?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The efficacy of infection control interventions against Acinetobacter baumannii remains unclear, despite such information being critical for effective prevention of the transmission of this pathogen. Mathematical modeling offers an alternative to clinical trials, which may be prohibitively expensive, unfeasible or unethical, in predicting the impact of interventions. Furthermore, it allows the ability to ask key “what if” questions to evaluate which interventions have the most impact. We constructed a transmission dynamic model to quantify the effects of interventions on reducing A. baumannii prevalence and the basic reproduction ratio (R0) in intensive care units (ICUs). We distinguished between colonization and infection, and incorporated antibiotic exposure and transmission from free-living bacteria in the environment. Under the assumptions and parameterization in our model, 25% and 18% of patients are colonized and infected with A. baumannii, respectively; and R0 is 1.4. Improved compliance with hand hygiene (≥87%), enhanced environmental cleaning, reduced length of ICU stay of colonized patients (≤ 10 days), shorter durations of antibiotic treatment of A. baumannii (≤6 days), and isolation of infected patients combined with cleaning of isolation rooms are effective, reducing R0 to below unity. In contrast, expediting the recovery of the intestinal microbiota (e.g. use of probiotics) is not effective. This study represents a biologically realistic model of the transmission dynamics of A. baumannii, and the most comprehensive analysis of the effectiveness of interventions against this pathogen. Our study provides important data for designing effective infection control interventions.
Abbreviations
| CFU | = | colony-forming unit |
| ICU | = | intensive care unit |
| LoS | = | length of stay |
Introduction
Acinetobacter baumannii is a leading cause of severe infections, such as ventilator-associated pneumonia, bacteremia, urinary tract infections and meningitis, in patients in intensive care units (ICUs).Citation1 Infections caused by A. baumannii are difficult to treat because it is resistant to many antibiotics.Citation2 As such, limiting the emergence and spread of this pathogen is of paramount importance.
The acquisition and spread of A. baumannii is a complex and dynamic process determined by various inter-related factors. Exposure to antibiotics and the resultant disruption of the intestinal microbiota are known to predispose to A. baumannii acquisition.Citation3 Other major contributing factors for the acquisition of A. baumannii in ICUs include patient-related factors such as use of invasive procedures, and ICU-related factors such as transmission between patients within the ward (cross-transmission).Citation4,5 Furthermore, A. baumannii can remain viable in the hospital environment for a prolonged period of time, serving as an important reservoir and contributing to acquisition by susceptible patients (environment-patient transmission).Citation6,7 As such, a multifaceted approach which encompasses reducing cross-transmission, environment-patient transmission and antibiotic exposure could be required to limit the acquisition and spread of this pathogen. However, the relative contribution of each component remains unclear. Historically, such data can be obtained by conducting clinical and epidemiological studies. However, these studies are time-consuming and may be prohibitively expensive in the hospital setting. Operational and/or ethical constraints may further limit whether interventions can be evaluated in clinical studies. Additionally, these studies are inherently unable to capture the interdependence between individuals. As such, these studies only provide individual patient-level data and fail to fully characterize the transmission dynamics of the pathogen.
Population-level mathematical models, by providing a theoretical framework to conceptualize the dynamic interactions between interdependent variables, can overcome the aforementioned challenges.Citation8 They provide important insights into the underlying dynamics of an infection; and enable us to quantify the potential impact of various interventions without conducting those interventions.Citation8 Mathematical models also allow us to test “what-if” scenarios for the design of optimal intervention strategies.Citation8 While various models have investigated the effects of interventions against Gram-positive pathogens;Citation9-12 data on the population-level impact of interventions against A. baumannii (and other Gram-negative organisms alike) are scant. To date, there are only 2 modeling studies that investigate the transmission dynamics of A. baumannii.Citation4,7 Although both studies provide important insight into the spread of this pathogen, they have limitations. In our previous model, environment and interventions were not considered;Citation4 whereas the model by Wang et al.Citation7 did not distinguish between colonization and infection, nor did it take into account the effects of antibiotic exposure. Furthermore, nurse cohorting and nurse-patient ratio were the only interventions investigated in Wang et al.Citation7
Consequently, we have developed a comprehensive mechanistic model to describe the transmission dynamics of A. baumannii in ICUs, and to quantify the effects of various interventions on reducing A. baumannii transmission. Unlike most previous models,Citation9-11,13,14 we have differentiated between patients colonized and infected with A. baumannii, and incorporated the important role of antibiotic exposure and free-living bacteria in the environment.
Materials and Methods
Mechanistic transmission dynamic model
A mechanistic model was developed to describe the transmission dynamics of A. baumannii in a hypothetical 100-bed ICU (). In this model, patients were in 5 mutually exclusive states according to their infection status: uncolonized without or with antibiotic exposure (U and UA, respectively), colonized without or with antibiotic exposure (C and CA, respectively), or clinically infected (I). Antibiotic exposure was defined as currently receiving any systemic antibiotic or having received antibiotics within the last 30 d.Citation15 Colonized and infected patients harbor the pathogen; however only infected patients manifest clinical symptoms. Patients could be admitted to the ICU in any of these 5 states. Of new admissions, 57.6% and 36.8% were uncolonized without and with antibiotic exposure, respectively;Citation16 and 0.3% and 5.3% were colonized without and with antibiotic exposure, respectively.Citation16 New admissions that were already clinically infected with A. baumannii were 0%.Citation17 Patients could be discharged from any compartment, except for the infected compartment where they were manifesting symptoms.Citation18 Discharge occurred at a rate of γ per day, calculated as the inverse of the length of ICU stay (hereinafter referred to as length of stay, LoS) specific for each compartment. Uncolonized and colonized patients, irrespective of their antibiotic exposure status, stayed in the ICU for an average of 5.5 and 16.5 d, respectively.Citation19-22 We assumed that the ICU was fully occupied, and that new admissions balanced discharges, resulting in a constant population size of N = U + UA + C + CA + I = 100.Citation4,12
Figure 1. A compartmental model describing the transmission dynamics of A. baumannii in an intensive care unit. The solid arrows represent entry to and exit from the 5 compartments: C, colonized without antibiotic exposure; CA, colonized with antibiotic exposure; I, infected; U, uncolonized without antibiotic exposure; UA, uncolonized with antibiotic exposure. The broken arrows represent the shed of bacteria into the environment (E) from colonized and infected patients, and the transmission from free-living bacteria in the environment to susceptible (uncolonized) patients.
