Abstract
Cryptococcus neoformans (Cn) causes meningoencephalitis in immunocompromised individuals. This encapsulated fungus can be found interacting with environmental microbes in soil contaminated with pigeon excrement. Cn survival within polymicrobial and other challenging communities has been shown to affect the evolution of its virulence factors. We compared the survival of 10 serotype A and D strains after interaction with the soil bacterium, Acinetobacter baumannii (Ab). Although co-incubation with Ab stimulated virulence factors production by strains of both cryptococcal serotypes, on average, serotype A strains displayed significantly higher survival rate, number of metabolically active cells within biofilms, and capsular polysaccharide production and release than serotype D strains. Our findings suggest that interactions of Cn with other microorganisms influence the fungus' regulation and production of virulence factors, important elements needed for the successful colonization of the human host.
Introduction
Cryptococcus neoformans (Cn) is an encapsulated fungus responsible for life-threatening meningoencephalitis in HIV-infected individuals, resulting in ≥600,000 deaths per year worldwide.Citation1 This fungus is frequently found in soil contaminated with pigeon droppingsCitation2 and invades the host from the environment by inhalation of small fungal particles in the forms of desiccated, and poorly encapsulated yeast form or as basidiospores.Citation3,4 In healthy individuals, the fungus is controlled by local host responses and remains dormant in the lungs; however, immunosuppression may lead to reactivation, leading to fungal replication in the lung and subsequent dissemination via the bloodstream or lymphatics to other organs, especially the brain.
Cn is currently classified into 2 varieties: grubii (serotype A) and neoformans (serotype D),Citation5 based on biochemical, morphological, and genetic characteristics.Citation6,7 These serotype classifications are based off of antigenic differences resulting from structural variation of the major capsular polysaccharide glucuronoxylomannan (GXM), which is profusely released during infection.Citation8,9 Environmental serotype A and D isolates differ in geographic distribution.Citation10,11 Serotype A is found worldwide with the exception of certain European countries,Citation12 in which serotype D is endemic including Denmark, Italy, and limited regions of France.Citation10,11,13 Moreover, both serotypes differ in pathogenesis with an increased risk of serotype D infections for patients with skin lesionsCitation13 whereas serotype A strains are responsible for the vast majority of central nervous system infections particularly in HIV-infected patients.Citation14 Analysis of multiple strains from each serotype have revealed stark differences in thermal susceptibility, with serotype D strains being more susceptible to high temperatures than serotype A.Citation15 These findings may be an explanation for the geographic differencesCitation10,11 found between both serotype strains and the exclusive dermatotropism exhibited by many of serotype D isolates.Citation13 Furthermore, recent molecular evidence suggests the possible speciation of Cn serotypes A and D and underscores the careful characterization of strains belonging to these varieties.Citation16
Cn's interactions with countless soil predators influences the evolution of virulence factors associated with enhanced survival in competitive environments.Citation17 One example of a soil predator is Acinetobacter baumannii (Ab), a Gram negative, free-living saprophyte found ubiquitously in nature.Citation18 This coccobacillus forms biofilms, a crucial survival feature that imparts resistance to a myriad of stressors found in any given environmental niche.Citation19 Therefore, we assessed whether or not Ab places selective pressures on Cn serotypes A and D strains survival. We compared 20 strains from these serotypes (10 strains per serotype) to investigate the effect of Ab interactions on fungal biofilm formation and capsular production both are features that protect Cn in the environment. This study aims to provide additional data supporting the notion that the diverse colonization strategies of the human host use by strains of Cn serotypes A and D may be selected from interactions with other microbes in the environment.Citation20
Results
Cn serotype A strains are more resistant to killing by Ab than serotype D strains
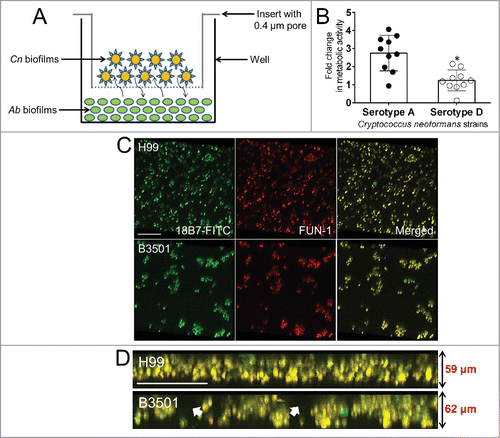
Cn is found in soil associated with pigeon excreta,Citation2 where the fungus is in contact with many soil predators.Citation17 These interactions influence the evolution of microbial virulence and the microbe's ability to survive in difficult environments.Citation17 Using colony forming units (CFU) assay, we explored the effect of Ab interaction on Cn serotype A and D strains' viability. On average, cells from serotype A strains demonstrated significantly higher survival percentages than serotype D strains (P < 0.05) after interaction with Ab strain 0057 (). There were no differences in Ab population size after incubation with strains of both serotypes ().
