing 1 × 107 conidia of the respective A. terreus isolates and incubated at 30°C. Survival was monitored every 24 h over 144 h (6 days). (ATR: T9 and T90; ATS: T77, 09-164 and 09-175). Kaplan-Meyer curves represent one out of 3 experiments.
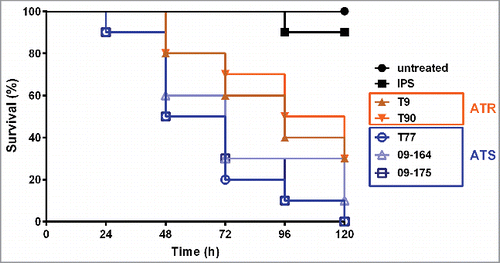
Figure 3. Histopathology of G. mellonella infected with A. terreus ATS strain T77, and ATR strain T90. Larvae were infected with IPS (left panel)), 1 × 107 spores per larvae of ATS T77 (middle panel) and of ATR T90 (right panel). Larvae were sacrificed at 12 h and 24 h after incubation at 37°C, conserved in formalin, embedded in paraffin. To identify the fungus more easily, tissue sections were stained with Grocott´s silver stain.
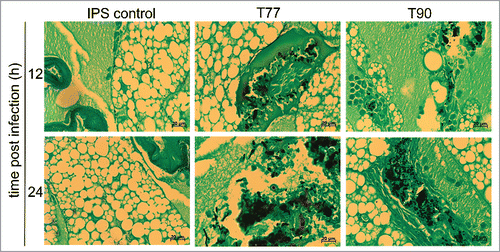
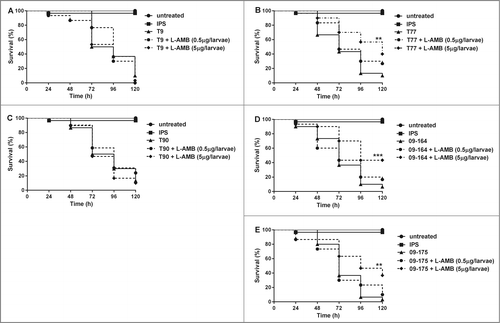
Figure 4. Ability of L-AMB to increase the time to death of G. mellonella challenged with ATR (A, C) and ATS strains (B, D, E). Larvae were infected with 1 × 106 spores 2 h before L-AMB (0.5 µg and 5 µg per larvae) was administered and incubated at 37°C. Survival was monitored every 24 h. Graphs represent one out of 3 single experiments. Results are significantly different (*p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001; Mantel Cox test) to the untreated infected larvae.
AMB efficacy against A. terreus infections in G. mellonella larvae correlates with in vitro susceptibility
In vitro susceptibility of ATR and ATS isolates against AMB and L-AMB was determined with microbroth dilution assay according to the EUCAST guildlines.Citation23 No significant differences in MICs between the different AMB formulations were determined (data not shown). Antifungal susceptibility testing demonstrated a MIC range of 2 – 4 µg/mL for both ATR isolates (geometric mean of 3.125 µg/mL), while all 3 AMB susceptible strains had a MIC range of 0.25 – 0.5 µg/mL (geometric mean of 0.378 µg/mL). As L-AMB is the therapeutic of choice, we used this formulation for all in vivo assays. For assessing AMB efficacy in vivo, a single dose of L-AMB at a concentration of 0.5 µg and 5 µg per larvae, chosen according to the MIC values of ATS strains plus a concentration 10 times higher, was administered to each larva, previously infected with 1 × 106 conidia of the respective A. terreus isolate and incubated at 37°C. In addition to untreated and IPS controls, further control groups were included receiving L-AMB (0.5 or 5 µg per larva) to rule out putative toxic effects of L-AMB. Survival was not less than 80% in any of the controls, indicating that L-AMB itself had no effect on survival at the concentrations used (data not shown).
Treatment with L-AMB only showed success in the test groups infected with ATS isolates (T77, 09-164, 09-175) (), reflecting in vitro data. In groups that received 5 µg of L-AMB, survival of larvae was significantly (p ≤ 0.01) prolonged compared to control groups that received IPS only. Although less pronounced, even the lower dose of 0.5 µg L-AMB per larva seemed to have a positive effect on survival, although statistically not significant. No significant increase in survival due to L-AMB application could be detected for the 2 ATR isolates T9 and T90 ().