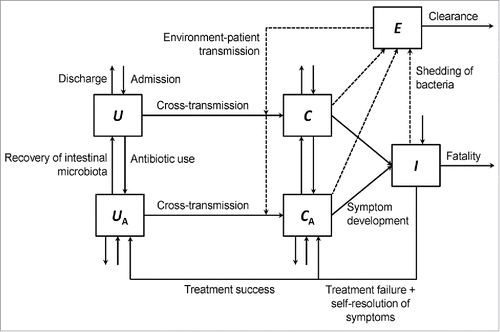
The movement from uncolonized or colonized without antibiotic exposure (U, C, respectively) to the corresponding compartments with antibiotic exposure (UA, CA, respectively) occurred at a rate of ϵ = 0.12 per day (antibiotic prescribing rate), which calibrated to 59.5% of ICU patients being prescribed antibiotics (for reasons other than treatment of A. baumannii infection) at any time during their stay.Citation23,24 The reverse process (moving from UA to U, and from CA to C) occurred when a patient's intestinal microbiota recovered after discontinuation of antibiotics, which was assumed to take 35 d.Citation25 The recovery rate of the intestinal microbiota, λ, was calculated as the inverse of this recovery time (λ = 0.03 per day). The use of probiotics was assumed to act by expediting this recovery process.Citation26
Uncolonized patients could become colonized via cross-transmission between patients within the ICU. This acquisition process was determined by the cross-transmission coefficient, β = 50 × 10−4 per colonized per susceptible per day, which was estimated in our previous model.Citation4 This cross-transmission coefficient incorporates both direct transmission between patients and transmission between patients via transiently contaminated hands of healthcare workers.Citation4 Of note, the latter is responsible for most cross-transmission because ICU patients are not moving in the ward.Citation4 Cross-transmission could be reduced by improving the rate of compliance with hand hygiene, h, which was 82% at baseline.Citation27 Hand hygiene was assumed to be 100% effective.Citation28 Uncolonized patients with antibiotic exposure (UA) were assumed to be 1.67 times more susceptible than uncolonized patients without antibiotic exposure (U); whereas colonized patients with antibiotic exposure (CA) and infected patients (I) were assumed, respectively, to be 1.67 and 2 times more infectious than colonized patients without antibiotic exposure (i.e. infectivity of CA relative to C, Ω1 = 1.67; infectivity of I relative to C, Ω2 = 2).Citation29
Colonized and infected patients shed bacteria into the environment (i.e., ICU), which could survive for a prolonged period of time, serving as another important source of transmission (environment-patient transmission). This transmission process was determined by the environment-patient transmission coefficient, ζ = 4 × 10−6 (per colony-forming unit [CFU] per susceptible per day).Citation30 Hand hygiene among healthcare workers or cleaning of the ICU (environmental cleaning), which occurred at a rate of r = 0.7 per day, would reduce this transmission source.Citation30 Environmental cleaning was assumed to eradicate 55% of the bacteria (environmental cleaning efficacy, α).Citation31 Free-living bacteria were assumed to be uniformly distributed in the environment and modeled in our study as another compartment (E).Citation30
Colonized patients with antibiotic exposure became infected (movement from CA to I) at a rate of θA = 0.11 per day.Citation5 Colonized patients without antibiotic exposure were assumed to be 5-times less likely to become infected than those with antibiotic exposure.Citation32 Antibiotic treatment of patients infected with A. baumannii was assumed to take 13 d (τ−1 = 13) with a successful clearance rate of σ = 0.76 per treated patient.Citation33,34 Infected patients who were successfully treated and cleared of the pathogen returned to the uncolonized with antibiotic exposure compartment; whereas the remaining treated patients returned to the colonized with antibiotic exposure compartment. Fifteen percent of infected patients had self-resolving symptoms and returned to the colonized with antibiotic exposure compartment;Citation35 and 14% of infected patients died as a result of the disease.Citation36 summarizes the input values of the model variables with their definitions and references. The system of ordinary differential equations that describe the transition between compartments is as follows:
Table 1. Baseline input values of model variables
The model was simulated for one year (365 days). The following outcome measures were estimated: prevalence of colonized and infected patients, and the basic reproduction ratio, R0. Briefly, R0 is the average number of secondary colonized cases resulting from one single infectious individual in a totally susceptible population.Citation37 It is an important predictor of whether and how quickly an infection will spread. The aim of any intervention is to reduce R0 to below unity. The next generation matrix method (described in Appendix 1) was used to estimate R0 in our study.Citation38
Interventions
Simulations were performed to evaluate the efficacy of various interventions in reducing the prevalence of colonized and infected patients, and R0. The following interventions were investigated: (1) improved compliance with hand hygiene (h); (2) reduced antibiotic prescribing rate (ϵ); (3) reduced antibiotic treatment duration in patients infected with A. baumannii ; (4) improved recovery rate of the intestinal microbiota (λ, via use of probiotics); (5) reduced LoS of colonized patients
following evidence that LoS is a major risk factor for A. baumannii;Citation39 (6) increased environmental cleaning rate (r, more frequent cleaning); (7) improved environmental cleaning efficacy (α, e.g., more effective cleaning products, training for cleaning teams); and (8) isolation of infected patients combined with cleaning of isolation rooms.
