Sepsis, defined as the systemic inflammatory response syndrome (SIRS) in the setting of suspected infection, is directly responsible for at least 200,000 deaths per year in the United States,Citation1 accounting for more attributable deaths than breast cancer, colon cancer, leukemia, lymphoma, and ovarian cancer combined.Citation2 By consensus, sepsis is defined as infection combined with the systemic inflammatory response syndrome (SIRS).Citation3 The pathophysiology of sepsis is complex, and the relative contributions of host-mediated responses and the specific causative pathogen to poor patient outcomes are unclear. In a patient population with clearly defined bloodstream infections, we sought to determine whether acute bloodstream infection with different pathogens was associated with different concentrations of host biomarkers reflective of different physiologic processes.
From the standpoint of the host response, there are substantial differences between the degree of acute inflammation induced by specific bacterial species in pre-clinical animal model systems with controlled inocula of pathogen.Citation4–8 In pediatric populations with hematologic malignancy, differences were observed in inflammatory cytokine production in patients with gram-positive cocci when compared to gram-negative bacilli infections.Citation9 In adults, higher levels of pro-inflammatory IL-6 have been reported in patients with gram-negative vs. gram-positive bacteremia when measured during routine clinical care.Citation10 Higher levels of the pro-inflammatory cytokines IL1β, IL-6, and IL18 responses have been reported in human leukocytes stimulated with LPS when compared with heat-killed S. aureus, a finding mirrored in the elevations of the serum of patients with unspecified “infections” from gram-positive pathogens.Citation11 The in vitro differences are contrasted by in vivo findings from a different group who found no difference in IL-6 between patients with sepsis from pure gram-positive infection when compared to pure gram-negative infections.Citation12 No differences were found at the level of mRNA expression of neutrophils from patients with gram-positive versus gram-negative infections.Citation13 In terms of clinical outcomes using administrative databases, authors have reported either no difference in outcome Citation14 or a roughly 6% crude mortality difference with respect to the inciting pathogen.Citation15
In experimental systems, the acute hyperinflammatory response has traditionally been thought to be followed by a subsequent compensatory antiflammatory response syndrome (CARS) where patients are thought to be at risk from pathogens of normally low virulence.Citation16,17 This two-phase response has been brought into question recently as markers associated with both inflammation and an anti-inflammatory response (e.g. IL-10) are elevated simultaneously in both routine clinical care and experimental systems.Citation18,19 We are aware of no data examining the host response to pathogens of normally low virulence.
Immunologic signals are thought to mediate organ dysfunction by contributing to disruption of endothelial integrity.Citation20–22 Soluble TNF receptor (sTNFR-1) is considered to play an anti-inflammatory role by binding TNF and preventing activation of the membrane form of TNFR on target cells.Citation23,24 sTNFR-1 has been associated with outcomes in sepsis but has not been investigated in the context of different pathogens.Citation25 The Ang-1-Ang-2 axis has emerged as a strong candidate biomarker for measurement endothelial physiology. Ang-1 is present at stable, detectable levels in the blood of healthy volunteers and serves to maintain endothelial integrity.Citation26 Ang-2 is not normally present in the circulation of healthy individuals but acute inflammation induces the release of Ang- 2 from endothelial cells.Citation27 Ang-2 competes with Ang-1 for binding to the tyrosine kinase receptor, Tie-2, promoting microvascular leak in preclinical mouse models.Citation28 The response of the angiopoietin system to different classes of endovascular bacterial infection has not been investigated to date.
Septic patients also have an increase in cellular apoptosis, primarily occurring in the spleen and lymphatic organs.Citation29,30 Signaling via the canonical death receptor FAS (CD95) receptor is a key event in the one of the major pathways that induces apoptosis.Citation31,32 Gram-negative bacteria contain a conserved Type III secretion system that targets this pathway, which is absent in gram-positive bacteria.Citation33 It is unknown if there are differences in FAS mediated responses with respect to different pathogens but sFAS has been associated with the development of sepsis after trauma.Citation34
Since there is complexity in the host response to severe infection, biomarker panels have been proposed as a method to capture composite information.Citation35 If different responses could be observed early between bacterial class, differential patterns of biomarker concentrations could be used to stratify patients for novel interventions that targeted various elements of the host response. Efforts to understand the different host response to various pathogens are complementary to current efforts to improve the rapidity of microbiologic identification using molecular techniques.Citation36,37 For example, if low virulence organisms were associated with an altered ratio of inflammatory to anti-inflammatory markers, it would suggest that immunostimulatory strategies should be pursued.