A. terreus strains induce different cellular immune responses in G. mellonella
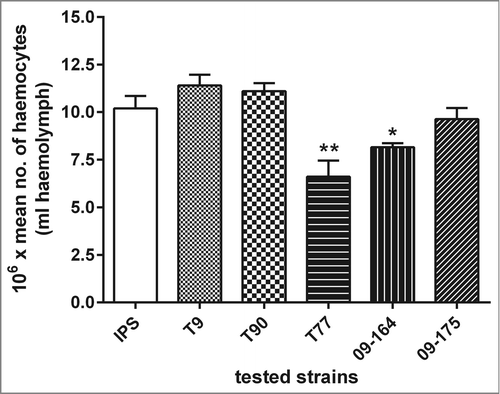
To determine if differences in survival rates could be attributed to different killing activity of haemocytes against hyphae of the different isolates, the extent of fungal damage induced by larval haemocytes was measured by XTT-assay. Fungal damage caused by isolated haemocytes was higher in the 2 ATR isolates (T9 and T90) than in the ATS strains (T77, 09-164 and 09-175) (data not shown). These results correspond to the data obtained in virulence assays (), were ATS strains demonstrated higher killing ability of larvae compared to ATR strains, which might be due to less damage by haemocytes. Highest fungal damage was measured for ATR isolate T90 (65.26%; SD ± 12.36), that also showed the lowest virulence potential. Interestingly, all ATS strains exhibited 20% (SD ± 6.3) less damage caused by larval haemocytes compared to the resistant strain T90. Furthermore, haemocyte densities of infected larvae were determined by calculating the concentration of haemocytes per mL of haemolymph. Haemocyte densities of infected larvae revealed comparable numbers for larvae infected with ATR strains and the control, while larvae infected with the ATS strains exhibited a decreased density of circulating haemocytes in the haemolymph (). This reduction was most prominent in larvae infected with the ATS strain T77 (35.29%, SD ± 10.94), suggesting that infection with T77 induced haemocyte destruction.
Figure 5. Larval haemocyte densities in response to A. terreus infections. Larvae were injected with a non lethal dose of different A. terreus spores (ATR: T9, T90; ATS: T77, 09-164, 09-175) or IPS buffer and incubated at 30°C for 48 h and haemocyte counts were carried out relative to the IPS control. Results are significantly different (*p ≤ 0.05; **p ≤ 0.01; Students t-test, 2 tail) to the IPS control.
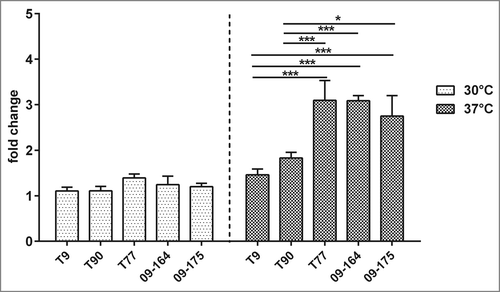
Melanization is one of the first humoral responses of the larvae and occurs quickly after infection.Citation24 Larvae infected with ATS strains showed higher melanization than ATR strains (). Quantification of melanization revealed significant differences (p < 0.05) in melanin accumulation between ATS and ATR strains 24 h post infection, the effect being apparent at 37°C, resulting in a 3 times higher melanization rate for T77 than the IPS control. ATR strains T9 and T90 showed only little difference in melanization compared to the IPS control (). These data strengthen the hypothesis that A. terreus ATR and ATS strains induce different cellular immune responses in G. mellonella, which might contribute to virulence potential.
Figure 6. Melanization of G. mellonella larvae infected with A. terreus. (A) Fold change of melanization rate (OD 405nm) normalized to the IPS control at 30°C (white) and 37°C (gray). (B) Visual appearance of larvae infected with different A. terreus strains at a dose of 1 × 107 spores after 24 h. Error bars represent standard deviation out of 3 independent experiments. (* p ≤ 0.05; *** p ≤ 0.001: 2 way ANOVA).
