abstract
Candida albicans is a polymorphic fungus which is the predominant cause of superficial and deep tissue fungal infections. This microorganism has developed efficient strategies to invade the host and evade host defense systems. However, the host immune system will be prepared for defense against the microbe by recognition of receptors, activation of signal transduction pathways and cooperation of immune cells. As a consequence, C. albicans could either be eliminated by immune cells rapidly or disseminate hematogenously, leading to life-threatening systemic infections. The interplay between Candida albicans and the host is complex, requiring recognition of the invaded pathogens, activation of intricate pathways and collaboration of various immune cells. In this review, we will focus on the effects of innate immunity that emphasize the first line protection of host defense against invaded C. albicans including the basis of receptor-mediated recognition and the mechanisms of cell-mediated immunity.
Introduction
Candida albicans is an opportunistic pathogen that can not only live as a common benign commensal fungus in immunocompetent individuals but also cause mucocutaneous and systemic infections in immune compromised patients. The determinant of friendly colonization or invasive dissemination is the balance of fungal proliferation and host defense. The imbalanced interplay between the host and C. albicans can lead to mucosal and life-threatening deep-seated fungal infection, especially in immunocompromised individuals caused by cancer chemotherapy, human immunodeficiency virus (HIV) infection, organ transplantation or indwelling medical devices.Citation1 However, due to the limited effectiveness of existing therapies and increasing resistance of the pathogen to antifungal agents, new therapies are urgently needed to prevent high morbidity and mortality in candidemia. To overcome the increasing emergence of antifungal resistance, it is necessary to gain insights into mechanisms by which Candida species invade the host and the host responds to the invasion.
The innate immune system is typically the first-line defense of host defense encountered by invading pathogens.Citation2 The sophisticated pathogen invasion is initiated by impairing the host physical barrier that consists of the skin and mucosa. The morphological transition of the yeast to hyphal form has been considered as a contributing factor to the invasion of C. albicans.Citation3 Breach of the mucosal barrier enables C. albicans to gain access to the underlying host tissues. To access distant tissues and organs, the organism must cross the host endothelium in order to reach the vasculature and disseminate in the blood.Citation4,5 Although C. albicans hyphae are recognized as a crucial virulence factor for both penetrating epithelium and piercing phagocytes, the yeast form of the fungus is believed to be required for dissemination during systemic infection.Citation6
More specifically, host combat against fungal disease requires a series of complex molecular mechanisms involving the recognition of evolutionarily conservative fungal cell wall components, the activation of host immune cell signaling cascades, and the release of cytokines and chemokines.Citation7 Being the core of the immune response, professional immune cells act as the most effective weapon for ingesting and killing the pathogens, though complement system and antimicrobial peptides are the other 2 components of innate immune defense mechanisms.Citation8 In this review, we will concentrate on the innate immune protection during the process of infection. Therefore, it's useful to depict the recognition of C. albicans by the host, as well as the interactions between C. albicans and versatile host cell types. Understanding these mechanisms will be a great benefit to fungal infectionprevention and new treatment development.
Receptor-mediated recognition of Candida albicans
The interaction between C. albicans and the host immune system is initially achieved by detection of fungal cell wall components, predominantly carbohydrate polymers and proteins. According to the current knowledge, the C. albicans cell wall is a rigid structure consisting of an outer layer of mannoproteins, an inner layer of β-1,3- and β-1,6-glucans, as well as the innermost layer of chitin.Citation9,10 Pattern recognition receptors (PRRs) are responsible for recognizing these conserved microbial chemical signatures, also called pathogen-associated molecular patterns (PAMPs), which activate intracellular signaling pathways, elicit innate immune responses and help develop adaptive immunity. Groups of PRRs demonstrated to participate in sensing different pathogens include TLRs (Toll-like receptors),Citation11 CLRs (C-type lectin receptors),Citation12 NLRs (nucleotide-binding domain leucine-rich repeat-containing receptors),Citation13 and RLRs (retinoic acid-inducible gene-I (RIG-I) receptors),Citation14 among which TLRs and CLRs are known to play a central role in the antifungal immune response (). The NLRs and RLRs have not been proved to directly participate in fungal recognition.Citation13
Figure 1. Recognition of C. albicans by pattern recognition receptors (PRRs). CLRs and TLRs are major membrane receptors for recognizing C. albicans. (A) Fungal recognition by dectin-1 can activate Syk, NFAT, ERK and NF-κB or trigger reactions through Raf-1 independent of Syk. The activation of dectin-1 results in the production of several inflammatory factors, including IL-2, IL-6, IL-10, TNF-α and COX2. (B) Dectin-1 collaborates with TLR2 in producing ROS and activating NLRP3 inflammasome that facilitates IL-1β maturation. (C) Dectin-2 and dectin-3 form a heterodimer to detect microbial infections and initiate Syk-mediated activation of NF-κB. FcRγ is recruited to help dectin-2 and mincle to activate intracellular signaling cascades. Dectin-3 is also demonstrated to associate with FcRγ. Both TLR2 and TLR4 are vital receptors for pro-inflammatory reactions mediated by MyD88 and Mal. (D) TLR2 binding enhances the amount of IL-10 and TGFβ (Tumor growth factor β) through the ERK-cFos pathway, which will cause immunesupression and the inhibition of pro-inflammatory signals. (E) TLR4 is efficient in mediating pro-inflammatory reactions, releasing IL-8, IL-12 and TNF. Besides individual effects, the combination of TLR1/TLR2 and TLR2/TLR6 will enhance the production of cytokines.
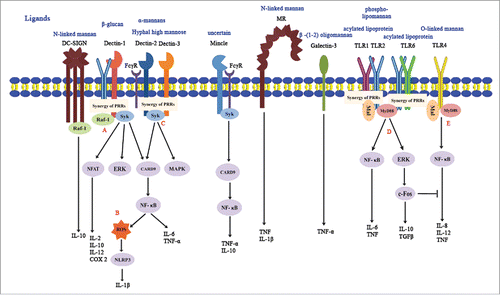
Accumulated evidence shows that TLR2 recognizes PLM (phospholipomannan) components,Citation15 while TLR4 prefers short linear structures O-bound mannan and α-linked mannose structures.Citation16 TLR9 is distinct from other TLRs because of its ability to detect intracellular DNA (CpG-oligodeoxynucleotides) of C. albicans.Citation17 More recently, studies have focused on the synergies of different PRRs. TLR2 is reported to heterodimerize with 2 other family members, TLR1 or TLR6 and to recognize acylated lipoprotein as dimers.Citation18 The recognition by TLRs is followed by the activation of different pathways, which contributes to the production of cytokines and chemokines. Both the TLR2 and TLR4 bindings contribute to the induction of pro-inflammatory signals in immune cells via MyD88 (myeloid differentiation factor 88), Mal-mediated pathways, and further NF-κB (nuclear factor κB) pathway.Citation19 The CLRs are another major family of PRRs implicated in C. albicans recognition. The family includes dectin-1, dectin-2, dectin-3, Mincle (macrophage-inducible C-type lectin), MR (mannose receptor), and DC-SIGN (dendritic cell specific intercellular adhesion molecule-3-grabbing non-integrin). Dectin-1 is an important CLR that can recognize β-glucans in the fungal cell wall and initiate a series of cellular responses via the Syk/CARD9 and Raf-1, Syk-independent signaling pathways.Citation20,21 Owing to substantial differences in the composition and nature of fungal cell walls, dectin-1 has recently been surprisingly demonstrated to be strain-specific during infection in vivo.Citation22 Dectin-2 can recognize α-mannan and high-mannose structures in hyphae.Citation23,24 However, both yeast and hyphal forms of C. albicans can be recognized by dectin-2.Citation25 Also, dectin-2 can form a heterodimeric PRR with dectin-3, a recently identified CLR, to sense fungal infection and show stronger binding to α-mannan, leading to potent inflammatory responses against fungal infections.Citation23 Dectin-2 signal transduction was demonstrated to interact with FcγR (Fcγ receptor) chain and activate Syk-CARD9-NF-κB signaling pathways to induce the production of cytokines such as TNF (tumor necrosis factor),Citation25,26 though the exact mechanism remains to be elucidated. Like dectin-2, mincle also recognizes α-mannose residues of C. albicans but it plays a significant role in the defense against yeast C. albicans.Citation27,28 Mincle is highly expressed in macrophages and contributes to innate inflammatory response by associating with FcγR chain and recruiting Syk.Citation29 Other CLRs, MR and DC-SIGN recognize N-linked mannan and specifically mediate C. albicans binding and internalization via phagocytes.Citation30-32 Although MR lacks signaling motifs, it performs endocytosis, mediates internalization of their ligands and also contributes to antigen presentation.Citation33 Galectin-3, an S-type lectin receptor, recognizes β -(1-2) oligomannan and efficiently helps defend against disseminated C. albicans in a mouse model.Citation19,34
NLRP1, a subset of NLRs, cytosolic receptors were able to assemble and oligomerize into a common structure which collectively activated the caspase-1 cascade, thereby leading to the production of pro-inflammatory cytokines especially IL-1β and IL-18.Citation35 This NLRP1 multi-molecular complex was referred to as the “inflammasome”.Citation36 NLRP subfamily members contain a central nucleotide-binding domain (NACHT), an N-terminal pyrin domain (PYD) and C-terminal leucine rich repeats (LRR) which function as ligand sensor.Citation13,36 Inflammasome formation is initiated through activation of a nod-like receptor protein (NLRP1, NLRP3, or NLRC4) and the recruitment of the adaptor molecule ASC (apoptosis-associated speck-like protein containing a CARD). This process is followed by the activation of caspase-1 and the cleavage of pro-IL-1β into active mature IL-1β.Citation37 NLRP3 and NLRC4 inflammasomes have been demonstrated to play an important role in defense against dissemination of mucosal infection and mortality in vivo.Citation38 NLRP10 has no effect on innate immune responses in disseminated C. albicans infection but influences the adaptive responses independent of the NLRP3 inflammasome and the production of IL-1β.Citation13
Epithelial cells
The mucosal epithelium is commonly recognized as the first line of host defense after the initial contact with invading pathogens. The interaction between the epithelia and the microorganism either causes commensalism or violation of the superficial barrier on mucosal surfaces. The infection process of C. albicans consists of adhesion, invasion and cell damage. During the initial adherence of C. albicans to human epithelial surfaces, a great number of specialized adhesins are needed to build the attachment to the host, such as Hwp1p,Citation39-42 Eap1p,Citation43,44 Iff4p,Citation45,46 Ssa1pCitation47,48 and Als proteins mainly involving Als1-7p and Als9p.Citation39,49-52 Adhesion can only enable C. albicans to develop their virulence. In order to establish the infection and dissemination, deep and fast penetration into the epithelium is necessary.Citation53 C. albicans has been demonstrated to gain entry into host epithelial cells through 2 mechanisms: induced endocytosis and active penetration.Citation54,55 Endocytosis is mediated by adhesion by forcing epithelial cell actin to aggregate around the invading microorganism to produce pseudopods, which act like nets to catch the pathogen. Research has shown that, during fungal invasion, both Als1 and Als3 induce endocytosis via binding to E-cadherin on oral epithelial cells.Citation51,52 Active penetration is another way for C. albicans to invade oral epithelial cells. However, invasion into stomach and intestine has only been observed via active penetration. Collectively, invasion by C. albicans may depend on the epithelial cell type and the differentiation stage of epithelial cells, indicating the presence of different susceptibilities between various epithelial cells. Both adhesion and active penetration result in the final damage to epithelial cells. Recent studies have demonstrated that 3 C. albicans gene families, SAP (secreted aspartyl proteinase), PL (phospholipases) and LIP (lipases), play crucial roles in producing a number of extracellular hydrolytic enzymes contributing to the damage to host cells structures. SAPs have been most widely studied among all these hydrolases and the function of SAP5 has already been demonstrated to destroy epithelial cell junctions on oral epithelial cells.Citation56 Recently, SAP4-6 were discovered to bind integrins on epithelial cells via their RGD/KGD amino acid motifs.Citation57 In summation, C. albicans invades the host epithelial cells via the combined effect of contact-sensing, directed hyphal extension, active penetration and expression of pathogenicity factors to promote inter-epithelial invasion and dissemination, ultimately causing damage to host cells.
