Abstract
Microbial pathogens are known to express an array of specific signaling molecules referred as Pathogen Associated Molecular Patterns (PAMPs), which are recognized by Pattern Recognition Receptors (PRRs), present on the surface of the host cells. Interactions between PAMPs and PRRs on the surface of the host cells lead to signaling events which could culminate into either successful infection or clearance of the pathogens. Here, we summarize how these events may generate novel host based as well as pathogen based molecular targets for designing effective therapeutic strategies against infections.
Introduction
A pathogen interacts with the host and causes infection, leading to the development of disease in the host. A pathogen may be any harmful microbial agent such as a bacterium, virus, protozoa, fungus or helminth etc. When the pathogen enter the host cell, it has to face strong panoply of immune defense system inside, that further confine and eliminate the pathogen.Citation1
At the interface of host-pathogen interaction, when a pathogen-ligand interacts with its specific host cell receptor, it results in its activation and ultimately leads to the recruitment of signaling molecules via signaling cascades.Citation2 In immune cells, signal transduction cassettes consist of specific surface bound membrane receptors like B-cell receptors, T-cell receptors, co-stimulatory receptors and cytokine receptors, that on activation lead to recruitment of various regulatory proteins and effector signaling elements. These cassettes detect, amplify and integrate the external signals generated from ligand-receptor binding to trigger the appropriate and adequate effector responses of the immune system in order to achieve complete removal of pathogens and limit the host damage to the minimum.Citation3 However, some of the pathogens utilize these communication pathways as key targets to modulate the host immune response and promote their survival and multiplication.Citation4
Research focused on host-pathogen interactions have provided a better understanding of the infectious diseases and various mechanisms being adopted by the pathogens to cause infection. It has provided new discernment about the elemental aspects of the microbial pathogenicity and proven to be an aid in the development of better treatment and prevention of infectious diseases. In this review, we will discuss various aspects of the interaction of pathogen ligands with their cognate receptors on the host cell membrane and how the interaction modulate subsequent events that may either support or eliminate the pathogen from the host. We will further discuss how the host-pathogen interactions generate strategic hotspots at the interface between them and how they may be targeted for the development of novel and effective therapeutics including drugs and vaccines. We have also discussed some of the known off-target effects of some widely prescribed antimicrobials.
Receptors Involved in the Host-Pathogen Interactions
Mechanism of pathogenesis or disease development involves transmission of pathogen to a susceptible host, adherence to appropriate target tissue, invasion, colonization, damage to the host while evading robust protective suite of immune system and finally exit from the body of the host followed by survival outside the host for getting transmitted to another host. Pathogens are characterized by specific arrangements of key molecules called PAMPs which are recognized by the cells of innate immune system of the host. The PAMPs represent small molecular motifs conserved within a class of the pathogen. PAMPs are highly specific and vary across the microbial pathogens. Bacterial PAMPs include lipoteichoic acid, peptidoglycan, lipoproteins, DNA, flagellin and lipopolyssacharides. Coat proteins and nucleic acids act as PAMPs in case of viruses, whereas, parasites have Glycosyl-Phosphatidylinositol anchors (GPI) and yeast express zymosan as PAMPs.Citation5 The cells of innate immune system express PRRs to recognize the PAMPs in order to detect and bind pathogens. PRRs also identify endogenous molecules released from damaged cells known as Damage-Associated-Molecular-Patterns (DAMPs).Citation5 The PRRs are found on many cells of the innate immune system including epithelial cells, granulocytes, macrophage-monocytes, dendritic cells and mast cells.Citation1 PRRs can be classified as membrane bound, cytosolic and secreted PRRs () ().
Figure 1. PAMP-PRR interactions and innate immune signaling. TLR and CLR are strategically localized over the cell surface to recognize the conserved PAMPs of extracellular pathogenic microbes such as bacteria, fungi, protozoan and viruses. TLRs are also present inside the cells in endosomes. RLR and NLR are present in the cell cytosol where they sense PAMPs of intracellular pathogens like bacteria and viruses and secreted PRRs like pentraxins and Lipopolysaccharide-Binding Protein (LBP) secreted in host tissue fluid recognize extracellular bacterial pathogens. After recognition, these receptors coordinate the activation of signaling pathways by inducing the NF-кβ which activates and increases the transcription of immune response genes for the anti-microbial defense.
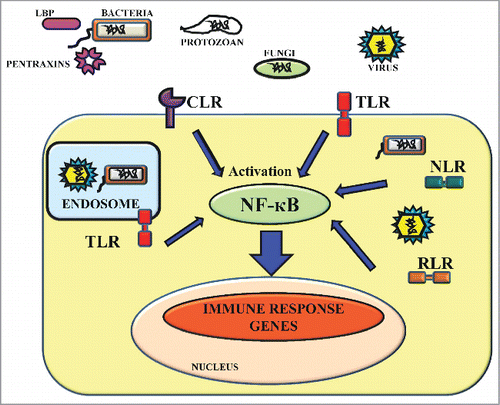
Table 1. PRRs and PAMPs in the host and respective microbes
Membrane bound PRRs
These are the membrane-bound receptors localized on the cellular or endosomal membranes. They recognize extracellular pathogens like bacteria and fungi. In the endosomes they sense for the intracellular invaders such as viruses. There are several membrane bound PRRs like Toll-like receptors (TLRs), C-type lectin receptors (CLRs), scavengers, complement and mannose receptors etc. (). Following are the main types of membrane bound PRRs which exist in the host cells.
Toll-like receptors (TLRs)
The TLRs are found in most of the higher organisms. These are responsible for sensing the pathogens that intrude into the cells from outside. Each TLR contains an ecto-domain comprised of Leucine-Rich Repeats (LRR), a transmembrane domain, and a cytoplasmic Toll/IL-1 Receptor Homology (TIR) domain. The transmembrane domain is involved in the recognition of pathogen product whereas, TIR domain recruits different signaling molecules that will in turn activate the transcription of genes involved in inflammation and in the anti-microbial defense. In humans, there are over 10 different TLRs while in mice there are 12 different TLRs with different ligand-binding specificities ().Citation5 The phenomenon of the microbial ligand binding to the TLRs may either lead to the release of pro-inflammatory cytokines, phagocytosis or up-regulation of APC's co-stimulatory molecules and the maturation and transformation of naive dendritic cells. TLR engagement with the microbial ligands is thus a fundamental step in both the activation of the acquired immune system and acute inflammatory response.Citation1
C-type lectin receptors (CLRs)
CLRs are the most important and significant PRRs for the recognition of the microbial agents such as viruses, bacteria and fungi by the host cells. CLRs are a diverse family of Ca2+-dependent glycan-binding transmembrane proteins. These also contain one or more C-type Lectin-Like Domain (CTLD) and are found in all organisms. In the Carbohydrate-Recognition Domains (CRDs), these have been found to show primary and secondary structural homology. With CRDs these recognize various polysaccharide structures present on the surface of the microbial agents and mediates the activation of various intracellular signaling pathways to regulate the immune response by regulating the gene expression of TLRs.Citation5 There are some other CLRs which have a CTLD fold but no CRDs and they do not bind any carbohydrate molecules. The large family of CLRs includes selectins, collectins, proteoglycans, mannose and endocytic receptors. Some of these CLR proteins are transmembrane and others are secretory proteins. They often oligomerize to form homodimers, homotrimers or higher-ordered oligomers. This oligomerization eventually increases their binding interactions with multivalent ligands. Although CLRs share structural homology, these usually differ significantly in the binding specificities of different types of carbohydrate moieties. These proteins are reported to act as adhesion and signaling receptors during immune response events such as inflammation and cytotoxic killing of tumorous and virally-infected cells.Citation21 Dectin-1 and Dectin-2 that are well-characterized CLRs have been reported to assist transcription factor Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B Cells (NF-кB) mediated signaling by activating Spleen Tyrosine Kinase (SYK) and a multiprotein complex of Mucosa-Associated Lymphoid Tissue (MALT1), Caspase-Recruitment Domain (CARD9), and B-cell lymphoma 10 (Bcl−10). Citation19
Scavenger and Complement receptors
The Scavenger Receptors (SR) consists of a large family of transmembrane cell surface glycoproteins which are structurally diverse and restricted to dendritic cells (DC), macrophages and endothelial cells.Citation22,23 It has been reported that many SR, including Macrophage Scavenger Receptors (MARCO), Surfactant Protein A I (SR-AI), Surfactant Protein A II (SR-AII) and CD36 play an important role in the innate immune defense and act as PRRs against many bacterial pathogens. SRs expressed on DCs and macrophages act as phagocytic receptors and mediate the non-opsonic phagocytosis of various pathogenic microbes. Some of the SRs act as co-receptors to TLRs, and modulate the inflammatory response against the TLR agonists. In case of bacteria, the SR ligands have commonly been reported to be binding the lipoteichoic acid or lipopolysaccharides (LPS) as well as to the intact bacteria and act as receptors of phagocytosis for cleaning tissue sites with invading pathogenic microbes. Citation24 Whereas, Complement Receptors (CR) are the membrane proteins expressed on the surface of various immune cells. They specifically interact with complement factors of the complement system and lead to the antigen removal from the circulation. These are the part of the complement mediated innate immune system. The complement system promotes microbe destruction and exerts an influence on the immune activities of the immune cells. The complement system has functions like promotion of particle phagocytosis, clearance of soluble immune complexes etc. These functions are reported to be exerted through specific interactions of complement factors C5, C3 or C1 activation products with specific CRs present on the responding cells. These CRs are divided into 3 different categories: (1) CRs for collagenous lectins and C1q, (2) CRs binding C3b (the active C3 fragment), and its degradation products, C3dg and iC3b (3) CRs recognizing the anaphylotoxic polypeptides C5a-desarg, C5a and C3a.Citation25
Formyl-peptide receptors (FPR)
These receptors with 7 transmembrane domains are G-proteins coupled receptors and are present on the monocytes and neutrophils membranes.Citation26 These receptors bind and interact with formyl–Methionine-Leucy L-Phenylalanine (fMLF), a peptide produced by pathogenic bacteria, and are in part accountable for the drawing of neutrophils via chemotaxis to the sites of tissue invasion. Complementally, endogenous ligands have been recognized for these receptors, implicating their function in modulation of the inflammatory responses. The FPR activation triggers the rearrangement of the cytoskeleton to facilitate the cell migration and chemokine synthesis. These are also involved in regulation of many important signaling pathways like G-protein triggered activation of phospholipase C which ultimately leads to the breakdown of the membrane constituent phospholipid, phosphatidylinositol (4,5)-bisphosphate (PIP2) into diacyl glycerol (DAG) and phosphatidylinositol (3,4,5)-trisphosphate (IP3). Further, IP3 acts as an effective inducer for opening Ca2+ channels to increase Ca2+ from cytoplasmic pools and outside the cell. A continued increased level of Ca2+ is required for directed migration of immune cells.Citation1
Cytosolic PRRs
Mammals have developed a distinctive set of recognition receptors known as cytosolic PRRs situated inside the cell cytoplasm that can trigger an immune response against intracellular pathogens. These activate the inflammatory cytokines and assembly of inflammasome components during microbial infection. There are several cytosolic PRRs like NOD-like receptors, RIG like receptors, intracellular receptors etc. (). Important types of cytosolic PRRs are described below.