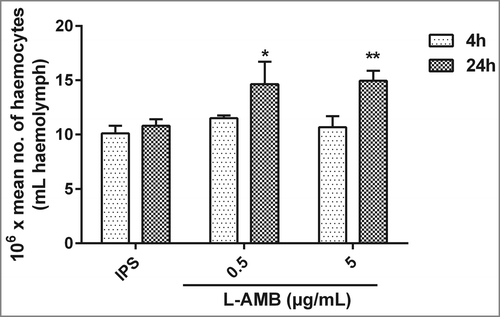
Effect of L-AMB adiminstration on haemocyte density in G. mellonella larvae
Previous work demonstrated that administration of caspofungin to larvae primes the larval immune response by elevating haemocyte density.Citation21 L-AMB administration of 5 µg resulted in a significant (p < 0.05) increase of 38.38%; SD ± 5.14 in haemocyte density 24 h after administration. Additionally, administration of 0.5 µg of L-AMB demonstrated same increase in haemocyte denisity after 24 h injection (35.61%; SD ± 14). Interestingly, no significant alterations in haemocyte densities could be observed 4 h post-treatment ().
Figure 7. Effect of L-AMB on the haemocyte density in G. mellonella. Haemocyte density in larvae injected with IPS or L-AMB (0.5 or 5 µg/mL) and incubated at 37°C was assessed after 4 h and 24 h. Gray bars, 4 h; black bars, 24 h. Results are significantly different (*p ≤ 0.05; **p ≤ 0.01; Students t-test, 2 tail) to the IPS control.
Discussion
The use of G. mellonella larvae to study the pathogenicity of various microbial pathogens, including fungi, has been widely reported in the recent years.Citation15,25 Dose-dependent mortality observed in this study was expected, as it has also been shown in studies carried out with A. fumigatus.Citation25 This, plus the obvious development of hyphae inside the larval body, indicates that A. terreus kills larvae through an active infection mechanism rather than a merely physical effect of the injected conidia. Similar to what has been shown in 2 different mouse models and embryonated chicken eggs, higher doses of A.terreus conidia were necessary to cause same lethality compared to A. fumigatus in larvae.Citation26 The differences in virulence potential of A. terreus in G. mellonella as observed here, correlate closely with previously published data obtained with Balb/c mice, in which the ATS strain T77 also exhibited higher pathogenicity than the ATR strain T90.Citation13 These findings make G. mellonella a valid model for A. terreus infections.
Adaption of a fungal pathogen to different micro-environmental stresses is necessary to allow germination and growth within the host environment and strongly influences virulence. Petraitis et al.Citation22 demonstrated a correlation between virulence potential in vivo with species-dependent differences in germination rate in vitro. In contrast, investigating putative differences in virulence potential of A. fumigatus color mutants did not reveal any correlation between faster germination and higher pathogenicity.Citation25 Different outcome of infection is also linked to variable response of immune cells, such as killing of intruding pathogens by phagocytic cells, as shown in this study. Reasons therefore could be explained by variations in the outer layers of the isolates or to a difference in resistance to killing by reactive oxygen species.
Another factor contributing to virulence is the secretion of secondary metabolites such as mycotoxins, that influence host immune system or harm host tissue. A well studied example is A. fumigatus gliotoxin which is known to induce apoptosis and prevents NF-κB activation by inhibition of the proteasome and suppression of angiogenesis. Citation27-31 Additionally, fumagillin is also produced by A. fumigatus during hyphal growth and has been identified as an inhibitor of angiogenesis through a covalent interaction with methionine aminopeptidase-2 and suppresses tumor growth.Citation32,33 The production of gliotoxin and fumagillin was also proved in infected G. mellonella larvae.Citation31,34 A study by Lewis et al.Citation35 detected gliotoxin in culture medium of A. terreus, but as we did not test gliotoxin production, or any other secondary metabolites, of the 5 different isolates we can only speculate its contribution to differences in virulence potential. The same effect may account for the variable production of proteases by the ATR and ATS strains. A linear relationship between the haemocyte density and the survival rate of the larvae infected with Candida spp. was demonstrated by Bergin et al.Citation36 and these findings correlate with the results presented here, demonstrating lower haemocyte density in larvae infected with ATS. Additionally, our results agree with those presented previously,Citation37 demonstrating correlation between high increase in melanin and higher mortality rates in larvae infected with different Klebsiella strains. Together, the observed differences in resistance to haemocyte killing and melanin production might explain the differences in virulence potential in addition to physiological attributes of the tested strains.