The goals of our pilot study were to determine: 1) whether sepsis caused by specific classes of bacterial pathogens is associated with differences in concentrations of biomarkers reflective of the host response, and 2) whether bloodstream infection with different pathogens is associated with variations in clinical outcomes in sepsis.
Subjects: The Harborview SIRS cohort prospectively enrolled subjects meeting at least 2 SIRS criteria within 24 hours of enrollment who were admitted to the ICU and clinically suspected to have infection from December 2006-December 2010, as previously described.Citation38 Exclusion criteria included known immunosuppression, HIV infection, current diagnosis of cancer, major trauma, or intracranial hemorrhage. For all subjects, plasma samples were obtained within 24 hours of enrollment. The cohort was initially restricted to Caucasians to minimize the potential for confounding due to underlying genetic differences. Of this larger prospective cohort, we retrospectively identified a subset of 100 patients who had a defined bloodstream infection within 24 hours of when the plasma sample was obtained. Bloodstream infection was defined according to Centers for Disease Control and Prevention (CDC) criteria.Citation39 In concordance with CDC criteria, we excluded patients with microbiologic growth of an alternate pathogen at any other site, and each infection was classified as “gram-positive cocci,” “gram-negative bacilli” or “low virulence” on the basis of the pathogen's reported ability to cause clinical sepsis in immunocompetent hosts.Citation40 We applied strict criteria to the low virulence class that included at least two positive cultures and an absence of an alternative diagnosis at any other site, given the fact that many of the organisms in this class (e.g.,, coagulase negative staphylococci) are common colonizers of the skin. See supplementary Table 1 for full details of the organisms identified. APACHEIII scores were calculated using data from the first 24 hours after admission.
Protein measurement: Frozen plasma samples were thawed no more than twice and concentrations of IL-6, IL-8, G-CSF, sTNFR-1, Ang-1, Ang-2, sVCAM-1 and sFAS were measured using a multiplex immunoassay (Meso Scale Discovery, Rockville, MD). To fit the samples to a standard curve, samples were diluted. The dynamic ranges of the assays were as follows: IL-6, IL-8, sTNFR-1, G-CSF (0.08–2500 pg/mL); Ang-1 (3–100,000 pg/mL); Ang-2 (0.5 to 10,000 pg/mL); and sVCAM-1 (0.05 to 1000ng/mL). In 9 patients, there were insufficient sample volume for testing of IL-6, IL-6, G-CSF, or sTNFR-1. Samples above or below the range of the assay were assigned the upper or lower limit of the assay, respectively.
Statistical analysis
We used the Mann-Whitney U test for comparison between the groups “alive at 28 days” and “dead at 28 days.” The data were not normally distributed, so we transformed biomarker concentrations using log-2 transformation in order to apply linear models. We generated a Spearman correlation matrix in order to determine the degree of correlation between each biomarker when compared to each other biomarker. Weak correlation was defined as a Spearman correlation of less than 0.5.
Our initial analysis utilized unadjusted logistic regression to test for associations with biomarkers and 28-day mortality and hypotension. Hypotension was defined as a systolic blood pressure below 90 mmHg with at least 500 cc of crystalloid fluid administered or receipt of a vasopressor.Citation41 Since hypotension is included in the APACHE III definition, we adjusted for we then adjusted for the potential covariates Age, Gender, Chronic Renal Insufficiency, Smoking status, and Diabetes Mellitus. We then incorporated the Acute Physiology and Chronic Health Evaluation (APACHE) III score Citation42 into our model in order to adjust for potential confounders with respect to mortality.
We also tested for unadjusted associations with each class of bacteria with respect to 28-day mortality or hypotension, using gram-positive cocci as the reference. As above, we then proceeded to use multivariable logistic regression models to adjust for covariates and acute illness. We tested for differences between biomarker concentrations in patients with bloodstream infection using bacterial class as a categorical variable using a Mann Whitney U test, considering p < 0.05 to represent statistical significance.
Statistical analysis was performed using R 3.00 and graphics were generated in R unless otherwise specified. The dot plots graphs for each biomarker were generated using Prism 6 (Graphpad Software).