Figure 1. Cryptococcus neoformans (Cn) serotype A strains displayed higher survival percentage than serotype D strains after interactions with Acinetobacter baumannii (Ab). (A) Percentage survival of Cn serotype A and D strains after interaction with Ab. (B) Percentage survival of Ab after interaction with Cn strains. For A and B, bars are the averages of the results for 10 strains (each symbol represents an individual cryptococcal strain) per serotype, and error bars denote standard deviations (SDs). Asterisk denotes P-value significance (P < 0.05) calculated by Student's t-test. Each experiment was performed thrice with similar results obtained.
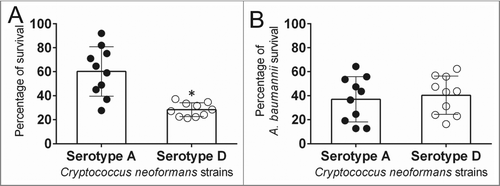
Ab stimulates the metabolic activity of cells within Cn serotype A biofilms
Biofilms is the phenotype shown by microbes in the environment, a mode of growth that allows microorganisms to tolerate hostile environments.Citation21 Hence, bacterial and fungal biofilms were grown separately and co-incubated using a microtiter transwell system that permits chemotactic exchange through the supernatant (). Using the XTT reduction assay, our results demonstrated that on average serotype A strains formed more robust biofilms than serotype D strains (P < 0.05) after interaction with Ab (). There were no differences in Ab biofilm metabolic activity after incubation with strains of both serotypes (data not shown; serotype A: 0.227 ± 3.69; serotype D: 0.211 ± 3.01). We used confocal microscopy to associate the XTT reduction assay findings with the visual properties on biofilm metabolism and architecture (). Regions of red fluorescence (FUN-1) represent metabolically active cells, and the green fluorescence (monoclonal antibody [MAb] 18B7-fluorescein isothiocyanate [FITC]-conjugated goat anti-mouse [GAM] IgG1) indicates GXM. In this regard, the size of Cn serotype A (59 μm) and D (62 μm) biofilms were similar with a thickness of ∼60 μm (). However, there were variations in the biofilm morphologies showcased by strains from both serotypes. Cn serotype A strain H99 cells co-incubated with bacteria displayed uniform biofilms across the field with high metabolic activity on yeasts surrounded by vast amounts of GXM (). In contrast, Cn serotype D strain B3501 exhibited scattered biofilms in a mushroom-like arrangement with highly metabolically active fungal cells encased in massive quantities of GXM ().
Figure 2. Cn serotype A strains form more metabolically active biofilms than serotype D strains after interactions with Ab. (A) Graphic representation of Cn and Ab biofilms interaction assay performed in this study. Fungal and bacterial biofilms were grown separately (Cn, 0.4 μm pore insert; Ab, bottom of a well) and co-incubated using a microtiter transwell system that permits chemotactic exchange through the supernatant. (B) Biofilm formation was determined by measuring the metabolic activity by XTT reduction assay. Bars are the averages of the results for 10 strains (each symbol represents an individual cryptococcal strain) per serotype, and error bars denote SDs. Asterisk denotes P-value significance (P < 0.05) calculated by Student's t-test. Each experiment was performed thrice with similar results obtained. (C) Confocal microscopy of Cn H99 and B3501 strain biofilms after interaction with Ab. Images of mature fungal biofilms showed metabolically active (red; FUN-1-stained) cells embedded in the polysaccharide extracellular material (green; stained with MAb 18B7-FITC-conjugated GAM IgG1). Images were obtained after 48 h co-incubation of the fungal cells to Ab. (D) The thickness and morphology of the cryptococcal biofilms can be observed in the Z-stack reconstruction. White arrows denote separation between Cn B3501strain biofilm aggregates. For C and D, the pictures were taken at a magnification of ×63. Bars, 20 µm. The results are representative of 2 distinct experiments.