NOD-like receptors (NLRs)
Nucleotide-binding, Oligomerization Domain (NOD)-Like Receptors (NLRs) are a type of the cytosolic receptors. These act as a second line of defense against the invading pathogens. Two important functions of the NLRs against the intracellular pathogens during host defense are the identification of bacterial peptidoglycans (by NOD1 and NOD2) and turning on the inflammasome, an innate immune signaling complex. On recognition of an endogenous dangerous signal or a microbial ligand (e.g. Adenosine Triphosphate or Uric acid liberated from damaged epithelial cells) the inflammasome induces activation of Caspase-1 and processes pro-IL-18 and pro-IL-1β into active cytokines. The NLR family consists of cytoplasmic pathogen sensors composed of a central nucleotide-binding domain and C-terminal LRR. The NLR protein family consists of twenty-two members in humans and thirty-four members in mouse. Citation27
RIG like receptors (RLRs)
Retinoic Acid-Inducible Gene-I (RIG-I)-Like Receptors (RLRs) are a subset of cytosolic PRRs and recognize double stranded RNA containing viruses. The RLR family consists of Laboratory of Genetics and Physiology 2 (LGP2), Melanoma Differentiation Associated Gene 1 (MDA5) and RIG-I. MDA5 and RIG -I contain 2 N-terminal Caspase-Recruitment Domain (CARDs), a C-terminal repressor/RNA-binding domain and an internal RNA helicase domain. These RLRs are reported to recognize viral replication by recognizing the double stranded RNA, which is produced by RNA viruses to form their genome during their replication cycle inside the host.Citation17
Intra-cellular receptors
Intra-cellular receptors are located in the cytoplasm of the cells and interact with hydrophobic ligand molecules. Intra-cellular receptors such as Protein Kinase R (PKR), activated by double stranded RNA present in the host cells to detect and respond to invading viral pathogens. PKR also upregulates Mitogen-Activated Protein (MAP) kinase and NF-кB and results in synthesis of interferons. Recently innate intra-cytoplasmic receptors such as NOD have emerged as the targets of immense interest for the researchers.Citation18 They consist of a nucleotide-binding domain, N-terminal CARD and LRR domain similar to TLRs. During the activation of NF-кB, LRR acts as a negative regulator. The intra-cytoplasmic innate receptors are: NOD1 (CARD4), NOD2 (CARD15), CARD 12, NALP-1 and NALP-2 (NACHT, LRR and PYD Pyrin Domains-Containing Protein). Interestingly, CARD15 polymorphisms have been connected to Crohn's disease implicating defects in recognition of LPS followed by inflammatory activation.Citation28
AIM2 (Absent in Melanoma 2)-like receptors (ALRs)
The ALRs are the cytosolic PRRs that act as a scaffold and promote the assembly of the inflammasome. The activation of the inflammasome is a crucial step for the clearance of many intracellular bacteria like Francisella novicida, Shigella flexneri, Legionella pneumophila, Listeria monocytogenes, and Salmonella typhimurium.Citation29 These ALRs directly bind with the cytoplasmic double-stranded DNA of the pathogen through their HIN-200 domain to form a large inflammasome complex. The completely assembled ALR inflammasome further recruits and subsequently oligomerizes ASC (Apoptosis-associated speck-like protein containing a CARD) which is a caspase-1-activating adaptor protein. It bridges ALRs to caspase-1, leading to their activation and production of the mature cytokines like interleukin 1β (IL-1β) and IL-18 which subsequently leads to cell death.Citation30
STING (Stimulator of Interferon Genes)
STING also known as ERIS/MPYS/MITA is encoded by gene TMEM173. It is a transmembrane protein predominantly localized in the endoplasmic reticulum. In response to the pathogenic DNA and even certain viral RNA, STING has been reported as crucial for recognizing the cytoplasmic DNA. It activates the transcription of innate immune genes and triggers type I IFNs (Interferons) immune response.Citation31
Secreted PRRs
A number of PRRs do not remain associated with the cell surface and may be secreted out into the bloodstream and tissue fluid of the host like collectins, complement proteins, LBP, CD14 etc. (). Some of the secreted PRRs are explained in the following text.
The Collectins
The collectins are the proteins that bind specifically to the unique carbohydrate moieties on the surface of the microbial cells. These belong to the Ca2+-dependent (C-type) lectin superfamily consisting of the C- type Carbohydrate Recognition Domain (CRD). They play important role in the clearance and elimination of the pathogens. Collectins have a similar domain architecture comprising of 4 regions namely, N-terminal cysteine-rich domain, collagen-like region, α-helical neck domain and a CRD. The collectin family consists of 9 members, conglutinin, collectin CL−P1, CL−L1, CL−43, CL−46, CL−K1, mannose-binding lectin (MBL), surfactant protein (SP)-A and SP-D. On recognition of the pathogen, the collectins operate using various effector mechanisms like opsonization, neutralization, agglutination, complement activation and phagocytosis to restraint the microbial growth.Citation32
The Complement cascade
The complement cascade being a proteolytic cascade system constitutes the most important effector arm of the humoral branch of the immune defense system. It modulates several adaptive immune responses and contributes to the destruction of the encroaching pathogen. The complement cascade gets activated through 3 different routes namely, the classical pathway, the alternative pathway and the lectin pathway. The activation of complement classical pathway is initiated by immune complexes. It requires antibody for its activation and can also be activated by C-reactive protein (CRP) and is considered as a representative of acquired immunity. The MBL and the MASPs play significant role in activating the complement lectin cascade. MBL on binding to mannose residues induces conformational changes resulting in the cleavage of C2 and C4 to C4b2a or C3 convertase, whereas, MASPs directly cleave C3, resulting in membrane attack complex formation. The complement alternative pathway begins with the slow deposition and activation of C3 on the cell surface. It is considered as a part of the innate immune system.Citation33
Lipopolysaccharide Binding Protein (LBP) and CD14
Lipopolysaccharide (LPS) is an important PAMP and may swiftly bind to LBP in the serum.Citation34 LBP is a soluble acute-phase protein that binds to bacterial LPS to elicit immune responses by presenting the LPS to CD14 and TLR4, the cell surface PRRs. CD14 is found in soluble form and membrane bound form. The membrane-bound form of CD14 lacks intra-cytoplasmic signaling domain and fails to transmit a signal inside the cell. Thus, as soon as LPS is bind to CD14 through the LBP, the complex connects simultaneously to additional cell-linked receptors known as TLR4 which eventually directs intra-cellular signaling and secretion of cytokine by the macrophages.Citation1,34
The pentraxins
The pentraxins are an evolutionary conserved family of PRRs characterized by pentraxin protein domain having a cyclic pentameric structure.Citation35 Proteins of this family are found to be involved in acute immunological responses. The family contains the short pentraxins such as Serum Amyloid P Component (SAP) and CRP and the long pentraxins.Citation35 SAP and CRP are acute phase proteins produced by the liver as a result of the acute inflammatory response. CRP binds to mannose residues and phosphorylcholine found on the fungal and the bacterial cell walls and both SAP and CRP act as opsonins. They can activate the classical pathway of complement system by binding to C1q (one of the interacting subunit of C1 enzyme). C1q characteristically has a bouquet-like structure and consists of 6 identical heterotrimeric proteins. Each of these has a collagen-like domain forming triple helix structure. These six triple helices associate together to form an N-terminal “stalk” and diverge to form individual triple helix “stems.” These “stems” of C1q terminate into a heterotrimeric globular “head” which contains the antibody recognition function.Citation36 CRP interacts with the collagen-binding component rather than the antibody-binding component of C1q. The Fc receptors on surface of phagocytic cells, which recognize Fc region of antibodies, may take up short pentraxins. They are involved in clearance of pathogens and of apoptotic host cells. The long pentraxins have a long amino-terminal domain attached to the pentraxin domain reported to target Aspergillus antigens. These long pentraxins are synthesized by a variety of epithelial and immune cells.Citation1
Response by the Host for Pathogen Clearance
The interaction between pathogens and the host is mediated initially via an interaction between PRRs and PAMPs. The receptor-ligand binding initiates intracellular signal transduction cascades which result in the activation of various transcription factors and other proteins to regulate different processes such as gene induction, apoptosis, phagocytosis, secretion and proliferation of immune cells for pathogen removal. Citation37 Important host response mechanisms against the invading microbes are explained below.
Immune cell signaling events
The innate immune system recognizes infection by identifying well conserved PAMPs through PRRs. Different PRRs recognize highly specific PAMPs from different pathogens such as bacteria, fungi, viruses and protozoan parasites. After recognition, these receptors coordinate the activation of signaling pathways by activating the transcription of genes involved in inflammation and in the anti-microbial defense (). These pathways tend to generate immunological responses against the PAMPs expressed by the pathogens. They are independent of immunological memory and transmit the signals to induce inflammatory cytokines and boost the host defense mechanisms resulting in clearance of the microbial pathogens. In the following text we are explaining the major immune signaling events.
Figure 2. Immune cell signaling. (A) Antigen receptor signaling: Ligand binding moiety, sIg, present on the membrane recognize the antigen and transduce intracellular signals initiated by phosphorylation of signal transduction moieties, Ig-α/β dimmers, which span the plasma membrane and has cytoplasmic tail. Ig-α/β dimmers are associated with sIg forming BCR complex. The activated BCR complex recruits adaptor and signaling proteins which trigger the activation of various signaling cascades resulting in gene induction leading to the promotion of B cell survival, proliferation and differentiation. (B) Cytokine receptor signaling: ligation of cytokines to the cytokine receptors leads to activation of JAKs. JAKs have tyrosine kinase activity. Activated JAKs phosphorylate the tyrosine residues in the cytokine receptor and create phosphotyrosine-binding SH2 domains on these receptors. This enables SH2-domain containing STAT recruitment and binding to these phosphotyrosine residues on the receptors. STATs are then tyrosine phosphorylated by the JAKs and further act as binding sites for other STATs mediating their dimerization. These hetero- or homodimer STATs are translocated to the nucleus followed by binding to GAS motifs. This eventually leads to gene induction. (C) TLR signaling: Pathogen binding to the TLRs initiates recruitment of various adaptor molecules resulting in activation of NF-kβ, MAP kinase and IRF-3. These further induce the production of IL-12, IL-6, TNF-α and IFN-β. The surface diagrams of sIg, cytokine receptor and TLR structures were generated in PyMOL using PDBs: 1IGT (Structure of Immunoglobulin), 2B5I (Cytokine Receptor Complex) and 2Z7X (Crystal structure of the TLR1-TLR2 heterodimer which is formed due to binding of a tri-acylated lipopeptide) and after that inserted in the lipid bilayer cartoon.