Sensitivity analysis
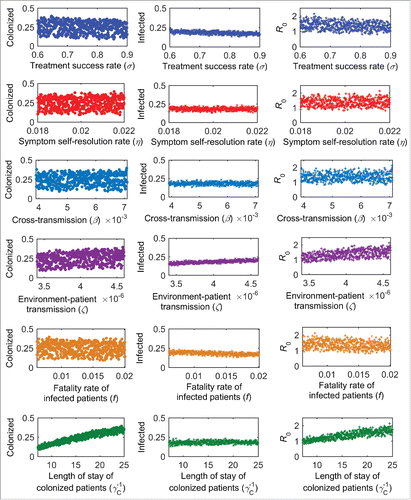
Multivariate sensitivity analysis was carried out to investigate the key drivers of the outcome measures and the sensitivity of model outputs to changes in model inputs. The following variables were assessed: success rate of A. baumannii treatment (σ), rate of self-resolution of symptoms (η); cross-transmission coefficient (β); environment-patient transmission coefficient (ζ); fatality rate of infected patients (f ); and LoS of colonized patients . The range of each variable is shown in . The Latin hypercube sampling method was performed. Partial rank correlation coefficients were calculated to evaluate the strength of the correlation between each outcome measure and each variable.Citation40 All analysis was performed using MATLAB (version R2015a, MathWorks, Natick, MA, USA). The differential equations were solved using the ode45 solver.
Table 2. Variation range for variables evaluated in sensitivity analysis
Results
Baseline scenario
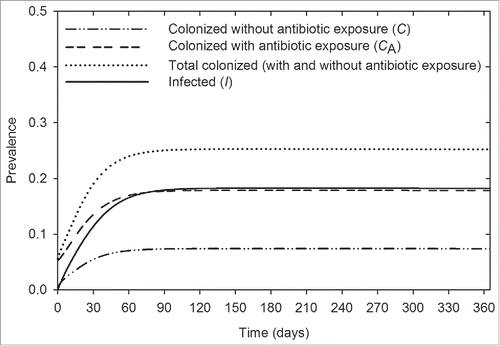
Using the baseline parameters (), we estimate that 25% of patients are colonized, and 18% are infected with A. baumannii (). Acquisition is predominantly caused by within-ward transmission (98%), rather than colonization already present on admission. R0 is estimated to be 1.4.
The impact of interventions
shows the predicted effects of individual interventions on reducing the prevalence of A. baumannii and R0. The relative effects among the interventions investigated are shown in . Compliance with hand hygiene (h) is the most effective intervention whereby a modest improvement in compliance rate, from 82% (baseline) to 87%, reduces R0 from 1.4 to unity, and reduces the prevalence of colonized and infected patients by 6% (from 25% to 19%) and 4% (from 18% to 14%), respectively (). In an idealized scenario where compliance rate is 100%, R0 is reduced to zero; however, A. baumannii always persists at a low level (). This is because of the constant admission of colonized patients into the ICU, with 5.6% of admitted patients already colonized at baseline. shows that when colonized patients are not admitted, A. baumannii will become extinct when R0 is below unity. When R0 is above unity, A. baumannii is always endemic irrespective of whether colonized patients are admitted ().
Figure 3. Effects of individual interventions on the prevalence of colonization (long-dashed lines), infection (short-dashed lines), total colonization and infection (solid lines), and the basic reproduction ratio, R0 (dotted lines). The following interventions were investigated: compliance with hand hygiene (A), environmental cleaning rate (B), environmental cleaning efficacy (C), length of stay of colonized patients (D), antibiotic prescribing rate (E), treatment duration of infected patients (F), and recovery of intestinal microbiota (G).
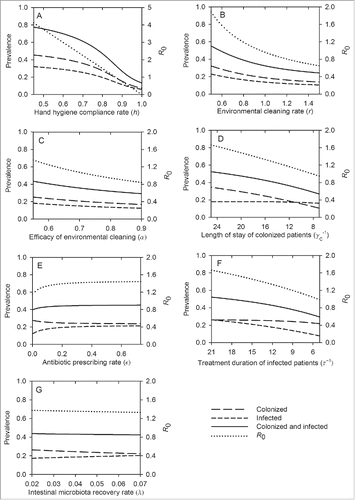
Figure 4. Relative effects of different interventions on the total prevalence of colonized and infected patients (A), and the basic reproduction ratio, R0 (B).
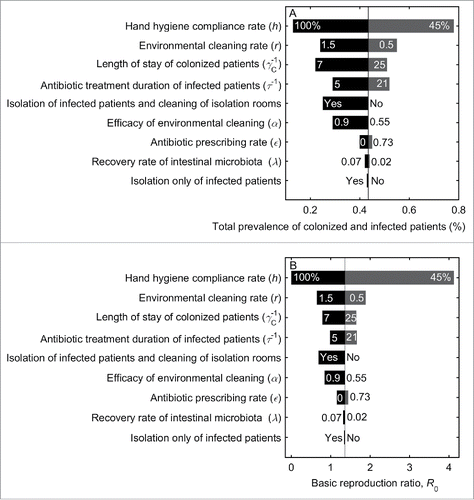
Figure 5. Prevalence of A. baumannii when the basic reproduction ratio, R0 < 1 (A) and R0 > 1 (B). When R0 <1, A. baumannii persists when colonized and infected patients are admitted (solid lines), and will die out if there is no admission of colonized and infected patients (broken lines). When R0 >1, A. baumannii always persists, once it has been introduced into the ward, irrespective of whether colonized and infected patients are admitted.