Given the contribution of Sap and Als proteins to fungal virulence, researchers have considered them as candidates for vaccine targets. As protein vaccines, Sap2p, Als1p and Als3p not only have defined amino acid sequences and structure, but also relatively safer compared with live attenuated fungal strains.Citation58,59 Sap2p, a common expressed Sap is proved to be effective against vulvovaginal candidiasis (VVC) in vivo and now has already gone through Phase I clinical trials against C. albicans infection.Citation60,61 Three Als vaccines, rAls1p-N (recombinant N-terminus of Als1p),Citation62 rAls3p-N (recombinant N-terminus of Als3p)Citation63,64 and NDV-3 (recombinant N-terminus of Als3p formulated with alum adjuvant)Citation65,66 have been explored to prevent candidal infections. Compared with rAls1p-N, rAls3p-N vaccine showed equal efficacy against disseminated candidiasis but was more superior in treating oropharyngeal or vaginal candidiasis.Citation64 NDV-3, as a vaccine candidate against Candida, has proven effective against VVC through both humoral and adaptive immune responses and has already been tested in a Phase I clinical trial.Citation66 Thus, fully understanding the interaction between the pathogen and host does provide new ideas for the discovery of new drug targets and the invention of more antifungal therapies.
The recognition of fungal pathogens by epithelial cells depends on the interaction between PAMPs and PRRs. Up to now, only certain TLRs and CLRs expressed on epithelial cells have been demonstrated to detect C. albicans. The exact distribution and composition of PRRs on epithelial cells remain unclear.Citation67 Different groups of TLRs such as TLR2, TLR4, TLR6, and TLR9 are functionally expressed on the oral epithelium, highlighting the essential status of TLRs in oral defense.Citation68,69 TLR2 and TLR4 have been found to be elevated inside the inflamed gingivae.Citation70 TLR4 is found to be involved in the vaginal PMN recruitment response initiated by S100A8 alarmin.Citation71 Also, human epithelial TLR4 has been shown to participate in defense against fungal infection in oral mucosa via a process mediated by (polymorphonuclear leukocytes) PMNs.Citation72 As for CLRs, dectin-1 is the only receptor that shows some effects in the recognition of human gingival epithelia.Citation69 Ligation of epithelial PRRs by invading pathogens is usually followed by the activation of cascade signaling, and the subsequent production of pro-inflammatory, growth factors and antimicrobial factors. It was found that oral and vaginal epithelial cells activate NF-κB and the biphasic MAPK (mitogen-activated protein kinase) pathway in response to the stimulation caused by C. albicans.Citation73,74 Once a sufficient fungal burden and hypha-associated surface moieties are detected, the MAPK/MKP1/c-Fos pathway is activated, triggering release of pro-inflammatory cytokines. A new study has demonstrated that PI3K-Akt-mTOR signaling plays a key role in epithelial immunity for the protection against cell damage without the help of MAPK or NF-κB signaling.Citation75 However, the pro-inflammatory response in various epithelial cells is quite different for the production of cytokines. C. albicans strongly induces the production of cytokines such as G-CSF (granulocyte colony-stimulating factor), GM-CSF (granulocyte-macrophage colony-stimulating factor), TNF-α(tumor necrosis factor-α), IL-6(interlukin-6), IL-8, IL-1α and IL-1β in oral epithelia.Citation76-79 Vaginal epithelial cells have a low level of IL-6 production and almost no G-CSF or CCL20 (Chemokine (C-C Motif) Ligand 20).Citation74 IL-1β is an important cytokine associated with the activation of NLRP3 and NLRC4 inflammasomes, both of which have been demonstrated to protect against C. albicans infection in the oral cavity.Citation37,38
Additionally, upon recognition of C. albicans, epithelial cells are regulated to secret antimicrobial peptides, such as defensins, cathelicidins, and histatins to clear the invading pathogen directly.Citation6 These host defense peptides have been recognized as important antimicrobial effectors in innate immune responses but may result in different respective activities if released into different local microbial environments.Citation80 It is well worth mentioning the protective effect of Th17 responses which mediate the production of IL-17 and IL-22. IL-17 and IL-22 both contribute to clear mucosal candidal infections by upregulating the expression the antimicrobial peptides by oral epithelial cells.Citation81,82 IL-17 has been shown to enhance the production of IL-8 and GM-CSF in oral epithelial infection and afterwards perform the recruitment of neutrophils.Citation76 As mentioned above, epithelium plays an important role in the inhibition of C. albicans dissemination by activating pathways to induce the production of cytokines that recruit immune cells.
Endothelial cells
In immune compromised hosts, C. albicans can disseminate hematogenously and migrate from circulation into the tissues to cause extensive organ damage and systemic candidiasis. Blood-borne C. albicans that fails to be eliminated by phagocytes and fungicidal factors must adhere to and penetrate into endothelial cells before they can disseminate in deep-seated organs. As the initiation of hematogenous infections, vascular endothelium acts as a barrier to prevent the pathogen dissemination. To get entry into the tissues successfully, C. albicans must conquer 2 main difficulties: adhering to endothelial cells and gaining access to endothelial layers. In the first step, C. albicans needs to find a way to adhere to the endothelium. There has been controversy over the necessity of morphogenetic change for the yeast form to adhere to the endothelial surface. Collectively, ample evidence indicates that morphogenetic transformation certainly occurs during the adhesion process, but no clear sign has proved it to be a prerequisite so far. Research has demonstrated that both yeast and the hyphal form of C. albicans can adhere under the condition of flowing pressure, which is similar to capillary blood pressure.Citation83 Although PRRs are predominantly involved in the recognition of correspondent cell wall structure during the host immune defense, they are also helpful in mediating the adhesion of C. albicans to endothelial cells of blood vessels. It has been demonstrated that TLR2 and TLR4 are expressed on endothelial cells.Citation84,85 MR, a mannan receptor, and galectins including galectin-1,-3 and -9 are also found on the endothelium. In addition, C. albicans uses different mediators to help the adhesion process. Recently, Gpm1 (Phosphoglyceratemutase 1), a candida surface protein, has been shown to mediate fungal adhesion to human endothelial cells by binding to vitronectin.Citation86 There are 2 other mediators (integrin αvβ3 and αvβ5-like adhesin) that use vitronectin as a surface ligand. Getting across the endothelial barrier is the second difficulty to overcome. C. albians transmigrates endothelial cell lines by inducing their own endocytosis with the help of the expressed invasins (Als3 and Ssa1) on their surface.Citation50 New research has found that N-cadherin expressed on the endothelium binds to C. albicans Als3 and Ssa1 in a complex that also contains an intracellular GTP-binding protein, septin-7.Citation87,88 This binding process induces endothelial cell microfilaments, thereby producing pseudopods that engulf the organism.
