Abstract
P-selectin glycoprotein ligand-1 (PSGL-1) has been proved to serve as the functional receptor for enterovirus 71 (EV71). We found the abundant expression of PSGL-1 on monocyte-derived dendritic cells (MDDCs). However, we have previously demonstrated that MDDCs did not support efficient replication of EV71. Dendritic cells (DCs) have been described to be subverted by various viruses including EV71 for viral dissemination, we thus explore the potential contribution of PSGL-1 on DC-mediated EV71 transmission. We found that the cell-surface-expressing PSGL-1 on MDDCs mediated EV71 binding, and intriguingly, these loaded-viruses on MDDCs could be transferred to encountered target cells; Prior-treatment with PSGL-1 antibodies or interference with PSGL-1 expression diminished MDDC-mediated EV71 transfer and rescued virus-induced cell death. Our data uncover a novel role of PSGL-1 in DC-mediated EV71 spread, and provide an insight into blocking primary EV71 infection.
Introduction
Enterovirus 71 (EV71) belongs to the family Picornaviridae, and is the main causative agent of hand, foot and mouth disease in young children.Citation1-3 There are no antiviral agents or vaccines available currently.Citation4,5 EV71 invasion usually occurs via the oral pharyngeal or intestinal mucosa, and the initial viral expansion mainly takes place in the lymphoid tissues and regional lymph nodes.Citation6-8 However, the specific molecular process through which EV71 infects human cells is poorly understood, particularly the specific cell type(s) that EV71 preferentially infects and how the virus is transported to lymphoid tissues during the initial viral expansion.
Dendritic cells (DCs) resident in the sub-mucosa play crucial roles in regulating the innate and adaptive immune responses against invading pathogens.Citation9,10 However, the migratory property of DCs can be subverted by various enveloped viruses, leading to viral dissemination and subsequent infections.Citation11-13 We recently reported that monocyte-derived dendritic cells (MDDCs) bound to non-enveloped EV71 through the cell surface C-type lectin DC-specific ICAM-3-grabbing non-integrin (DC-SIGN), leading to viral spread and facilitating EV71 infection.Citation14
Several host receptors or attachment factors have been recently reported to mediate EV71 binding and infection. Enteroviruses may retain strain-specific usage of diverse receptors for infection.Citation15 P-selectin glycoprotein ligand-1 (PSGL-1) and scavenger receptor class B, membrane 2 (SCARB2) serve as EV71 functional receptors. Citation16,17 SCARB2 is widely expressed in various cell types, including neurons in the central nervous system. SCARB2 can be used by most EV71 strains as an entry receptor.Citation16 However, PSGL-1 is limitedly expressed on leukocytes and myeloid cells, and can be adopted by certain EV71 strains for binding and infection.Citation17,18 Based on residue 145 within the capsid protein VP1, there are 2 kinds of EV71 strains, PSGL-1-binding viruses had VP1-145G or Q, whereas PSGL-1-nonbinding viruses had VP1-145E.Citation19 EV71 binds to the N-terminus of PSGL-1,Citation20 and the binding can be blocked by PSGL-1 specific monoclonal antibody KPL1.Citation17,21 Additionally, DC-SIGN is another molecule that has been found to mediate partially EV71 entry into DCs.Citation22 The sialic-acid-linked glycans and annexin II proteins have also been reported to bind capsid protein for enhancing EV71 infection.Citation23,24
Here we show that the surface-expressing PSGL-1 on MDDCs mediates EV71 transmission. Prior-treatment with PSGL-1 antibody KPL1 or interference with PSGL-1 expression diminished MDDC-mediated EV71 transfer, and rescued virus-induced cell death. Our results uncover the crucial role of PSGL-1 in DC-mediated EV71 spread and provide a novel insight into blocking primary EV71 infection.