The demographics of the patients who survived to 28 days did not differ statistically from those who died within 28 days (). The population was composed primarily of patients admitted to the medicine service (83%), and had a median age of 53.5 years. Roughly half of all patients experienced hypotension within 72 hours. The mean APACHEIII score was 53.7 (+/−27.6) in patients who survived to 28 days and 80.2 +/− 38.7 in patients who died. In our population, 17% of all patients in the cohort died within 28 days. Because of low sample volume, we were unable to perform biomarker analysis on nine samples.
Table 1. Demographics of HMS SIRS study populations.
Correlations among pro-inflammatory biomarkers
We evaluated the correlation of each biomarker to each other biomarker using a Spearman matrix (Supplementary Table 2). In general, biomarkers were weakly correlated across pre-established categories and more strongly correlated within categories. Notable exceptions included the following: weak correlation (Spearman correlation <0.5) within the category of “endothelial homeostasis” (Ang-1, Ang-2, and sVCAM-1); strong correlation (Spearman correlation >.5) between sFAS and sTNFR-1 (0.68); and sFAS and sVCAM-1 (0.56).
Biomarker association with 28 day mortality
There were differences in the mean of biomarker concentrations between patients who survived and patients who did not, as shown in . In unadjusted logistic regression analysis (), all biomarkers were associated with 28-day mortality with a p value < 0.05 (with the exception of Ang-1 and G-CSF). The ratio of Ang-2/Ang-1 was associated with 28-day mortality with an odds ratio of 1.26 (1.00–1.61). All biomarkers were associated with the development of hypotension in unadjusted analysis (data not shown).
Table 2. Comparison of plasma biomarkers and 28-day mortality.
Table 3. Multivariate analysis of plasma biomarkers association with clinical outcomes.
Since tobacco use, age, chronic renal insufficiency and diabetes could influence the development of hypotension and or the expression of biomarkers, we then adjusted for these covariates via logistic regression adjustment for age, gender, smoking, and presence of diabetes (after log-2 transformation). Only sVCAM-1 and sFAS remained independently associated with the development of hypotension.
The APACHEIII score does not account for tobacco use, chronic renal insufficiency, diabetes or gender,Citation42 so we then included both covariates of gender, smoking, chronic renal insufficiency, and presence of diabetes in combination with APACHEIII in a logistic model, and we determined that sFAS remained independently associated with mortality with an odds ratio of 2.89 (1.10–7.56).
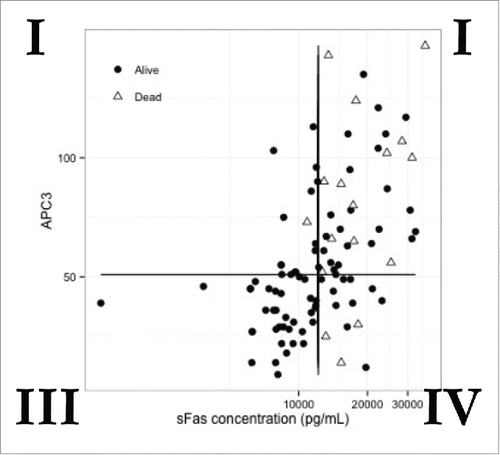
We identified a population of patients with high sFAS and APACHEIII <50 with poor outcomes (quadrant IV of ). The median sFAS concentration in our population was 12168 pg/mL. Only one patient with sFAS measurement slightly below the median (10884 pg/mL) did not survive to 28 days.
Bacterial class and association with 28-day mortality and hypotension
We classified patients with bloodstream infections into “gram-positive cocci,” “gram-negative bacilli,” and “low virulence organisms.” “Gram-positive cocci” infections were largely Staphylococcus aureus, whereas “gram-negative bacilli” infections were composed of enteric pathogens (mostly Escherichia coli). Low virulence organisms were predominantly coagulase negative Staphylococci (Table S1). Using “gram-positive” infections as the reference, no single organism class was uniquely associated with 28-day mortality or development of septic shock. Adjustment for either covariates or severity of illness (APACHEIII) did not change the association results ().
Table 4. Multivariate analysis of organism type association with clinical outcomes.
Bacterial class and biomarker concentrations
We then examined whether bacterial class was associated with different levels of host biomarker expression. There was no statistically appreciable difference between biomarker concentrations with respect to sVCAM-1, sTNFR-1, IL-6, IL-8, or sFAS (). However, individuals with “gram-negative bacilli” infections had higher circulating plasma concentrations of Ang-2/Ang-1 when compared to individuals with “gram-positive cocci” (p = 0.0184) or “low virulence” infections (p = 0.0088). Individuals with “gram-negative bacilli” infections had higher concentrations of G-CSF when compared to individuals with “low virulence” infections (p = 0.0096).