Polymorphonuclear neutrophils
PMNs (Polymorphonuclear neutrophils) are predominant phagocytes in host defense and are the first line of protection during engulfing the Candida pathogen in the innate cellular immune system.Citation89,90 TLR2, TLR4 and dectin-1 are all participating in the recognition of C. albicans and the highly expressed CR3 (complement receptor 3) and FcγR assist in the process of opsonization.Citation91 Investigations have clearly shown that several pro-inflammatory cytokines facilitate the migration of PMNs to the site of infection, such as IL-6,Citation92 IL-8Citation93 IL-17Citation94 and TNF-α.Citation95 Also, GM-CSF and G-CSF have been demonstrated to induce the recruitment of PMNs.Citation96 As effective phagocytes, PMNs internalize pathogens and clear them by producing ROS (Reactive oxygen species) and lysosomal enzymes.Citation97 New evidence shows that PMNs cannot only perform their role of phagocytosis but act as modulators during inflammation. PMNs can weaken pro-inflammatory response after C. albicans stimulation by releasing neutrophil-derived proteases responsible for degrading cytokines such as IL-1β and TNF-α.Citation98
As an indispensable defender for the host, neutrophils have both intra- and extracellular antifungal activities. The mechanism of killing the invading fungal pathogen by phagocytes is quite complex involving the production of reactive oxygen and nitrogen intermediates, the expression of antimicrobial peptides, the release of hydrolases, and nutrient limitation as well.Citation2 In addition to killing pathogens by phagocytosis, neutrophils can also catch invading pathogens in the extracellular space. In fact, C. albicans can induce neutrophils to release chromatin fibers, also known as NETs (neutrophil extracellular traps), which are capable of killing both yeast-form and hyphal cellsCitation99 by releasing calprotectin, an antifungal peptide.Citation90 Although the comprehension about the function of NETs is growing, factors that orchestrate the formation of NETs remain unclear. Research has confirmed that as an important component of the secretory machinery of azurophilic granule, the Rab family small GTPase, Rab27 in neutrophils plays an important role in NET formation triggered by C. albicans through up-regulating the ROS production mediated by NADPH oxidase.Citation100
Monocytes and macrophages
Monocytes and macrophages are vital detectors of PAMPs. They participate in the process of defending the host against fungal infection and work together to summon neutrophils to the inflammatory site.Citation101 Vice versa, the influx of neutrophils also has the effect on recruiting monocytes and modulating cytokine release of activated macrophage.Citation101 After being recruited by neutrophils, monocytes differentiate into macrophages and continue to participate in immune responses. New evidence has shown that Candida stimuli induces the differentiation of macrophages from pro-inflammatory phenotype (M1, classically activated macrophages) into a more anti-inflammatory phenotype (M2, alternatively activated macrophages), and thus may enhance fungal survival and reduce the infection damage.Citation102 As to receptors mediating the recognition of C. albicans, studies have shown high levels of TLRs on the surface of monocytes.Citation103 CLRs, such as dectin-1 and mincle, also play an important role in mediating monocytes recognition of C. albicans.Citation28 Recent studies have proposed that monocytes as well as natural killer cells (NK cells) could display innate immune memory, which challenges the dogma of only adaptive immunity being specific and having immunological memory. C. albicans and its cell wall β-glucans can train monocytes to develop an enhanced and lasting response through dectin-1 receptor/Raf-1 pathway, which induces the activation of signaling molecules such as p38.Citation104 The receptors mediating signaling modulation and epigenetic histone modifications lead to increased production of pro-inflammatory cytokines and antifungal effects.Citation105 In-depth research of the trained immunity may provide important evidence for vaccine design especially about the strength and duration of induced trained immunity.Citation106
Macrophages are effective in the host immune defense because they can not only control C. albicans burden but also recruit other immune cells to help clear the pathogens. Pathogen detection triggers several intracellular signaling pathways containing MAPK and NF-κB, which leads to the production of pro-inflammatory cytokines, essential to combat the aggressive pathogen. Dectin-1 has been shown to coordinate the antifungal response in macrophages. However, the protective functions are dependent on fungal morphology. The filamentous morphology is not recognized efficiently due to the lack of exposed β-glucan.Citation107 Recently, BTK (Bruton's Tyrosine Kinase) and Vav1, 2 new interactors localized to the Candida-containing phagocytic cup, are found to contribute to dectin-1-mediated phagocytosis in macrophages.Citation108 Although dectin-1 signals are sufficient to trigger phagocytosis, collaboration with TLR2 signals does help up-regulate NF-κB responses and cytokine production (IL-6, TNF-α). The recognition signal of dectin-1, transferred through its ITAM-like motif, usually results in the activation of Src and Syk, and the release of IL-2, IL-10 and IL-1β in macrophages.Citation109,110 The activated Syk signaling pathway is one of the prerequisites for the production of ROS due to its antifungal activity.Citation111,112 NFAT (Nuclear Factor of Activated T-cells), a regulator of T cell activation, is also modulated by the collaboration of dectin-1 and TLR or by dectin-1 independently.Citation113 The activation of NFAT transcription factors in macrophages promotes both pro-inflammatory (IL-2, IL-12 and COX-2) and anti-inflammatory (IL-10) responses.Citation109 Also, the activation of NFAT in DCs produces a similar result. Mincle is highly expressed on macrophages and shown to mediate macrophage response to yeast C. albicans and induce the production of the inflammatory cytokine TNF-α.Citation28
Upon mediation by several opsonic and nonopsonic receptors on macrophages, phagocytosis of C. albicans occurs. After ingestion, the activation of macrophages will cause the production of antimicrobial effectors, including ROS (reactive oxygen species) and RNS (reactive nitrogen species). Although some C. albicans can be easily killed, many still survive. They have successfully developed escape mechanisms. To cope with these oxidative anti-fungal mechanisms, most pathogens including C. albicans can encode SODs (superoxide dismutases) to detoxify excessive ROS species.Citation114 Some pathogens actively suppress the generation of toxic compounds to protect themselves. New research has revealed that C. albicans can actively block NO (nitric oxide) production of macrophage via a secreted mediator that functions as the inhibitor of iNOS (inducible nitric oxide synthase).Citation115 Related studies have shown that in addition to physically bursting out of macrophages, the hyphae of C. albicans can also initiate pyroptotic cell death in macrophages as an additional means of escape following phagocytosis.Citation116,117 In the presence of C. albicans, PRRs on the macrophage, such as TLR2, dectin-1 and dectin-2, activate the transcription and translation of NLRP3 inflammasome, pro-IL-1β as well as pro-IL-18.Citation110 The activation of cysteine protease caspase-1, mediated by NLRP3, contributes to cleave pro-IL-1β and pro-IL-18 into their bioactive forms. This process is beneficial to host survival from systemic candidiasis, because the release of IL-1β and IL-18 is important for the recruitment of additional phagocytes and the initiation of Th1 and Th17 adaptive immune responses in the prevention of candidiasis dissemination.Citation110,118 Knowing that PMNs and macrophages are both professional phagocytes for clearing invading pathogens, a question about which one is more effective arises. Co-culture of PMNs and macrophages showed that macrophages had higher ability of engulfing hyphae while PMNs played a predominant role in clearing yeast form C. albicans.Citation89 But when they were incubated separately, PMNs showed lower overall phagocytic capacity but higher susceptibility as compared with macrophages.Citation89 Taken together, it is hard to judge which phagocyte is more efficient for the lack of researches especially in vivo. New evidence also reveals that human macrophages may take up apoptotic neutrophils, so that they can own the antimicrobial peptides or acquire neutrophil granules to promote their antimicrobial activity.Citation119
Dendritic cells
It is essential to have a deeper comprehension about the interaction between DCs and invading pathogenic fungi, for DCs maturation is believed to be the link of innate and adaptive immunity. DCs are known to play a central role in a series of processes, such as detecting fungal pathogens in the surroundings through PRRs localized on their surfaces, secreting cytokines, engulfing pathogen particles by phagocytosis, and finally inducing adaptive immune reaction via the presentation of antigens to T cells.Citation120 The immune response is equally initiated with the recognition of PAMPs by PRRs expressed on DCs surface or inside them. Because of the great role that they play during the process of antigen presentation, DCs have most of PRRs for the recognition of C. albicans, such as TLRs, CLRs, and FcγR.Citation121 Among them, MR and DC-SIGN, as 2 subgroups of CLRs, are vitally important in mediating the recognition and internalization of C. albicans by human DCs.Citation122 Research also found that the recognition of C. albians by DC-SIGN was a time and concentration-dependent process.Citation123 C. albicans enters DCs via DC-SIGN but not via dectin-1, which results in inhibition of the NADPH oxidative and ROS production.Citation124 In contrast to DC-SIGN, dectin-1 plays a role in mediating NADPH oxidase activation which leads to Candida-killing activity of DCs. Dectin-1 can directly activate NF-κB in dendritic cells via the signaling adaptor molecule CARD9 and DCs maturation.Citation125 The dectin-1 and TLRs of dendritic cells have a synergistic effect on mediating the production of cytokines such as IL-12 and TNF-α126. Finally, after being internalized by DCs, the antigen is delivered into endosomes and lysosomes, and is finally presented to T cells.
Natural killer cells
NK cells are also essential modulators of the innate immune system and have influence on adaptive immune responses. NK cells have been demonstrated as lymphocytes in the innate immune system. They exhibit not only cytotoxicity against viral infection and bacteria invasion but also anti-tumor effects.Citation127 Studies have shown the inhibitory effect of NK cells on a variety of fungi including Cryptococcus neoformans,Citation128 Candida albicans,Citation129 Aspergillus fumigatusCitation130 and Rhizopusoryzae.Citation131 More progress has been made in understanding the function of NK cells in candidiasis. NK cells have been demonstrated to be important in immunosupressed hosts against C. albicans, and to be the cause of hyperinflammation in immunocompetent hosts.Citation132 As for the specific action, studies have reported that NK cells in both humans and mice have fungicidal activity against C. albicans and other pathogens mainly through 2 different ways. In a direct and efficient way, NK cells exert antifungal activity through secreting granule content such as perforin. Also, NK cells contribute to the immune response indirectly via the generation of corresponding cytokines (GM-CSF, IFN-γ and TNF-α), which may recruit and activate specialized immune cells such as PMNs, dendritic cells, macrophages and other T cells.Citation133 Conversely, they are also regulated by the cytokines produced by other immune cells, thus forming an interactive process.