A. terreus isolates are known to show an intrinsic resistance to AMB with MIC values ≥2 µg/mL.Citation10,11,38 No clinical beakpoints for AMB are defined for A. terreus species, but regarding the EUCAST breakpoints for other Aspergillus spp, isolates with MIC values >2 µg/mL are considered to be clinically resistant against AMB.Citation39 Data from in vitro studies correlate well with murine studies.Citation12 Only few A. terreus isolates have been found so far that showed lower MIC values, making them AMB susceptible from a clinical point of view. One of these isolates has already been tested in a murine model, and treatment success was consistent with in vitro data.Citation12 In the larval model, the same outcome was shown for the 3 ATS strains and 2 ATR isolates, indicating that the larval model is a useful tool to test for L-AMB efficacy in vivo, and could also be employed with other fungal pathogens.
Administration of external agents into the insect haemocoel was shown to result in an unspecific antimicrobial immune response.Citation21,34,40 A study by Kelly et al.Citation21 demonstrated that caspofungin administration primes the immune response of G. mellonella larvae and induces a non-specific antimicrobial response. Increased haemocyte numbers due to L-AMB seem not to be enough to cure the infection, otherwise one would expect increased survival rates also in larvae infected with ATR strains. Nevertheless, for ATS the positive effect on haemocyte numbers might be an additional effect in prolonging survival.
In conclusion, this study demonstrates for the first time that the G. mellonella larval model is useful to investigate the in vivo antifungal efficacy of L-AMB against A. terreus isolates and it is also an effective tool to screen for differences in virulence potential. Immuno-stimulatory effects of antifungal agents should be taken into consideration when interpreting results from in vivo treatment studies employing Galleria, but as they were shown to be marginal for AMB, G. mellonella larvae can be employed as a rapid, inexpensive and reliable way to evaluate AMB efficacy in vivo.
Materials and methods
Fungal strains, growth conditions and susceptibility testing
All five A. terreus isolates tested were derived from clinical specimens of either the University Hospital Innsbruck, Austria, the University of Milan and Rome, Italy or the Stanford University, USA, ATS strains are named T77, 09-164 and 09-175 and ATR strains T9 and T90. Species identification was performed by sequencing the internal transcribed spacer region (ITS 3–4), gene regions of β-tubulin, calmodulin, enolase and cytochrome C. Fungi were grown on Aspergillus Complete MediaCitation41 for 7–14 days at 37°C to obtain conidia. Minimum inhibitory concentration (MIC) of AMB and L-AMB were determined for all strains following EUCAST guidelines 9.2.Citation23 Antifungal susceptibility testing was performed in triplicates on 3 separate occasions. MIC was defined as the minimal concentration of AMB resulting in complete growth inhibition.
Virulence assay and antifungal treatment studies in G. mellonella
Sixth instar larvae of G. mellonella (Biologische Wurmzucht, Langenzersdorf, Austria) were stored in wood shavings in the dark at 18°C prior to use. Larvae weighing 0.3 – 0.4 g were selected for experimental use. Inoculum preparation, larval infection and monitoring of survival were carried out as described previously.Citation42 Larvae injected with sterile insect physiological salin (IPS; 150 mM NaCl, 5 mM KCl, 10 mM EDTA and 30 mM sodium citrate in 0.1 M Tris–HCl, pH 6.9)Citation18 and untouched larvae served as controls. To utilize G. mellonella larvae as a model for A. terreus infections to compare killing ability of different A. terreus isolates, larvae were first infected with different concentrations of conidia (1 × 105, 1 × 106 and 1 × 107 conidia per larva) of an A. terreus patient isolate. Survival was monitored in parallel at 30°C or 37°C over 144 h. To determine different virulence potential of ATR and ATS, larvae were infected with 1 × 107 conidia per larvae and survival was monitored over 120 h at 30°C. Additionally, survival rates were determine at 37°C with an infection dose of 1 × 106 condida per larvae. To verify that mortality ist due to active growth, larvae were infected with heat-inactivated spores and survival was monitored at 30°C and 37°C.
To assess efficacy of L-AMB in vivo, larvae were infected with 1 × 106 conidia per larva, and injected with 0.5 (1.6 mg/kg), 5 µg L-AMB (16.6 mg/kg) per larva, or IPS, respectively, 2 hours post-infection. For evaluation of antifungal treatment larvae were incubated at 37°C to make comparison to vertebrate hosts possible.
Haemocyte isolation and determination of haemocyte density
G. mellonella larvae were infected and treated as described above. Haemocytes were isolated as described in Citation21 from 3 larvae of each group, 4 h, 24 h or 48 h post injection of either pathogen or L-AMB. Larvae were incubated at 30°C or 37°C. Cell density was determined by enumeration using a haemocytometer.