Results
Surface expressing PSGL-1 on MDDCs binds EV71
PSGL-1 is mainly expressed on leukocytes and myeloid cells,Citation18 and has been identified as one of the EV71 functional receptors.Citation17 We therefore first confirmed the expression of PSGL-1 on the surface of MDDCs by flow cytometry after immunostaining with PSGL-1 antibodies KPL1, followed by FITC-conjugated secondary antibodies. MDDCs showed the expression of PSGL-1 (), and were able to capture EV71 particles on the cell surface (). This concurred with our previous results that the entry of EV71 was partially mediated by viral binding through DC-SIGN.Citation14 Since PSGL-1 is a functional receptor for EV71,Citation17 MDDCs might also capture EV71 through viral binding to PSGL-1. EV71 staining (using anti-VP0 polyclonal antibodies) in MDDCs was seen to overlap with that of PSGL-1, as observed under confocal microscopy (). The genome of EV71 is a single-stranded, positive sense RNA, which encodes a single large polyprotein that can be divided into structural P1 and non-structural P2 and P3 regions,Citation25 and the structural P1 region can be processed by viral proteinase to yield the subunit proteins VP0, VP1 and VP3, all of which co-assemble to form empty capsids, and the further cleavage of VP0 into VP2 and VP4 by autocatalytic mechanism yields the infectious mature virions.Citation26 The subunit protein-specific polyclonal antibodies against VP0, VP1 and VP3 have been generated and have been used for recognizing EV71 subunits.Citation14,26
Figure 1 (See previous page). PSGL-1 mediates EV71 binding on MDDCs. (A) PSGL-1 expression on MDDCs was detected by flow cytometry. Anti-PSGL-1 monoclonal antibodies of KPL1 were used for immunostaining and followed by staining with secondary antibodies. The percentage of cells positive for immunostaining was noted. (B) The overlay of EV71 with PSGL-1. MDDCs were incubated with EV71 viruses (MOI=1) for 1 h at 4°C, after washing, cells were fixed for immunostaining with KPL1 or anti-VP0 polyclonal antibodies, followed by staining with secondary antibodies. Cells were observed under confocal microscopy. Nuclei were stained with DAPI (blue). DIC, differential interference contrast. (C) KPL1 blocks EV71 binding. MDDCs were prior-treated with 10 μg/ml of KPL1 for 30 min before addition of EV71 (MOI=1) for additional 1 h incubation at 4°C. Anti-VP0 polyclonal antibodies were used for immunostaining as above and viral binding were detected by flow cytometry. The positive percents for immunostaining and MFI (mean fluorescence intensity) were noted, and 10,000 counts were calculated for each sample in flow cytometry assay. (D) The knocking-down of PSGL-1 in MDDCs by specific siRNAs. (E) The knocking-down of PSGL-1 blocks EV71 binding, as detected with quantified (RT)-PCR of EV71 mRNA. β-Actin was used for normalization of the expression of EV71 mRNA. (F) The concentration gradient of KPL1 in blocking EV71 binding. (G) The prior-blocking with antibody reduces EV71 binding on MDDCs, as quantified EV71 mRNA as above. The normalized percentage for EV71 binding was noted. (H) Generation of MDDCs. MDDCs were differentiated from CD14+ monocytes in presence with cytokines, and MDDC phenotype was monitored by flow cytometry with specific antibodies. (D, E) The same donor was used and the experiments were done simultaneously. (A, C, D, E, F and G) One representative from at least 3 donors was shown. Data are mean ± SD. **P <0.01 and ***P<0.001, were considered as significant difference in paired t-test.
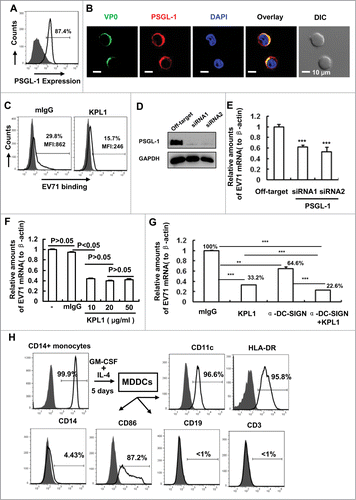
To determine the role of PSGL-1 in EV71 binding to MDDCs, MDDCs were pretreated with anti-PSGL-1 antibodies or control mouse IgG (mIgG). Prior-treatment with KPL1 reduced EV71 binding to the MDDCs from 29.8% to 15.7% (), in keeping with a previous report that KPL1 inhibited EV71 replication in Jurkat T cells. Citation17,20
To confirm the role of PSGL-1 in mediating the binding of EV71 to MDDCs, the expression of PSGL-1 in MDDCs was knocked down by 2 separate small interfering RNAs (siRNA) (). The PSGL-1 knocking-down significantly decreased, but did not completely abolish, the binding of EV71 to MDDCs, as quantified EV71 genome mRNA ().
We previously showed that DC-SIGN mediated EV71 binding.Citation14 To compare the relative contributions of DC-SIGN and PSGL-1 to EV71 binding, the concentration gradient of KPL1 was applied in EV71 binding assay. We found that 10-20 μg/ml of KPL1 could reach maximum inhibition (). We also found that 15-20 μg/ml of anti-DC-SIGN can reach maximum inhibition (data not shown). MDDCs were pretreated with 20 μg/ml of either KPL1 or anti-DC-SIGN antibodies followed with viral binding, and the differences were analyzed statistically using the paired t-test. PSGL-1 blocking with KPL1 decreased EV71 binding to MDDCs to a greater extent than that with anti-DC-SIGN (33.2% versus 64.6% remaining). Moreover, PSGL-1 and DC-SIGN had a synergistic effect on EV71 binding (). However, we could not exclude other factor(s) that might also mediate EV71 binding, because the pretreatment with a combination of KPL1 and anti-DC-SIGN antibodies did not fully abolish EV71 binding (22.6% remaining).