The interplay between NK cells and fungus similarly begins with the detection of PAMPs by the receptors on the host cells which may moderate the function and intrinsic stability of NK cells. NK cells have a diverse array of receptors to recognize pathogens and balance the signal transduction. These receptors, include NCRs (natural cytotoxicity receptors), KIRs (killer-cell-Ig-like-receptors), and TLRs.Citation134,135 Among so many receptors identified, NKp30 receptor, one of the NCRs family members, has been demonstrated to be vital in the recognition of fungal pathogens and antifungal cytotoxicity.Citation135,136 While, other NK cell receptors mentioned above are mainly involved in killing tumor and virus-infected cells. Although NKp30 is considered to mediate fungal killing, there is no clear evidence regarding the identity of the associated ligands. What is known is that NK cells can be activated via the PI3K/AKT and ERK pathways during fungal infection, leading to the formation of a microbial synapse between the pathogen and NK cell.Citation137 It is plausible that the synapse leads to a polarization of the receptors on NK cells, followed by degranulation, the release of perforin, granzyme, granulysin or other effector molecules.Citation129,136,138 Perforin has been proved to play an important role in direct antifungal process.Citation129 Perforin displays its cytotoxicity by perforating the cell membrane and finally inducing lysis of the target cell.Citation139 High levels of Granzyme B have been similarly observed in NK cells invaded by C. albicans. Moreover, human NK cells activated by C. albicans can secret GM-CSF, IFN-γ, and TNF-α.Citation140,141 However, previous studies showed that the production of IFN-γ by murine NK cells was down-regulated by C. albicans.Citation142 IFN-γ is generally recognized as an important modulator for promoting candidacidal activity of PMNs and macrophages.Citation143,144 TNF-α and GM-CSF have effects on potentiating antifungal activity of PMNs.Citation145 Paradoxically, new research results have shown that the activation of NK cells, specifically their contact with pathogen, is inhibited in the presence of PMNs. Meanwhile, the presence of activated NK cells can activate more PMNs and increase PMNs anti-fungal activity by increasing the production of ROS and delaying the apoptosis of PMNs.Citation146,147 This interplay between NKs and PMNs could be a mechanism to prevent the overreaction of the immune system and the damage of surrounding tissues caused by the overreaction. DCs can also act on NK cells by producing inflammatory mediators, resulting in NK cells releasing another mediator, GM-CSF. Vice versa, DC maturation is largely attributed to IFN-γ and TNF released by activated NK cells.Citation148 Additionally, NK cells can be activated by soluble factors such as IL-12, IL-15, IL-18 and type I IFNs produced by activated macrophages and dendritic cells. Thus, DCs, macrophages and PMNs in turn form the basis of NK cell immunity initiation.Citation149 The reciprocal impact of immune cells and cytokines and chemokines helps to highlight the interaction between different immune cells.
Conclusion
During the past decades, significant progress has been made in our understanding of the interplay between C. albicans and the host immune system. The interplay mechanisms include how the host innate immune system recognizes, responds to and clears the invading pathogen. Additionally, fungal pathogens have developed virulence factors to invade the host and contribute to pathogenesis. Moreover, virulence factors have become targets for developing Candida vaccines against disseminated candidiasis.
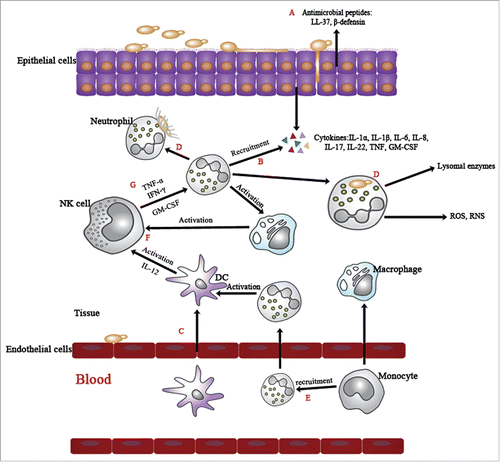
The recognition between the PRRs and fungal PAMPs is the fundamental step to initiate an immune response. Although lots of PRRs and fungal PAMPs have been identified, new evidence of unexplored interaction between them is still anxiously expected for its importance in new drug invention. As the defender of the host, the innate immune system is a huge complex network equipped with different kinds of immune cells. Epithelial and endothelial cells are not classical immune cells. But they are important barriers for infection agents and have indispensable effects on directing antifungal responses. Professional phagocytes are definitely main components in host defense responses. Moreover, the interaction and cooperation among different phagocytes provide effective protection against disseminated C. albicans (). Since, traditional antifungal therapies are confronted with the emergence of resistance and the rise of toxic side effects, new therapies, such as vaccines are urgently needed. However, as a potential fungal treatment, vaccines still have problems in several aspects and require further clinical validation. In summary, all the perspective antifungal strategies will be based on a better understanding of innate immune cell responses to fungi.
Figure 2. Innate cell response network against C. albicans infection. C. albicans interacts with epithelia by the process: adherence, invasion and cell damage. (A) To clear the invading pathogen, epithelial cells secret antimicrobial agents as the first defending step. In healthy circumstances, PMNs are strictly regulated and controlled by chemokines. (B) Upon Candida infection, the release of several chemoattractants and cytokines recruits PMNs to the site of infection. (C) Immune cells that receive the recruitment signals traverse the endothelial cells to participate in immune defense. (D) The activated PMNs clear the invading pathogen by 2 ways: phagocytosis by forming neutrophil extracellular traps (NETs). (E) They are indispensable for recruiting monocytes and facilitating their differentiation into macrophages. (F) Macrophages and DCs both contribute to the activation of NK cells. (G) Afterwards, the active NK cells can reinforce the immunological function of PMNs through the release of TNF-α, TNF-γ and GM-CSF.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by National Key Basic Research Program of China (No 2013CB531602), National Science Foundation of China (No 81173100, 81273556), Shanghai Science and Technology Major Project (11JC1415400).
References
- Wang YC, Tsai IC, Lin C, Hsieh WP, Lan CY, Chuang YJ, Chen BS. Essential functional modules for pathogenic and defensive mechanisms in Candida albicans infections. BioMed Res Int 2014; 2014:136130; PMID:24757665
- Brown GD. Innate antifungal immunity: the key role of phagocytes. Annu Rev Immunol 2011; 29:1-21; PMID:20936972; http://dx.doi.org/10.1146/annurev-immunol-030409-101229
- Berman J, Sudbery PE. Candida Albicans: a molecular revolution built on lessons from budding yeast. Nat Rev Genet 2002; 3:918-30; PMID:12459722; http://dx.doi.org/10.1038/nrg948
- Orozco AS, Zhou X, Filler SG. Mechanisms of the proinflammatory response of endothelial cells to Candida albicans infection. Infect Immun 2000; 68:1134-41; PMID:10678917; http://dx.doi.org/10.1128/IAI.68.3.1134-1141.2000
- Klotz SA. Fungal adherence to the vascular compartment: a critical step in the pathogenesis of disseminated candidiasis. Clin Infect Dis: Off Publ Infect Dis Soc America 1992; 14:340-7; PMID:1571448; http://dx.doi.org/10.1093/clinids/14.1.340
- Cheng SC, Joosten LAB, Kullberg BJ, Netea MG. Interplay between Candida albicans and the Mammalian Innate Host Defense. Infect Immun 2012; 80:1304-13; PMID:22252867; http://dx.doi.org/10.1128/IAI.06146-11
- Brown GD, Denning DW, Levitz SM. Tackling human fungal infections. Science 2012; 336:647; PMID:22582229; http://dx.doi.org/10.1126/science.1222236
- Zipfel PF, Skerka C, Kupka D, Luo S. Immune escape of the human facultative pathogenic yeast Candida albicans: the many faces of the Candida Pra1 protein. Int J Med Microbiol: Indian J Med Microbiol 2011; 301:423-30; PMID:21565550; http://dx.doi.org/10.1016/j.ijmm.2011.04.010
- Gow NA, Hube B. Importance of the Candida albicans cell wall during commensalism and infection. Curr Opin Microbiol 2012; 15:406-12; PMID:22609181; http://dx.doi.org/10.1016/j.mib.2012.04.005
- Hall RA, Gow NA. Mannosylation in Candida albicans: role in cell wall function and immune recognition. Mol Microbiol 2013; 90:1147-61; PMID:24125554; http://dx.doi.org/10.1111/mmi.12426
- Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 2011; 34:637-50; PMID:21616434; http://dx.doi.org/10.1016/j.immuni.2011.05.006
- Hardison SE, Brown GD. C-type lectin receptors orchestrate antifungal immunity. Nat Immunol 2012; 13:817-22; PMID:22910394; http://dx.doi.org/10.1038/ni.2369
- Joly S, Eisenbarth SC, Olivier AK, Williams A, Kaplan DH, Cassel SL, Flavell RA, Sutterwala FS. Cutting edge: Nlrp10 is essential for protective antifungal adaptive immunity against Candida albicans. J Immunol 2012; 189:4713-7; PMID:23071280; http://dx.doi.org/10.4049/jimmunol.1201715
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell 2006; 124:783-801; PMID:16497588; http://dx.doi.org/10.1016/j.cell.2006.02.015