Determination of hyphal damage caused by haemocytes
A. terreus conidia (1 × 104 conidia per 100 µL) were incubated in RPMI1640 at 37°C for 16 h to allow formation of hyphae, before adding freshly isolated haemocytes in a ratio of 1:1 and further incubation at 30°C. Wells containing either fungus or haemocytes alone served as controls. After 4 h haemocytes were lysed by ice-cold 2% Triton X-100 and the viability of remaining hyphae was quantified by the use of the In Vitro Toxicology Assay Kit, (XTT assay; Sigma Aldrich) according to the manufacturer´s description. The percentage of fungal damage was calculated with the following formula: (1- [OD450 of fungi with haemocytes -OD450 of haemocytes alone]/[OD450 of fungi alone]) × 100.
Histology of larvae
Infected larvae plus control larvae (injected with IPS), incubated at 37°C, were conserved as a whole in formalin for 10 days, embedded in paraffin, cut at 5 µm and stained with Grocott´s silver stain to detect fungal elements.Citation43
Quantification of melanization
Larvae were infected with 1 × 10×7 conidia per larva, and incubated at either 30°C or 37°C, before haemolymph was collected at 3 h and 24 h post infection. Haemolymph was diluted 1:10 in IPS buffer and 100 µL of each sample were placed in 96 well microdilution plates and quantification of melanization assays were performed.Citation14 Optical density of haemolymph from infected larvae was normalized to that of control larvae.
Statistical analysis
All experiments were performed on at least 3 independent occasions. Results are expressed as the mean ± SD. Survival rates were evaluated using Kaplan Meier survival curves and analyzed with the log rank (Mantel-Cox) method utilizing GraphPad Prism software. Further, Tukey Test (one way ANOVA) or Students t-test were used to determine differences. Differences were considered significant at *p ≤ 0.05.
Disclosure of Potential Conflicts of Interest
C. L-F has received grant support from the Austrian Science Fund (FWF), MFF Tirol, Astellas Pharma, Gilead Sciences, Pfizer, Schering Plough, and Merck Sharp & Dohme. She has been an advisor/consultant to Gilead Sciences, Merck Sharp & Dohme, Pfizer, and Schering Plough. She has received travel/accommodation expenses from Gilead Sciences, Merck Sharp & Dohme, Pfizer, Astellas, and Schering Plough and has been paid for talks on behalf of Gilead Sciences, Merck Sharp & Dohme, Pfizer, Astellas, and Schering Plough. All other authors have no conflicts of interest to declare.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
1045183_supplemental_files.doc
Download MS Word (1.9 MB)Acknowledgments
We are grateful to Dr. D. Stevens, Dr. M. Sanguinetti and Dr. A.M. Tortorano for sharing A. terreus isolates with us, and we thank C. Hörtnagl for technical assistance.
Funding
This work was financially supported by the Medical University of Innsbruck (MUI start grant number 19970) to U.B., an “i-med Auslandstipendium” short-time fellowship to E.M. NB is the recipient of a Hume Scholarship from Maynooth University and CS is funded by a studentship from the Irish Research Council.