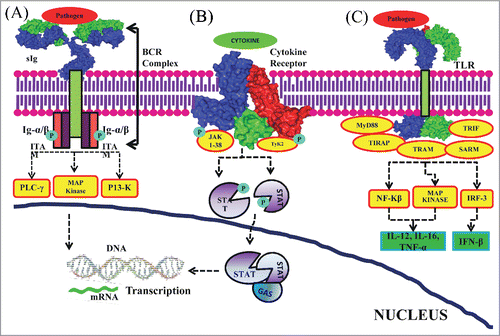
B cell receptor signaling
Specific antigen recognition by B cells is carried out by surface-bound immunoglobulin (sIg). Signaling is initiated by recruitment of Ig-α/β dimers spanning the membrane and possessing Intracellular Tyrosine-based Activatory Motifs (ITAMs) in their cytoplasmic tails. On antigen recognition by the ligand binding moeity, sIg, transduces intracellular signals initiated with tyrosine phosphorylation of Ig-α/β dimers and association with sIg to form the activated B Cell Receptor (BCR) complex. A signaling scaffold is formed around the activated BCR complex followed by the recruitment of adaptors/signaling proteins. These interactions trigger the activation of various signaling cascades including the PLC-γ, PI3K and MAP kinase pathways eventually resulting in gene induction, promotion of B cell survival, proliferation and differentiation ().Citation38
Cytokine receptor signaling
Cytokine receptor signaling is achieved through MAPK, PI3K, PLC and Janus kinase (JAK-STAT) pathways. Following binding of cytokines to their cytokine receptors, tyrosine kinases of the JAK family (Tyk2 and JAK1–3) are recruited. Activated JAK proteins phosphorylate the tyrosine residues in the β and γ signaling cytokine receptor chains. This enables the SH2 containing Signal Transducers and Activators of Transcription (STAT1–6) and other proteins recruitment. These STATs are then phosphorylated at designated tyrosine residue by the JAKs and further act as binding sites for other STATs mediating their dimerization. Phosphorylated STAT proteins form homo- or heterodimers and translocate into the nucleus. These bind to the GAS elements and act as transcription factors resulting in the promotion of gene induction ().
TLR signaling
As soon as the pathogen derived products bind with the LRR domain of TLRs, signaling is initiated via the recruitment of adaptor proteins including TIR-containing Adaptor Protein/MyD88 adaptor like (TIRAP/Mal), Myeloid Differentiation Factor 88 (MyD88), TIR-Containing Adaptor Molecule-1/TIR-Containing Adaptor Inducing IFN-b/(TICAM-1/TRIF), Sterile α and HEAT-Armadillo Motifs (SARM) and TRIF-related adaptor molecule (TRAM).Citation39,40,41 Association of TLRs with various adaptor proteins triggers different downstream events e.g., MyD88-dependent NF-kβ activation, TRIF-dependent phosphorylation and activation of the transcription factor IRF-3 inducing IFN-β production().Citation40
Autophagocytosis
Autophagy being an intracellular process is considered as critical response of the host against invading bacterial pathogens. It has a protective role against many intracellular pathogens (e.g., Shigella flexneri, Listeria monocytogenes, Salmonella Typhimurium). It delivers the cytoplasmic bacterial pathogen in the form of autophagosome to lysosome for its degradation and elimination. Autophagy performs multitier immunological function during various bacterial infections by interacting with the different PRRs. It contributes to both adaptive and innate immunity. The autophagic machinery delivers the PAMPs to TLRs and NLRs and enhances the TLR and NLR recognition of PAMPs during bacterial infection. Conversely, many of the TLRs like TLR2, TLR3, TLR4, TLR5 and TLR7 have also been found to induce autophagy. Citation41 In macrophages, NOD1 and NOD2, the NLRs, have also been found to interact with Atg16L1 and signals to induce autophagy. Citation42 Similarly, RLRs have also been reported to induce autophagy. Citation43 Autophagy receptors such as Neighbor of Breast cancer gene 1 (NBR1), p62 (Sequestosome 1 or SQSTM1), Optineurin (OPTN) and Nuclear Dot Protein, 52 kDa (NDP52) also called as Sequestosome 1/p62-Like Receptors (SLRs) recognize ubiquitinated substrates and interact with Autophagy-Related Protein 8 (ATG8) family proteins to recruit membranes for autophagosome and eliminate the pathogen from the cytoplasm.Citation44
Macroautophagy contributes to antigen presentation by presenting the products of lysosomal proteolysis to MHC class II molecules. These in turn present these products to CD4 (+) T cells and help in pathogen recognition and elimination. In some of the MHC class II-positive cells, including epithelial cells, B cells and dendritic cells, autophagosomes have been reported to fuse with multivesicular MHC class II loading compartments. This targeting of the antigens to autophagosomes enhances their MHC class II presentation to CD4 (+) T cells and results in their successful elimination.Citation45
Anti-Immune Strategies of the Pathogens
During the long lasting coexistence with the hosts, microbial pathogens have evolved with a myriad of molecular mechanisms to overcome the host immune response. Various strategies are utilized by the microbial pathogens for the host immune evasion like destroying various elements of host immune system, interference with the functions of host immune system and hiding from the host immune system. These strategies include: killing immune cells or blocking the antimicrobial small molecules, intrinsic cellular pathways and acquired immunity by blocking antigen presentation or inhibiting the complement, cytokines, interferons and chemokines, or modulating the apoptosis and autophagy or by interfering with expression of various receptors by hijacking or preventing their recognition. While some pathogens evade immune surveillance by either the direct engagement or coupling of host cell surface receptors stimulating downstream effector molecules or specialized bacterial proteins involved in targeting proteins known to play important role in cytoskeletal remodelling. Both strategies conclude with the formation of cell surface projections that further promote efficient attachment, colonisation or the eventual phagocytosis of the invading bacteria. Some pathogens have also evolved with various strategies for example antigenic variation among the surface exposed proteins which are the key signals to trigger microbial clearance in order to conquer the host immune responses.Citation46 While some, especially the viral and bacterial pathogens are involved in secretion of virulence factors like toxins and immune modulators like ligand-mimics and proteases which are utilized by them to escape the host immune surveillance. Here, we are highlighting some of the mechanisms involved in the anti-immune evasion strategies.
Impairment of antigen presentation pathways
Many viral and some of the bacterial pathogens have evolved with various strategies to down-regulate the antigen presentation by the host. The host MHC Class I antigen presentation pathway plays a very important role in the detection of virally infected cells. However, in turn, many adenoviruses and retroviruses have evolved with proteins that interfere with the various stages of the MHC class I antigen presentation pathway to further prevent the display of viral peptides and escape CTL lysis. These viral proteins have been found to be exploiting the bottlenecks in MHC Class I pathway and degrade or mislocalize the MHC Class I molecules. These strategies involve turning off the TAP or promoting the MHC Class I heavy chain degradation by throwing it with cellular garbage out of the ER into the cytosol or preventing MHC Class I transport from ER to the plasma membrane. TAP is a heterodimer with an N-terminal membrane domain and a C-terminal Nucleotide Binding Domain (NBD). The membrane domain has peptide binding site and a pore through which peptides translocate and NBD domain energizes this peptide translocation. The Herpes Simplex Virus (HSV) infected cell protein (ICP)47Citation47 and Human cytomegalovirus (HCMV) unique short (US) region US6Citation48 gene products targets and inhibits this TAP function and in turn antigen presentation by the host. HCMV also encodes 2 proteins: US2 and US11 which targets and dislocates the MHC Class I heavy chain into the cytosol for degradation. The adenoviral protein E3/19K(E19) interacts with MHC Class I in the ER and inhibits its trafficking. A dilysine motif is present in the cytosolic tail of the E19 which acts as an ER retrieval motif and inhibits the trafficking of MHC Class I.Citation49 Similarly US3 and US10 proteins have been found to be affecting the MHC Class I export to Golgi.Citation50 The Kaposi's sarcoma-associated herpesvirus (KSHV) encodes K3 and K5 proteins which down-regulates the MHC Class I molecules from the plasma membrane by exploiting clathrin-dependent endocytosis pathway. The MHC Class I molecules are internalized in an endocytic acidic compartment for degradation by acidic proteases ().Citation51
Figure 3. Impairment of the antigen presentation pathways by microbial pathogens. (A) Viral regulation of MHC Class I antigen presentation pathway: viruses have gradually developed a number of proteins specifically interfering with the antigen presentation pathway of MHC Class I. The HSV ICP47 Citation47 and HCMV US6Citation48 proteins inhibit peptide translocation function of TAP. HCMV US2 and US11 proteins dislocate the MHC Class I heavy chain from ER into the cytosol for degradation. Adenovirus E19 protein inhibits MHC Class I trafficking.Citation49 US3 and US10 inhibit the transport of the MHC Class I-antigen peptide complex to be exported to the Golgi.Citation50 The KSHV encoded K3 and K5 proteins internalize the MHC Class I-antigen peptide complex into lysosomes, which lead to its degradation and impairment of the antigen presentation.Citation51 (B) Bacterial regulation of CD1 antigen presentation pathway: The monocytes generally differentiate into immature DCs and then to mature DCs which further express CD1 glycoproteins on their surface, which present antigens to T cells. However, the exact mechanism of CD1 antigen presentation pathway is yet to be discovered. It has been reported that in M.tuberculosis infected DCs MAPK is phosphorylated and activated to down-regulate the expression of CD1 on mature DCs resulting in immune evasion.Citation52,53
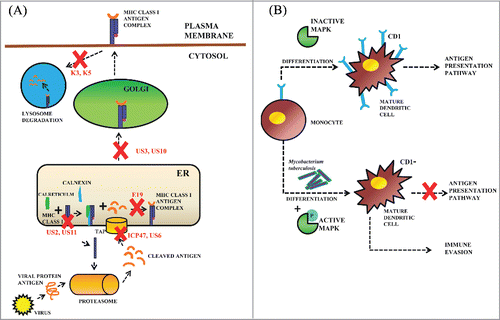
Group I CD1 (CD1a, CD1b and CD1c) antigen presenting proteins expressed on the dendritic cells present non-peptide antigens to the T cells. The monocytes are differentiated to form mature dendritic cells expressing CD1 glycoproteins. The CD1 restricted T cells recognize the mycobacterial lipid antigens. However, Mycobacterium tuberculosis has developed a successful tactic to down-regulate the expression of CD1 glycoproteins in order to evade the CD1 dependent immuntiy.Citation52 The M. tuberculosis was shown to induce human monocytes to differentiate into CD1 dendritic cells which lose the ability to present the mycobacterial lipid antigens to the T cells. The p38 mitogen-activated protein kinase (MAPK) is inactivated during differentiation of monocytes to dendritic cells. M. tuberculosis was found to induce the phosphorylation of p38 and in turn inhibits the surface expression of CD1 on the dendritic cells ().Citation53
Actin remodelling