Figure 1. Scatter Plots of Circulating Plasma Biomarker Concentrations According to Bacterial Class IL-6, IL-8, sTNFR-1, granulocyte-colony stimulating factor, soluble FAS, soluble vascular cell adhesion molecule-1, and Ang2/Ang1 were measured in the plasma of 100 subjects meeting CDC criteria for bloodstream infection and sepsis within 24 of admission to the ICU. The graphs depict individual subjects (symbols) and a horizontal line representing the mean with vertical lines representing the standard error measures for each biomarker. Mann-Whitney U test was used to compare the medians of each biomarker. *:p < 0.05, ** p < 0.01.
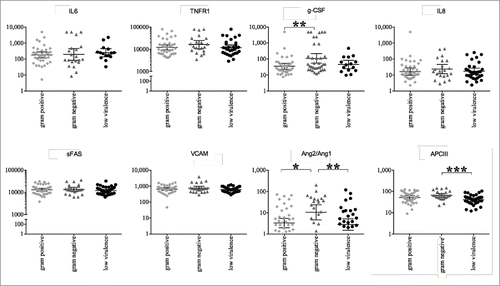
Figure 2. sFAS Discriminates a Subset of Patients with Low APACHEIII who Subsequently Died of Sepsis.The measured values for soluble FAS and APACHEIII score were calculated for individual patients with sepsis from defined bloodstream infection. The values were plotted for each individual patient on an X and Y axis. The population was divided into four quadrants with the APACHEIII divided into populations above and below the clinically meaningful value of 50. Survivors are depicted with circles and nonsurvivors with triangles.
In our population of patients with sepsis caused by bacterial bloodstream infection, elevated circulating levels of sFAS were strongly associated with 28-day mortality. Bacterial class, however, was not associated with the clinical outcome of hypotension or 28 day-mortality or substantial difference in the pattern of putative biomarker concentrations.
Our report is consistent with prior reports that also found no evidence for differences in clinical outcome by pathogen.Citation43 We are the first group to demonstrate that in a restricted cohort of patients with bacteremia, the host biomarker profile does not vary substantially by the infectious etiology.
The lack of association of bacterial class with mortality is similar to a study by Abe et al. of a Japanese population with bloodstream infection.Citation10 Our findings contrast with those of Abe et al. with respect to IL-6, as their group identified higher IL-6 levels in patients with gram-negative bacteremia when compared to gram-positive cocci infections. This difference could be due to patient populations, given that their population was entirely of Japanese descent whereas our cohort was uniformly Caucasian. Also, there are potential differences in the underlying methodology. The population in the Abe et al. study were drawn from a larger inpatient population that had IL-6 levels measured as part of routine clinical care (and was identified retrospectively), rather than enrolled as part of a prospective observational cohort of patients with suspected sepsis.
In summary, our data demonstrate that candidate biomarkers reflective of the host response to sepsis were associated with mortality in bacteremic patients whereas the type of bacterial class was not associated with mortality or the development of shock. Host biomarkers have been associated with clinical outcomes previously.Citation9–12, 19, 25, 46
The association with host-derived sFAS remained following adjustment for covariates, acuity of illness (APACHEIII score) or a combination of acuity of illness and covariates (). Only one patient with sFAS slightly below the median did not survive to 28 days (). These data suggest that circulating sFAS in patients with bacteremic sepsis provides may provide clinically informative insight into a process not currently captured by standard information or the APACHEIII score. Importantly, increased Ang-2/Ang-1 ratios were commonly observed in this population along with elevations in biomarkers reflective of inflammation (IL-6, IL-8). Elevations in all of these markers were associated with 28-day mortality in unadjusted analysis, but the association did not remain after incorporating clinical information into our model (). The latter finding is most likely due to the relatively low number of mortality events (only 17 deaths) in this cohort.