References
- Latge JP. Aspergillus fumigatus and aspergillosis. Clin Microbiol Rev 1999; 12:310-50; PMID:10194462
- Abad A, Fernandez-Molina JV, Bikandi J, Ramirez A, Margareto J, Sendino J, Hernando FL, Pontón J, Garaizar J, Rementeria A. What makes Aspergillus fumigatus a successful pathogen? Genes and molecules involved in invasive aspergillosis. Rev Iberoam Micol 2010; 27:155-82; PMID:20974273; http://dx.doi.org/10.1016/j.riam.2010.10.003
- Marr KA, Carter RA, Boeckh M, Martin P, Corey L. Invasive aspergillosis in allogeneic stem cell transplant recipients: changes in epidemiology and risk factors. Blood 2002; 100:4358-66; PMID:12393425; http://dx.doi.org/10.1182/blood-2002-05-1496
- Blum G, Perkhofer S, Grif K, Mayr A, Kropshofer G, Nachbaur D, Kafka-Ritsch R, Dierich MP, Lass-Flörl C. A 1-year Aspergillus terreus surveillance study at the University Hospital of Innsbruck: molecular typing of environmental and clinical isolates. Clin Microbiol Infect 2008; 14:1146-51; PMID:19076844; http://dx.doi.org/10.1111/j.1469-0691.2008.02099.x
- Lass-Florl C, Grif K, Kontoyiannis DP. Molecular typing of Aspergillus terreus isolates collected in Houston, Texas, and Innsbruck, Austria: evidence of great genetic diversity. J Clin Microbiol 2007; 45:2686-90; PMID:17581930; http://dx.doi.org/10.1128/JCM.00917-07
- Bruggemann RJ, Alffenaar JW, Blijlevens NM, Billaud EM, Kosterink JG, Verweij PE, Burger DM. Clinical relevance of the pharmacokinetic interactions of azole antifungal drugs with other coadministered agents. Clin Infect Dis 2009; 48:1441-58; PMID:19361301; http://dx.doi.org/10.1086/598327
- Anaissie E. Opportunistic mycoses in the immunocompromised host: experience at a cancer center and review. Clin Infect Dis 1992; 14 Suppl 1:S43-53; PMID:1562695; http://dx.doi.org/10.1093/clinids/14.Supplement_1.S43
- Loo AS, Muhsin SA, Walsh TJ. Toxicokinetic and mechanistic basis for the safety and tolerability of liposomal amphotericin B. Expert Opin Drug Saf 2013; 12(6):881-95; PMID:23931455
- Azanza Perea JR, Barberan J. ; Liposomal amphotericin B: a unique pharmacokinetic profile. An unfinished story. Rev Esp Quimioter 2012; 25:17-24; PMID:22488537
- Lass-Florl C, Griff K, Mayr A, Petzer A, Gastl G, Bonatti H, Freund M, Kropshofer G, Dierich MP, Nachbaur D. Epidemiology and outcome of infections due to Aspergillus terreus: 10-year single centre experience. Br J Haematol 2005; 131:201-7; PMID:16197450; http://dx.doi.org/10.1111/j.1365-2141.2005.05763.x
- Walsh TJ, Petraitis V, Petraitiene R, Field-Ridley A, Sutton D, Ghannoum M, Sein T, Schaufele R, Peter J, Bacher J, et al. Experimental pulmonary aspergillosis due to Aspergillus terreus: pathogenesis and treatment of an emerging fungal pathogen resistant to amphotericin B. J Infect Dis 2003; 188:305-19; PMID:12854088; http://dx.doi.org/10.1086/377210
- Blum G, Hortnagl C, Jukic E, Erbeznik T, Pumpel T, Dietrich H, Nagl M, Speth C, Rambach G, Lass-Flörl C. New insight into amphotericin B resistance in Aspergillus terreus. Antimicrob Agents Chemother 2013; 57:1583-8; PMID:23318794; http://dx.doi.org/10.1128/AAC.01283-12
- Speth C, Blum G, Hagleitner M, Hortnagl C, Pfaller K, Posch B, Ott HW, Würzner R, Lass-Flörl C, Rambach G. Virulence and thrombocyte affectation of two Aspergillus terreus isolates differing in amphotericin B susceptibility. Med Microbiol Immunol 2013; 202(5):379-89; PMID:23722593
- Scorzoni L, de Lucas MP, Mesa-Arango AC, Fusco-Almeida AM, Lozano E, Cuenca-Estrella M, Mendes-Giannini MJ, Zaragoza O. Antifungal efficacy during Candida krusei infection in non-conventional models correlates with the yeast in vitro susceptibility profile. PloS one 2013; 8:e60047; PMID:23555877; http://dx.doi.org/10.1371/journal.pone.0060047