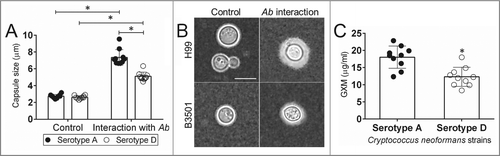
Ab increases capsular size and release in Cn serotype A strains
GXM is the major component of Cn polysaccharide capsule and extensively released in culture and during infection.Citation9,22 We investigated how Ab and Cn interaction alters fungal capsular size and production. India ink staining demonstrated that strains of both serotypes increase their polysaccharide capsule after interaction with bacteria relative to unexposed controls (). Serotype A strains exhibited significantly larger capsules than their serotype D counterparts (P < 0.05) (). Similarly, Cn serotype A strains displayed significantly higher amounts of GXM released in culture than D strains incubated in the presence of Ab (P < 0.05) ().
Figure 3. Impact of Ab interaction on Cn capsular size and GXM released. (A) Capsule size measurements of Cn serotype A and D strains were performed for yeasts grown in the absence and presence of Ab. Bars are the averages of the results for 25 cell measurements at each condition, and error bars denote SDs. (B) Representative India ink images displaying the effect of Ab interaction on the capsule size of Cn H99 (serotype A) and B3501 (serotype D). The pictures were taken using a ×100-power field. Scale bar, 2 µm. The experiments were performed thrice, and similar results were obtained. (C) GXM concentration in the supernatant of Cn serotype A and D strain cultures was determined by capture ELISA. Bars are the averages of the results for 10 strains per serotype, and error bars denote SDs. For A and C, asterisk denote P-value significance (P < 0.05) calculated by Student's t-test. Each experiment was performed thrice with similar results obtained.
Discussion
Limited information is available about the effect of environmental microbial interactions on the evolution of virulence, especially soil inhabitants.Citation17,23 In this study, we showed that the interaction of fungal pathogen Cn with other soil species such as Ab may have influenced differences found in virulence and infection of the human host between serotypes A and D. We demonstrated that Cn serotype A strains had significantly higher survival rates than serotype D strains after interaction with Ab. Likewise, using the XTT reduction assay, we established that serotype A strains formed stronger biofilms than serotype D strains after interactions with bacteria. This is important because increased biofilm formation with metabolically active cells might explain the survival of these strains in harsh and competitive environmental conditions.
Confocal microscopic images demonstrated that even though the biofilm thickness of both serotypes was similar, morphologically there were significantly different. For example, Cn serotype A H99 strain exhibited a uniform distribution of cells throughout the imaged field whereas serotype D B3501 strain displayed aggregates of cells scattered in the imaged field, in both cases surrounded by substantial amounts of GXM. The structural cluster-like organization observed in serotype D strain B3501 might provide the yeast cells with a protected niche against environmental predators,Citation24 immune cells,Citation25 and shear forces.Citation26 These findings suggest that physical differences in Cn serotypes A and D biofilms may be critical for fungal survival and might reflect the predilection of some serotype D strains for peripheral tissue (e.g. skin) whereas the hermetic architecture of serotype A biofilms may select and shelter these strains in immunologically fit battleground tissues such as the lungs. In fact, it is plausible that metabolically active cryptococci within the serotype A strain H99 biofilms may have an inclination to detach or disperse as single or aggregates of cells, enter into the blood stream, and disseminate to the host's central nervous system. Studies in the commensal fungus Candida albicans indicate that dispersed fungal cells from biofilms are more metabolically active than their planktonic counterparts.Citation27 Furthermore, the dispersion process in bacteria and fungi appears to be regulated by either extracellular stimuli,Citation28 intracellular signals,Citation27,29 or intercellular messengers.Citation30
The production and release of Cn capsular polysaccharide is extensively regulated during infection protecting the fungus against clearance by phagocytic cells.Citation31,32 GXM released is also essential for Cn biofilm formation, promoting the degree and magnitude of fungal cells attachment.Citation33 Cn induces extrusion from macrophages in a microcolony-like fashion after accumulation of the polysaccharide material in the phagosome.Citation34 Thus, we evaluated differences in capsule production and release of Cn serotype A and D strains after interaction with Ab. We observed that survival and biofilm formation by cryptococcal strains were directly related with capsule size and GXM released. GXM is actively produced during infection altering immunological and cellular processes.Citation35 It is feasible to think that the modulation of the capsule during infection evolved from Cn interactions with other environmental species. Previous studies have shown that standard serotype D strains B3501 and 24067 form stronger biofilms than serotype A strain H99 in vitro.Citation33 However, controlled laboratory conditions do not necessarily mimic infection or environmental settings where microbes are exposed to selective pressures that influence them to adapt according to their circumstances for survival. These results indicate that the polysaccharide capsule has defensive properties against other microbes and presumably functions in a protective role to prevent killing of yeast cells by bacteria.