The MDDCs were generated by the stimulation of purified CD14+ monocytes with granulocyte-macrophage colony-stimulating factor and interleukin-4 for 5 days, as described previously.Citation27 MDDCs showed the phenotype with CD11c+ HLA-DR+ CD86+ CD14low (). Taken together, these data show that PSGL-1 expressed on MDDCs can bind EV71 particles.
PSGL-1 mediates EV71 transmission
We have previously reported that surface-bound EV71 could be transferred to susceptible human rhabdomyosarcoma (RD) cells for robust viral replication, and that the cell surface DC-SIGN partially accounted for this type of viral transmission.Citation14 Using the same experimental system, we found that pretreatment with KPL1 antibodies significantly impaired MDDC-mediated viral transfer to co-cultured RD cells () or Jurkat T cells (). Similarly, the knocking-down of PSGL-1 obviously reduced viral transmission, as measured by the levels of EV71 viral RNA ().
Figure 2. MDDC-mediated EV71 transmission can be diminished with KPL1 antibodies or PSGL-1 specific siRNAs. (A, B) Prior-treatment with KPL1 before EV71 incubation blocks MDDC-mediated viral transmission to RD cells (A) or Jurkat T cells (B). MDDCs were pretreated with KPL1 antibodies (10 μg/ml) or mouse IgG isotype control for 30 min, then were incubated with different amounts of EV71 for 1 h at 37°C, and after washing, EV71-loaded MDDCs were cocultured or not (DC alone) with RD cells or Jurkat cells for 24 h. Viral replication was determined by quantifying EV71 mRNA. (C) PSGL-1 specific siRNAs impairs MDDC-mediated EV71 transmission to RD cells. (D) The separation of MDDCs with Jurkat cells abolishes EV71 transmission. (E) The formation of virological synapses between virus-loaded MDDCs with Jurkat cells. White arrows indicate the representative synapses between MDDCs (CD11c-labeled) and Jurkat cells. One representative from at least 3 donors or repeats was shown (A, B, C, D and E). Data are mean ± SD. *P < 0.05 and ***P < 0.001, were considered as significant difference in paired t-test.
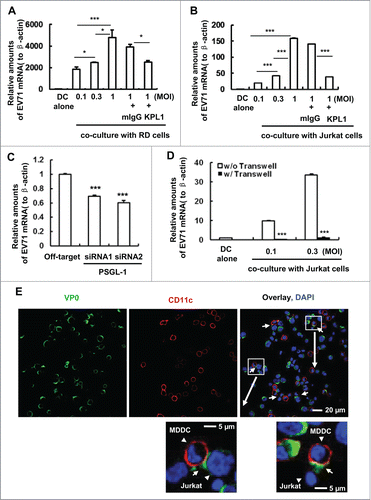
To determine whether direct cell-cell contact between MDDCs and co-cultured target cells is required for the efficient transfer of EV71, we separated virus-loaded MDDCs from Jurkat T cells using a transwell plate (insert membrane size of 0.4 μm) and found that the separation abolished EV71 transmission (). To support this, we used confocal microscopy to observe the formation of infectious synapses between MDDCs and Jurkat T cells, and the localization of viral particles at the synapses (). These data imply that DC-T cell cross-talk can favor viral spread, and the PSGL-1 can mediate EV71 transmission.