- Thomas RJ, Hamblin KA, Armstrong SJ, Muller CM, Bokori-Brown M, Goldman S, Atkins HS, Titball RW. Galleria mellonella as a model system to test the pharmacokinetics and efficacy of antibiotics against Burkholderia pseudomallei. Int J Antimicrob Agents 2013; 41:330-6; PMID:23402703; http://dx.doi.org/10.1016/j.ijantimicag.2012.12.009
- Slater JL, Gregson L, Denning DW, Warn PA. Pathogenicity of Aspergillus fumigatus mutants assessed in Galleria mellonella matches that in mice. Med Mycol 2011; 49 Suppl 1:S107-13; http://dx.doi.org/10.3109/13693786.2010.523852
- Tojo S, Naganuma F, Arakawa K, Yokoo S. Involvement of both granular cells and plasmatocytes in phagocytic reactions in the greater wax moth, Galleria mellonella. J Insect Physiol 2000; 46:1129-35; PMID:10817839; http://dx.doi.org/10.1016/S0022-1910(99)00223-1
- Bergin D, Reeves EP, Renwick J, Wientjes FB, Kavanagh K. Superoxide production in Galleria mellonella hemocytes: identification of proteins homologous to the NADPH oxidase complex of human neutrophils. Infect Immun 2005; 73:4161-70; PMID:15972506; http://dx.doi.org/10.1128/IAI.73.7.4161-4170.2005
- Renwick J, Reeves EP, Wientjes FB, Kavanagh K. Translocation of proteins homologous to human neutrophil p47phox and p67phox to the cell membrane in activated hemocytes of Galleria mellonella. Dev Comp Immunol 2007; 31:347-59; PMID:16920193; http://dx.doi.org/10.1016/j.dci.2006.06.007
- Lavine MD, Strand MR. Insect hemocytes and their role in immunity. Insect Biochem Mol Biol 2002; 32:1295-309; PMID:12225920; http://dx.doi.org/10.1016/S0965-1748(02)00092-9
- Kelly J, Kavanagh K. Caspofungin primes the immune response of the larvae of Galleria mellonella and induces a non-specific antimicrobial response. J Med Microbiol 2011; 60:189-96; PMID:20947665; http://dx.doi.org/10.1099/jmm.0.025494-0
- Petraitis V, Petraitiene R, Antachopoulos C, Hughes JE, Cotton MP, Kasai M, Harrington S, Gamaletsou MN, Bacher JD, Kontoyiannis DP, et al. Increased virulence of Cunninghamella bertholletiae in experimental pulmonary mucormycosis: correlation with circulating molecular biomarkers, sporangiospore germination and hyphal metabolism. Med Mycol 2013; 51:72-82; http://dx.doi.org/10.3109/13693786.2012.690107
- Arendrup M. C. C-EM, Lass-Flörl C, Hope W, Howard S.J. and the Subcommittee on Antifungal Susceptibility Testing (AFST) of the ESCMID European Committeefor Antimicrobial Susceptibility Testing (EUCAST) EUCAST DEFINITIVE DOCUMENT EDef 9.2: Method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for conidia forming moulds. 2014.
- Bidla G, Hauling T, Dushay MS, Theopold U. Activation of insect phenoloxidase after injury: endogenous versus foreign elicitors. J Innate Immun 2009; 1:301-8; PMID:20375588; http://dx.doi.org/10.1159/000168009
- Jackson JC, Higgins LA, Lin X. Conidiation color mutants of Aspergillus fumigatus are highly pathogenic to the heterologous insect host Galleria mellonella. PloS one 2009; 4:e4224; PMID:19156203; http://dx.doi.org/10.1371/journal.pone.0004224
- Slesiona S, Ibrahim-Granet O, Olias P, Brock M, Jacobsen ID. Murine infection models for Aspergillus terreus pulmonary aspergillosis reveal long-term persistence of conidia and liver degeneration. J Infect Dis 2012; 205:1268-77; PMID:22438397; http://dx.doi.org/10.1093/infdis/jis193
- Ben-Ami R, Lewis RE, Leventakos K, Kontoyiannis DP. Aspergillus fumigatus inhibits angiogenesis through the production of gliotoxin and other secondary metabolites. Blood 2009; 114:5393-9; PMID:19843884; http://dx.doi.org/10.1182/blood-2009-07-231209
- Pahl HL, Krauss B, Schulze-Osthoff K, Decker T, Traenckner EB, Vogt M, Myers C, Parks T, Warring P, Mühlbacher A, et al. The immunosuppressive fungal metabolite gliotoxin specifically inhibits transcription factor NF-kappaB. J Exp Med 1996; 183:1829-40; PMID:8666939; http://dx.doi.org/10.1084/jem.183.4.1829
