ABSTRACT
Acinetobacter baumannii is a nosocomial pathogen that has a considerable ability to survive in the hospital environment partly due to its capacity to form biofilms. The first step in the process of establishing an infection is adherence of the bacteria to target cells. Chaperone-usher pili assembly systems are involved in pilus biogenesis pathways that play an important role in adhesion to host cells and tissues as well as medically relevant surfaces. After screening a collection of strains, a biofilm hyper-producing A. baumannii strain (MAR002) was selected to describe potential targets involved in pathogenicity. MAR002 showed a remarkable ability to form biofilm and attach to A549 human alveolar epithelial cells. Analysis of MAR002 using transmission electron microscopy (TEM) showed a significant presence of pili on the bacterial surface. Putative protein-coding genes involved in pili formation were identified based on the newly sequenced genome of MAR002 strain (JRHB01000001/2 or NZ_JRHB01000001/2). As assessed by qRT-PCR, the gene LH92_11085, belonging to the operon LH92_11070-11085, is overexpressed (ca. 25-fold more) in biofilm-associated cells compared to exponential planktonic cells. In the present work we investigate the role of this gene on the MAR002 biofilm phenotype. Scanning electron microscopy (SEM) and biofilm assays showed that inactivation of LH92_11085 gene significantly reduced bacterial attachment to A549 cells and biofilm formation on plastic, respectively. TEM analysis of the LH92_11085 mutant showed the absence of long pili formations normally present in the wild-type. These observations indicate the potential role this LH92_11085 gene could play in the pathobiology of A baumannii.
Introduction
Acinetobacter baumannii is a non-fermentative, oxidase negative, non-flagellated, Gram-negative coccobacillus that has emerged as an important hospital pathogen causing severe infections in compromised patients, including bacteremia and pneumonia.Citation1-3 The remarkable ability of this pathogen to develop antibiotic resistance and persist in stressful or otherwise unfavorable conditions makes the prevention and treatment of the infections caused by this pathogen difficult. Recently, outbreaks caused by multiresistant strains of A. baumannii have emerged, causing serious health problems.Citation4-6 Consequently, A. baumannii was recently listed as one of the six most dangerous opportunistic pathogens worldwide.Citation3,7 The ability of A. baumannii to form biofilms may explain its extreme resistance to antibiotics and many other antimicrobial agents as well as its ability to evade host defenses.Citation8-10 A bacterial biofilm is an organized community of bacteria embedded within a self-produced matrix made of extracellular polymeric substances.Citation11,12 The A. baumannii colonization of mucosal surfaces or contamination of medical devices, such as intravascular catheters or endotracheal intubation devices may result in biofilm formation, increasing the risk of bloodstream and respiratory infections.Citation13,14 Processes involved in biofilm formation by A. baumannii are controlled by complex regulatory networks, including those based on the presence of antibiotic resistance genes, environmental conditions, or cell density.Citation15-18 A recent study established the complete transcriptome profiles of planktonic and biofilm cells of A. baumannii ATCC 17978 revealing 1,621 genes over-expressed in biofilm cells compared to planktonic cells and 55 genes only expressed in sessile bacteria.Citation19 To date, several gene products have been shown to be involved in adhesiveness and biofilm formation by A. baumannii. The CsuA/BABCDE pilus chaperone-usher assembly system, which is regulated by the two-component regulatory system BfmS/BfmR,Citation20 is essential for the attachment to abiotic surfaces by A. baumannii ATCC 19606T strain.Citation21 In addition, the 854-kDa outer membrane protein homolog of the staphylococcal biofilm-associated protein (Bap) was described as involved in the stabilization of A. baumannii biofilms.Citation22 Actually, BAP-like proteins 1 and 2 (BLP1 and BLP2) were shown to be involved in the abilities of A. baumannii AYE strain to form biofilm and to adhere to epithelial cells.Citation23 The outer membrane protein OmpA has also been described in A. baumannii to be involved in the development of robust biofilms on plastic and it has been characterized as essential for bacterial attachment to A549 human alveolar epithelial cells.Citation24 Pili play an important role in adhesion to biotic or abiotic surfaces and in biofilm formation. Pili are 1- to 3-µm-long hair-like bacterial appendages with diameters between 2 and 8 nm built by protein subunits called pilins. Chaperone-usher assembly systems are involved in pilus biogenesis pathways that harbor genes coding for usher, chaperone and fimbrial/pilus subunit proteins. The chaperone and usher proteins are the accessory proteins needed to assemble subunits into a pilus and secrete the final assembled appendage.Citation25 In 2008, Tomaras et al.Citation20 correlated the expression of the csuA/BABCDE chaperone-usher pilus assembly operon with the presence of pili around the cell surface. Chabane et al.Citation26 studied the pellicle formation of A. baumannii ATCC 17978 and found three subunits of pili encoded by genes A1S_2218 (CsuA/B), A1S_1510 and A1S_2091, assembled by chaperone-usher systems and coding for the most abundant proteins embedded within the pellicle matrix. The recent genome analysis of A. baumannii strain MAR002Citation27 allowed us to identify the LH92_11085 gene which has a role in adherence and biofilm formation. In the present work the LH92_11085 gene has been functionally characterized for the first time. We demonstrated that this gene is necessary for mature biofilm formation and adherence to eukaryotic cells being implicated in the virulence of this microorganism.