- Jouault T, Ibata-Ombetta S, Takeuchi O, Trinel P-A, Sacchetti P, Lefebvre P, Akira S, Poulain D. Candida albicans phospholipomannan is sensed through toll-like receptors. J Infect Dis 2003; 188:165-72; PMID:12825186; http://dx.doi.org/10.1086/375784
- Netea MG, Gow NA, Munro CA, Bates S, Collins C, Ferwerda G, Hobson RP, Bertram G, Hughes HB, Jansen T, et al. Immune sensing of Candida albicans requires cooperative recognition of mannans and glucans by lectin and Toll-like receptors. J Clin Investigat 2006; 116:1642-50; PMID:16710478; http://dx.doi.org/10.1172/JCI27114
- Miyazato A, Nakamura K, Yamamoto N, Mora-Montes HM, Tanaka M, Abe Y, Tanno D, Inden K, Gang X, Ishii K, et al. Toll-like receptor 9-dependent activation of myeloid dendritic cells by deoxynucleic acids from Candida albicans. Infect Immun 2009; 77:3056-64; PMID:19433551; http://dx.doi.org/10.1128/IAI.00840-08
- Inoue M, Shinohara ML. Clustering of pattern recognition receptors for fungal detection. PLoS Pathogens 2014; 10:e1003873; PMID:24586145; http://dx.doi.org/10.1371/journal.ppat.1003873
- Netea MG, Marodi L. Innate immune mechanisms for recognition and uptake of Candida species. Trends Immunol 2010; 31:346-53; PMID:20705510; http://dx.doi.org/10.1016/j.it.2010.06.007
- Kerrigan AM, Brown GD. Syk-coupled C-type lectin receptors that mediate cellular activation via single tyrosine based activation motifs. Immunolog Rev 2010; 234:335-52; PMID:20193029; http://dx.doi.org/10.1111/j.0105-2896.2009.00882.x
- Drummond RA, Brown GD. The role of Dectin-1 in the host defence against fungal infections. Curr Opin Microbiol 2011; 14:392-9; PMID:21803640; http://dx.doi.org/10.1016/j.mib.2011.07.001
- Marakalala MJ, Vautier S, Potrykus J, Walker LA, Shepardson KM, Hopke A, Mora-Montes HM, Kerrigan A, Netea MG, Murray GI, et al. Differential adaptation of Candida albicans in vivo modulates immune recognition by dectin-1. PLoS Pathogens 2013; 9:e1003315; PMID:23637604; http://dx.doi.org/10.1371/journal.ppat.1003315
- Zhu L-L, Zhao X-Q, Jiang C, You Y, Chen X-P, Jiang Y-Y, Jia XM, Lin X. C-type lectin receptors Dectin-3 and Dectin-2 form a heterodimeric pattern-recognition receptor for host defense against fungal infection. Immunity 2013; 39:324-34; PMID:23911656; http://dx.doi.org/10.1016/j.immuni.2013.05.017
- McGreal EP, Rosas M, Brown GD, Zamze S, Wong SY, Gordon S, Martinez-Pomares L, Taylor PR. The carbohydrate-recognition domain of Dectin-2 is a C-type lectin with specificity for high mannose. Glycobiology 2006; 16:422-30; PMID:16423983; http://dx.doi.org/10.1093/glycob/cwj077
- Saijo S, Ikeda S, Yamabe K, Kakuta S, Ishigame H, Akitsu A, Fujikado N, Kusaka T, Kubo S, Chung SH, et al. Dectin-2 recognition of alpha-mannans and induction of Th17 cell differentiation is essential for host defense against Candida albicans. Immunity 2010; 32:681-91; PMID:20493731; http://dx.doi.org/10.1016/j.immuni.2010.05.001
- Saijo S, Iwakura Y. Dectin-1 and Dectin-2 in innate immunity against fungi. Int Immunol 2011; 23:467-72; PMID:21677049; http://dx.doi.org/10.1093/intimm/dxr046
- Osorio F, Reis e Sousa C. Myeloid C-type lectin receptors in pathogen recognition and host defense. Immunity 2011; 34:651-64; PMID:21616435; http://dx.doi.org/10.1016/j.immuni.2011.05.001
- Wells CA, Salvage-Jones JA, Li X, Hitchens K, Butcher S, Murray RZ, Beckhouse AG, Lo YL, Manzanero S, Cobbold C, et al. The macrophage-inducible C-type lectin, Mincle, is an essential component of the innate immune response to Candida albicans. J Immunol 2008; 180:7404-13; PMID:18490740; http://dx.doi.org/10.4049/jimmunol.180.11.7404
- Yamasaki S, Ishikawa E, Sakuma M, Hara H, Ogata K, Saito T. Mincle is an ITAM-coupled activating receptor that senses damaged cells. Nat Immunol 2008; 9:1179-88; PMID:18776906; http://dx.doi.org/10.1038/ni.1651
- Porcaro I, Vidal M, Jouvert S, Stahl PD, Giaimis J. Mannose receptor contribution to Candida albicans phagocytosis by murine E-clone J774 macrophages. J Leukocyte Biol 2003; 74:206-15; PMID:12885937; http://dx.doi.org/10.1189/jlb.1202608
- Poulain D, Jouault T. Candida albicans cell wall glycans, host receptors and responses: elements for a decisive crosstalk. Curr Opin Microbiol 2004; 7:342-9; PMID:15358252; http://dx.doi.org/10.1016/j.mib.2004.06.011
- McKenzie CG, Koser U, Lewis LE, Bain JM, Mora-Montes HM, Barker RN, Gow NA, Erwig LP. Contribution of Candida albicans cell wall components to recognition by and escape from murine macrophages. Infect Immun 2010; 78:1650-8; PMID:20123707; http://dx.doi.org/10.1128/IAI.00001-10
- Heinsbroek SE, Taylor PR, Martinez FO, Martinez-Pomares L, Brown GD, Gordon S. Stage-specific sampling by pattern recognition receptors during Candida albicans phagocytosis. PLoS Pathogens 2008; 4:e1000218; PMID:19043561; http://dx.doi.org/10.1371/journal.ppat.1000218
- Linden JR, De Paepe ME, Laforce-Nesbitt SS, Bliss JM. Galectin-3 plays an important role in protection against disseminated candidiasis. Med Mycol 2013; 51:641-51; PMID:23488971; http://dx.doi.org/10.3109/13693786.2013.770607
- Chavarria-Smith J, Vance RE. The NLRP1 inflammasomes. Immunol Rev 2015; 265:22-34; PMID:25879281; http://dx.doi.org/10.1111/imr.12283
- Schroder K, Tschopp J. The inflammasomes. Cell 2010; 140:821-32; PMID:20303873; http://dx.doi.org/10.1016/j.cell.2010.01.040
- Hise AG, Tomalka J, Ganesan S, Patel K, Hall BA, Brown GD, Fitzgerald KA. An essential role for the NLRP3 inflammasome in host defense against the human fungal pathogen Candida albicans. Cell Host Microbe 2009; 5:487-97; PMID:19454352; http://dx.doi.org/10.1016/j.chom.2009.05.002
- Tomalka J, Ganesan S, Azodi E, Patel K, Majmudar P, Hall BA, Fitzgerald KA, Hise AG. A novel role for the NLRC4 inflammasome in mucosal defenses against the fungal pathogen Candida albicans. PLoS Pathogens 2011; 7:e1002379
- Zordan R, Cormack B. Adhesins on opportunistic fungal pathogens. Candida and Candidiasis: Washington, DC: ASM Press, 2012:243-59.
- Staab JF, Datta K, Rhee P. Niche-Specific Requirement for Hyphal Wall protein 1 in Virulence of Candida albicans. PloS One 2013; 8:e80842
- Staab JF, Bradway SD, Fidel PL, Sundstrom P. Adhesive and mammalian transglutaminase substrate properties of Candida albicans Hwp1. Science 1999; 283:1535-8; PMID:10066176; http://dx.doi.org/10.1126/science.283.5407.1535
- Sundstrom P, Balish E, Allen CM. Essential role of the Candida albicans transglutaminase substrate, hyphal wall protein 1, in lethal oroesophageal candidiasis in immunodeficient mice. J Infect Dis 2002; 185:521-30; PMID:11865405; http://dx.doi.org/10.1086/338836
- Li F, Palecek SP. EAP1, a Candida albicans gene involved in binding human epithelial cells. Eukaryotic Cell 2003; 2:1266-73; PMID:14665461; http://dx.doi.org/10.1128/EC.2.6.1266-1273.2003
- Li F, Palecek SP. Distinct domains of the Candida albicans adhesin Eap1p mediate cell-cell and cell-substrate interactions. Microbiology 2008; 154:1193-203; PMID:18375812; http://dx.doi.org/10.1099/mic.0.2007/013789-0
- Kempf M, Cottin J, Licznar P, Lefrançois C, Robert R, Apaire-Marchais V. Disruption of the GPI protein-encoding gene IFF4 of Candida albicans results in decreased adherence and virulence. Mycopathologia 2009; 168:73-7; PMID:19347602; http://dx.doi.org/10.1007/s11046-009-9201-0
- Kempf M, Apaire-Marchais V, Saulnier P, Licznar P, Lefrancois C, Robert R, Cottin J. Disruption of Candida albicans IFF4 gene involves modifications of the cell electrical surface properties. Colloid Surface B 2007; 58:250-5; PMID:17481864; http://dx.doi.org/10.1016/j.colsurfb.2007.03.017
- Sun JN, Solis NV, Phan QT, Bajwa JS, Kashleva H, Thompson A, Liu Y, Dongari-Bagtzoglou A, Edgerton M, Filler SG. Host cell invasion and virulence mediated by Candida albicans Ssa1. PLoS Pathog 2010; 6:e1001181; PMID:21085601
- Puri S, Edgerton M. Candida albicans Ssa: An Hsp70 Homologue and Virulence Factor. Moonlighting Cell Stress Proteins in Microbial Infections: Springer, 2013:223-35.
- Zhao X, Pujol C, Soll DR, Hoyer LL. Allelic variation in the contiguous loci encoding Candida albicans ALS5, ALS1 and ALS9. Microbiology 2003; 149:2947-60; PMID:14523127; http://dx.doi.org/10.1099/mic.0.26495-0
- Liu Y, Filler SG. Candida albicans Als3, a multifunctional adhesin and invasin. Eukaryotic Cell 2011; 10:168-73; PMID:21115738; http://dx.doi.org/10.1128/EC.00279-10
- Sheppard DC, Yeaman MR, Welch WH, Phan QT, Fu Y, Ibrahim AS, Filler SG, Zhang M, Waring AJ, Edwards JE Jr. Functional and structural diversity in the Als protein family of Candida albicans. J Biol Chem 2004; 279:30480-9; PMID:15128742; http://dx.doi.org/10.1074/jbc.M401929200
- Phan QT, Myers CL, Fu Y, Sheppard DC, Yeaman MR, Welch WH, Ibrahim AS, Edwards JE Jr, Filler SG. Als3 is a Candida albicans invasin that binds to cadherins and induces endocytosis by host cells. Plos Biol 2007; 5:543-57; PMID:17311474; http://dx.doi.org/10.1371/journal.pbio.0050064
- Verma S, Heffernan M. Superficial fungal infection: Dermatophytosis, onychomycosis, tinea nigra, piedra. Fitzpatrick's dermatology in general medicine 7th ed New York: McGraw-Hill 2008; 1815.