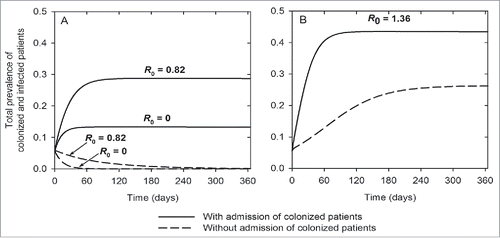
Frequent ward cleaning (r) is also an effective intervention (). Increasing environmental cleaning rate from 0.7 (baseline) to 1 (daily cleaning) reduces the prevalence of colonized and infected patients from 25% to 19% and from 18% to 14%, respectively. Daily ward cleaning also reduces R0 to unity. Similar reductions can be achieved when the efficacy of environmental cleaning (α) is improved from 55% (baseline) to 75% (). show the combined effects of enhanced cleaning rate and cleaning efficacy. Combining the 2 interventions yields improved benefits compared to each intervention alone. For example, daily ward cleaning combined with improved cleaning efficacy (from 55% to 75%) reduces the total prevalence of colonization and infection by 18%, compared to 10–11% reduction achievable with each intervention alone. Isolation of infected patients has modest effects (). However, isolation combined with cleaning of isolation rooms is effective, significantly reducing the prevalence of colonized patients from 25% (baseline) to 14%, and the prevalence of infected patients from 18% (baseline) to 11%. With this strategy, R0 is 0.7 ().
Figure 6. Effects of different combinations of interventions on the total prevalence of colonization and infection (A, C, E), and the basic reproduction ratio, R0 (B, D, F).
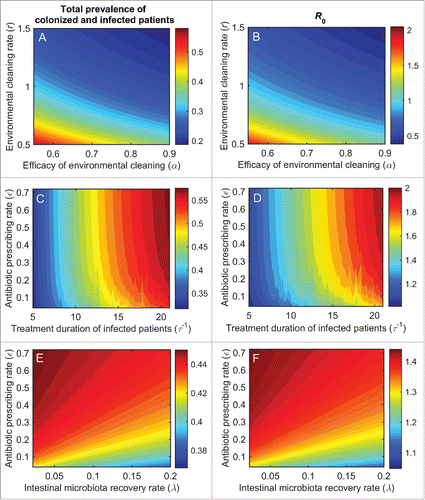
shows the effects of reducing the LoS of colonized patients . The prevalence of A. baumannii decreases with shorter LoS (increased discharge rate). Specifically, when the LoS is reduced from the baseline of 16.5 d to 10 days, R0 reduces to below unity, and the prevalence of colonization and infection reduces by 7% (from 25% to 18%) and 5% (from 18% to 13%), respectively.
When antibiotic prescribing rate (ϵ) among ICU patients (for reasons other than treatment of A. baumannii infection) and antibiotic treatment duration of infected patients are investigated separately, only the latter is effective. Reducing antibiotic prescribing rate from 0.12 (baseline) to 0 (no antibiotic use) only results in a 4% reduction in the total prevalence of colonized and infected patients (). In contrast, similar reductions in antibiotic treatment duration of infected patients (
, from 13 d to 5 days) decrease the total prevalence of colonized and infected patients by 14% (from 43% to 29%) and R0 to unity (). This intervention has greater effects on the prevalence of infected patients than on colonized patients because it specifically targets the former (). Combining the 2 interventions fails to yield any improvements in reducing A. baumannii prevalence compared to each intervention alone (). For example, simultaneously reducing antibiotic prescribing rate from 0.12 to 0 and antibiotic treatment duration of infected patients from 13 d to 5 d only results in a 14% reduction in the total prevalence of colonized and infected patients. Comparable reduction would be achievable with reduced antibiotic treatment duration of infected patients alone.
The use of probiotics to expedite the recovery of the intestinal microbiota (λ, 1/recovery time) is not effective. Reducing the recovery time from 35 d to 14 d only reduces the total prevalence of colonization and infection by 1% (). No enhanced effects are achieved when probiotics are used in conjunction with reduced antibiotic prescribing rate ().
Varying transmission coefficients
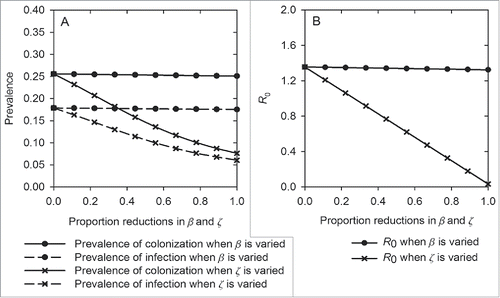
shows the effects of varying the cross-transmission coefficient (β) or environment-patient transmission coefficient (ζ) on the prevalence of A. baumannii and R0. Contaminated environment is the predominant acquisition source compared to cross-transmission. Complete elimination of environment-patient transmission (ζ = 0) generates substantial benefits, reducing the prevalence of colonization from 25% to 7% and infection from 18% to 6%. Eliminating this transmission source reduces R0 to zero. In contrast, only modest impact occurs when cross-transmission is limited.
Sensitivity analysis
shows the sensitivity of the outcome measures to changes in model inputs. The outcome measures are most sensitive to LoS of colonized patients , A. baumannii treatment success rate (σ), environment-patient transmission coefficient (ζ) and fatality rate of infected patients (f ). The correlation coefficients are shown in .
Table 3. Correlation coefficients
Discussion
Our study represents the most comprehensive transmission dynamic model for A. baumannii. Unlike previous studies,Citation7,9-11,13,14,41 we differentiated between colonized and infected patients because of their differences in clinical characteristics, particularly in pathogenesis, infectivity and infection control management. Under the baseline values found from review of the literature, we predict that A. baumannii is likely to be endemic in ICUs. The estimated prevalence of colonization and infection is 25% and 18%, respectively. Although we did not calibrate our model to any outcome values, our estimates are comparable with the mean values of the prevalence of A. baumannii colonization (21%)Citation4,16,42,43 and infection (16%)Citation5,42,44 reported in the literature. We found that A. baumannii transmission continues even when R0 is below unity owing to admission of already colonized patients to the ICU. Our finding suggests that active screening on admission and subsequent isolation of positive cases is an essential component of any infection control policy to eliminate the transmission of this pathogen. Although this strategy may be effective in controlling A. baumannii, active screening is currently not in place in most hospitals worldwide. This may be due in part to concerns about the high costs of implementing such programs.Citation8 Nevertheless, a comprehensive cost-effectiveness analysis of active screening programs is needed.