Cn capsule physical changes respond to environmental factors, including iron levels and CO2 concentrations.Citation36,37 Our findings demonstrate that Cn becomes defensive by producing a larger capsule in presence of Ab cells. Even though several signaling pathways that may contribute to capsular size regulation by the fungus have been explored,Citation38-41 the structural variations of the capsule in response to environmental fluctuations are poorly understood. Aspects that contribute to increase capsule synthesis by Cn are multifactorial and may include increase polysaccharide shedding, complex polysaccharide assembly, and production of physically modified or larger polysaccharide fibers.Citation9,42,43
Our study provides additional evidence that environmental interaction among microbes influences virulence in fungi.Citation17,23 The restricted geographical distribution of Cn serotype D strains, common in Europe, but infrequently found in other regions of the world compared to the ubiquitous distribution of serotype A strainsCitation10,11,13 suggests that fungal strains belonging to these serotypes possibly do not interact equally with microbes outside of their environmental niche. For example, while not isolated from the same sample, Cn and Ab strains have been recovered from soil in countries presenting different climatic conditions and are capable to colonize multiple hosts.Citation14,44 This may provide an adaptive advantage to serotype A strains when interacting with other microbes or colonizing the human host. The differences in virulence of Cn are a consequence of adaptations that have evolved for protection against environmental predators such as bacteria and amoebae,Citation17,23 providing an explanation for the broad prevalenceCitation10,11 and systemic infectionsCitation14 caused by serotype A strains of this accidental pathogenic fungus. Further studies are warranted to elucidate the results of complex Cn interactions with other microbes in the environment and the impact of these symbioses in the evolution of virulence and survival mechanisms developed by this fungus.
Materials and Methods
Cn
Cn strains were inoculated in sabouraud dextrose broth (Sab; Difco, MI) and incubated at 30°C for 24 h in a rotary shaker set at 150 rpm (Cole-Parmer, IL). A total of 20 isolates (Serotype A strains: H99, 55, 57, 59, 68, 72, 83, 88, 116, 129; Serotype D strains: B3501, 24067, J9, J22, JEC21, 11, 13, 14, 16, 114) were included in this study.
Ab
Ab 0057, a clinical isolate acquired from Mark D. Adams (Cleveland, OH), was chosen for this study. Test organisms were grown in a tryptic soy broth (TSB; MP Biomedicals, LLC, Solon, OH) overnight at 37 °C using a rotary shaker set at 150 rpm. Growth was monitored by measuring the optical density at 600 nm using a microtiter reader (Bio-Tek, Winooski, VT).
Single fungal and bacterial cell interaction assays
Cn serotype A or D strains were mixed with Ab cells in a 2 mL micro centrifuge tube to yield a 1:1 effector-to-target ratio (108cells/mL) and incubated at 37°C for 2 h. Importantly, a 1:1 effector-to-target ratio was used because replication rates are significantly different (∼20 min Ab vs. ≥ 2 h Cn). After interaction, yeast and bacterial cells suspension was serially diluted and plated onto Sab agar supplemented with amikacin (64 μg/mL; Thermo Fisher Scientific, Waltham, MA) to inhibit bacterial growth whereas TS agar plates were supplemented with amphotericin B (0.125 μg/mL; Sigma, St. Louis, MO) to inhibit fungal growth. Sab and TS agar plates were incubated at 30°C for 48 h and 37°C for 24 h, respectively. Unmixed bacteria and yeast were used as control to determine the percentage of survival by CFU assay.