- Dalle F, Wachtler B, L'Ollivier C, Holland G, Bannert N, Wilson D, Labruère C, Bonnin A, Hube B. Cellular interactions of Candida albicans with human oral epithelial cells and enterocytes. Cell Microbiol 2010; 12:248-71; PMID:19863559; http://dx.doi.org/10.1111/j.1462-5822.2009.01394.x
- Zhu W, Filler SG. Interactions of Candida albicans with epithelial cells. Cell Microbiol 2010; 12:273-82; PMID:19919567; http://dx.doi.org/10.1111/j.1462-5822.2009.01412.x
- Villar CC, Kashleva H, Nobile CJ, Mitchell AP, Dongari-Bagtzoglou A. Mucosal tissue invasion by Candida albicans is associated with E-cadherin degradation, mediated by transcription factor Rim101p and protease Sap5p. Infect Immun 2007; 75:2126-35; PMID:17339363; http://dx.doi.org/10.1128/IAI.00054-07
- Wu H, Downs D, Ghosh K, Ghosh AK, Staib P, Monod M, Tang J. Candida albicans secreted aspartic proteases 4–6 induce apoptosis of epithelial cells by a novel Trojan horse mechanism. FASEB J 2013; 27:2132-44; PMID:23430844; http://dx.doi.org/10.1096/fj.12-214353
- Cassone A, Casadevall A. Recent progress in vaccines against fungal diseases. Curr Opin Microbiol 2012; 15:427-33; PMID:22564747; http://dx.doi.org/10.1016/j.mib.2012.04.004
- Wang XJ, Sui X, Yan L, Wang Y, Cao YB, Jiang YY. Vaccines in the treatment of invasive candidiasis. Virulence 2015; 6:309-15; PMID:25559739; http://dx.doi.org/10.1080/21505594.2014.1000752
- Pericolini E, Gabrielli E, Amacker M, Kasper L, Roselletti E, Luciano E, Sabbatini S, Kaeser M, Moser C, Hube B, et al. Secretory aspartyl proteinases cause vaginitis and can mediate vaginitis caused by Candida albicans in mice. MBio 2015; 6:e00724-15; PMID:26037125; http://dx.doi.org/10.1128/mBio.00724-15
- De Bernardis F, Amacker M, Arancia S, Sandini S, Gremion C, Zurbriggen R, Moser C, Cassone A. A virosomal vaccine against candidal vaginitis: immunogenicity, efficacy and safety profile in animal models. Vaccine 2012; 30:4490-8; PMID:22561143; http://dx.doi.org/10.1016/j.vaccine.2012.04.069
- Spellberg BJ, Ibrahim As Fau-Avenissian V, Avenissian V, Fau-Filler SG, Filler Sg Fau-Myers CL, Myers Cl Fau-Fu Y, Fu Y, Fau-Edwards JE Jr. The anti-Candida albicans vaccine composed of the recombinant N terminus of Als1p reduces fungal burden and improves survival in both immunocompetent and immunocompromised mice.
- Lin L, Ibrahim AS, Xu X, Farber JM, Avanesian V, Baquir B, Fu Y, French SW, Edwards JE Jr, Spellberg B. Th1-Th17 cells mediate protective adaptive immunity against Staphylococcus aureus and Candida albicans infection in mice. PLoS Pathogens 2009; 5:e1000703; PMID:20041174; http://dx.doi.org/10.1371/journal.ppat.1000703
- Spellberg BJ, Ibrahim AS, Avanesian V, Fu Y, Myers C, Phan QT, Filler SG, Yeaman MR, Edwards JE Jr. Efficacy of the anti-Candida rAls3p-N or rAls1p-N vaccines against disseminated and mucosal candidiasis. J Infect Dis 2006; 194:256-60; PMID:16779733; http://dx.doi.org/10.1086/504691
- Schmidt CS, White CJ, Ibrahim AS, Filler SG, Fu Y, Yeaman MR, Edwards JE Jr, Hennessey JP Jr. NDV-3, a recombinant alum-adjuvanted vaccine for Candida and Staphylococcus aureus, is safe and immunogenic in healthy adults. Vaccine 2012; 30:7594-600; PMID:23099329; http://dx.doi.org/10.1016/j.vaccine.2012.10.038
- Ibrahim AS, Luo GPS, Gebremariam T, Lee H, Schmidt CS, Hennessey JP, French SW, Yeaman MR, Filler SG, Edwards JE Jr. NDV-3 protects mice from vulvovaginal candidiasis through T- and B-cell immune response. Vaccine 2013; 31:5549-56; PMID:24063977; http://dx.doi.org/10.1016/j.vaccine.2013.09.016
- Naglik J, Moyes D. Epithelial cell innate response to Candida albicans. Adv Dental Res 2011; 23:50-5; PMID:21441481; http://dx.doi.org/10.1177/0022034511399285
- Mahanonda R, Pichyangkul S. Toll-like receptors and their role in periodontal health and disease. Periodontol 2000 2007; 43:41-55; PMID:17214834
- Weindl G, Wagener J, Schaller M. Epithelial cells and innate antifungal defense. J Dental Res 2010; 89:666-75; PMID:20395411; http://dx.doi.org/10.1177/0022034510368784
- Sugawara Y, Uehara A, Fujimoto Y, Kusumoto S, Fukase K, Shibata K, Sugawara S, Sasano T, Takada H. Toll-like receptors, NOD1, and NOD2 in oral epithelial cells. J Dent Res 2006; 85:524-9; PMID:16723649; http://dx.doi.org/10.1177/154405910608500609
- Yano J, Palmer GE, Eberle KE, Peters BM, Vogl T, McKenzie AN, Fidel PL Jr. Vaginal epithelial cell-derived S100 alarmins induced by Candida albicans via pattern recognition receptor interactions are sufficient but not necessary for the acute neutrophil response during experimental vaginal candidiasis. Infect Immun 2014; 82:783-92; PMID:24478092; http://dx.doi.org/10.1128/IAI.00861-13
- Weindl G, Naglik JR, Kaesler S, Biedermann T, Hube B, Korting HC, Schaller M. Human epithelial cells establish direct antifungal defense through TLR4-mediated signaling. J Clin Invest 2007; 117:3664-72; PMID:17992260
- Moyes DL, Runglall M, Murciano C, Shen C, Nayar D, Thavaraj S, Kohli A, Islam A, Mora-Montes H, Challacombe SJ, et al. A biphasic innate immune MAPK response discriminates between the yeast and hyphal forms of Candida albicans in epithelial cells. Cell Host Microbe 2010; 8:225-35; PMID:20833374; http://dx.doi.org/10.1016/j.chom.2010.08.002
- Moyes DL, Murciano C, Runglall M, Islam A, Thavaraj S, Naglik JR. Candida albicans yeast and hyphae are discriminated by MAPK signaling in vaginal epithelial cells. Plos One 2011; 6:e26580; PMID:22087232; http://dx.doi.org/10.1371/journal.pone.0026580
- Moyes DL, Shen CG, Murciano C, Runglall M, Richardson JP, Arno M, Aldecoa-Otalora E, Naglik JR. Protection against epithelial damage during Candida albicans infection is mediated by PI3K/Akt and mammalian target of rapamycin signaling. J Infect Dis 2014; 209:1816-26; PMID:24357630; http://dx.doi.org/10.1093/infdis/jit824
- de Repentigny L, Goupil M, Jolicoeur P. Oropharyngeal candidiasis in HIV infection: analysis of impaired mucosal immune response to Candida albicans in mice expressing the HIV-1 transgene. Pathogens 2015; 4:406-21; PMID:26110288; http://dx.doi.org/10.3390/pathogens4020406
- Schaller M, Mailhammer R, Grassl G, Sander CA, Hube B, Korting HC. Infection of human oral epithelia with Candida species induces cytokine expression correlated to the degree of virulence. J Investigat Dermatol 2002; 118:652-7; PMID:11918712; http://dx.doi.org/10.1046/j.1523-1747.2002.01699.x
- Feller L, Khammissa RA, Chandran R, Altini M, Lemmer J. Oral candidosis in relation to oral immunity. J Oral Pathol Med: Off Pub Int Assoc Oral Pathologists Am Acad Oral Pathol 2014; 43:563-9; PMID:24118267; http://dx.doi.org/10.1111/jop.12120
- Steele C, Fidel PL, Jr. Cytokine and chemokine production by human oral and vaginal epithelial cells in response to Candida albicans. Infect Immun 2002; 70:577-83; PMID:11796585; http://dx.doi.org/10.1128/IAI.70.2.577-583.2002
- Diamond G, Beckloff N, Ryan LK. Host defense peptides in the oral cavity and the lung: similarities and differences. J Dental Res 2008; 87:915-27; PMID:18809744; http://dx.doi.org/10.1177/154405910808701011
- Conti HR, Shen F, Nayyar N, Stocum E, Sun JN, Lindemann MJ, Ho AW, Hai JH, Yu JJ, Jung JW, et al. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J Exp Med 2009; 206:299-311; PMID:19204111; http://dx.doi.org/10.1084/jem.20081463
- De Luca A, Zelante T, D'Angelo C, Zagarella S, Fallarino F, Spreca A, Iannitti RG, Bonifazi P, Renauld JC, Bistoni F, et al. IL-22 defines a novel immune pathway of antifungal resistance. Mucosal Immunol 2010; 3:361-73; PMID:20445503; http://dx.doi.org/10.1038/mi.2010.22
- Wilson D, Hube B. Hgc1 mediates dynamic Candida albicans-endothelium adhesion events during circulation. Eukaryotic Cell 2010; 9:278-87; PMID:20023069; http://dx.doi.org/10.1128/EC.00307-09
- Opitz B, Hippenstiel S, Eitel J, Suttorp N. Extra-and intracellular innate immune recognition in endothelial cells. Thromb Haemost 2007; 98:319; PMID:17721613