Hand hygiene is the most effective intervention in our study because it limits both the transmission between patients and, between patients and the environment. The rate of compliance with hand hygiene was set at 82% at baseline in line with Australian data.Citation27 Although it is effective, inexpensive and simple, compliance with hand hygiene remains obstinately low in some settings, with estimated compliance rates as low as 50%.Citation45 When this compliance rate was considered, the prevalence of colonization, infection, and R0 were estimated to be 44%, 31%, and 3.7, respectively. Using , predictions of A. baumannii prevalence and R0 could be obtained for any level of hand hygiene compliance. Our model predicts that a modest improvement in compliance rate, from 82% to 87%, will reduce R0 to below unity. Previous studies have shown that such improvements would be achievable with increased education and training, increased availability of alcohol-based hand rubs, workplace reminders, and performance feedback.Citation45
Our model also shows that shorter durations of antibiotic treatment in patients infected with A. baumannii are associated with lower prevalence of A. baumannii. This suggests that treating patients as quickly and effectively as possible may be an effective strategy. Indeed, previous clinical studies have shown that the durations of antibiotic therapy could be safely reduced to ≤7 d based on clinical, laboratory and radiologic measures without any adverse effects on patient's outcomes while reducing the prevalence of infection and resistance.Citation46-48 These findings emphasize the importance of effective antimicrobial stewardship programs in reducing the duration of antibiotic therapy. However, it should be noted that the translation of the findings of these studies into clinical practice is currently unknown; and that the optimal durations of antibiotic treatment for many common infections remain to be established. Effective antimicrobial stewardship programs can also lead to reductions in antibiotic usage.Citation49 However, we found that reducing antibiotic prescribing rate among patients who are not infected with A. baumannii has a modest impact on A. baumannii prevalence, consistent with previous studies.Citation12,18 This is because this strategy only targets the small subset of patients who are uncolonized or colonized without prior antibiotic exposure in our model.
Clinical evidence on the efficacy of probiotics in reducing infections is inconclusive. It has been shown to be beneficial in some studies;Citation50 while others have shown minimal effects.Citation51 Our model suggests that using probiotics has a modest impact on the population-level transmission dynamics of A. baumannii, consistent with previous clinical studies.Citation51 Nevertheless, our finding does not necessarily negate the health benefits of these products on an individual-patient level.Citation26
We found that reducing LoS is effective in attenuating A. baumannii, in support of evidence from clinical studies and previous models.Citation10,18,52 This is because this strategy reduces patient's risk factors for acquiring A. baumannii in the ICU.Citation16 Our finding is in contrast with Cooper et al.Citation28 who demonstrated in their model that shorter LoS resulted in higher within-ward colonization.Citation28 This may be explained by the increased number of susceptible patients admitted to the ward as a result of reduced LoS (increased discharge rate). Nevertheless, it should be noted that LoS of patients should be based on their clinical characteristics and response to treatment. As such, reducing LoS is likely to be impractical in reality.
The current model incorporates transmission from free-living bacteria in the environment, which has been ignored in the majority of previous models.Citation4,9-12,18,53 We found that contaminated environment is the major source of acquisition. Our finding reinforces the importance of environmental cleaning in controlling health-care associated infections as previously shown in clinical studies.Citation31,54 We found that improving the effectiveness of environmental cleaning would substantially reduce A. baumannii. This could be achieved by using more effective cleaning products, training and monitoring the efficacy of decontamination with feedback to cleaning teams. In our model, free-living bacteria in the ICU were assumed to be uniformly distributed. In fact, bacterial density may be different between places in the ICU (e.g., environment around infectious patients versus computer keyboards or door knobs). Future models that take into account such heterogeneity in contamination status are needed. Direct transmission between patients and free-living bacteria in the environment should also be considered in future studies.
Our study represents a biologically realistic model for the transmission dynamics of A. baumannii in ICUs. It provides a comprehensive analysis of the impact of interventions against this pathogen. We have incorporated crucial factors to the epidemiology of this pathogen such as antibiotic exposure, transmission from contaminated environment and, distinguishing colonization and infection. Like most biologically realistic simulation models, we were unable to simulate a specific setting because there is no single clinical study that provides complete parameterization for our model. Importantly, our model provides a framework that can be easily adjusted when such studies become available. Given that the epidemiology of A. baumannii varies considerably over time and between different settings, the intention of our model is not to provide numerical estimates that apply to every setting. However, our model can be adjusted to incorporate institution-specific data to guide infection control. Our model can be modified to integrate greater complexity such as co-morbidities, immune status, antibiotic resistance, co-infection with other pathogens, and the effects of stochasticity.
Disclosure of Potential Conflicts of Interest
DCM.K. has sat on advisory boards of Pfizer, Merck Sharp & Dohme, and receives financial/travel support (unrelated to the current work) from Pfizer, Roche, Merck Sharp & Dohme, Novartis and Gilead Sciences. C.M.J.K has undertaken collaborative research projects unrelated to the current work with Roche, Pfizer, CSL and d3 Medicine. All other authors have no potential conflicts of interest to disclose.
Funding
T.N.D. is a recipient of the Monash Graduate Scholarship and Monash International Postgraduate Research Scholarship. E.S.M. has received financial support from the Australian National Health and Medical Research Council (NHMRC) Career Development Fellowship. All other authors: none to declare.