Results
Biofilm formation in Acinetobacter baumannii in a clinical collection
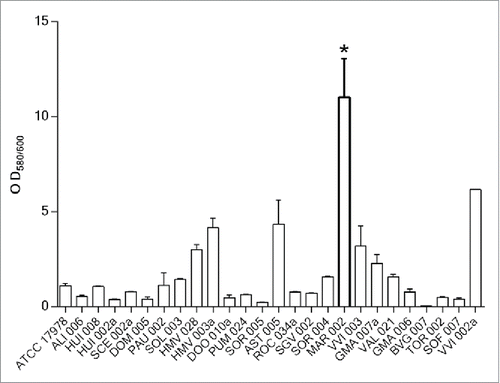
The biofilm formation abilities of 172 A. baumannii clinical isolates were investigated (data not shown). shows the data obtained for 25 of these clinical isolates, revealing that most of them had an OD580/600 ratio between 0.23 and 3.2. In contrast, the MAR002 isolate demonstrated a hyper-producing biofilm phenotype by being able to form ca. 10 times more biofilm compared to an ATCC-type strain such as A. baumannii 17978. Quantitative analysis of biofilms formed on an abiotic surface was performed and demonstrated that the clinical isolate named MAR002, assigned to the ST271 genotype, emerged with a remarkable capacity to form biofilm on a plastic surface (P value < 0.0001). Therefore, the MAR002 isolate was selected from the clinical strains collection for studying potential targets involved in pathogenesis.
Figure 1. Quantification of biofilm formation in 25 A. baumannii clinical isolates selected from a collection of 172 hospital-acquired strains during the 2nd Spanish Study of colonization/infection caused by A. baumannii (GEIH/REIPI-Ab2010). Experiments were performed in triplicate and each bar represents the mean ± standard deviation (* P value < 0.0001).
Expression level of genes coding for pili in the MAR002 strain
Since pili proteins play an important role in adhesion to biotic and abiotic surfaces, we examined the differential transcription of 11 predicted genes potentially involved in pili formation according to the analysis of the MAR002 genome Citation27 by qRT-PCR. shows the expression level of these genes in planktonic exponential and biofilm-associated cells. During this screening, the gene LH92_11085 was detected as overexpressed: ca. 25-fold more in biofilm-associated cells compared to exponential planktonic bacteria (). The expression of the LH92_11085 gene from planktonic and sessile cells of ATCC 17978 was investigated and compared with MAR002. Gene LH92_11085, annotated in ATCC 17978 as A1S_2091, was also overexpressed (ca. 10-fold more) in ATCC 17978 biofilm-associated cells compared to exponential planktonic cells. In addition, the expression level of this gene in biofilm-associated cells was higher (ca. 7.5-fold more) in the MAR002 strain compared to ATCC 17978.
Table 1 Expression level of genes involved in pili formation detected in planktonic and biofilm-associated cells of the MAR002 strain, assessed by qRT-PCR.