- Faure E, Equils O, Sieling PA, Thomas L, Zhang FX, Kirschning CJ, Polentarutti N, Muzio M, Arditi M. Bacterial lipopolysaccharide activates NF-κB through toll-like receptor 4 (TLR-4) in cultured human dermal endothelial cells differential expression of TLR-4 and TLR-2 in endothelial cells. J Biolog Chem 2000; 275:11058-63; PMID:10753909; http://dx.doi.org/10.1074/jbc.275.15.11058
- Lopez CM, Wallich R, Riesbeck K, Skerka C, Zipfel PF. Candida albicans uses the surface protein Gpm1 to attach to human endothelial cells and to keratinocytes via the adhesive protein vitronectin. PloS One 2014; 9:e90796; PMID:24625558; http://dx.doi.org/10.1371/journal.pone.0090796
- Phan QT, Fratti RA, Prasadarao NV, Edwards JE, Filler SG. N-cadherin mediates endocytosis of Candida albicans by endothelial cells. J Biol Chem 2005; 280:10455-61; PMID:15632157; http://dx.doi.org/10.1074/jbc.M412592200
- Phan QT, Eng DK, Mostowy S, Park H, Cossart P, Filler SG. Role of endothelial cell septin 7 in the endocytosis of Candida albicans. MBio 2013; 4:e00542-13; PMID:24345743; http://dx.doi.org/10.1128/mBio.00542-13
- Rudkin FM, Bain JM, Walls C, Lewis LE, Gow NAR, Erwig LP. Altered dynamics of Candida albicans phagocytosis by macrophages and PMNs when both phagocyte subsets are present. MBio 2013; 4:e00810-13; PMID:24169578; http://dx.doi.org/10.1128/mBio.00810-13
- Urban CF, Ermert D, Schmid M, Abu-Abed U, Goosmann C, Nacken W, Brinkmann V, Jungblut PR, Zychlinsky A. Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathogens 2009; 5:e1000639; PMID:19876394; http://dx.doi.org/10.1371/journal.ppat.1000639
- Miramon P, Dunker C, Windecker H, Bohovych IM, Brown AJ, Kurzai O, Hube B. Cellular responses of Candida albicans to phagocytosis and the extracellular activities of neutrophils are critical to counteract carbohydrate starvation, oxidative and nitrosative stress. Plos One 2012; 7:e52850; PMID:23285201; http://dx.doi.org/10.1371/journal.pone.0052850
- Gabay C. Interleukin-6 and chronic inflammation. Arthritis Res Ther 2006; 8:S3; PMID:16899107; http://dx.doi.org/10.1186/ar1917
- Balish E, Wagner RD, Vazquez-Torres A, Jones-Carson J, Pierson C, Warner T. Mucosal and systemic candidiasis in IL-8Rh-/-BALB/c mice. J Leukocyte Biol 1999; 66:144-50
- Huang W, Na L, Fidel PL, Schwarzenberger P. Requirement of interleukin-17A for systemic anti-Candida albicans host defense in mice. J Infect Dis 2004; 190:624-31; PMID:15243941; http://dx.doi.org/10.1086/422329
- Schaller M, Boeld U, Oberbauer S, Hamm G, Hube B, Korting HC. Polymorphonuclear leukocytes (PMNs) induce protective Th1-type cytokine epithelial responses in an in vitro model of oral candidosis. Microbiology 2004; 150:2807-13; PMID:15347740; http://dx.doi.org/10.1099/mic.0.27169-0
- Wood AJ, Lieschke GJ, Burgess AW. Granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor. New Engl J Med 1992; 327:28-35; PMID:1375975; http://dx.doi.org/10.1056/NEJM199207023270106
- Wright HL, Moots RJ, Bucknall RC, Edwards SW. Neutrophil function in inflammation and inflammatory diseases. Rheumatology (Oxford) 2010; 49:1618-31.
- Gresnigt MS, Joosten LA, Verschueren I, van der Meer JW, Netea MG, Dinarello CA, van de Veerdonk FL. Neutrophil-mediated inhibition of proinflammatory cytokine responses. J Immunol 2012; 189:4806-15; PMID:23053514; http://dx.doi.org/10.4049/jimmunol.1103551
- Urban CF, Reichard U, Brinkmann V, Zychlinsky A. Neutrophil extracellular traps capture and kill Candida albicans yeast and hyphal forms. Cell Microbiol 2006; 8:668-76; PMID:16548892; http://dx.doi.org/10.1111/j.1462-5822.2005.00659.x
- Kawakami T, He J, Morita H, Yokoyama K, Kaji H, Tanaka C, Suemori S, Tohyama K, Tohyama Y. Rab27a is essential for the formation of neutrophil extracellular traps (NETs) in neutrophil-like differentiated HL60 cells. PloS One 2014; 9:e84704; PMID:24404184; http://dx.doi.org/10.1371/journal.pone.0084704
- Amulic B, Cazalet C, Hayes GL, Metzler KD, Zychlinsky A. Neutrophil function: from mechanisms to disease. Ann Rev Immunol 2012; 30:459-89; PMID:22224774; http://dx.doi.org/10.1146/annurev-immunol-020711-074942
- Reales-Calderon JA, Aguilera-Montilla N, Corbi AL, Molero G, Gil C. Proteomic characterization of human proinflammatory M1 and anti-inflammatory M2 macrophages and their response to Candida albicans. Proteomics 2014; 14:1503-18; PMID:24687989; http://dx.doi.org/10.1002/pmic.201300508
- Netea MG, Brown GD, Kullberg BJ, Gow NA. An integrated model of the recognition of Candida albicans by the innate immune system. Nat Rev Microbiol 2008; 6:67-78; PMID:18079743; http://dx.doi.org/10.1038/nrmicro1815
- Quintin J, Saeed S, Fau-Martens JHA, Martens Jh, Fau-Giamarellos-Bourboulis EJ, Giamarellos-Bourboulis Ej, Fau-Ifrim DC, Ifrim Dc, Fau-Logie C, Logie C, et al. Candida albicans infection affords protection against reinfection via functional reprogramming of monocytes. Cell Host Microbe 2012; 12:223-32; PMID:22901542; http://dx.doi.org/10.1016/j.chom.2012.06.006
- Quintin J, Cheng SC, van der Meer JWM, Netea MG. Innate immune memory: towards a better understanding of host defense mechanisms. Curr Opin Immunol 2014; 29:1-7; PMID:24637148; http://dx.doi.org/10.1016/j.coi.2014.02.006
- Bowdish DM, Loffredo MS, Mukhopadhyay S, Mantovani A, Gordon S. Macrophage receptors implicated in the “adaptive” form of innate immunity. Microbes Infect / Institut Pasteur 2007; 9:1680-7; PMID:18023392; http://dx.doi.org/10.1016/j.micinf.2007.09.002
- Gantner BN, Simmons RM, Underhill DM. Dectin-1 mediates macrophage recognition of Candida albicans yeast but not filaments. EMBO J 2005; 24:1277-86; PMID:15729357; http://dx.doi.org/10.1038/sj.emboj.7600594
- Strijbis K, Tafesse FG, Fairn GD, Witte MD, Dougan SK, Watson N, Spooner E, Esteban A, Vyas VK, Fink GR, et al. Bruton's Tyrosine Kinase (BTK) and Vav1 contribute to Dectin1-dependent phagocytosis of Candida albicans in macrophages. PLoS Pathogens 2013; 9:e1003446; PMID:23825946; http://dx.doi.org/10.1371/journal.ppat.1003446
- Goodridge HS, Simmons RM, Underhill DM. Dectin-1 stimulation by Candida albicans yeast or zymosan triggers NFAT activation in macrophages and dendritic cells. J Immunol 2007; 178:3107-15; PMID:17312158; http://dx.doi.org/10.4049/jimmunol.178.5.3107
- Krysan DJ, Sutterwala FS, Wellington M. Catching fire: Candida albicans, macrophages, and pyroptosis. PLoS Pathogens 2014; 10:e1004139; PMID:24967821
- Rogers NC, Slack EC, Edwards AD, Nolte MA, Schulz O, Schweighoffer E, Williams DL, Gordon S, Tybulewicz VL, Brown GD, et al. Syk-dependent cytokine induction by Dectin-1 reveals a novel pattern recognition pathway for C type lectins. Immunity 2005; 22:507-17; PMID:15845454; http://dx.doi.org/10.1016/j.immuni.2005.03.004
- Underhill DM, Rossnagle E, Lowell CA, Simmons RM. Dectin-1 activates Syk tyrosine kinase in a dynamic subset of macrophages for reactive oxygen production. Blood 2005; 106:2543-50; PMID:15956283; http://dx.doi.org/10.1182/blood-2005-03-1239
- Macian F. NFAT proteins: key regulators of T-cell development and function. Nat Rev Immunol 2005; 5:472-84; PMID:15928679; http://dx.doi.org/10.1038/nri1632
- Frohner IE, Bourgeois C, Yatsyk K, Majer O, Kuchler K. Candida albicans cell surface superoxide dismutases degrade host-derived reactive oxygen species to escape innate immune surveillance. Mol Microbiol 2009; 71:240-52; PMID:19019164; http://dx.doi.org/10.1111/j.1365-2958.2008.06528.x
- Collette JR, Zhou H, Lorenz MC. Candida albicans suppresses nitric oxide generation from macrophages via a secreted molecule. PloS One 2014; 9:e96203
- Uwamahoro N, Verma-Gaur J, Shen HH, Qu Y, Lewis R, Lu J, Bambery K, Masters SL, Vince JE, Naderer T, et al. The pathogen Candida albicans hijacks pyroptosis for escape from macrophages. MBio 2014; 5:e00003-14; PMID:24667705; http://dx.doi.org/10.1128/mBio.00003-14