Prior-treatment of MDDCs with KPL1 rescues the death of RD cells induced by transferred viruses
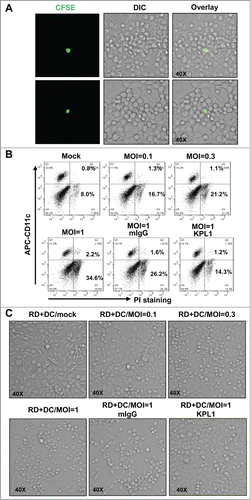
EV71 infection can induce the apoptosis of multiple types of host cells.Citation28-31 Therefore, we investigated whether the transferred EV71 could lead to the same effect. EV71-inoculated MDDCs were labeled with CFSE and then co-cultured with RD cells at a ratio of 1:103. We observed the apparent cytopathic effect of RD cells around the CFSE-labeled MDDCs, which showed the productive infection of RD cells induced by direct viral transmission of MDDCs (). We also found that EV71 preferentially induced the death of RD cells (CD11− population), but not MDDCs (CD11+ population), during the 24-h incubation (). Pretreatment with KPL1 to block the viral capture and subsequent viral transfer by MDDCs protected the RD cells from death, as the PI+ RD cell population decreased from 26.2% to 14.3% (). The death of RD cells induced by the replication of transferred EV71 was also identified by light microscopy. Similarly, the pretreatment of MDDCs with KPL1 inhibited viral transfer, resulting in the decreased death of RD cells (). Taken together, these data demonstrate that prior-treatment with PSGL-1 antibodies KPL1 blocks viral capture by MDDCs and thus prevents the death of target cells induced by transferred viruses.
Figure 3. Prior-treatment of MDDCs with KPL1 rescues the RD cells from death induced by transferred viruses. (A) RD cells around virus-loaded MDDCs (CFSE-labeled) show cytopathic effect. Two representative fields were shown. (B) Cell death detection by flow cytometry. PI-exclusion staining was used to examine cell death, and MDDCs (CD11c+) and RD cells (CD11c−) were distinguished by CD11c immunostaining. (C) Cell death observed under light microscopy. (A, B, C) One representative from at least 3 repeats was shown, and 10,000 counts were calculated for each samples in flow cytometry assay.
Discussion
DCs play pivotal roles in host defense by initiating innate immunity and bridging adaptive immunity.Citation32-34 DCs are widely distributed in the sub-mucosa, yet have been believed to be hijacked by several enveloped viruses such as Measles virus, HCV and HIV, etc, for viral binding and subsequent transfer to target cells for robust infection.Citation11-14 Our recent study has demonstrated that the surface-bound EV71 on MDDCs could be transferred to target cells, and the cell surface molecule DC-SIGN partially accounted for viral transmission.Citation14 Here we prove the role of DCs in EV71 dissemination and expansively demonstrate the contribution of PSGL-1 in viral capture and spread.
The direct contact between EV71-loaded MDDCs and target cells appears essential for viral transfer, as the separation abolished EV71 transmission. The infectious synapses, at which numerous intact viral particles can be recruited, provide an efficient pathway for viral spread.Citation27,35 Whether the infectious synapses are critical for EV71 transmission deserves further investigation.
We have proved that the uptake of EV71 by MDDCs did not lead to an efficient viral replication, instead, these loaded-EV71 particles in MDDCs undergo a rapid degradation.Citation14 A recent report showed that the binding and endocytosis of EV71 viral like particles (VLPs) to MDDCs induced cell maturation, and EV71/VLP-stimulated MDDCs increased cytokines production and enhanced the capacity for driving naïve T cell differentiation.Citation36 EV71/VLP treatment might facilitate DC-mediated presentation of VLPs antigen to T cells for generating a memory immune for protection in EV71/VLP immunized animals.Citation37,38
Our results demonstrate a novel role of PSGL-1 in the MDDC-mediated capture and spread of EV71, and emphasize the crucial role of DCs in the early steps of EV71 infection. The administration of anti-α4β7 monoclonal antibodies in rhesus macaques prior to and during acute infection of SIVmac251 was recently reported to successfully block viral mucosal transmission and maintain CD4+ T cell numbers in both lood and gut-associated lymphoid tissue.Citation39 In this study, we show that PSGL-1 antibody KPL1 blocks EV71 capture by MDDCs and prevents the virus-induced death of target cells, which might offer a potential means of combating viral infection by PSGL-1-adaptive EV71 strains.
Methods and Materials
Cells
Human peripheral blood mononuclear cells (PBMCs) were purchased from the Blood Center of Shanghai, China. CD14+ monocytes were purified from PBMCs using anti-CD14 specific antibody-coated microbeads (Miltenyi Biotec). Monocyte-derived DCs (MDDCs) were generated by stimulation of CD14+ monocytes with 50 ng/ml granulocyte–macrophage colony-stimulating factor (GM-CSF) (R&D Systems) and interleukin (IL)-4 (R&D Systems) for 5 days, and MDDCs show the phenotype of CD11c+HLA-DR+CD86+CD14lowCD3−CD19−. RD cells (rhabdomyosarcoma) (ATCC# CCL−136) were maintained in Dulbecco's Modified Eagle's Medium (Hyclone) containing 10% fetal bovine serum (FBS; Hyclone) with 100 U/ml penicillin and 100 μg/ml streptomycin at 37°C under 5% CO2. Jurkat T (ATCC# TIB-152) cells were grown in RPMI 1640 medium supplemented with 10% FBS (Hyclone), 100 U/ml penicillin and 100 μg/ml of streptomycin at 37°C under 5% CO2.