References
- Consales G, Gramigni E, Zamidei L, Bettocchi D, De Gaudio AR. A multidrug-resistant Acinetobacter baumannii outbreak in intensive care unit: antimicrobial and organizational strategies. J Crit Care 2011; 26:453-9; PMID:21439763; http://dx.doi.org/10.1016/j.jcrc.2010.12.016
- Maragakis LL, Perl TM. Acinetobacter baumannii: epidemiology, antimicrobial resistance, and treatment options. Clin Infect Dis 2008; 46:1254-63; PMID:18444865; http://dx.doi.org/10.1086/529198
- Panda S, El khader I, Casellas F, Lopez Vivancos J, Garcia Cors M, Santiago A, Cuenca S, Guarner F, Manichanh C. Short-term effect of antibiotics on human gut microbiota. PLoS One 2014; 9:e95476; PMID:24748167; http://dx.doi.org/10.1371/journal.pone.0095476
- Doan TN, Kong DCM, Marshall C, Kirkpatrick CMJ, McBryde ES. Characterising the transmission dynamics of Acinetobacter baumannii in intensive care units using hidden Markov models. PLoS One 2015; 10:e0132037; PMID:26131722; http://dx.doi.org/10.1371/journal.pone.0132037
- Jung JY, Park MS, Kim SE, Park BH, Son JY, Kim EY, Lim JE, Lee SK, Lee SH, Lee KJ, et al. Risk factors for multi-drug resistant Acinetobacter baumannii bacteremia in patients with colonization in the intensive care unit. BMC Infect Dis 2010; 10:228; PMID:20670453; http://dx.doi.org/10.1186/1471-2334-10-228
- Jawad A, Seifert H, Snelling AM, Heritage J, Hawkey PM. Survival of Acinetobacter baumannii on dry surfaces: comparison of outbreak and sporadic isolates. J Clin Microbiol 1998; 36:1938-41; PMID:9650940
- Wang X, Chen Y, Zhao W, Wang Y, Song Q, Liu H, Zhao J, Han X, Hu X, Grundmann H, et al. A data-driven mathematical model of multi-drug resistant Acinetobacter baumannii transmission in an intensive care unit. Sci Rep 2015; 5:9478; PMID:25804674; http://dx.doi.org/10.1038/srep09478
- Doan TN, Kong DCM, Kirkpatrick CMJ, McBryde ES. Optimizing hospital infection control: the role of mathematical modeling. Infect Control Hosp Epidemiol 2014; 35:1521-30; PMID:25419775; http://dx.doi.org/10.1086/678596
- Wang J, Wang L, Magal P, Wang Y, Zhuo J, Lu X, Ruan S. Modelling the transmission dynamics of meticillin-resistant Staphylococcus aureus in Beijing Tongren hospital. J Hosp Infect 2011; 79:302-8; PMID:22033439; http://dx.doi.org/10.1016/j.jhin.2011.08.019
- McBryde ES, Pettitt AN, McElwain DL. A stochastic mathematical model of methicillin resistant Staphylococcus aureus transmission in an intensive care unit: predicting the impact of interventions. J Theor Biol 2007; 245:470-81; PMID:17188714; http://dx.doi.org/10.1016/j.jtbi.2006.11.008
- McBryde ES, Pettitt AN, Cooper BS, McElwain DL. Characterizing an outbreak of vancomycin-resistant enterococci using hidden Markov models. J R Soc Interface 2007; 4:745-54; PMID:17360254; http://dx.doi.org/10.1098/rsif.2007.0224
- D'Agata EM, Horn MA, Ruan S, Webb GF, Wares JR. Efficacy of infection control interventions in reducing the spread of multidrug-resistant organisms in the hospital setting. PLoS One 2012; 7:e30170.
- Christopher S, Verghis RM, Antonisamy B, Sowmyanarayanan TV, Brahmadathan KN, Kang G, Cooper BS. Transmission dynamics of methicillin-resistant Staphylococcus aureus in a medical intensive care unit in India. PLoS One 2011; 6:e20604; PMID:21750700; http://dx.doi.org/10.1371/journal.pone.0020604
- Pressley J, D'Agata EM, Webb GF. The effect of co-colonization with community-acquired and hospital-acquired methicillin-resistant Staphylococcus aureus strains on competitive exclusion. J Theor Biol 2010; 264:645-56; PMID:20347850; http://dx.doi.org/10.1016/j.jtbi.2010.03.036
- Patel N, McNutt LA, Lodise TP. Relationship between various definitions of prior antibiotic exposure and piperacillin-tazobactam resistance among patients with respiratory tract infections caused by Pseudomonas aeruginosa. Antimicrob Agents Chemother 2008; 52:2933-6; PMID:18519718; http://dx.doi.org/10.1128/AAC.00456-08
- Arvaniti K, Lathyris D, Ruimy R, Haidich AB, Koulourida V, Nikolaidis P, Matamis D, Miyakis S. The importance of colonization pressure in multiresistant Acinetobacter baumannii acquisition in a Greek intensive care unit. Crit Care 2012; 16:R102; PMID:22694969; http://dx.doi.org/10.1186/cc11383
- Thom KA, Harris AD, Johnson JA, Furuno JP. Low prevalence of Acinetobacter baumannii colonization on hospital admission. Am J Infect Control 2010; 38:329-31; PMID:20189683; http://dx.doi.org/10.1016/j.ajic.2009.10.006
- Yakob L, Riley TV, Paterson DL, Clements AC. Clostridium difficile exposure as an insidious source of infection in healthcare settings: an epidemiological model. BMC Infect Dis 2013; 13:376; PMID:23947736; http://dx.doi.org/10.1186/1471-2334-13-376
- Weingarten CM, Rybak MJ, Jahns BE, Stevenson JG, Brown WJ, Levine DP. Evaluation of Acinetobacter baumannii infection and colonization, and antimicrobial treatment patterns in an urban teaching hospital. Pharmacotherapy 1999; 19:1080-5; PMID:10610015; http://dx.doi.org/10.1592/phco.19.13.1080.31597
- Lee BY, McGlone SM, Doi Y, Bailey RR, Harrison LH. Economic impact of Acinetobacter baumannii infection in the intensive care unit. Infect Control Hosp Epidemiol 2010; 31:1087-9; PMID:20804376; http://dx.doi.org/10.1086/656378
- Ntusi NB, Badri M, Khalfey H, Whitelaw A, Oliver S, Piercy J, Raine R, Joubert I, Dheda K. ICU-associated Acinetobacter baumannii colonisation/infection in a high HIV-prevalence resource-poor setting. PLoS One 2012; 7:e52452; PMID:23300673; http://dx.doi.org/10.1371/journal.pone.0052452
- Abbo A, Carmeli Y, Navon-Venezia S, Siegman-Igra Y, Schwaber MJ. Impact of multi-drug-resistant Acinetobacter baumannii on clinical outcomes. Eur J Clin Microbiol Infect Dis 2007; 26:793-800; PMID:17701063; http://dx.doi.org/10.1007/s10096-007-0371-8
- MacDougall C, Polk RE. Variability in rates of use of antibacterials among 130 US hospitals and risk-adjustment models for interhospital comparison. Infect Control Hosp Epidemiol 2008; 29:203-11; PMID:18257689; http://dx.doi.org/10.1086/528810
- Polk RE, Fox C, Mahoney A, Letcavage J, MacDougall C. Measurement of adult antibacterial drug use in 130 US hospitals: comparison of defined daily dose and days of therapy. Clin Infect Dis 2007; 44:664-70; PMID:17278056; http://dx.doi.org/10.1086/511640
- Rafii F, Sutherland JB, Cerniglia CE. Effects of treatment with antimicrobial agents on the human colonic microflora. Ther Clin Risk Manag 2008; 4:1343-58; PMID:19337440
- Oudhuis GJ, Bergmans DC, Verbon A. Probiotics for prevention of nosocomial infections: efficacy and adverse effects. Curr Opin Crit Care 2011; 17:487-92; PMID:21900768; http://dx.doi.org/10.1097/MCC.0b013e32834a4bab
- Hand Hygiene Australia. October 2014. Available at http://www.hha.org.au/LatestNationalData.aspx [accessed in April 2015].