Many invading bacterial pathogens survive inside the host by utilizing an arsenal of highly sophisticated mechanisms that modify the cellular actin cytoskeleton to trigger their localization into normally non-phagocytic host cells in order to escape the humoral immune defense. This invasion of non-phagocytic cells by stimulating some of the endogenous uptake processes which includes macropinocytosis and phagocytosis. Actin polymerization plays a central role in both of these uptake processes which drives the plasma membrane extensions to engulf external cargo. There are some groups of pathogens (e.g., pathogenic-Helicobacter pylori and E. coli) which do not invade their host cells but intentionally damage or divert the actin cytoskeleton from outside with the aim to establish colonies and specialized niches in the gut and stomach of the host and to compete over commensals with less refined adhesion mechanisms.Citation54
Altered membrane physicochemical properties
In the cell membrane there is a close-fitting interaction between cholesterol and sphingolipids which triggers the generation of the different membrane domains. These are named as rafts since they float in the “ocean” of phospholipids. Different lipid rafts containing proteins in the cell membrane recruit proteins and result in lateral organization of the cell membrane upon application of the suitable stimulus. These lipid rafts are involved in regulation of different signal transduction mechanisms. These act as signaling platforms to co-localize the required components and facilitate their interaction to support signaling. These lipid rafts have also been reported to play important role by regulating G protein-coupled receptors and receptor tyrosine kinases. Citation55 Erich et al. (2003) proposed a mechanism by which rafts might be converted to create a larger membrane platform that tend to initiate signal transduction through receptor molecules in addition to the infection of mammalian cells.Citation56 They have exemplified that stimulation via CD40 and CD95 triggers the intake of Sphingomyelin to ceramide in rafts.Citation57 The generation of ceramide culminates in the merging of small rafts to an enormous ceramide rich membrane platform that tends to group together and signal CD40 and CD95. This concept was shown for invigoration of CD40 and CD95 using physiological ligands and stimulatory antibodies.Citation57 The molecular function of ceramide-enriched membrane platforms and rafts appears to be the reorganization of receptor, resulting in altered intracellular signaling molecules in the cell membrane enabling the interaction of the pathogen with the cells.Citation58
Chlamydia pneumoniae, an intracellular prokaryote, requires some essential lipids which it cannot synthesize and these lipids have been shown to play important fluidizing roles in plasma membrane. C. pneumoniae brings about the depletion of macrophage membrane phosphatidylinositol, cardiolipin and cholesterol but increases phosphotidylcholine resulting in a comparative increase in total phospholipid content as reported in the earlier studies by Azenabor et al.Citation59 They have reported that C. pneumoniae infected macrophages have altered membrane physicochemical characteristics. Macrophages play a very important role in atherogenic process and altered membrane properties may render them atherogenic, particularly with lesional focus formation marked by early events like enhanced stickiness of macrophages to vascular endothelium.Citation59
Plasmodium spp., the malaria causing parasite, during its development in the erythrocytes, modifies the lipid and protein compositions and various other properties of the plasma membrane of the host cells. Fatty acid modifications are not observed in all lipid classes. Modifications in the fatty acid composition of phosphatidyl ethanolamine and phosphatidyl choline of erythrocyte membranes were reported for Plasmodium knowlezi by Simoes et al.Citation60 Intracellular parasite Leishmani major disrupts the membrane rafts of the host cells by depleting the membrane cholesterol and increasing the membrane fluidity. This eventually results in reduced NK cell-mediated target cell lysis as well as T-cell mediated cytotoxicity. Citation61 Modifications in fatty acid composition of host cell lipids are not confined to only parasitic infections. Changes in phospholipid fatty acid composition as observed after infection with Bacillus Calmette-Guérin, an attenuated mutant of Mycobacterium bovis, in mouse tissues, while infection with Human Immunodeficiency Virus-1 (HIV) of cultured lymphocytes resulted in modification of the fatty acid composition of the membrane lipids of the lymphocytes. Citation62
Adhesins interaction with the host receptors
Majority of commensal and pathogenic bacteria have adhesin molecules on their surface which interact with the host cell receptors and promote host-pathogen interaction. Some of the bacterial class adhesins include flagella, type IV pili, chaperon usher assembled pili, Trimeric Autotransporter Adhesins (TAAs), curli and Serine-Rich Repeat Protein (SRRP), sortase assembled pili. Citation63 These adhesin molecules act as an aid in bacterial attachment to the host cells during bacterial colonization. This attachment is beneficial but sometimes it may result in elimination of bacterial pathogen by eliciting an immune response and phagocytosis by the host. It has become clear that the bacterial adhesins depend on ligand interactions to mediate a series of signaling events that may affect bacterial uptake or invasion and/or promote anti- or pro-inflammatory events by affecting the host innate immune receptors.Citation64 To escape the arduous circumstances, some bacterial pathogens have expressed their adhesions on polymeric structures that extend out from the cell surface, allowing for the initial host interactions at a “safe” distance or producing a surface layer that prevents immune recognition and leads to dodging of immune response.Citation65 Taken together, the interaction between bacterial adhesins and phagocytic cells may lead to invasion of host cells and subsequent immune escape where bacterial survivors may disseminate in the tissues or killing of the bacteria by the host defense system.Citation65
Immune quiescence through the Extracellular Polymeric Matrix
Colonizing pathogenic infections mediate diverse set of inflammatory reactions by the host. These are host specific responses and result in pathogenic clearance. During pathogenic clearance, immune response affects the entire physiology of the host. It includes an early Type I Interferon response, remodelling of the basement membrane, proliferation of T-cell, antigen-specific CD4+T-cell response development that includes regulatory T cells (Tregs) and the production of capsular polysaccharides and opsonizing antibodies against pathogenic proteins.Citation66 However, there are some bacterial pathogens that can evade immune response with the help of biofilm formation. Many of the survival strategies ascribed to biofilms formation in bacteria appear from the structure of the biofilm itself and presence of the Extracellular Polymeric Matrix (EPM). Enclosing of pathogenic bacterial cells within the EPM enables them to evade a variety of host immune effectors of both the adaptive and innate branches of the response (e.g., phagocytosis is repressed when organisms are growing within a biofilm). Moreover, various species show increased resistance against many of the host-derived antimicrobial peptides like defensins, when grown in a biofilm. This would most likely result in reduced release of lipoteichoic cell wall components of the Gram-positive bacteria and proinflammatory LPS from the Gram-negative bacteria. The EPM also limits the release of bacterial components resulting in normal bacterial death. Citation67
Lipid membrane mimicry
Lipids are the biological amphiphilic molecules present in cell membrane. Earlier it was believed that lipids merely have structural role. The pioneering work of Irvine and Berridege in 1980s showed lipids being involved in intracellular signaling. Citation68 Lipids present in the host cell membranes as well as pathogen cell membranes play important role in the ability of the pathogens to escape from the host immune system. Many highly pathogenic viruses such as HIV, influenza virus and vaccinia virus are enveloped with host originated lipids and virus encoded proteins, covering nucleocapsid with the viral genome. All lipids originated from the host are recruited from the host membranes. Thus, the lipid composition of the virus envelope is similar to that of the host cell membrane.Citation69 This form of imitation may allow viruses to escape immune detection.
Targeting Novel Biomarkers in the Host and Pathogen for Modern Vaccine and Drug Design
Biomarkers are naturally occurring biological molecules (DNA, RNA or Protein), which could help us in diagnosing a particular pathological or physiological condition i.e. some metabolic disease or infection of a microbial pathogen. These are unique and specific for the disease and hence could also act as novel targets against which, the future therapeutic interventions may be carried out. Identification of novel biomarkers could lead to the development of novel vaccines, drugs and diagnostic assays against infectious diseases. In response to pathogen-ligand interaction with host cell receptors, it triggers a number of signaling cascades and various adaptor proteins in the host which can prove to be useful molecular targets for in vitro point-of-care diagnostics and also the improved design of novel potential vaccines to combat infectious diseases. Characterization and analysis of host immune response pathways and mechanisms across multiple infectious agents during host pathogen interaction will enable rapid identification of novel targets against existing and re-emerging biological threats caused by pandemic and resistant pathogens.
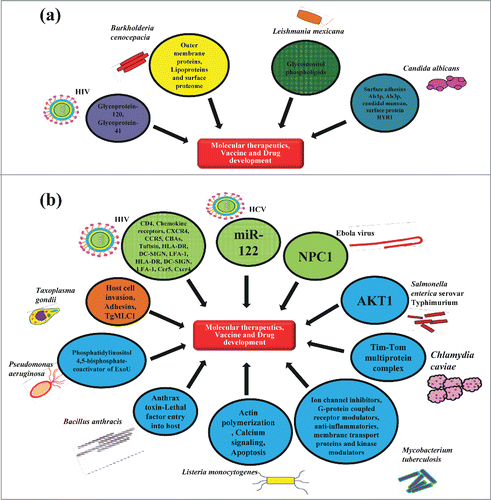
Another promising approach is to target PAMPs expressed by pathogens participating in host-pathogen interaction and mechanism of pathogenesis for suitable biomarkers for drug development and future broad spectrum molecular therapeutics. Current biomarkers include targeting of both the pathogen factors as well as the host factors generated in response to pathogenic infection (). Here in the following text, we are discussing some of the specific microbial biomarkers which have been exploited in the past or can be explored in the future for invention of novel vaccines and other therapeutic strategies.
Figure 4. Host and pathogen derived targets. (A) Pathogen derived factors: The arrows indicate some of the targets from pathogenic viruses, bacteria, protozoan and fungus which may help in the development of novel molecular therapeutics, vaccines and drugs against them. (B) Host derived factors against pathogens: The arrows indicate host based targets for pathogenic viruses, bacteria, protozoan and fungus that contribute, either positively or negatively to the establishment of infections and can be targeted for discovering novel therapeutic strategies to fight against them.