- Pardo J, Urban C, Galvez EM, Ekert PG, Muller U, Kwon-Chung J, Lobigs M, Müllbacher A, Wallich R, Borner C, et al. The mitochondrial protein Bak is pivotal for gliotoxin-induced apoptosis and a critical host factor of Aspergillus fumigatus virulence in mice. J Cell Biol 2006; 174:509-19; PMID:16893972; http://dx.doi.org/10.1083/jcb.200604044
- Dhingra S, Andes D, Calvo AM. VeA regulates conidiation, gliotoxin production, and protease activity in the opportunistic human pathogen Aspergillus fumigatus. Eukaryotic Cell 2012; 11:1531-43; PMID:23087369; http://dx.doi.org/10.1128/EC.00222-12
- Reeves EP, Messina CG, Doyle S, Kavanagh K. Correlation between gliotoxin production and virulence of Aspergillus fumigatus in Galleria mellonella. Mycopathologia 2004; 158:73-9; PMID:15487324; http://dx.doi.org/10.1023/B:MYCO.0000038434.55764.16
- Ingber D, Fujita T, Kishimoto S, Sudo K, Kanamaru T, Brem H, Folkman J. Synthetic analogues of fumagillin that inhibit angiogenesis and suppress tumour growth. Nature 1990; 348:555-7; PMID:1701033; http://dx.doi.org/10.1038/348555a0
- Mitchell CG, Slight J, Donaldson K. Diffusible component from the spore surface of the fungus Aspergillus fumigatus which inhibits the macrophage oxidative burst is distinct from gliotoxin and other hyphal toxins. Thorax 1997; 52:796-801; PMID:9371210; http://dx.doi.org/10.1136/thx.52.9.796
- Fallon JP, Reeves EP, Kavanagh K. The Aspergillus fumigatus toxin fumagillin suppresses the immune response of Galleria mellonella larvae by inhibiting the action of haemocytes. Microbiology 2011; 157:1481-8; PMID:21349977; http://dx.doi.org/10.1099/mic.0.043786-0
- Lewis RE, Wiederhold NP, Lionakis MS, Prince RA, Kontoyiannis DP. Frequency and species distribution of gliotoxin-producing Aspergillus isolates recovered from patients at a tertiary-care cancer center. J Clin Microbiol 2005; 43:6120-2; PMID:16333108; http://dx.doi.org/10.1128/JCM.43.12.6120-6122.2005
- Bergin D, Brennan M, Kavanagh K. Fluctuations in haemocyte density and microbial load may be used as indicators of fungal pathogenicity in larvae of Galleria mellonella. Microbes Infect 2003; 5:1389-95; PMID:14670452; http://dx.doi.org/10.1016/j.micinf.2003.09.019
- Insua JL, Llobet E, Moranta D, Perez-Gutierrez C, Tomas A, Garmendia J, Bengoechea JA. Modelling Klebsiella pneumoniae pathogenesis by infecting the wax moth Galleria mellonella. Infect Immun 2013; 81(10):3552-65; PMID:23836821
- Binder U, Maurer E, Lackner M, Lass-Florl C. Effect of reduced oxygen on the antifungal susceptibility of clinically relevant aspergilli. Antimicrob Agents Chemother 2015; 59:1806-10; PMID:25547350; http://dx.doi.org/10.1128/AAC.04204-14
- Arendrup MC, Cuenca-Estrella M, Lass-Florl C, Hope WW. EUCAST technical note on Aspergillus and amphotericin B, itraconazole, and posaconazole. Clin Microbiol Infect 2012; 18:E248-50; PMID:22540149; http://dx.doi.org/10.1111/j.1469-0691.2012.03890.x
- Mowlds P, Coates C, Renwick J, Kavanagh K. Dose-dependent cellular and humoral responses in Galleria mellonella larvae following beta-glucan inoculation. Microbes Infect 2010; 12:146-53; PMID:19925881; http://dx.doi.org/10.1016/j.micinf.2009.11.004
- Cove DJ. The induction and repression of nitrate reductase in the fungus Aspergillus nidulans. Biochimica et biophysica acta 1966; 113:51-6; PMID:5940632; http://dx.doi.org/10.1016/S0926-6593(66)80120-0
- Beckmann N, Schafferer L, Schrettl M, Binder U, Talasz H, Lindner H, Haas H. Characterization of the Link between Ornithine, Arginine, Polyamine and Siderophore Metabolism in Aspergillus fumigatus. PloS one 2013; 8:e67426; PMID:23825660; http://dx.doi.org/10.1371/journal.pone.0067426
- Cowen LE, Singh SD, Kohler JR, Collins C, Zaas AK, Schell WA, Aziz H, Mylonakis E, Perfect JR, Whitesell L, et al. Harnessing Hsp90 function as a powerful, broadly effective therapeutic strategy for fungal infectious disease. Proc Natl Acad Sci U S A 2009; 106:2818-23; PMID:19196973; http://dx.doi.org/10.1073/pnas.0813394106