- Cooper BS, Medley GF, Scott GM. Preliminary analysis of the transmission dynamics of nosocomial infections: stochastic and management effects. J Hosp Infect 1999; 43:131-47; PMID:10549313; http://dx.doi.org/10.1053/jhin.1998.0647
- Chamchod F, Ruan S. Modeling methicillin-resistant Staphylococcus aureus in hospitals: transmission dynamics, antibiotic usage and its history. Theor Biol Med Model 2012; 9:25; PMID:22738359; http://dx.doi.org/10.1186/1742-4682-9-25
- Wang X, Xiao Y, Wang J, Lu X. A mathematical model of effects of environmental contamination and presence of volunteers on hospital infections in China. J Theor Biol 2012; 293:161-73; PMID:22024632; http://dx.doi.org/10.1016/j.jtbi.2011.10.009
- Xu H, Jin H, Zhao L, Wei X, Hu L, Shen L, Wei L, Xie L, Kong Q, Wang Y, et al. A randomized, double-blind comparison of the effectiveness of environmental cleaning between infection control professionals and environmental service workers. Am J Infect Control 2015; 43:292-4; PMID:25556049; http://dx.doi.org/10.1016/j.ajic.2014.11.009
- Baran G, Erbay A, Bodur H, Onguru P, Akinci E, Balaban N, Cevik MA. Risk factors for nosocomial imipenem-resistant Acinetobacter baumannii infections. Int J Infect Dis 2008; 12:16-21; PMID:17513154; http://dx.doi.org/10.1016/j.ijid.2007.03.005
- Lee NY, Lee HC, Ko NY, Chang CM, Shih HI, Wu CJ, Ko WC. Clinical and economic impact of multidrug resistance in nosocomial Acinetobacter baumannii bacteremia. Infect Control Hosp Epidemiol 2007; 28:713-9; PMID:17520546; http://dx.doi.org/10.1086/517954
- Chan JD, Graves JA, Dellit TH. Antimicrobial treatment and clinical outcomes of carbapenem-resistant Acinetobacter baumannii ventilator-associated pneumonia. J Intensive Care Med 2010; 25:343-8; PMID:20837632; http://dx.doi.org/10.1177/0885066610377975
- Raz R, Alroy G, Sobel JD. Nosocomial bacteremia due to Acinetobacter calcoaceticus. Infection 1982; 10:168-71; PMID:7107014; http://dx.doi.org/10.1007/BF01640769
- Daniels TL, Deppen S, Arbogast PG, Griffin MR, Schaffner W, Talbot TR. Mortality rates associated with multidrug-resistant Acinetobacter baumannii infection in surgical intensive care units. Infect Control Hosp Epidemiol 2008; 29:1080-3; PMID:18837670; http://dx.doi.org/10.1086/591456
- Keeling MJ, Danon L. Mathematical modelling of infectious diseases. Br Med Bull 2009; 92:33-42; PMID:19855103; http://dx.doi.org/10.1093/bmb/ldp038
- Diekmann O, Heesterbeek JA, Roberts MG. The construction of next-generation matrices for compartmental epidemic models. J R Soc Interface 2010; 7:873-85; PMID:19892718; http://dx.doi.org/10.1098/rsif.2009.0386
- Jang TN, Lee SH, Huang CH, Lee CL, Chen WY. Risk factors and impact of nosocomial Acinetobacter baumannii bloodstream infections in the adult intensive care unit: a case-control study. J Hosp Infect 2009; 73:143-50; PMID:19716203; http://dx.doi.org/10.1016/j.jhin.2009.06.007
- Marino S, Hogue IB, Ray CJ, Kirschner DE. A methodology for performing global uncertainty and sensitivity analysis in systems biology. J Theor Biol 2008; 254:178-96; PMID:18572196; http://dx.doi.org/10.1016/j.jtbi.2008.04.011
- D'Agata EM, Webb G, Horn M. A mathematical model quantifying the impact of antibiotic exposure and other interventions on the endemic prevalence of vancomycin-resistant enterococci. J Infect Dis 2005; 192:2004-11; PMID:16267774; http://dx.doi.org/10.1086/498041
- Rodriguez-Bano J, Cisneros JM, Fernandez-Cuenca F, Ribera A, Vila J, Pascual A, Martinez-Martinez L, Bou G, Pachon J. Clinical features and epidemiology of Acinetobacter baumannii colonization and infection in Spanish hospitals. Infect Control Hosp Epidemiol 2004; 25:819-24; PMID:15518022; http://dx.doi.org/10.1086/502302
- Playford EG, Craig JC, Iredell JR. Carbapenem-resistant Acinetobacter baumannii in intensive care unit patients: risk factors for acquisition, infection and their consequences. J Hosp Infect 2007; 65:204-11; PMID:17254667; http://dx.doi.org/10.1016/j.jhin.2006.11.010
- Vincent JL, Rello J, Marshall J, Silva E, Anzueto A, Martin CD, Moreno R, Lipman J, Gomersall C, Sakr Y, et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA 2009; 302:2323-9; PMID:19952319; http://dx.doi.org/10.1001/jama.2009.1754
- Magiorakos AP, Leens E, Drouvot V, May-Michelangeli L, Reichardt C, Gastmeier P, Wilson K, Tannahill M, McFarlane E, Simon A. Pathways to clean hands: highlights of successful hand hygiene implementation strategies in Europe. Euro Surveill 2010; 15:19560; PMID:20460091