Viral stock
EV71 (G08-2 strain) was propagated in RD cells as previously described. Citation26,40 RD cells with 80% confluence were inoculated with EV71 at 37°C for 1 h with occasional shaking, and were incubated after washing in DMEM supplemented with 2% FBS until a 90% cytopathic effect was reached. Cell pellets were harvested and subjected to 3 freeze–thaw cycles to release the intracellular virus particles. Viral aliquots were stored at −80°C, and virus titers were determined by microtitration using RD cells, which were expressed as the 50% tissue culture infectious dose (TCID50), according to the Reed–Muench method.
Flow cytometry
The following antibodies or isotype-matched IgG were used (clone numbers and resources are given in parentheses): phycoerythrin (PE)-conjugated CD3 (UCHT1; eBioscience); APC-Alexa Fluor750-CD11c (B-ly6; BD Biosciences PharMingen); PE-CD14 (61D3; eBioscience); PE-CD19 (H1B19; eBioscience); PE-CD86 (IT2.2; eBioscience) and APC-cy7-HLA-DR (LN3; eBioscience) and purified anti-PSGL-1 monoclonal antibody (KPL-1; BD Pharmingen), followed by staining with PE-conjugated anti-mouse antibody. Stained cells were detected with an LSRII flow cytometer (BD Biosciences PharMingen) and analyzed with FlowJo 7.6.1 software (TreeStar Inc.).
Viral binding
For the binding assay, MDDCs (2×105/sample) were incubated with EV71 (4×105 TCID50) for 1 h at 4°C. The bound viruses were then stained with VP0 polyclonal antibody,Citation26 followed by Alexa Fluor 568-goat anti-rabbit IgG (Invitrogen). Stained cells were detected with an LSRII flow cytometer and analyzed with FlowJo 7.6.1 software.
Real-time PCR
Total RNAs from differently treated MDDCs, RD or Jurkat T cells were extracted by TRIzol Reagent (Invitrogen), and were reverse transcribed into cDNA by performing the ReverseAid™ First strand cDNA synthesis Kit (Fermentas) according to the manufacturer's instructions. Two microliters of cDNA reaction was amplified using forward and reverse primers for either EV71 genome or β-actin. Primers were listed as below: EV71-F, 5′-CCCTGAATGCGGCTAATCC-3′, and EV71-R, 5′-ATTGTCACCATAAGCAGCC A-3′; β-Actin-F, 5′-GGGAAATCGTGCG TGACAT-3′, and β-Actin-R, 5′-GTCAGGC AGCTCGTAGCTCTT-3′. Real- time PCR was carried out by using the Thunderbird SYBR qPCR Mix (TOYOBO) with the thermal cycling conditions: initial denaturation at 95°C for 10 min, 40 cycles of denaturation at 95°C for 15 s, primer annealing at 60°C for 15 s, and extension at 72°C for 30 s, followed by final extension at 72°C for 6 min. The level of EV71 RNA was expressed as fold change relative to β-actin.
EV71 transmission
MDDCs (3×105) were inoculated with different amount of infectious EV71 for 1 h at 37°C. Cells were washed to remove cell-free viruses and cocultured with RD or Jurkat T cells (8×105) for an additional 24 h (for RD) or 48 h (for Jurkat). In some samples, a Transwell plate with an insert membrane size of 0.4 μm was used to separate MDDCs (upper chamber) from Jurkat T cells (lower chamber). Viral transmission was quantified by detecting EV71 mRNA level relative to β-actin. In some samples, the EV71-inoculated MDDCs were pre-labeled with 2 μM of CFSE (Carboxyfluorescein succinimidyl ester) (Invitrogen), and then co-cultured with RD cells (at a 1:103 ratio of MDDCs:RD cells) for 24 h. Cell morphology was observed under a light microscope (Leica), and cell killing was measured by using propidium iodide (PtdIns) (Invitrogen) exclusion staining via flow cytometry. MDDCs and RD cells were distinguished by immunostaining with APC-Alexa Fluor750-CD11c (B-ly6; BD Biosciences PharMingen).