Pathogen based factors
Targeting Viral moieties
The surface glycoprotein-120 (gp120) is present in the outer envelope of HIV. Gp120 together with transmembrane protein gp41 facilitates the entry of the viruses into the CD4+ T-cells and macrophages by binding to the CD4 receptor. Gp120 and gp41 can be the attractive targets for devising the potential therapeutic strategy such as designing anti-retroviral drugs and vaccines, since these are exposed on the surface of the HIV infected cells. An immunoliposomal carrier enclosing the protease inhibitor P11 with anti-gp120 antibody present on its surface was reported to show excellent specificity for HIV infected cells. It was due to binding of the carrier anti-gp120 to the surface exposed domains of gp120 present on the HIV infected cells. Citation70
Targeting bacterial moieties
Surface associated proteins like Outer Membrane Proteins (OMPs) are the integral β-barrel membrane proteins present in the outer membrane of the Gram-negative bacteria and some of the Gram-positive bacteria like Mycobacterium spp, having atypical cell wall consisting of outer membrane.Citation71 These OMPs are reported to play crucial role during the host cell invasion by the pathogen and its survival within the macrophages. These OMPs have been targeted as potential antigenic candidates for identifying suitable epitopes for the formulation of vaccines against the Mycobacterium spp.Citation72 Some of the bacterial pathogens have the ability to resist the effects of antimicrobial compounds like antibiotics used against the bacterial infections. It is often mediated by other surface associated proteins like the Integral Membrane Proteins (IMPs) commonly expressed as ATP-binding cassette transporters (ABC) like protein, TonB dependent siderophore receptors and Multi-Drug Resistance (MDR) Efflux system protein which represent promising targets against drug resistant bacterial infections.Citation73 In most of the Gram-negative bacteria, high affinity iron acquisition requires membrane localized proteins that bind iron chelates at the cell surface and promote their uptake. Transport of bound chelates across the outer membrane reported to be dependent upon 3 membrane-spanning proteins TonB-ExbB-ExbD in Flavobacterium psychrophilum. Citation74 Alvarez et al (Citation2008) showed that a mutant in one of 2 ExbD loci of a TonB system in F. psychrophilum resulted in attenuated virulence and conferred protection in rainbow trout against cold water disease representing it as a suitable target to generate live attenuated vaccines.Citation74 Recently, a number of antimicrobial resistant bacterial strains have emerged. One of the main mechanisms where the bacteria develop resistance against antimicrobial agents includes modification of the drug targets, enzymatic inactivation and reduction in intracellular drug concentration by changing the membrane permeability or by expressing the efflux pump proteins.Citation75 Therefore, these efflux pumps prove to be viable antibacterial targets. The identification and development of potent efflux pump inhibitors (EPIs) is a promising and valid strategy against these drug resistant bacterial infections. A number of EPIs have been developed against Gram-positive bacteria S. aureus infections like chalcones, N-cinnamoylphenalkylamides, piperine-like compounds or citral amide derivatives targeting the NorA efflux pump.Citation76 Certain bacterial pathogens tend to recruit some host complement inhibitors like factor H binding protein (fHbp) in order to evade the host immune system. These bacterial complement inhibitor-binding molecules may prove to be the promising vaccine targets which may elicit antibodies that neutralize this bacterial defense mechanism. fHbp is one of the major antigen used in a multi-component meningococcal vaccine which is recently licensed in Europe against serogroup B diseases. fHbp recruits the factor H, a complement down regulator, to the bacterial surface. It enables the organism to escape the complement-mediated bacteriolysis. Citation77
Targeting protozoan moieties
Leishmania mexicana, an intracellular protozoan parasite is known to cause a chronic disease in mice and requires host FccRIII and IL-10 for its survival. When Leishmania amastigotes are released intracellularly from cells, the surface bound IgG induces IL-10 and suppresses IL-12 generation from macrophages. The surface glycolipid glycoinositol phospholipids (GIPLs) consisting of a branched mannose structure of L. mexicana ligates and interacts with IgG1 in mice on infection. This GIPL is expressed abundantly on the surface of L. mexicana amastigotes and rarely in stationary-phase promastigotes, whereas, it is not present in L. major which is persistent with a role for antibodies for GIPLs in chronic disease. Monoclonal anti-GIPL IgG in mouse has been shown to recognize GIPLs on the surface of L. mexicana surface and inducing IL-10 in macrophages. Similarly, in humans infected with L. mexicana having localized and diffused zoonotic cutaneous leishmaniasis, antibodies recognizing GIPLs and binding to the surface of amastigotes can induce IL-10 from human leukocytes. These surface expressed glycolipids interacting with the host receptors can prove to be an important therapeutic target for drug and vaccine development. Citation78
Targeting fungal moieties
In Candida albicans, the Agglutinin-Like Sequence (ALS) protein family is generally used for development of the candidal vaccines. On vaccination with the recombinant N-terminus of the ALS1p (rALS1p-N) or ALS3p (rALS3p-N) candidal hyphal-specific surface adhesins were found to protect the mice from lethal disseminated bloodstream candidiasis. It was also found to decrease the severity of fungal infection in a vaginitis model and oropharyngeal candidosis model treated with steroids.Citation79 Other anticandidal vaccines that have been assessed and published in preclinical studies have principally focused on using peptides conjugants with fungal mannans, candidal heat shock proteins and various candidal surface proteins like Hyphal cell wall protein (HYR1) resulting in remarkable protection in systemic infection models.Citation80 Characterizing these molecules will help in building up the fundamental knowledge for the effective and protective immune response against Candida albicans, that will eventually lead to the development of a clinically potential vaccine for the disease.
Host based factors
Targeting host derived factors against viruses
Another attractive approach to target pathogen infected cells is to identify unique biomarkers from the infected host cells. In HIV infections, majority of the targets identified are the host cell-based targets as compared to the pathogen based targets. Host cell-based targets include the main host co-receptors such as chemokine receptors, CD4 (receptor for HIV), Human Chemokine Receptor 5 (CCR5) and CXCR4 (co-receptors for HIV), tuftsin, Carbohydrate-Binding Antigens (CBAs), Dendritic Cell specific HIV-1 receptor (DC- SIGN), Human Leukocyte Antigen (HLA-DR) and Leukocyte Function Antigen (LFA-1), that are used by HIV for its entry into the cells. Citation81
The miRNA 122 (miR-122) is the host encoded liver specific antiviral target, that is crucial for efficient Hepatits C virus (HCV) RNA replication in cultured mammalian cells infected with HCV. Santaris Pharma has developed a Locked Nucleic Acid (LNA) modified oligonucleotide also known as SPC3649 or miravirsen. It is a modified RNA, which contains an oxymethylene bridge formed between 4′ carbon and 2′ oxygen in the ribose ring. This bridge results in the formation of bi-cyclic structure which locks the ribose conformation. It is the reason for the high stability of the LNA and its affinity to the complementary RNA sequence. When this oligonucleotide binds to the target mRNA and microRNA sequences, it creates a stretch of dsRNA which prevents its further translation.Citation82 Using various assays like surface plasmon resonanace (SPR) binding assay and minimalistic Dicer enzymatic assay, SPC3649 was found to be highly suppressive for the biogenesis of miR-122 as compared to the other candidate drugs like AMO-122, SPcon and AMO con.Citation83 Thus, miR-122 holds promise as a potential target for devising effective diagnostic and therapeutic strategies against HCV infection. Moreover, a number of other agents targeting different host molecules are currently in the development pipeline for the HCV treatment. These include agents blocking the host molecules involved at different steps in the life cycle of HCV including attachment, invasion, replication, assembly and release of the viral particles.
Infection caused by the Marburg and Ebola filoviruses result in fatal hemorrhagic fever among the humans. The recent Ebola epidemic in 2014 is the seventh unprecedented outbreak affecting multiple countries worldwide with more than 3,400 deaths so far.Citation84 Currently there are no approved antivirals available against these deadly pathogens. Filovirus entry into the host cells is mediated by the viral envelope spike Ebola virus (EBOV) and Marburg virus (MARV) glycoproteins (GP), which help in attachment of viruses to the host cell surface, deliver and catalyze fusion between endosomal and virus membranes. For GP mediated fusion of virus envelope, a cholesterol transporter namely, the Niemann-Pick C1 (NPC1) protein is required for cellular entry.Citation85 With the current 2014 Ebola epidemic, researchers have started working on the experimental vaccines cAd3-EBOV (cAd3) and rVSVΔG-EBOV-GP (rVSV) against Ebola. In both these vaccines, wild-type strain is genetically engineered to express immunogenic viral envelope glycoprotein which could provoke an immune response against them.Citation86
Targeting host derived factors against bacteria
In view of the currently emerging deadly bacterial infections around the globe, there is a growing demand for developing improved anti-bacterial intervention strategies targeting the host specific biochemical pathways required for bacterial survival and growth inside the host. During Chlamydia caviae infection of Drosophila cells, a multiprotein complex, tim-tom, is targeted by C. caviae for its survival inside the host. This tim-tom complex is required for the proper trafficking of nucleus encoded proteins into the mitochondria.Citation87 Using RNA interference screen, it was found that some of the tim-tom complex components like Tom 22 and Tom40 depletion has inhibited C. caviae infection in mammalian cells. Depletion of these Tom complex proteins in the mitochondria resulted in reduction of mitochondrial recruitment to the C. caviae inclusions and specifically modulated the mitochondrial functions impairing C. caviae intracellular replication.Citation88
Kinase inhibitors with antibiotic properties have been developed that prevent intracellular growth of unrelated pathogens such as Mycobacterium tuberculosis and Salmonella enterica serovar Typhimurium. Bacterial host targets like protein kinase B (PKB) or serine/threonine protein kinase AKT have been identified, that are exploited by the bacterial pathogens during the intracellular survival inside the host cells. Inhibitors of AKT were developed which showed therapeutic potential as an antibiotic in mice.Citation89 Because, in many human tumors AKT is found to be activated, various AKT inhibitors are already in clinical trials as anticancer drugs in humans. It implies that kinases may be used to yield novel antibiotics that halt host pathways activated by bacterial pathogens for survival.Citation90 The host-targeting inhibitors namely, ion channel inhibitors, G-protein coupled receptor modulators, anti-inflammatories, membrane transport proteins and kinase modulators have been identified that restrict the growth of Mycobacterium tuberculosis particularly in the macrophage infection. It was reported that fluoxetine, a serotonin specific reuptake inhibitor and gefitinib, an Epidermal Growth Factor Receptor (EGFR) inhibitor induce autophagy and enhance the secretion of proinflammatory cytokine TNF-α in infected macrophages restricting bacterial growth. These agents proved to be the new anti-tubercular agents that act by modulating host pathways.Citation91 Although, these antimicrobics offer many advantages during treatment of Mycobacterium infection but these come with some cellular side-effects too like fluoxetine has been observed with side effects such as nausea, seizures, anxiety, sleepiness and serious allergic reactions. It targets and affects various cellular processes which include protein localization, establishment of cell polarity and cytoskeleton biogenesis and organization.Citation92
For Listeria monocytogenes, an intracellular Gram-positive bacterium, 20 one compounds targeting cell functions like calcium signaling, actin polymerization and apoptosis were identified that decreased infection efficiency of L. monocytogenes. After internalization of L. monocytogenes in membrane-bound vacuoles of the host, the pore-forming listeriolysin O (LLO), cytolysin and a Phosphoinositide-Specific Phospholipase C (PI-PLC) mediate efficient lysis of the vacuoles and release the pathogens into cytosol of the host cell. L. monocytogenes manipulates the host cell by polymerizing host actin and propels itself into other adjacent host cells to continue their life cycle and infection. The anti-psychotic drug pimozide used to treat schizophrenia and Tourette's syndrome was found to decrease internalization of L. monocytogenes and other bacterial species including Salmonella typhimurium, Bacillus subtilis and E. coli. It decreased invasion, escape from vacuole and subsequent spread from cell-to-cell of L. monocytogenes inside the host.Citation93 Pimozide was found to act as calcium channel antagonist however, later it was reported that, pimozide activity was independent of calcium.Citation93 Therefore, it is still unclear how pimozide mediates the inhibition of L. monocytogenes infection. Other antipsychotic drugs like thioridazine and the calcium channel blocker bepridil were shown to exhibit dose dependent inhibition of vacuole escape and intracellular replication during L. monocytogenes infection of murine macrophages.Citation94 These represent the drug candidates with potential to treat infectious diseases with broad spectrum anti-microbial applications targeting host based factors.
Targeting host derived factors against protozoans
Parasites also employ host cells for completing their life cycle consisting of attachment, invasion, multiplication and host cell lysis during infection. To discover inhibitors for Taxoplasma gondii, a protozoan intracellular human parasite, 24 non-cytotoxic inhibitors were identified inhibiting various aspects of the infection process which include host cell adhesins secretion and gliding motility. Among these inhibitors, tachypleginA causes a post-translational modification of the T. gondii Myosin Light Chain-1 (TgMLC1). This component drives parasite mobility and host cell penetration.Citation95 Similarly, various other factors involved during the host-pathogen interactions can be targeted for efficient drug and vaccine development against T. gondii infection.