- Pugh R, Grant C, Cooke RP, Dempsey G. Short-course versus prolonged-course antibiotic therapy for hospital-acquired pneumonia in critically ill adults. Cochrane Database Syst Rev 2011; 10:CD007577; PMID:21975771
- Hayashi Y, Paterson DL. Strategies for reduction in duration of antibiotic use in hospitalized patients. Clin Infect Dis 2011; 52:1232-40; PMID:21507920; http://dx.doi.org/10.1093/cid/cir063
- Hedrick TL, McElearney ST, Smith RL, Evans HL, Pruett TL, Sawyer RG. Duration of antibiotic therapy for ventilator-associated pneumonia caused by non-fermentative gram-negative bacilli. Surg Infect 2007; 8:589-97; PMID:18171118; http://dx.doi.org/10.1089/sur.2006.021
- Cisneros JM, Neth O, Gil-Navarro MV, Lepe JA, Jimenez-Parrilla F, Cordero E, Rodriguez-Hernandez MJ, Amaya-Villar R, Cano J, Gutierrez-Pizarraya A, et al. Global impact of an educational antimicrobial stewardship programme on prescribing practice in a tertiary hospital centre. Clin Microbiol Infect 2014; 20:82-8; PMID:23517432; http://dx.doi.org/10.1111/1469-0691.12191
- Kotzampassi K, Giamarellos-Bourboulis EJ, Voudouris A, Kazamias P, Eleftheriadis E. Benefits of a synbiotic formula (Synbiotic 2000Forte) in critically ill trauma patients: early results of a randomized controlled trial. World J Surg 2006; 30:1848-55; PMID:16983476; http://dx.doi.org/10.1007/s00268-005-0653-1
- McNaught CE, Woodcock NP, Anderson AD, MacFie J. A prospective randomised trial of probiotics in critically ill patients. Clin Nutr 2005; 24:211-9; PMID:15784480; http://dx.doi.org/10.1016/j.clnu.2004.08.008
- Spoorenberg V, Hulscher ME, Akkermans RP, Prins JM, Geerlings SE. Appropriate antibiotic use for patients with urinary tract infections reduces length of hospital stay. Clin Infect Dis 2014; 58:164-9; PMID:24158412; http://dx.doi.org/10.1093/cid/cit688
- Yakob L, Riley TV, Paterson DL, Marquess J, Clements A. Assessing control bundles for Clostridium difficile: a review and mathematical model. Emerg Microbes and Infect 2014; 3:e43; PMID:26038744; http://dx.doi.org/10.1038/emi.2014.43
- Wilson AP, Smyth D, Moore G, Singleton J, Jackson R, Gant V, Jeanes A, Shaw S, James E, Cooper B, et al. The impact of enhanced cleaning within the intensive care unit on contamination of the near-patient environment with hospital pathogens: a randomized crossover study in critical care units in two hospitals. Crit Care Med 2011; 39:651-8; PMID:21242793; http://dx.doi.org/10.1097/CCM.0b013e318206bc66
- Falagas ME, Bliziotis IA, Siempos, II. Attributable mortality of Acinetobacter baumannii infections in critically ill patients: a systematic review of matched cohort and case-control studies. Crit Care 2006; 10:R48; PMID:16563184; http://dx.doi.org/10.1186/cc4869
Appendix 1 The next generation matrix for estimating the basic reproduction number, R0
The next generation matrix method involves linearizing the original nonlinear ordinary differential equations at disease-free equilibrium.Citation38 At the disease-free equilibrium, X*, the numbers of individuals in each compartment are given by:
Let F be the transmission matrix, describing the production of new infections; and V be the transition matrix, describing changes in state (including removal by discharge or death). F and V are defined as follows:where Fi(X) is the number of new infections in the ith compartment from Xj infectious individuals; and Vi(X) is the net change of individuals in the ith compartment by any other means. The rates are evaluated at the disease-free equilibrium, X*. The next generation matrix, K, is then given by K = −FV−1. The (i,j) element of the K matrix represents the number of secondary infected cases in compartment i produced by individuals in compartment j. The basic reproduction number, R0, is given by the spectral radius of the K matrix.
For our model, the transmission and transition matrices in the case with only cross-transmission between patients (FP and VP, respectively) are given by:
The next generation matrix with only cross-transmission is then given by .
The transmission and transition matrices in the case with only environment-patient transmission (FE and VE, respectively) are given by:
The next generation matrix with only environment-patient transmission, KE, is then given by
The next generation matrix of the model is then given by ; and R0 is given by the spectral radius of the K matrix. The high-dimension of the K matrix makes analytical solution for R0 intractable. R0 therefore was derived numerically in our model. Detailed discussion of the next generation matrix method for compartmental epidemic models can be found in Diekmann et al.Citation38