Fungal and bacterial biofilm interaction assays
Cryptococcal biofilms of serotype A or D strains were grown on 24-well tissue culture inserts with a pore size of 0.4 μm for 48 h at 37°C in minimal media (MgSO4.7H2O 1 M, CaCl2 1 M, Thiamine-HCl 10 g/L and dH2O). Following incubation, the inserts were washed with phosphate buffered saline (PBS) to get rid of non-adherent cells. Then, inserts were placed onto 24-well polystyrene plate containing Ab biofilms and incubated at 37°C for 24 h. Fungal and bacterial metabolic activity was quantified using the 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)carbonyl]-2H-tetrazolium-hydroxide (XTT; Sigma) reduction assay as described elsewhere.Citation33,45 Briefly, XTT tetrazolium salt is converted to XTT formazan salt by mitochondrial dehydrogenases in fungi and the electron transport system in the cellular membrane of live bacteria resulting in a colorimetric change. The optical density was measured in a microtiter reader at 492 nm.
Capsule measurement
An aliquot of 10 µL of unexposed or exposed yeast cells to Ab was mixed with India ink and visualized with light microscopy as described previously.Citation46 Briefly, the capsule size of 25 cells per strain was measured in these images using ImageJ 1.39u software (NIH, Bethesda, MD). Capsule size was defined as the difference between the diameter of the total cell (capsule included) and the cell body diameter, defined by the cell wall.
GXM quantification
To evaluate the effect of Ab interaction on polysaccharide release by Cn strains, exposed and unexposed fungal cells were separated from the supernatant by centrifugation and 1 mL of the supernatant was collected after 24 h co-incubation utilized fresh or frozen at −20°C until analyzed, which usually occurred within a day or 2. GXM concentrations in supernatant were then measured by capture ELISA as previously described.Citation47 Briefly, microtiter polystyrene plates were coated with GAM IgM (1 μg/mL) and blocked with 1% bovine serum albumin (BSA) in PBS. Next, the IgM GXM binding mAb 2D10 (2 μg/mL) was added as a capture antibody, and the plate was incubated for 1 h. The solution to be tested for GXM was then added, serially diluted on the plate, and incubated for 1 h. The ELISA was completed by adding, in successive steps, MAb 18B7 (2 μg/mL) in PBS (1% BSA), 1 μg of alkaline phosphatase-labeled GAM IgG1/mL, and 50 μL of p-nitrophenyl phosphate (5 mg/mL) in substrate buffer. Between every step, the wells were washed with 0.05% Tween 20 in Tris-buffered saline. All incubations were done at 37 for 1 h or 4°C overnight.
Confocal microscopy
Cn biofilms were incubated for 45 min in 75 µl of PBS containing the fluorescent stain FUN-1 (10 µM). Then, wells were blocked with PBS (1% BSA). mAb 18B7 (2 µg/mL) was added, and the plate was incubated. FITC-conjugated GAM IgG1 at a 1-µg/mL concentration in PBS (1% BSA) was applied. Between steps, the wells were washed with 0.05% Tween 20 in TBS. All incubations were done at 37°C for 1 h. FUN-1 (excitation wavelength, 470 nm; emission, 590 nm) is converted to orange-red cylindrical intravacuolar structures by metabolically active cells, while MAb 18B7, when bound by FITC-conjugated GAM IgG1 (excitation wavelength, 488 nm; emission, 530 nm), labels GXM and fluoresces green. Microscopic examinations of biofilms formed in microtiter plates were performed with confocal microscopy using an inverted Leica TCS SP5 confocal laser scanning microscope (Leica, Wetzlar, Germany) as previously described.Citation46 To determine the structure of the biofilms, a series of horizontal (xy) optical sections with a thickness of 1.175 μm were taken throughout the full length of the biofilm. Confocal images of green (mAb-18B7-FITC) and red (FUN-1) fluorescence were conceived simultaneously using a multichannel mode. Z-stack images and measurements were corrected utilizing Leica Application Suite Advanced Fluorescence (LCS AF) software-deconvolution mode (Leica).
Statistical analysis
Data were analyzed using Prism (GraphPad, LaJolla, CA). Analyses of CFU, metabolic activity, capsule size, and GXM determinations were done using Student's t-test. P values of < 0.05 were considered significant.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Authorship
All authors contributed to the design of the experiments, analysis of the data, and writing of the manuscript. A.F.A. performed the survival, capsule size, and GXM determination assays. H.H.L. performed the confocal microscopy. M.A. performed the XTT reduction assays.
Acknowledgements
We thank Mr. J Christian Belisario for his constructive suggestions.
Funding
LRM is supported by the NYIT College of Osteopathic Medicine Start-up funds.