Confocal microscopy
MDDCs were incubated with EV71 as above. Cells were washed and seeded on the poly-L-lysine-coated microscope slides (Polyscience). In some experiments, virus-loaded MDDCs were co-cultured with Jurkat T cells for 30 min at 37°C before seeding on the slides. Cells were then fixed with 4% paraformaldehyde (Sigma-Aldrich) or acetone for 1h at 4°C. Slides of cells were immunostained with VP0 polyclonal antibody and PSGL-1 antibody KPL1 or CD11c antibody (B-ly6; BD Biosciences PharMingen). The secondary antibodies of Alexa 546- or 488-labeled goat anti-mouse IgG or goat anti-rabbit IgG (2 μg/ml; Invitrogen) were used. Nuclei were indicated with DAPI. Slides were mounted with Fluorescent Mounting Medium (Dako) and observed using a laser scanning confocal microscope (Leica SP5).
siRNA interference
MDDCs were transfected with specific siRNA duplex of PSGL-1 or off-target control (GenePharma, China). Amaxa human dendritic cell nucleofector kit (Amaxa) was used for transfection according to the manufacturer's instructions. Cells were harvested to confirm the knockdown of target gene with Western-blotting. Viral binding and transmission were investigated as described above. The sequences of siRNA duplex were listed as below: Negative control (Off-target) siRNA: 5′-UUCUCCGAACGUGUCACGUTT-3′ (sense), 5′-ACGUGACACGUUCGGAGA
AdTdT-3′ (antisense); PSGL-1 siRNA-1: 5′-GCAGCUAUGGAGAUACAGATT-3′ (sense), 5′-UCUGUAUCUCCAUAGC -UGCTT-3′ (antisense); PSGL-1 siRNA-2: 5′-CCAGCAAUUUGUCCGUCAATT-3′ (sense), 5′-UUGACGGACAAAUUGCUGGT T-3′ (antisense).
Statistical analysis
Statistical analysis was performed using a paired t test with SigmaStat 2.0 (Systat Software, San Jose, CA, USA).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
H.-B. W thanks the grant supports from Youth Innovation Promotion Association, the Chinese Academy of Sciences, and Natural Science Foundation of Shanghai (No.13ZR1445400). This work was also supported by grant received by J.-H. W from the Interdisciplinary and Collaboration Team of the Chinese Academy of Sciences.
References
- Puenpa J, Mauleekoonphairoj J, Linsuwanon P, Suwannakarn K, Chieochansin T, Korkong S, Theamboonlers A, Poovorawan Y. Prevalence and characterization of enterovirus infections among pediatric patients with hand foot mouth disease, herpangina and influenza like illness in Thailand, 2012. PLoS One 2014; 9:e98888; PMID:24887237; http://dx.doi.org/10.1371/journal.pone.0098888
- Gao LD, Hu SX, Zhang H, Luo KW, Liu YZ, Xu QH, Huang W, Deng ZH, Zhou SF, Liu FQ, et al. Correlation analysis of EV71 detection and case severity in hand, foot, and mouth disease in the Hunan Province of China. PLoS One 2014; 9:e100003; PMID:24941257; http://dx.doi.org/10.1371/journal.pone.0100003
- Chen L, Mou X, Zhang Q, Li Y, Lin J, Liu F, Yuan L, Tang Y, Xiang C. Detection of human enterovirus 71 and coxsackievirus A16 in children with hand, foot and mouth disease in China. Mol Med Rep 2012; 5:1001-4; PMID:22218731
- Chong P, Liu CC, Chow YH, Chou AH, Klein M. Review of enterovirus 71 vaccines. Clin Infect Dis 2015; 5:797-803; PMID:25352588
- Pourianfar HR, Grollo L. Development of antiviral agents toward enterovirus 71 infection. J Microbiol Immunol Infect 2015; 1:1-8; PMID:24560700
- Chang LY. Enterovirus 71 in Taiwan. Pediatr Neonatol 2008; 49:103-12; PMID:19054914; http://dx.doi.org/10.1016/S1875-9572(08)60023-6
- Ooi MH, Wong SC, Lewthwaite P, Cardosa MJ, Solomon T. Clinical features, diagnosis, and management of enterovirus 71. Lancet Neurol 2010; 9:1097-105; PMID:20965438; http://dx.doi.org/10.1016/S1474-4422(10)70209-X
- Solomon T, Lewthwaite P, Perera D, Cardosa MJ, McMinn P, Ooi MH. Virology, epidemiology, pathogenesis, and control of enterovirus 71. Lancet Infect Dis 2010; 10:778-90; PMID:20961813; http://dx.doi.org/10.1016/S1473-3099(10)70194-8