The strengths of our study include prospective enrollment, a rigorous definition of bloodstream infection, and early measurement of candidate biomarkers that coincides with the onset of clinical deterioration. Some of the limitations are that our population is derived from a single center with a uniformly Caucasian population, and there were a relatively low number of total outcomes (only 17), so our findings require replication in larger, more heterogeneous cohorts. Furthermore, we can only speculate as to the causal basis for the association with an increase in observed mortality. Our hypothesis is that the source of sFAS is primarily lymphocytes based upon autopsy studies of septic patients that have demonstrated an increased in immunohistochemical markers of apoptosis in the spleen.Citation44 Lymphocyte apoptosis could affect the host ability to respond to secondary infections, consistent with our observation that 41% (7/17) of patients died after seven days of hospitalization. Alternatively, elevations in sFAS could represent a proxy for susceptibility to other forms of end-organ damage. A previous investigation using this cohort determined that polymorphisms in sFAS were associated with development of acute lung injury.Citation38
This study is the first to document an association with mortality and sFAS levels in severe infection and to demonstrate that sFAS concentration was associated with 28-day mortality even after adjustment for available clinical information. Another group did not find an association with sFAS and 28-day mortality in patients with bacteremia.Citation45 We suspect that key differences in patient populations account for this discrepancy. Specifically, our population was drawn prospectively from patients experiencing physiologic disturbance leading to ICU admission, whereas Huttunen et al. selected their study population from a mixed inpatient group on the basis of bacterial growth in blood cultures drawn during routine clinical care. Furthermore, our population had sampling of plasma for biomarkers within 24 hours of the blood cultures, such that the biological information captured by the measurement of biomarkers was at or before the time of bloodstream infection.
sFAS levels below the median early in the development of sepsis were strongly associated with protection from mortality at 28-days. Elevations in the plasma concentration of the endothelial markers sVCAM-1 and Ang-2/Ang-1, the anti-nflammatory signal sTNFR-1, and the proinflammatory signals G-CSF, IL-6, IL-8 were also associated with mortality. We have recently demonstrated similar associations in a broader population of patients with SIRS.Citation46 Because the host response in our population was relatively uniform irrespective of pathogen (with the exception of Ang-2/Ang-1 and G-CSF), our findings support the importance of endothelial dysfunction and/or apoptosis in the pathophysiology of sepsis irrespective of pathogen. Our findings support the general concept that host response to infection rather than the causative pathogen per se determines patient outcome in clinical sepsis.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Author contributions
WOH, CM, JH, MMW, and WCL designed the study. CM and SHB performed and analyzed the experiments. BLP and RK performed statistical analysis. WOH, MMH and WCL interpreted the results and drafted the manuscript. All authors reviewed, revised and approved the manuscript for submission.
KVIR_A_S1144003.docx
Download MS Word (13.3 KB)Funding
Funding was provided by NIH T32AI007044-39 (WOH), NIH K23HL120896, and Parker B Francis Fellowship (CM), and Department of Medicine Research Fund (WCL).
References
- Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 2001; 29:1303–10; PMID:11445675; http://dx.doi.org/10.1097/00003246-200107000-00002
- SEER Cancer Stat Fact Sheets [Internet]. [cited 2014 Oct 3]; Available from: http://seer.cancer.gov/statfacts/
- Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 2013; 41:580–637; PMID:23353941; http://dx.doi.org/10.1097/CCM.0b013e31827e83af
- Kimbrell MR, Warshakoon H, Cromer JR, Malladi S, Hood JD, Balakrishna R, Scholdberg TA, David SA. Comparison of the immunostimulatory and proinflammatory activities of candidate Gram-positive endotoxins, lipoteichoic acid, peptidoglycan, and lipopeptides, in murine and human cells. Immunol Lett 2008; 118:132–41; PMID:18468694; http://dx.doi.org/10.1016/j.imlet.2008.03.009
- Branger J, Knapp S, Weijer S, Leemans JC, Pater JM, Speelman P, Florquin S, van der Poll T. Role of Toll-like receptor 4 in gram-positive and gram-negative pneumonia in mice. Infect Immun 2004; 72:788–94; PMID:14742522; http://dx.doi.org/10.1128/IAI.72.2.788-794.2004
- Elson G, Dunn-Siegrist I, Daubeuf B, Pugin J. Contribution of Toll-like receptors to the innate immune response to Gram-negative and Gram-positive bacteria. Blood 2007; 109:1574–83; PMID:17038528; http://dx.doi.org/10.1182/blood-2006-06-032961
- Björk L, Andersson J, Ceska M, Andersson U. Endotoxin and Staphylococcus aureus enterotoxin A induce different patterns of cytokines. Cytokine 1992; 4:513–9; PMID:1292633; http://dx.doi.org/10.1016/1043-4666(92)90013-H
- McConnell KW, McDunn JE, Clark AT, Dunne WM, Dixon DJ, Turnbull IR, Dipasco PJ, Osberghaus WF, Sherman B, Martin JR, et al. Streptococcus pneumoniae and Pseudomonas aeruginosa pneumonia induce distinct host responses. Crit Care Med 2010; 38:223–41; PMID:19770740; http://dx.doi.org/10.1097/CCM.0b013e3181b4a76b
- Xu X-J, Tang Y-M, Liao C, Song H, Yang S-L, Xu W-Q, Shi S-W, Zhao N. Inflammatory cytokine measurement quickly discriminates gram-negative from gram-positive bacteremia in pediatric hematology/oncology patients with septic shock. Intensive Care Med 2013; 39:319–26; PMID:23179333; http://dx.doi.org/10.1007/s00134-012-2752-4
- Abe R, Oda S, Sadahiro T, Nakamura M, Hirayama Y, Tateishi Y, Shinozaki K, Hirasawa H. Gram-negative bacteremia induces greater magnitude of inflammatory response than Gram-positive bacteremia. Crit Care 2010; 14:R27; PMID:20202204; http://dx.doi.org/10.1186/cc8898
- Feezor RJ, Oberholzer C, Baker HV, Novick D, Rubinstein M, Moldawer LL, Pribble J, Souza S, Dinarello CA, Ertel W, et al. Molecular characterization of the acute inflammatory response to infections with gram-negative vs. gram-positive bacteria. Infect Immun 2003; 71:5803–13; PMID:14500502; http://dx.doi.org/10.1128/IAI.71.10.5803-5813.2003
- Kinasewitz GT, Yan SB, Basson B, Comp P, Russell JA, Cariou A, Um SL, Utterback B, Laterre PF, Dhainaut JF, et al. Universal changes in biomarkers of coagulation and inflammation occur in patients with severe sepsis, regardless of causative micro-organism [ISRCTN74215569]. Crit Care 2004; 8:R82–90; PMID:15025782; http://dx.doi.org/10.1186/cc2459
- Tang BMP, McLean AS, Dawes IW, Huang SJ, Cowley MJ, Lin RCY. Gene-expression profiling of gram-positive and gram-negative sepsis in critically ill patients. Crit Care Med 2008; 36:1125–8; PMID:18379237; http://dx.doi.org/10.1097/CCM.0b013e3181692c0b
- Zahar JR, Timsit JF, Garrouste-Orgeas M, Français A, Vesin A, Vesim A, Descorps-Declere A, Dubois Y, Souweine B, Haouache H, et al. Outcomes in severe sepsis and patients with septic shock: pathogen species and infection sites are not associated with mortality. Crit Care Med 2011; 39:1886–95; PMID:21516036; http://dx.doi.org/10.1097/CCM.0b013e31821b827c
- Ani C, Farshidpanah S, Bellinghausen Stewart A, Nguyen HB. Variations in organism-specific severe sepsis mortality in the United States: 1999-2008. Crit Care Med 2015; 43:65–77; PMID:25230374; http://dx.doi.org/10.1097/CCM.0000000000000555
- Osuchowski MF, Welch K, Yang H, Siddiqui J, Remick DG. Chronic sepsis mortality characterized by an individualized inflammatory response. J Immunol 2007; 179:623–30; PMID:17579084; http://dx.doi.org/10.4049/jimmunol.179.1.623