Targeting host derived factors against Fungus
Various cell-death regulating pathways referred to as Programmed Cell Death (PCD) or apoptosis are important for the development and pathogen interactions. These play very important role in mediating hosts immune defense against pathogenic fungi. Similarly, fungal PCD, especially the anti-PCD machinery is also important for fungal virulence. Since PCD plays a central role in every type of fungus-host interactions, the PCD machinery and the pathways that regulate these in both the pathogen and host can be the putative targets for antifungal therapies. Citation96
Off-target Effects of Antimicrobials
The desired activity of any antimicrobial is to kill or inhibit the growth of offending pathogens. Some of these antimicrobials developed using in vitro assays against the pathogenic infections may have some other unknown targets and show various unanticipated cellular adverse effects too in vivo. Generalized adverse events shown by most of the antimicrobials are common, but some of them show specific effects. Some of the common off-target effects include photosensitivity, anaphylactoid and ototoxicity reactions. In the absence of co-drug therapy, the altered drug metabolism could also be treated as mild. Other side effects can be severe or even proven to be fatal such as: nephrotoxicity associated with aminoglycoside antimicrobial agents, the Stevens-Johnson syndrome related to sulfonamide antimicrobial agents, hepatitis caused by many antimicrobial drugs such as isoniazid, aplastic anemia from chloramphenicol and neuromuscular blockade associated with aminoglycoside. All of these off-target effects have unique etiologies based on the unique chemical nature of these antimicrobial agents.Citation97 For example, the oxazolidinones and aminoglycosides are inhibitors of bacterial ribosomes but some of their off-target effects appear to be directly based on inhibition of human mitochondrial ribosomes.Citation98 The mechanism behind this is the secondary structure of the A site with the A1555G or C1494T human mitochondrial mutation mimics the analogous secondary structure of the 30S bacterial ribosome A site.Citation99 Similarly, ionophores have been shown to induce ion channel dysregulation in myocytes associated with adverese effects such as myopathies.Citation100 Another antimicrobial agent, Amphotericin B (AmB), one of the crucial antifungal agents against serious systemic fungal infections, has been associated with well-known chronic adverse effects like nephrotoxicity, anemia, hyperbilirubinemia and hyperphosphatemia. The mechanisms suggested for AmB nephrotoxicity in the literature include direct toxic effects caused to the afferent tubules and arterioles leading to direct systemic and renal vasoconstriction.Citation101 In an effort to improve the efficacy and reduce the side effects of the antimicrobial agents, new generations of drugs and derivatives should be developed with fewer side effects.
Conclusions
Many studies have been endeavored in order to explain the biochemical basis of pathogenic microbes interacting with their hosts. These studies furnished us with information and compelling us to understand and perceive the implications of different infectious diseases. These have further helped the scientific community to better understand the outcome of the microbial infections. The efforts from ongoing work in this field have given insight into the biology of the pathogen and the host. Ultimate goal of pathogen and host sustaining pathogen is continuation of existence. During pathogenesis, a pathogen adheres itself to the host tissue via its ligands which are projections, that are attached on their cognate host cell receptors and triggers a series of alarm signals resulting in signaling cascades eliciting an immune response. It eventually kills and eliminates the pathogen but sometimes the pathogen outmanoeuvres these systems of defense. During their long side-by-side coexistence, the pathogens have developed diverse number of complex strategies to endure the dynamic immune response of the host like altering the host cell receptor conformation, modulating lipid and protein contents of the host cell membrane, surviving in the phagocytes or avoiding phagocytic recognition by producing the capsule envelops. Resistance to anti-microbial drugs is an emerging public health problem threatening the treatment and control of infectious diseases caused by various microbes ranging from virus, bacteria, and fungi to protozoan. Drug-resistant microbial pathogens can no longer be effectively treated with available anti-infective drugs. The risk is great because of the drug resistance many more people will die from some of these infectious diseases. The tremendous funding and the urgent need to treat the resistant infections lead to the rapid progress on the discovery and characterization of potential new drug targets. Novel inventions are being made that increase our understanding of the pathogenesis of infectious microbes. There is ample technological advancement also being made to accelerate and expedite the discovery processes of the new drugs, vaccines and the diagnostic techniques against these diseases. Engagement in the research focused on the biology at the interface of the host-pathogen interactions from the ligand-receptor levels to the physiological outcomes of these interactions have provided researchers with a better insight for a number of novel strategies for the vaccine and drug development, resulting in the effective immunisation and devise new therapies for the deadly diseases showing antibiotic resistance. Better characterization of the host response pathways and non-essential host factors hijacked by the pathogen might be the key to find novel targets for the development of new therapies and molecular diagnostics based on a mechanistic understanding of infectious disease pathogenesis.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
A. Rana acknowledges a Senior Research Fellowship from Indian Council of Medical Research, Govt. of India (ICMR). Research in M. Ahmed's lab is supported by Science and Engineering Research Board, DST, Govt. of India (SERB) and University Grant Commission, Govt. of India (UGC). Research in A. Rub's lab is supported by grants from Indian National Science Academy, UGC, and SERB. Research in Y. Akhter's lab is supported by extramural research funds from UGC, ICMR and SERB.
References
- Basset C, Holton J, O'Mahony R, Roitt I. Innate immunity and pathogen–host interaction. Vaccine 2003; 21:S12-S23; PMID:12763678; http://dx.doi.org/10.1016/S0264-410X(03)00195-6
- Carabeo R. Bacterial Subversion of Host Actin Dynamics at the Plasma Membrane. Cell Microbiol 2011; 13:1460-1469; PMID:21790944; http://dx.doi.org/10.1111/j.1462-5822.2011.01651.x
- Aderem A, Ulevitch RJ. Toll-like receptors in the induction of the innate immune response. Nature 2000; 406:782-787; PMID:10963608; http://dx.doi.org/10.1038/35021228
- Cdllb CR, Ishibashi BY, Claus S, Relman DA. Bordetella pertussis filamentous hemagglutinin interacts with a leukocyte signal transduction complex and stimulates bacterial adherence to monocyte CR3 (CDllb/CD18). J. Exp. Med 1994; 180:1225-33.
- Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell 2010; 140:805-20; PMID:20303872; http://dx.doi.org/10.1016/j.cell.2010.01.022
- Lien E, Sellati TJ, Yoshimura A, Flo TH, Rawadi G, Finberg RW, Carroll JD, Espevik T, Ingalls RR, Radolf JD, Golenbock DT. Toll-like Receptor 2 functions as a pattern recognition receptor for diverse bacterial products. J. Biol. Chem. 1999; 274:33419-33425; PMID:10559223; http://dx.doi.org/10.1074/jbc.274.47.33419
- Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll. Nature 2001; 413:732-8; PMID:11607032; http://dx.doi.org/10.1038/35099560
- Beutler B. Tlr4 : central component of the sole mammalian LPS sensor. Curr Opin Immunol. 2000; 12:20-6; PMID:10679411; http://dx.doi.org/10.1016/S0952-7915(99)00046-1
- Hayashi F, Smith KD, Ozinsky A, Hawn TR, Yi EC, Goodlett DR, Eng JK, Akira S, Underhill DM, Aderem A. Hayashi F, et al. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 2001; 410:1099-103; PMID:11323673; http://dx.doi.org/10.1038/35074106
- Hemmi H, Kaisho T, Takeuchi O, Sato S, Sanjo H, Hoshino K, Horiuchi T, Tomizawa H, Takeda K, Akira S. Small anti-viral compounds activate immune cells via the TLR7 MyD88- dependent signaling pathway. Nat. Immunol. 2002; 3:196-200; PMID:11812998; http://dx.doi.org/10.1038/ni758
- Hemmi H, Kaisho T, Takeuchi O, Sato S, Sanjo H, Hoshino K, Matsumoto M, Hoshino K, Wagner H, Takeda K et al. A Toll-like receptor recognizes bacterial DNA. Nature 2000; 408:740-5; PMID:11130078; http://dx.doi.org/10.1038/35047123
- Blasius AL, Beutler B. Intracellular toll-like receptors. Immunity 2010; 32:305-15; PMID:20346772; http://dx.doi.org/10.1016/j.immuni.2010.03.012
- Zhang D, Zhang G, Hayden MS, Greenblatt MB, Bussey C, Flavell RA, Ghosh S. A toll-like receptor that prevents infection by uropathogenic bacteria. Science 2004; 303:1522-6 ; PMID:15001781; http://dx.doi.org/10.1126/science.1094351
- Koblansky AA, Jankovic D, Oh H, Hieny S, Sungnak W, Mathur R, Hayden MS, Akira S, Sher A, Ghosh S. Recognition of profilin by Toll-like Receptor 12 is critical for host resistance to Toxoplasma gondii. Immunity 2013; 38:119-30; PMID:23246311; http://dx.doi.org/10.1016/j.immuni.2012.09.016
- Lee BL, Moon JE, Shu JH, Yuan L, Newman ZR, Schekman R, Barton GM. UNC93B1 mediates differential trafficking of endosomal TLRs. Elife 2013; 2:e00291; PMID:23426999
- Yoneyama M, Fujita T. RNA recognition and signal transduction by RIG-I-like receptors. Immunol. Rev. 2009; 227:54-65; PMID:19120475; http://dx.doi.org/10.1111/j.1600-065X.2008.00727.x
- Loo YM, Gale M Jr. Immune signaling by RIG-I-like receptors. Immuntiy 2011; 34:680-692; http://dx.doi.org/10.1016/j.immuni.2011.05.003
- Janeway CA, Medzhitov R. Innate immune recognition. Immunology 2002; 20:197-216; http://dx.doi.org/10.1146/annurev.immunol.20.083001.084359
- Kingeter LM, Lin X. C-type lectin receptor-induced NF-κB activation in innate immune and inflammatory responses. Cell. Mol. Immunol. 2012; 9:105-12; PMID:22246129; http://dx.doi.org/10.1038/cmi.2011.58
- Yamasaki S, Sho Matsumoto M, Takeuchi O, Matsuzawa T, Ishikawa E, Sakuma M, Tateno H, Uno J, Hirabayashi J, et al. C-type lectin Mincle is an activating receptor for pathogenic fungus, Malassezia. PNAS 2009; 106:1897-902; PMID:19171887; http://dx.doi.org/10.1073/pnas.0805177106
- Cummings RD, McEver RP. C-type Lectins. In: Essentials of Glycobiology. 2nd ed. Cold Spring Harbor, NY: 2009; Chap. 31.