- Barroca P, Calado M, Azevedo-Pereira JM. HIV/Dendritic Cell Interaction: Consequences in the Pathogenesis of HIV Infection. AIDS Rev 2014; 16:223-35; PMID:25350531
- Bieber K, Autenrieth SE. Insights how monocytes and dendritic cells contribute and regulate immune defense against microbial pathogens. Immunobiology 2015; 2:215-216; PMID:25468558
- Koethe S, Avota E, Schneider-Schaulies S. Measles virus transmission from dendritic cells to T cells: formation of synapse-like interfaces concentrating viral and cellular components. J Virol 2012; 86:9773-81; PMID:22761368; http://dx.doi.org/10.1128/JVI.00458-12
- Cormier EG, Durso RJ, Tsamis F, Boussemart L, Manix C, Olson WC, Gardner JP, Dragic T. L-SIGN (CD209L) and DC-SIGN (CD209) mediate transinfection of liver cells by hepatitis C virus. Proc Natl Acad Sci U S A 2004; 101:14067-72; PMID:15371595; http://dx.doi.org/10.1073/pnas.0405695101
- Wu L, KewalRamani VN. Dendritic-cell interactions with HIV: infection and viral dissemination. Nat Rev Immunol 2006; 6:859-68; PMID:17063186; http://dx.doi.org/10.1038/nri1960
- Ren XX, Ma L, Liu QW, Li C, Huang Z, Wu L, Xiong SD, Wang JH, Wang HB. The molecule of DC-SIGN captures enterovirus 71 and confers dendritic cell-mediated viral trans-infection. Virol J 2014; 11:47; PMID: 24620896; http://dx.doi.org/10.1186/1743-422X-11-47
- Tuthill TJ, Groppelli E, Hogle JM, Rowlands DJ. Picornaviruses. Curr Top Microbiol Immunol 2010; 343:43-89; PMID:20397067
- Yamayoshi S, Yamashita Y, Li J, Hanagata N, Minowa T, Takemura T, Koike S. Scavenger receptor B2 is a cellular receptor for enterovirus 71. Nat Med 2009; 15:798-801; PMID:19543282; http://dx.doi.org/10.1038/nm.1992
- Nishimura Y, Shimojima M, Tano Y, Miyamura T, Wakita T, Shimizu H. Human P-selectin glycoprotein ligand-1 is a functional receptor for enterovirus 71. Nat Med 2009; 15:794-7; PMID:19543284; http://dx.doi.org/10.1038/nm.1961
- Laszik Z, Jansen PJ, Cummings RD, Tedder TF, McEver RP, Moore KL. P-selectin glycoprotein ligand-1 is broadly expressed in cells of myeloid, lymphoid, and dendritic lineage and in some nonhematopoietic cells. Blood 1996; 88:3010-21; PMID:8874199
- Nishimura Y, Lee H, Hafenstein S, Kataoka C, Wakita T, Bergelson JM, Shimizu H. Enterovirus 71 binding to PSGL-1 on leukocytes: VP1-145 acts as a molecular switch to control receptor interaction. PLoS pathogens 2013; 9:e1003511; PMID:23935488; http://dx.doi.org/10.1371/journal.ppat.1003511
- Nishimura Y, Wakita T, Shimizu H. Tyrosine sulfation of the amino terminus of PSGL-1 is critical for enterovirus 71 infection. PLoS pathogens 2010; 6:e1001174; PMID:21079683; http://dx.doi.org/10.1371/journal.ppat.1001174
- Snapp KR, Ding H, Atkins K, Warnke R, Luscinskas FW, Kansas GS. A novel P-selectin glycoprotein ligand-1 monoclonal antibody recognizes an epitope within the tyrosine sulfate motif of human PSGL-1 and blocks recognition of both P- and L-selectin. Blood 1998; 91:154-64; PMID:9414280
- Lin YW, Wang SW, Tung YY, Chen SH. Enterovirus 71 infection of human dendritic cells. Exp Biol Med (Maywood) 2009; 234:1166-73; PMID:19596831; http://dx.doi.org/10.3181/0903-RM-116
- Yang B, Chuang H, Yang KD. Sialylated glycans as receptor and inhibitor of enterovirus 71 infection to DLD-1 intestinal cells. Virology journal 2009; 6:141; PMID:19751532; http://dx.doi.org/10.1186/1743-422X-6-141
- Yang SL, Chou YT, Wu CN, Ho MS. Annexin II binds to capsid protein VP1 of enterovirus 71 and enhances viral infectivity. J Virol 2011; 85:11809-20; PMID:21900167; http://dx.doi.org/10.1128/JVI.00297-11