References
- Park BJ, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, Chiller TM. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS 2009; 23:525-30; PMID:19182676; http://dx.doi.org/10.1097/QAD.0b013e328322ffac
- Littman ML, Schneierson SS. Cryptococcus neoformans in pigeon excreta in New York City. Am J Hyg 1959; 69:49-59; PMID:13626944
- Neilson JB, Ivey MH, Bulmer GG. Cryptococcus neoformans: size range of infectious particles fro aerosolized soil. Infect Immun 1977; 17:634; PMID:332630
- Levitz SM. The ecology of Cryptococcus neoformans and the epidemiology of cryptococcosis. Rev Infect Dis 1991; 13:1163; PMID:1775849; http://dx.doi.org/10.1093/clinids/13.6.1163
- Franzot SP, Salkin IF, Casadevall A. Cryptococcus neoformans var. grubii: separate varietal status for Cryptococcus neoformans serotype A isolates. J Clin Microbiol 1999; 37:838-40; PMID:9986871
- Bennett JE, Kwon-Chung KJ, Theodore TS. Biochemical differences between serotypes of Cryptococcus neoformans. Sabouraudia 1978; 16:167-74; PMID:360440; http://dx.doi.org/10.1080/00362177885380231
- Kwon-Chung KJ. A new genus, filobasidiella, the perfect state of Cryptococcus neoformans. Mycologia 1975; 67:1197-200; PMID:765816; http://dx.doi.org/10.2307/3758842
- Bhattacharjee AK, Bennett JE, Glaudemans CP. Capsular polysaccharides of Cryptococcus neoformans. Rev Infect Dis 1984; 6:619-24; PMID:6209768; http://dx.doi.org/10.1093/clinids/6.5.619
- Cherniak R, Sundstrom JB. Polysaccharide antigens of the capsule of Cryptococcus neoformans. Infect Immun 1994; 62:1507-12; PMID:8168912
- Bennett JE, Kwon-Chung KJ, Howard DH. Epidemiologic differences among serotypes of Cryptococcus neoformans. Am J Epidemiol 1977; 105:582-6; PMID:326036
- Kwon-Chung KJ, Bennett JE. Epidemiologic differences between the two varieties of Cryptococcus neoformans. Ame J Epidemiol 1984; 120:123-30; PMID:6377880
- Steenbergen JN, Casadevall A. Prevalence of Cryptococcus neoformans var. neoformans (Serotype D) and Cryptococcus neoformans var. grubii (Serotype A) isolates in New York City. J Clin Microbiol 2000; 38:1974-6; PMID:10790132
- Dromer F, Mathoulin S, Dupont B, Letenneur L, Ronin O. Individual and environmental factors associated with infection due to Cryptococcus neoformans serotype D. French Cryptococcosis Study Group. Clin Infect Dis 1996; 23:91-6; PMID:8816135; http://dx.doi.org/10.1093/clinids/23.1.91
- Casadevall A, Perfect JR. Cryptococcus neoformans. ASM Press; 1998.
- Martinez LR, Garcia-Rivera J, Casadevall A. Cryptococcus neoformans var. neoformans (serotype D) strains are more susceptible to heat than C. neoformans var. grubii (serotype A) strains. J Clin Microbiol 2001; 39:3365-7; PMID:11526180; http://dx.doi.org/10.1128/JCM.39.9.3365-3367.2001
- Desnos-Ollivier M, Patel S, Raoux-Barbot D, Heitman J, Dromer F, French Cryptococcosis Study G. Cryptococcosis Serotypes Impact Outcome and Provide Evidence of Cryptococcus neoformans Speciation. mBio 2015; 6; PMID:26060271; http://dx.doi.org/10.1128/mBio.00311-15
- Steenbergen JN, Shuman HA, Casadevall A. Cryptococcus neoformans interactions with amoebae suggest an explanation for its virulence and intracellular pathogenic strategy in macrophages. Proc Natl Acad Sci U S A 2001; 98:15245-50; PMID:11742090; http://dx.doi.org/10.1073/pnas.261418798
- Fournier PE, Richet H. The epidemiology and control of Acinetobacter baumannii in health care facilities. Clin Infect Dis 2006; 42:692-9; PMID:16447117; http://dx.doi.org/10.1086/500202