- Hotchkiss RS, Coopersmith CM, McDunn JE, Ferguson TA. The sepsis seesaw: tilting toward immunosuppression. Nat Med 2009; 15:496–7; PMID:19424209; http://dx.doi.org/10.1038/nm0509-496
- Osuchowski MF, Craciun F, Weixelbaumer KM, Duffy ER, Remick DG. Sepsis chronically in MARS: systemic cytokine responses are always mixed regardless of the outcome, magnitude, or phase of sepsis. J Immunol 2012; 189:4648–56; PMID:23008446; http://dx.doi.org/10.4049/jimmunol.1201806
- Tamayo E, Fernández A, Almansa R, Carrasco E, Heredia M, Lajo C, Goncalves L, Gómez-Herreras JI, de Lejarazu RO, Bermejo-Martin JF. Pro- and anti-inflammatory responses are regulated simultaneously from the first moments of septic shock. Eur Cytokine Netw 2011; 22:82–7; PMID:21628135
- Hutchins NA, Chung CS, Borgerding JN, Ayala CA, Ayala A. Kupffer cells protect liver sinusoidal endothelial cells from Fas-dependent apoptosis in sepsis by down-regulating gp130. Am J Pathol 2013; 182:742–54; PMID:23306157; http://dx.doi.org/10.1016/j.ajpath.2012.11.023
- Gais P, Reim D, Jusek G, Rossmann-Bloeck T, Weighardt H, Pfeffer K, Altmayr F, Janssen K-P, Holzmann B. Cutting edge: Divergent cell-specific functions of MyD88 for inflammatory responses and organ injury in septic peritonitis. J Immunol 2012; 188:5833–7; PMID:22586041; http://dx.doi.org/10.4049/jimmunol.1200038
- Lerolle N, Nochy D, Guérot E, Bruneval P, Fagon JY, Diehl JL, Hill G. Histopathology of septic shock induced acute kidney injury: apoptosis and leukocytic infiltration. Intensive Care Med 2010; 36:471–8; PMID:19924395; http://dx.doi.org/10.1007/s00134-009-1723-x
- Yagi H, Soto-Gutierrez A, Navarro-Alvarez N, Nahmias Y, Goldwasser Y, Kitagawa Y, Tilles AW, Tompkins RG, Parekkadan B, Yarmush ML. Reactive bone marrow stromal cells attenuate systemic inflammation via sTNFR1. Mol Ther 2010; 18:1857–64; PMID:20664529; http://dx.doi.org/10.1038/mt.2010.155
- Mohler KM, Torrance DS, Smith CA, Goodwin RG, Stremler KE, Fung VP, Madani H, Widmer MB. Soluble tumor necrosis factor (TNF) receptors are effective therapeutic agents in lethal endotoxemia and function simultaneously as both TNF carriers and TNF antagonists. J Immunol 1993; 151:1548–61; PMID:8393046
- Oberholzer A, Souza SM, Tschoeke SK, Oberholzer C, Abouhamze A, Pribble JP, Moldawer LL. Plasma cytokine measurements augment prognostic scores as indicators of outcome in patients with severe sepsis. Shock 2005; 23:488–93; PMID:15897799
- Gamble JR, Drew J, Trezise L, Underwood A, Parsons M, Kasminkas L, Rudge J, Yancopoulos G, Vadas MA. Angiopoietin-1 is an antipermeability and anti-inflammatory agent in vitro and targets cell junctions. Circ Res 2000; 87:603–7; PMID:11009566; http://dx.doi.org/10.1161/01.RES.87.7.603
- Benest AV, Kruse K, Savant S, Thomas M, Laib AM, Loos EK, Fiedler U, Augustin HG. Angiopoietin-2 is critical for cytokine-induced vascular leakage. PLoS One 2013; 8:e70459; PMID:23940579; http://dx.doi.org/10.1371/journal.pone.0070459
- Ziegler T, Horstkotte J, Schwab C, Pfetsch V, Weinmann K, Dietzel S, Rohwedder I, Hinkel R, Gross L, Lee S, et al. Angiopoietin 2 mediates microvascular and hemodynamic alterations in sepsis. J Clin Invest [Internet] 2013; 123(8):3436–3445
- Hotchkiss RS, Tinsley KW, Swanson PE, Schmieg RE, Jr, Hui JJ, Chang KC, Osborne DF, Freeman BD, Cobb JP, Buchman TG, et al. Sepsis-induced apoptosis causes progressive profound depletion of B and CD4+ T lymphocytes in humans. J Immunol 2001; 166:6952–63; PMID:11359857; http://dx.doi.org/10.4049/jimmunol.166.11.6952
- Marsik C, Halama T, Cardona F, Wlassits W, Mayr F, Pleiner J, Jilma B. Regulation of Fas (APO-1, CD95) and Fas ligand expression in leukocytes during systemic inflammation in humans. Shock 2003; 20:493–6; PMID:14625471; http://dx.doi.org/10.1097/01.shk.0000097248.97298.ea
- Hotchkiss RS, Osmon SB, Chang KC, Wagner TH, Coopersmith CM, Karl IE. Accelerated lymphocyte death in sepsis occurs by both the death receptor and mitochondrial pathways. J Immunol 2005; 174:5110–8; PMID:15814742; http://dx.doi.org/10.4049/jimmunol.174.8.5110