- McMinn PC. An overview of the evolution of enterovirus 71 and its clinical and public health significance. FEMS Microbiol Rev 2002; 26:91-107; PMID:12007645; http://dx.doi.org/10.1111/j.1574-6976.2002.tb00601.x
- Liu Q, Huang X, Ku Z, Wang T, Liu F, Cai Y, Li D, Leng Q, Huang Z. Characterization of enterovirus 71 capsids using subunit protein-specific polyclonal antibodies. J Virol Methods 2013; 187:127-31; PMID:23046991; http://dx.doi.org/10.1016/j.jviromet.2012.09.024
- Wang JH, Janas AM, Olson WJ, Wu L. Functionally distinct transmission of human immunodeficiency virus type 1 mediated by immature and mature dendritic cells. J Virol 2007; 81:8933-43; PMID:17567699; http://dx.doi.org/10.1128/JVI.00878-07
- Chen LC, Shyu HW, Chen SH, Lei HY, Yu CK, Yeh TM. Enterovirus 71 infection induces Fas ligand expression and apoptosis of Jurkat cells. J Med Virol 2006; 78:780-6; PMID:16628611; http://dx.doi.org/10.1002/jmv.20623
- Sun ZM, Xiao Y, Ren LL, Lei XB, Wang JW. ; Enterovirus 71 induces apoptosis in a Bax dependent manner. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi 2011; 25:49-52; PMID:21789855
- Shi W, Li X, Hou X, Peng H, Jiang Q, Shi M, Ji Y, Liu X, Liu J. Differential apoptosis gene expressions of rhabdomyosarcoma cells in response to enterovirus 71 infection. BMC Infect Dis 2012; 12:327; PMID:23191987; http://dx.doi.org/10.1186/1471-2334-12-327
- Zhang H, Li F, Pan Z, Wu Z, Wang Y, Cui Y. Activation of PI3K/Akt pathway limits JNK-mediated apoptosis during EV71 infection. Virus Res 2014; 192:74-84; PMID:25116390; http://dx.doi.org/10.1016/j.virusres.2014.07.026
- Wilkinson J, Cunningham AL. Mucosal transmission of HIV-1: first stop dendritic cells. Curr Drug Targets 2006; 7:1563-9; PMID:17168831; http://dx.doi.org/10.2174/138945006779025482
- Hansasuta P, Rowland-Jones SL. HIV-1 transmission and acute HIV-1 infection. Br Med Bull 2001; 58:109-27; PMID:11714627; http://dx.doi.org/10.1093/bmb/58.1.109
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature 1998; 392:245-52; PMID:9521319; http://dx.doi.org/10.1038/32588
- McDonald D, Wu L, Bohks SM, KewalRamani VN, Unutmaz D, Hope TJ. Recruitment of HIV and its receptors to dendritic cell-T cell junctions. Science 2003; 300:1295-7; PMID:12730499; http://dx.doi.org/10.1126/science.1084238
- Lin YL, Hu YC, Liang CC, Lin SY, Liang YC, Yuan HP, Chiang BL. Enterovirus-71 Virus-Like Particles Induce the Activation and Maturation of Human Monocyte-Derived Dendritic Cells through TLR4 Signaling. PLoS One 2014; 9:e111496; PMID:25360749; http://dx.doi.org/10.1371/journal.pone.0111496
- Lin YL, Yu CI, Hu YC, Tsai TJ, Kuo YC, Chi WK, Lin AN, Chiang BL. Enterovirus type 71 neutralizing antibodies in the serum of macaque monkeys immunized with EV71 virus-like particles. Vaccine 2012; 30:1305-12; PMID:22214888; http://dx.doi.org/10.1016/j.vaccine.2011.12.081
- Chung YC, Ho MS, Wu JC, Chen WJ, Huang JH, Chou ST, Hu YC. Immunization with virus-like particles of enterovirus 71 elicits potent immune responses and protects mice against lethal challenge. Vaccine 2008; 26:1855-62; PMID:18329759; http://dx.doi.org/10.1016/j.vaccine.2008.01.058
- Byrareddy SN, Kallam B, Arthos J, Cicala C, Nawaz F, Hiatt J, Kersh EN, McNicholl JM, Hanson D, Reimann KA, et al. Targeting alpha4beta7 integrin reduces mucosal transmission of simian immunodeficiency virus and protects gut-associated lymphoid tissue from infection. Nat Med 2014; 20:1397-400; PMID:25419708; http://dx.doi.org/10.1038/nm.3715
- Liu Q, Ku Z, Cai Y, Sun B, Leng Q, Huang Z. Detection, characterization and quantitation of coxsackievirus A16 using polyclonal antibodies against recombinant capsid subunit proteins. J Virol Methods 2011; 173:115-20; PMID:21295614; http://dx.doi.org/10.1016/j.jviromet.2011.01.016