- Mihu MR, Martinez LR. Novel therapies for treatment of multi-drug resistant Acinetobacter baumannii skin infections. Virulence 2011; 2:97-102; PMID:21321482; http://dx.doi.org/10.4161/viru.2.2.15061
- Wargo MJ, Hogan DA. Fungal–bacterial interactions: a mixed bag of mingling microbes. Curr Opin Microbiol 2006; 9:359-64; PMID:16777473; http://dx.doi.org/10.1016/j.mib.2006.06.001
- Hall-Stoodley L, Costerton JW, Stoodley P. Bacterial biofilms: from the natural environment to infectious diseases. Nat Rev Microbiol 2004; 2:95-108; PMID:15040259; http://dx.doi.org/10.1038/nrmicro821
- Goldman DL, Lee SC, Casadevall A. Tissue localization of Cryptococcus neoformans glucuronoxylomannan in the presence and absence of specific antibody. Infect Immun 1995; 63:3448-53; PMID:7642276
- Derengowski Lda S, Paes HC, Albuquerque P, Tavares AH, Fernandes L, Silva-Pereira I, Casadevall A. The transcriptional response of Cryptococcus neoformans to ingestion by Acanthamoeba castellanii and macrophages provides insights into the evolutionary adaptation to the mammalian host. Eukaryot Cell 2013; 12:761-74; PMID:23524994; http://dx.doi.org/10.1128/EC.00073-13
- Murga R, Forster TS, Brown E, Pruckler JM, Fields BS, Donlan RM. Role of biofilms in the survival of Legionella pneumophila in a model potable-water system. Microbiology 2001; 147:3121-6; PMID:11700362
- Jesaitis AJ, Franklin MJ, Berglund D, Sasaki M, Lord CI, Bleazard JB, Duffy JE, Beyenal H, Lewandowski Z. Compromised host defense on Pseudomonas aeruginosa biofilms: characterization of neutrophil and biofilm interactions. J Immunol 2003; 171:4329-39; PMID:NOT_FOUND; http://dx.doi.org/10.4049/jimmunol.171.8.4329
- Rijnaarts HH, Norde W, Bouwer EJ, Lyklema J, Zehnder AJ. Bacterial adhesion under static and dynamic conditions. Appl Environ Microbiol 1993; 59:3255-65; PMID:16349063
- Uppuluri P, Chaturvedi AK, Srinivasan A, Banerjee M, Ramasubramaniam AK, Kohler JR, Kadosh D, Lopez-Ribot JL. Dispersion as an important step in the Candida albicans biofilm developmental cycle. PLoS Pathog 2010; 6:e1000828.
- Stoodley P, Hall-Stoodley L, Lappin-Scott HM. Detachment, surface migration, and other dynamic behavior in bacterial biofilms revealed by digital time-lapse imaging. Methods Enzymol 2001; 337:306-19; PMID:11398439; http://dx.doi.org/10.1016/S0076-6879(01)37023-4
- Robbins N, Uppuluri P, Nett J, Rajendran R, Ramage G, Lopez-Ribot JL, Andes D, Cowen LE. Hsp90 governs dispersion and drug resistance of fungal biofilms. PLoS Pathog 2011; 7:e1002257; PMID:21931556; http://dx.doi.org/10.1371/journal.ppat.1002257
- Rice SA, Koh KS, Queck SY, Labbate M, Lam KW, Kjelleberg S. Biofilm formation and sloughing in Serratia marcescens are controlled by quorum sensing and nutrient cues. J Bacteriol 2005; 187:3477-85; PMID:15866935; http://dx.doi.org/10.1128/JB.187.10.3477-3485.2005
- Rivera J, Feldmesser M, Cammer M, Casadevall A. Organ-dependent variation of capsule thickness in Cryptococcus neoformans during experimental murine infection. Infect Immun 1998; 66:5027-30; PMID:9746613
- Tucker SC, Casadevall A. Replication of Cryptococcus neoformans in macrophages is accompanied by phagosomal permeabilization and accumulation of vesicles containing polysaccharide in the cytoplasm. Proc Natl Acad Sci U S A 2002; 99:3165-70; PMID:11880650; http://dx.doi.org/10.1073/pnas.052702799
- Martinez LR, Casadevall A. Specific antibody can prevent fungal biofilm formation and this effect correlates with protective efficacy. Infect Immun 2005; 73:6350-62; PMID:16177306; http://dx.doi.org/10.1128/IAI.73.10.6350-6362.2005