- Murphy JE, Tedbury PR, Homer-Vanniasinkam S, Walker JH, Ponnambalam S. Biochemistry and cell biology of mammalian scavenger receptors. Atherosclerosis 2005; 182:1-15; PMID:15904923; http://dx.doi.org/10.1016/j.atherosclerosis.2005.03.036
- Prabhudas M, Bowdish D, Drickamer K, Febbraio M, Herz J, Kobzik L, Krieger M, Loike J, Means TK, Moestrup SK, Post S, Sawamura T, Silverstein S, Wang XY, El Khoury J. Standardizing Scavenger Receptor Nomenclature. J Immunol. 2014; 192:1997-2006; PMID:24563502; http://dx.doi.org/10.4049/jimmunol.1490003
- Areschoug T, Gordon S. Scavenger receptors: Role in innate immunity and microbial pathogenesis. Cell. Microbiol. 2009; 11:1160-1169; PMID:19388903; http://dx.doi.org/10.1111/j.1462-5822.2009.01326.x
- Leslie RGQ, Hansen S. Complement Receptors. Complement Receptors. eLS 2009; http://dx.doi.org/10.1002/9780470015902.a0000512.pub2
- Le Y, Murphy PM, Wang JM. Formyl-peptide receptors revisited. Trends Immunol. 2002; 2:541-8; http://dx.doi.org/10.1016/S1471-4906(02)02316-5
- Boss JM, Howard I, Medica H. & Schreiber, S. The NLR gene family : An official nomenclature. Immunity 2009; 28:285-287
- Cruyssen BV, Peeters H, Hoffman IEa, Laukens D, Coucke P, Marichal D, Cuvelier C, Remaut E, Veys EM, Mielants H, et al. CARD15 polymorphisms are associated with anti-Saccharomyces cerevisiae antibodies in caucasian Crohn's disease patients. Clin. Exp. Immunol. 2005; 140:354-9; PMID:15807862; http://dx.doi.org/10.1111/j.1365-2249.2005.02759.x
- Crane DD, Bauler TJ, Wehrly TD, Bosio CM. Mitochondrial ROS potentiates indirect activation of the AIM2 inflammasome. Front Microbiol 2014; 5:438; PMID:25191316; http://dx.doi.org/10.3389/fmicb.2014.00438
- Fernandes-Alnemri T, Yu JW, Juliana C, Solorzano LWu J, Datta P, Margaret McCormick, Huang L, McDermott EM, Kang S, Eisenlohr L, et al. The AIM2 inflammasome is critical for innate immunity to Francisella tularensis. Nature immunology 2010; 11(5):385-93; PMID:20351693; http://dx.doi.org/10.1038/ni.1859
- Nishikawa Y, Matsuzaki Y, Nakano H, Sawamura D. Characterization of stimulator of interferon genes (sting) expression in human epidermal keratinocytes. Hirosaki Med J. 2013; 64(Suppl.):S58―S64.
- Gupta G, Surolia A. Collectins: sentinels of innate immunity. BioEssays 2007; 29:452-64 ; PMID:17450595; http://dx.doi.org/10.1002/bies.20573
- Gál P, Ambrus G. Structure and function of complement activating enzyme complexes: C1 and MBL-MASPs. Curr. Protein Pept. Sci. 2001; 2:43-59; http://dx.doi.org/10.2174/1389203013381242
- Chaby R. Lipopolysaccharide-binding molecules: transporters, blockers and sensors. Cell. Mol. Life Sci. 2004; 61:1697-713; PMID:15241548; http://dx.doi.org/10.1007/s00018-004-4020-4
- Bottazzi B, Vouret-Craviari V, Bastone A, Gioia LD, Matteucc C, Peri G, Spreafico F, Pausa M, D'Ettorre C, Gianazza E. Multimer formation and ligand recognition by the long pentraxin PTX3. J. Biol. Chem. 1997; 272:32817-32823; PMID:9407058; http://dx.doi.org/10.1074/jbc.272.52.32817
- Gaboriaud C, Thielens NM, Gregory LA, Rossi V, Fontecilla-Camps JC, Arlaud G. Structure and activation of the C1 complex of complement : unraveling the puzzle. Trends Immunol. 2004; 25:15207504; http://dx.doi.org/10.1016/j.it.2004.04.008
- Tracey KJ. Physiology and immunology of the cholinergic antiinflammatory pathway. The Journal of Clinical Investigation 2007; 117:289-296; PMID:17273548; http://dx.doi.org/10.1172/JCI30555
- Cantrell DA. Phosphoinositide 3-kinase signalling pathways. J. Cell Sci. 2001; 114:1439-45; PMID:11282020
- McGettrick AF, O'Neil LA. The expanding family of MyD88-like adaptors in Toll-like receptor signal transduction. Mol Immunol. 2004; 41:577-82; PMID:15219996; http://dx.doi.org/10.1016/j.molimm.2004.04.006
- Goodridge HS, Harnett MM. Introduction to immune cell signalling. Parasitology 2005; 130 Suppl:S3-9; http://dx.doi.org/10.1017/S0031182005008115
- Delgado MA, Elmaoued RA, Davis AS, Kyei G, Deretic V. Toll-like receptors control autophagy. EMBO J. 2008; 27:1110-21; PMID:18337753; http://dx.doi.org/10.1038/emboj.2008.31
- Balzola F, Bernstein C, Ho GT, Lees C. Nod1 and Nod2 direct autophagy by recruiting ATG16L1 to the plasma membrane at the site of bacterial entry. Inflamm. Bowel Dis. Monit. 2010; 10:140-141.
- Deretic V. Autophagy in immunity and cell-autonomous defense against intracellular microbes. Immunol Rev. 2011; 240:92-104; PMID:21349088; http://dx.doi.org/10.1111/j.1600-065X.2010.00995.x
- Deretic V. Autophagy as an innate immunity paradigm : expanding the scope and repertoire of pattern recognition receptors. Curr Opin Immunol 2012; 24:21-31; PMID:22118953; http://dx.doi.org/10.1016/j.coi.2011.10.006
- Eskelinen EL, Saftig P. Autophagy: a lysosomal degradation pathway with a central role in health and disease. Biochim. Biophys. Acta 2009; 1793:664-73.
- Akhter Y, Ehebauer MT, Mukhopadhyay S, Hasnain SE. The PE / PPE multigene family codes for virulence factors and is a possible source of mycobacterial antigenic variation : Perhaps more? Biochimie 2012; 94:110-116; http://dx.doi.org/10.1016/j.biochi.2011.09.026
- Galocha B, Hill A, Barnett BC, Dolan A, Raimondi A, Cook RF, Brunner J, McGeoch DJ, Ploegh HL. The active site of ICP47, a Herpes Simplex Virus–encoded inhibitor of the Major Histocompatibility Complex (MHC)-encoded Peptide Transporter Associated with Antigen Processing (TAP), maps to the NH 2 -terminal 35 Residues. J. Exp. Med 1997; 185:1565-1572.
- Ahn K, Gruhler A, Galocha B, Jones TR, Wiertz EJ, Ploegh HL, Peterson PA, Yang Y, Früh K. The ER-luminal domain of the HCMV glycoprotein US6 inhibits peptide translocation by TAP. Immunity 1997; 6:613-621; PMID:9175839; http://dx.doi.org/10.1016/S1074-7613(00)80349-0
- Menz B, Sester M, Koebernick K, Schmid R, Burgert, HG. Structural analysis of the adenovirus type 2 E3/19K protein using mutagenesis and a panel of conformation-sensitive monoclonal antibodies. Mol. Immunol. 2008; 46:16-26; PMID:18692902; http://dx.doi.org/10.1016/j.molimm.2008.06.019
- Park B, Spooner E, Houser BL, Strominger JL, Ploegh HL. The HCMV membrane glycoprotein US10 selectively targets HLA-G for degradation. J. Exp. Med. 2010; 207:2033-41; PMID:20713594; http://dx.doi.org/10.1084/jem.20091793
- Brulois K, Toth Z, Wong LY, Feng P, Gao SJ, Ensser A, Jung JU. Kaposi's sarcoma-associated herpesvirus K3 and K5 ubiquitin E3 ligases have stage-specific immune evasion roles during lytic replication. J Virol 2014; 88:9335-49; PMID:24899205; http://dx.doi.org/10.1128/JVI.00873-14
- Aquino A, Graziani G, Franzese O, Prete SP, Bonmassar E, Bonmassar L, D'Atri S. Exogenous control of the expression of Group I CD1 molecules competent for presentation of microbial nonpeptide antigens to human T lymphocytes. Clin. Dev. Immunol. 2011; 2011:790460; http://dx.doi.org/10.1155/2011/790460
- Gagliardi MC, Teloni R, Giannoni F, Mariotti S, Remoli ME, Sargentini V, Videtta M, Pardini M, De Libero G, Coccia EM, et al. Mycobacteria exploit p38 signaling to affect CD1 expression and lipid antigen presentation by human dendritic cells. Infect. Immun. 2009; 77:4947-52 ; PMID:19720761; http://dx.doi.org/10.1128/IAI.00607-09
- Gouin E, Cossart P. Actin-based motility of intracellular pathogens. Curr Opin Microbiol 2005; 8:35-45; PMID:15694855; http://dx.doi.org/10.1016/j.mib.2004.12.013
- Pike LJ. Lipid rafts: bringing order to chaos. J. Lipid Res. 2003; 44:655-67; PMID:12562849; http://dx.doi.org/10.1194/jlr.R200021-JLR200
- Grassmé H, Jendrossek V, Riehle A, von Kürthy G, Berger J, Schwarz H, Weller M, Kolesnick R, Gulbins E. Host defense against Pseudomonas aeruginosa requires ceramide-rich membrane rafts. Nat. Med. 2003; 9:322-30; http://dx.doi.org/10.1038/nm823
- Grassme H, Jekle A, Riehle A, Schwarz H, Berger J, Sandhoff K, Kolesnick R, Gulbins E. CD95 signaling via ceramide-rich membrane rafts. J Biol chem 2001; 276:20589-96; PMID:11279185; http://dx.doi.org/10.1074/jbc.M101207200
- Gulbins E, Dreschers S, Wilker B, Grassmé H. Ceramide, membrane rafts and infections. J. Mol. Med. (Berl). 2004; 82:357-63; PMID:15069600
- Azenabor AA, Job G, Adedokun OO. Chlamydia pneumoniae infected macrophages exhibit enhanced plasma membrane fluidity and show increased adherence to endothelial cells. Mol. Cell. Biochem. 2005; 269:69-84; PMID:15786718; http://dx.doi.org/10.1007/s11010-005-2537-y
- Simões AP, Moll GN, Beaumelle B, Vial HJ, Roelofsen B, Op den Kamp JA. Plasmodium knowlesi induces alterations in phosphatidylcholine and phosphatidylethanolamine molecular species composition of parasitized monkey erythrocytes. Biochim Biophys Acta 1990; 1022:135-45; PMID:2306451; http://dx.doi.org/10.1016/0005-2736(90)90107-Y
- Rub A, Dey R, Jadhav M, Kamat R, Chakkaramakkil S, Majumdar S, Mukhopadhyaya R, Saha B. Cholesterol depletion associated with Leishmania major infection alters macrophage CD40 signalosome composition and effector function. Nat. Immunol. 2009; 10:273-80; PMID:19198591; http://dx.doi.org/10.1038/ni.1705
- Klein A, Mercure L, Gordon P, Bruser B, Ramcharitar S, Malkin A, Wainberg MA. The effect of HIV-1 infection on the lipid fatty acid content in the membrane of cultured lymphocytes. AIDS 1990; 4:865-7; PMID:1979225; http://dx.doi.org/10.1097/00002030-199009000-00005