- Wellington M, Koselny K, Sutterwala FS, Krysan DJ. Candida albicans triggers NLRP3-mediated pyroptosis in macrophages. Eukaryotic Cell 2014; 13:329-40; PMID:24376002; http://dx.doi.org/10.1128/EC.00336-13
- Schmidt RL, Lenz LL. Distinct licensing of IL-18 and IL-1β secretion in response to NLRP3 inflammasome activation. PloS One 2012; 7:e45186
- Müller I, Munder M, Kropf P, Hänsch GM. Polymorphonuclear neutrophils and T lymphocytes: strange bedfellows or brothers in arms? Trends Immunol 2009; 30:522-30; PMID:19775938; http://dx.doi.org/10.1016/j.it.2009.07.007
- Ramirez-Ortiz ZG, Means TK. The role of dendritic cells in the innate recognition of pathogenic fungi (A. fumigatus, C. neoformans and C. albicans). Virulence 2012; 3:635-46; PMID:23076328; http://dx.doi.org/10.4161/viru.22295
- del Fresno C, Soulat D, Roth S, Blazek K, Udalova I, Sancho D, Ruland J, Ardavín C. Interferon-beta production via Dectin-1-Syk-IRF5 signaling in dendritic cells is crucial for immunity to C. albicans. Immunity 2013; 38:1176-86; PMID:23770228; http://dx.doi.org/10.1016/j.immuni.2013.05.010
- Cambi A, Netea MG, Mora-Montes HM, Gow NA, Hato SV, Lowman DW, Kullberg BJ, Torensma R, Williams DL, Figdor CG. Dendritic cell interaction with Candida albicans critically depends on N-linked mannan. J Biolog Chem 2008; 283:20590-9; PMID:18482990; http://dx.doi.org/10.1074/jbc.M709334200
- Cambi A, Gijzen K, de Vries IJM, Torensma R, Joosten B, Adema GJ, Netea MG, Kullberg BJ, Romani L, Figdor CG. The C-type lectin DC-SIGN (CD209) is an antigen-uptake receptor for Candida albicans on dendritic cells. Euro J Immunol 2003; 33:532-8; PMID:12645952; http://dx.doi.org/10.1002/immu.200310029
- Donini M, Zenaro E, Tamassia N, Dusi S. NADPH oxidase of human dendritic cells: role in Candida albicans killing and regulation by interferons, dectin-1 and CD206. Euro J Immunol 2007; 37:1194-203; PMID:17407098; http://dx.doi.org/10.1002/eji.200636532
- Gross O, Gewies A, Finger K, Schäfer M, Sparwasser T, Peschel C, Förster I, Ruland J. Card9 controls a non-TLR signalling pathway for innate anti-fungal immunity. Nature 2006; 442:651-6; PMID:16862125; http://dx.doi.org/10.1038/nature04926
- Kerrigan AM, Brown GD. Syk-coupled C-type lectin receptors that mediate cellular activation via single tyrosine based activation motifs. Immunol Rev 2010; 234:335-52; PMID:20193029; http://dx.doi.org/10.1111/j.0105-2896.2009.00882.x
- Zucchini N, Crozat K, Fau - Baranek T, Baranek T, Fau-Robbins SH, Robbins Sh Fau-Altfeld M, Altfeld M, Fau-Dalod M, Dalod M. Natural killer cells in immunodefense against infective agents. Expert Rev Anti Infect Ther 2008; 6:867-85; PMID:19053900; http://dx.doi.org/10.1586/14787210.6.6.867
- Murphy J, McDaniel D. In vitro reactivity of natural killer (NK) cells against Cryptococcus neoformans. J Immunol 1982; 128:1577-83; PMID:6120974
- Voigt J, Hunniger K, Bouzani M, Jacobsen ID, Barz D, Hube B, Löffler J, Kurzai O. Human natural killer cells acting as phagocytes against Candida albicans and mounting an inflammatory response that modulates neutrophil antifungal activity. J Infect Dis 2014; 209:616-26; PMID:24163416; http://dx.doi.org/10.1093/infdis/jit574
- Schmidt S, Tramsen L, Hanisch M, Latgé J-P, Huenecke S, Koehl U, Lehrnbecher T. Human natural killer cells exhibit direct activity against Aspergillus fumigatus hyphae, but not against resting conidia. J Infect Dis 2011; 203:430-5; PMID:21208932; http://dx.doi.org/10.1093/infdis/jiq062
- Schmidt S, Tramsen L, Perkhofer S, Lass-Flörl C, Hanisch M, Röger F, Klingebiel T, Koehl U, Lehrnbecher T. Rhizopus oryzae hyphae are damaged by human natural killer (NK) cells, but suppress NK cell mediated immunity. Immunobiology 2013; 218:939-44; PMID:23201314; http://dx.doi.org/10.1016/j.imbio.2012.10.013
- Quintin J, Voigt J, van der Voort R, Jacobsen ID, Verschueren I, Hube B, Giamarellos-Bourboulis EJ, van der Meer JW, Joosten LA, Kurzai O, et al. Differential role of NK cells against Candida albicans infection in immunocompetent or immunocompromised mice. Euro J Immunol 2014; 44:2405-14; PMID:24802993; http://dx.doi.org/10.1002/eji.201343828
- Djeu JY, Blanchard DK, Richards AL, Friedman H. Tumor necrosis factor induction by Candida albicans from human natural killer cells and monocytes. J Immunol 1988; 141:4047-52
- Sivori S, Carlomagno S, Pesce S, Moretta A, Vitale M, Marcenaro E. TLR/NCR/KIR: which one to use and when? Front Immunol 2014; 5:105
- Quintin J, Levitz SM. NKp30 enables NK cells to act naturally with fungi. Cell Host Microbe 2013; 14:369-71; PMID:24139394; http://dx.doi.org/10.1016/j.chom.2013.10.001
- Li SS, Kyei SK, Timm-McCann M, Ogbomo H, Jones GJ, Shi M, Xiang RF, Oykhman P, Huston SM, Islam A, et al. The NK receptor NKp30 mediates direct fungal recognition and killing and is diminished in NK cells from HIV-infected patients. Cell Host Microbe 2013; 14:387-97; PMID:24139398; http://dx.doi.org/10.1016/j.chom.2013.09.007
- Wiseman JC, Ma LL, Marr KJ, Jones GJ, Mody CH. Perforin-dependent cryptococcal microbicidal activity in NK cells requires PI3K-dependent ERK1/2 signaling. J Immunol 2007; 178:6456-64; PMID:17475875; http://dx.doi.org/10.4049/jimmunol.178.10.6456
- Orange JS. Formation and function of the lytic NK-cell immunological synapse. Nat Rev Immunol 2008; 8:713-25; PMID:19172692; http://dx.doi.org/10.1038/nri2381
- Law RH, Lukoyanova N, Voskoboinik I, Caradoc-Davies TT, Baran K, Dunstone MA, D'Angelo ME, Orlova EV, Coulibaly F, Verschoor S, et al. The structural basis for membrane binding and pore formation by lymphocyte perforin. Nature 2010; 468:447-51; PMID:21037563; http://dx.doi.org/10.1038/nature09518
- Bar E, Whitney PG, Moor K, Reis e Sousa C, LeibundGut-Landmann S. IL-17 regulates systemic fungal immunity by controlling the functional competence of NK cells. Immunity 2014; 40:117-27; PMID:24412614; http://dx.doi.org/10.1016/j.immuni.2013.12.002
- Whitney PG, Bar E, Osorio F, Rogers NC, Schraml BU, Deddouche S, LeibundGut-Landmann S, Reis e Sousa C. Syk signaling in dendritic cells orchestrates innate resistance to systemic fungal infection. PLoS Pathogens 2014; 10:e1004276; PMID:25033445; http://dx.doi.org/10.1371/journal.ppat.1004276
- Murciano C, Villamón E, O'Connor J-E, Gozalbo D, Gil ML. Killed Candida albicans yeasts and hyphae inhibit gamma interferon release by murine natural killer cells. Infect Immun 2006; 74:1403-6; PMID:16428793; http://dx.doi.org/10.1128/IAI.74.2.1403-1406.2006
- Ortega E, Algarra I, Serrano M, Alvarez de Cienfuegos G, Gaforio J. The use of 7-amino-actinomycin D in the analysis of Candida albicans phagocytosis and opsonization. J Immunolog Methods 2001; 253:189-93; PMID:11384680; http://dx.doi.org/10.1016/S0022-1759(01)00358-1
- Marodi L, Schreiber S, Anderson D, MacDermott R, Korchak H, Johnston Jr R. Enhancement of macrophage candidacidal activity by interferon-gamma. Increased phagocytosis, killing, and calcium signal mediated by a decreased number of mannose receptors. J Clin Investigat 1993; 91:2596-601; PMID:8390485; http://dx.doi.org/10.1172/JCI116498
- Djeu JY, Blanchard D, Halkias D, Friedman H. Growth inhibition of Candida albicans by human polymorphonuclear neutrophils: activation by interferon-gamma and tumor necrosis factor. J Immunol 1986; 137:2980-4; PMID:3093587
- Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol 2011; 11:519-31; PMID:21785456; http://dx.doi.org/10.1038/nri3024
- Bhatnagar N, Hong HS, Krishnaswamy JK, Haghikia A, Behrens GM, Schmidt RE, Jacobs R. Cytokine-activated NK cells inhibit PMN apoptosis and preserve their functional capacity. Blood 2010; 116:1308-16; PMID:20501895; http://dx.doi.org/10.1182/blood-2010-01-264903
- Gerosa F, Baldani-Guerra B, Nisii C, Marchesini V, Carra G, Trinchieri G. Reciprocal activating interaction between natural killer cells and dendritic cells. J Exp Med 2002; 195:327-33; PMID:11828007; http://dx.doi.org/10.1084/jem.20010938
- Lodoen MB, Lanier LL. Natural killer cells as an initial defense against pathogens.