- De Freitas I, Fernández-Somoza M, Essenfeld-Sekler E, Cardier JE. Serum levels of the apoptosis-associated molecules, tumor necrosis factor-α/tumor necrosis factor type-I receptor and Fas/FasL, in sepsis. Chest 2004; 125:2238–46; http://dx.doi.org/10.1378/chest.125.6.2238
- Li S, Zhang L, Yao Q, Li L, Dong N, Rong J, Gao W, Ding X, Sun L, Chen X, et al. Pathogen blocks host death receptor signalling by arginine GlcNAcylation of death domains. Nature 2013; 501:242–6; PMID:23955153; http://dx.doi.org/10.1038/nature12436
- Paunel-Görgülü A, Flohé S, Scholz M, Windolf J, Lögters T. Increased serum soluble Fas after major trauma is associated with delayed neutrophil apoptosis and development of sepsis. Crit Care 2011; 15:R20; PMID:21232130; http://dx.doi.org/10.1186/cc9965
- Ventetuolo CE, Levy MM. Biomarkers: diagnosis and risk assessment in sepsis. Clin Chest Med 2008; 29:591–603, vii; PMID:18954695; http://dx.doi.org/10.1016/j.ccm.2008.07.001
- Jordana-Lluch E, Giménez M, Quesada MD, Rivaya B, Marcó C, Domínguez MJ, Arméstar F, Martró E, Ausina V. Evaluation of the Broad-Range PCR/ESI-MS technology in blood specimens for the molecular diagnosis of bloodstream infections. PLoS One 2015; 10:e0140865; PMID:26474394; http://dx.doi.org/10.1371/journal.pone.0140865
- Haag H, Locher F, Nolte O. Molecular diagnosis of microbial aetiologies using SepsiTestTM in the daily routine of a diagnostic laboratory. Diagn Microbiol Infect Dis 2013; 76:413–8; PMID:23747029; http://dx.doi.org/10.1016/j.diagmicrobio.2013.04.027
- Glavan BJ, Holden TD, Goss CH, Black RA, Neff MJ, Nathens AB, Martin TR, Wurfel MM. ARDSnet Investigators. Genetic variation in the FAS gene and associations with acute lung injury. Am J Respir Crit Care Med 2011; 183:356–63; PMID:20813889; http://dx.doi.org/10.1164/rccm.201003-0351OC
- Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control 2008; 36:309–32; PMID:18538699; http://dx.doi.org/10.1016/j.ajic.2008.03.002
- Hirakata Y, Furuya N, Iwata M, Kashitani F, Ishikawa M, Yumoto S, Yasui K, Endoh H, Marui A, Kaku M, et al. Assessment of clinical significance of positive blood cultures of relatively low-virulence isolates. J Med Microbiol 1996; 44:195–8; PMID:8636936; http://dx.doi.org/10.1099/00222615-44-3-195
- Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med 2003; 31:1250–6; PMID:12682500; http://dx.doi.org/10.1097/01.CCM.0000050454.01978.3B
- Knaus WA, Wagner DP, Draper EA, Zimmerman JE, Bergner M, Bastos PG, Sirio CA, Murphy DJ, Lotring T, Damiano A. The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. Chest 1991; 100:1619–36; PMID:1959406; http://dx.doi.org/10.1378/chest.100.6.1619
- Opal SM, Garber GE, LaRosa SP, Maki DG, Freebairn RC, Kinasewitz GT, Dhainaut JF, Yan SB, Williams MD, Graham DE, et al. Systemic host responses in severe sepsis analyzed by causative microorganism and treatment effects of drotrecogin alfa (activated). Clin Infect Dis 2003; 37:50–8; PMID:12830408; http://dx.doi.org/10.1086/375593
- Hotchkiss RS, Swanson PE, Freeman BD, Tinsley KW, Cobb JP, Matuschak GM, Buchman TG, Karl IE. Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunction. Crit Care Med 1999; 27:1230–51; PMID:10446814; http://dx.doi.org/10.1097/00003246-199907000-00002
- Huttunen R, Syrjänen J, Vuento R, Laine J, Hurme M, Aittoniemi J. Apoptosis markers soluble Fas (sFas), Fas Ligand (FasL) and sFas/FasL ratio in patients with bacteremia: a prospective cohort study. J Infect 2012; 64:276–81; PMID:22207003; http://dx.doi.org/10.1016/j.jinf.2011.12.006
- Mikacenic C, Hahn WO, Price BL, Harju-Baker S, Katz R, Kain KC, Himmelfarb J, Liles WC, Wurfel MM. Biomarkers of Endothelial Activation Are Associated with Poor Outcome in Critical Illness. PLoS One 2015; 10:e0141251; PMID:26492036; http://dx.doi.org/10.1371/journal.pone.0141251