- Alvarez M, Saylor C, Casadevall A. Antibody action after phagocytosis promotes Cryptococcus neoformans and Cryptococcus gattii macrophage exocytosis with biofilm-like microcolony formation. Cell Microbiol 2008; 10:1622-33; PMID:18384661; http://dx.doi.org/10.1111/j.1462-5822.2008.01152.x
- Vecchiarelli A. Immunoregulation by capsular components of Cryptococcus neoformans. Med Mycol 2000; 38:407-17; PMID:11204878; http://dx.doi.org/10.1080/mmy.38.6.407.417
- Granger DL, Perfect JR, Durack DT. Virulence of Cryptococcus neoformans. Regulation of capsule synthesis by carbon dioxide. J Clin Invest 1985; 76:508-16; PMID:3928681; http://dx.doi.org/10.1172/JCI112000
- Vartivarian SE, Anaissie EJ, Cowart RE, Sprigg HA, Tingler MJ, Jacobson ES. Regulation of cryptococcal capsular polysaccharide by iron. J Infect Dis 1993; 167:186-90; PMID:8418165; http://dx.doi.org/10.1093/infdis/167.1.186
- Bahn YS, Kojima K, Cox GM, Heitman J. Specialization of the HOG pathway and its impact on differentiation and virulence of Cryptococcus neoformans. Mol Biol Cell 2005; 16:2285-300; PMID:15728721; http://dx.doi.org/10.1091/mbc.E04-11-0987
- Cramer KL, Gerrald QD, Nichols CB, Price MS, Alspaugh JA. Transcription factor Nrg1 mediates capsule formation, stress response, and pathogenesis in Cryptococcus neoformans. Eukaryot Cell 2006; 5:1147-56; PMID:16835458; http://dx.doi.org/10.1128/EC.00145-06
- Hu G, Steen BR, Lian T, Sham AP, Tam N, Tangen KL, Kronstad JW. Transcriptional regulation by protein kinase A in Cryptococcus neoformans. PLoS Pathog 2007; 3:e42; PMID:17367210; http://dx.doi.org/10.1371/journal.ppat.0030042
- Xue C, Bahn YS, Cox GM, Heitman J. G protein-coupled receptor Gpr4 senses amino acids and activates the cAMP-PKA pathway in Cryptococcus neoformans. Mol Biol Cell 2006; 17:667-79; PMID:16291861; http://dx.doi.org/10.1091/mbc.E05-07-0699
- Cordero RJ, Frases S, Guimaraes AJ, Rivera J, Casadevall A. Evidence for branching in cryptococcal capsular polysaccharides and consequences on its biological activity. Mol Microbiol 2011; 79:1101-17; PMID:21208301; http://dx.doi.org/10.1111/j.1365-2958.2010.07511.x
- Rodrigues ML, Nimrichter L, Oliveira DL, Frases S, Miranda K, Zaragoza O, Alvarez M, Nakouzi A, Feldmesser M, Casadevall A. Vesicular polysaccharide export in Cryptococcus neoformans is a eukaryotic solution to the problem of fungal trans-cell wall transport. Eukaryot Cell 2007; 6:48-59; PMID:17114598; http://dx.doi.org/10.1128/EC.00318-06
- Eveillard M, Kempf M, Belmonte O, Pailhories H, Joly-Guillou ML. Reservoirs of Acinetobacter baumannii outside the hospital and potential involvement in emerging human community-acquired infections. Int J Infect Dis 2013; 17:e802-5; PMID:23672981; http://dx.doi.org/10.1016/j.ijid.2013.03.021
- Orsinger-Jacobsen SJ, Patel SS, Vellozzi EM, Gialanella P, Nimrichter L, Miranda K, Martinez LR. Use of a stainless steel washer platform to study Acinetobacter baumannii adhesion and biofilm formation on abiotic surfaces. Microbiology 2013; 159:2594-604; PMID:24025603; http://dx.doi.org/10.1099/mic.0.068825-0
- Patel D, Desai GM, Frases S, Cordero RJ, DeLeon-Rodriguez CM, Eugenin EA, Nosanchuk JD, Martinez LR. Methamphetamine enhances Cryptococcus neoformans pulmonary infection and dissemination to the brain. mBio 2013; 4; PMID:AMBIGUOUS; http://dx.doi.org/10.1128/mBio.00400-13
- Martinez LR, Moussai D, Casadevall A. Antibody to Cryptococcus neoformans glucuronoxylomannan inhibits the release of capsular antigen. Infect Immun 2004; 72:3674-9; PMID:15155683; http://dx.doi.org/10.1128/IAI.72.6.3674-3679.2004