- Roy K, Hilliard GM, Hamilton DJ, Luo J, Ostmann MM, Fleckenstein JM. Enterotoxigenic Escherichia coli EtpA mediates adhesion between flagella and host cells. Nature 2009; 457:594-98; PMID:19060885; http://dx.doi.org/10.1038/nature07568
- Zhang P, Skurnik M, Zhang SS, Schwartz O, Kalyanasundaram R, Bulgheresi S, He JJ, Klena JD, Hinnebusch BJ, Chen T. Human dendritic cell-specific intercellular adhesion molecule-grabbing nonintegrin (CD209) is a receptor for Yersinia pestis that promotes phagocytosis by dendritic cells. Infec Immun. 2008; 76:2070-9; PMID:18285492
- Kline KA, Fälker S, Dahlberg S, Normark S, Henriques-Normark B. Bacterial adhesins in host-microbe interactions. Cell Host Microbe 2009; 5:580-92; PMID:19527885; http://dx.doi.org/10.1016/j.chom.2009.05.011
- Joyc, EA, Popper SJ, Falkow S. Streptococcus pneumoniae nasopharyngeal colonization induces type I interferons and interferon-induced gene expression. BMC Genomics 2009; 10:404; PMID:19712482; http://dx.doi.org/10.1186/1471-2164-10-404
- Blanchette KA, Orihuela CJ. Future perspective on host-pathogen interactions during bacterial biofilm formation within the nasopharynx. Fututre Microbiol 2012; 7:227-239; http://dx.doi.org/10.2217/fmb.11.160
- Berridge MJ, Irvine RF. Inositol phosphates and cell signalling. Nature 1989; 341:197-205; PMID:2550825; http://dx.doi.org/10.1038/341197a0
- Nayak DP, Hui EKW. The role of lipid microdomains in virus biology. Subcell. Biochem. 2004; 37:443-91; PMID:15376630
- Clayton R, Ohagen A, Nicol F, Del Vecchio AM, Jonckers TH, Goethals O, Van Loock M, Michiels L, Grigsby J, Xu Z. Sustained and specific in vitro inhibition of HIV-1 replication by a protease inhibitor encapsulated in gp120-targeted liposomes. Antivir. Res. 2009; 84:142-9; http://dx.doi.org/10.1016/j.antiviral.2009.08.003
- Rana A, Rub A, Akhter Y. Proteome-scale identification of outer membrane proteins in Mycobacterium avium subspecies paratuberculosis using a structure based combined hierarchical approach. Mol. Biosyst. 2014; 10:2329-2337; PMID:24950976; http://dx.doi.org/10.1039/C4MB00234B
- Rana A, Rub A, Akhter Y. Proteome-wide B and T cell epitope repertoires in outer membrane proteins of Mycobacterium avium subsp. paratuberculosis have vaccine and diagnostic relevance : a holistic approach. J Mol Recognit. 2015; PMID:25727233; http://dx.doi.org/10.1002/jmr.2458
- Liu H, Ibrahim M, Qiu H, Kausar S, Ilyas M, Cui Z, Hussain A, Li B, Waheed A, Zhu B, Xie G. Protein profiling analyses of the outer membrane of Burkholderia cenocepacia reveal a niche-specific proteome. Microb Ecol 2014; 69:75-83; http://dx.doi.org/10.1007/s00248-014-0460-z
- Alvarez B, Alvarez J, Menéndez A, Guijarro J. A mutant in one of two exbD loci of a TonB system in Flavobacterium psychrophilum shows attenuated virulence and confers protection against cold water disease. Microbiology 2008; 154:1144-51; PMID:18375806; http://dx.doi.org/10.1099/mic.0.2007/010900-0
- Sandhu P, Akhter Y. The internal gene duplication and interrupted coding sequences in the MmpL genes of Mycobacterium tuberculosis: Towards understanding the multidrug transport in an evolutionary perspective. Int. J. Med. Microbiol. 2015; 305(3):413-23; PMID:25841626
- Handzlik J, Matys A, Kieć-Kononowicz K. Recent Advances in Multi-Drug Resistance (MDR) efflux pump inhibitors of gram-positive bacteria S. aureus. Antibiotics 2013; 2:28-45; http://dx.doi.org/10.3390/antibiotics2010028
- Granoff DM, Ram S, Beernink PT. Does binding of complement factor H to the meningococcal vaccine antigen, factor H binding protein, decrease protective serum antibody responses? Clin. Vaccine Immunol. 2013; 20:1099-107; PMID:23740919; http://dx.doi.org/10.1128/CVI.00260-13
- Buxbaum LU. Leishmania mexicana infection induces IgG to parasite surface glycoinositol phospholipids that can induce IL-10 in mice and humans. PLoS Negl. Trop. Dis. 2013; 7:e2224; PMID:23675550; http://dx.doi.org/10.1371/journal.pntd.0002224
- Lin L, Ibrahim AS, Xu X, Farber JM, Avanesian V, Baquir B, Fu Y, French SW, Edwards JE Jr, Spellberg B. Th1-Th17 cells mediate protective adaptive immunity against Staphylococcus aureus and Candida albicans infection in mice. PLoS Pathog. 2009; 5:e1000703; PMID:20041174
- Luo G, Ibrahim AS, Spellberg B, Nobile CJ, Mitchell AP, Fu Y. Candida albicans Hyr1p confers resistance to neutrophil killing and is a potential vaccine target. J Infect Dis. 2010; 201:1718-1728; PMID:20415594; http://dx.doi.org/10.1086/652407
- Ramana LN, Anand AR, Sethuraman S, Krishnan UM. Targeting strategies for delivery of anti-HIV drugs. J. Control. Release 2014; 192:271-83; http://dx.doi.org/10.1016/j.jconrel.2014.08.003
- Weiss B, Davidkova G, Zhou LW. Antisense RNA gene therapy for studying and modulating biological. Cell Mol Life Sci. 1999; 55:334-58; PMID:10228554; http://dx.doi.org/10.1007/s000180050296
- Gebert LF, Rebhan MA, Crivelli SE, Denzler R, Stoffel M, Hall J. Miravirsen (SPC3649) can inhibit the biogenesis of miR-122. Nucleic Acids Res. 2014; 42:609-21; PMID:24068553; http://dx.doi.org/10.1093/nar/gkt852
- Maganga GD, Kapetshi J, Berthet N, Kebela Ilunga B, Kabange F, Mbala Kingebeni P, Mondonge V, Muyembe JJ, Bertherat E, Briand S. Ebola virus disease in the democratic republic of Congo. N Engl J Med 2014; 371:2083-91; http://dx.doi.org/NEJMoa1411099
- Carette JE, Raaben M, Wong AC, Herbert AS, Obernosterer G, Mulherkar N, Kuehne AI, Kranzusch PJ, Griffin AM, Ruthel G. Ebola virus entry requires the cholesterol transporter Niemann-Pick C1. Nature 2011; 477:340-3; PMID:21866103; http://dx.doi.org/10.1038/nature10348
- Dye C, Henao Restrepo AM, Fast P, Wood D, Dye C, Kieny MP, Moorthy V. Ebola vaccine-an urgent international priority. N Engl J Med 2014; 371:2249-51; http://dx.doi.org/10.1056/NEJMp1412166
- Rapaport D. How does the TOM complex mediate insertion of precursor proteins into the mitochondrial outer membrane? J. Cell Biol. 2005; 171:419-23; PMID:16260501; http://dx.doi.org/10.1083/jcb.200507147
- Derré I, Pypaert M, Dautry-Varsat A, Agaisse H. RNAi screen in Drosophila cells reveals the involvement of the Tom complex in Chlamydia infection. PLoS Pathog. 2007; 3:1446-58.
- Kuijl C, Savage ND, Marsman M, Tuin AW, Janssen L, Egan DA, Ketema M, van den Nieuwendijk R, van den Eeden SJ, Geluk A, et al. Intracellular bacterial growth is controlled by a kinase network around PKB/AKT1. Nature 2007; 450:725-30; PMID:18046412; http://dx.doi.org/10.1038/nature06345
- Granville CA, Memmott RM, Gills JJ, Dennis PA. Handicapping the race to develop inhibitors of the phosphoinositide 3- kinase / Akt / mammalian target of rapamycin pathway. Clin Cancer Res. 2006; 12:679-89; PMID:16467077; http://dx.doi.org/10.1158/1078-0432.CCR-05-1654
- Stanley SA, Barczak AK, Silvis MR, Luo SS, Sogi K, Vokes M, Bray MA, Carpenter AE, Moore CB, Siddiqi N. Identification of host-targeted small molecules that restrict intracellular Mycobacterium tuberculosis growth. PLoS Pathog. 2014; 10:e1003946; PMID:24586159; http://dx.doi.org/10.1371/journal.ppat.1003946
- Ericson E, Gebbia M, Heisler LE, Wildenhain J, Tyers M, Giaever G, Nislow C. Off-target effects of psychoactive drugs revealed by genome-wide assays in yeast. PLoS Genet. 2008; 4:e1000151; PMID:18688276; http://dx.doi.org/10.1371/journal.pgen.1000151
- Lieberman LA, Higgins DE. A small-molecule screen identifies the antipsychotic drug pimozide as an inhibitor of Listeria monocytogenes infection. Antimicrob. Agents Chemother. 2009; 53:756-64; PMID:19015342; http://dx.doi.org/10.1128/AAC.00607-08
- Lieberman LA, Higgins DE. Inhibition of Listeria monocytogenes infection by neurological drugs. Int J Antimicrob Agents. 2011; 35:1-10.
- Hong-geller E, Micheva-viteva S. Small Molecule Screens to identify inhibitors of infectious disease. (2013).
- Sharon A, Shlezinger N. Fungi infecting plants and animals: killers, non-killers, and cell death. PLoS Pathog. 2013; 9:e1003517; PMID:24009499; http://dx.doi.org/10.1371/journal.ppat.1003517
- Barnhill AE, Brewer MT, Carlson SA. Adverse effects of antimicrobials via predictable or idiosyncratic inhibition of host mitochondrial components. Antimicrob. Agents Chemother. 2012; 56:4046-51; PMID:22615289; http://dx.doi.org/10.1128/AAC.00678-12
- Lee SI, Lee JH, Lee SC, Lee JM, Lee JH. Calcium and neostigmine antagonize gentamicin, but augment clindamycin-induced tetanic fade in rat phrenic nerve-hemidiaphragm preparations. J anesth. 2008; 22:385-90; PMID:19011777; http://dx.doi.org/10.1007/s00540-008-0646-y
- Johnson RF, Cohen AP, Guo Y, Schibler K, Greinwald JH. Genetic mutations and aminoglycoside ¬ induced ototoxicity in neonates. Otolaryngol Head Neck Surg. 2010; 142:704-7; PMID:20416460; http://dx.doi.org/10.1016/j.otohns.2010.01.030
- Jurkat-Rott K, Weber MA, Fauler M, Guo XH, Holzherr BD, Paczulla A, Nordsborg N, Joechle W, Lehmann-Horn F. K+-dependent paradoxical membrane depolarization and Na+ overload, major and reversible contributors to weakness by ion channel leaks. Proc Natl Acad Sci USA 2009:106:4036-4041; http://dx.doi.org/10.1073/pnas.0811277106
- Laniado-Laborín R, Cabrales-Vargas MN. Amphotericin B: side effects and toxicity. Rev. Iberoam. Micol. 2009; 26:223-7; http://dx.doi.org/10.1016/j.riam.2009.06.003