Characterization of LH92_11070-11085 operon in A. baumannii MAR002
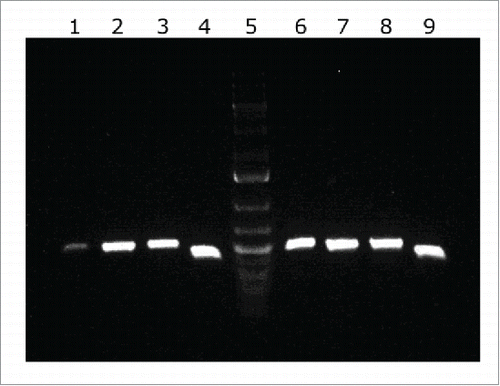
LH92_11085 is a 531-bp long gene that encodes a protein of 176 amino acids. This gene is part of a cluster containing 4 open reading frames. Total RNA was reverse transcribed and amplified to determine if genes from LH92_11070 to LH92_11085 formed a polycistronic operon. As shown in , all primer combinations amplified an intergenic fragment from cDNA, demonstrating that genes from LH92_11070 to LH92_11085 are co-transcribed as a single operon. Amplification fragments had the same length as those obtained from genomic DNA of A. baumannii MAR002. There was not any amplification from total RNA when used as negative control (data not shown). The operon was shown to be composed by 4 genes coding for a fimbrial protein subunit (LH92_11070), an usher (LH92_11075), a chaperone (LH92_11080) and a pilus rod (LH92_11085).
Figure 2. cDNA amplification of genes from the LH92_11070-11085 operon of A. baumannii MAR002 strain. The intergenic regions from genes LH92_11070-11075, LH92_11075-11080 and LH92_11080-11085 are shown in lanes 1, 2 and 3, respectively. Genomic DNA was used as template for positive control (lanes 6 to 8, respectively). Lanes 4 and 9 show the gyrB amplification from cDNA and DNA, respectively (positive controls). Lane 5 shows the GeneRuler 1 kb Plus DNA Ladder (Thermo Fisher Scientific).
Construction of the Δ11085 strain
Structure prediction analysis using Phyre2 Citation28 suggested that the product of the LH92_11085 gene may have a similar structure to the major type 1 pilus subunit FimA of Escherichia coli.Citation29 To investigate the role of this gene in cellular functions such as biofilm formation and adherence, a LH92_11085 knockout derivative of MAR002 (Δ11085) was constructed by deleting part of the LH92_11085 gene by double crossover recombination, using the plasmid pMo130.Citation30 The knockout derivative strain construction was confirmed by PCR. The expression level of each gene of the pili cluster was determine and the qRT-PCR results confirmed that only the LH92_11085 gene expression was null in the Δ11085 strain (data not shown).
Effect of LH92_11085 gene inactivation on biofilm formation
The MAR002 Δ11085 isogenic derivative described above was used to investigate the role of this gene in biofilm formation. Quantitative biofilm assays proved that there was a significant difference between the biofilms formed by the MAR002 parental strain and the Δ11085 mutant, which showed a biofilm deficient phenotype (). The inactivation of LH92_11085 gene led to a significantly reduced biofilm formation compared with that formed by the parental strain under the same experimental conditions (P value 0.0032) (). Complementation of the knockout strain with the parental allele (Δ11085 complemented) restored the wild-type biofilm formation phenotype ().
Figure 3. A) Quantification of biofilm formation by crystal violet staining. Eight independent replicates were done. Student´s t-test was done, values are means and bars indicate the standard deviation. B) SEM analysis of bacterial biofilm on plastic surface at the liquid-air interface of the A. baumannii strains a) ATCC 17978, b) MAR002, c) MAR002Δ11085 and d) MAR002Δ11085 complemented. All micrographs were taken at 5,000x magnification. Bars indicate the scale marks (2 μm).
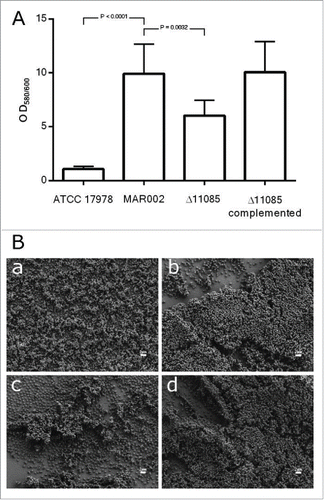
A deeper analysis of biofilm structures using SEM showed the role of LH92_11085 gene on biofilm architecture. Unlike strain ATCC 17978, which is a poor biofilm former (micrograph a, ), MAR002 showed a remarkable capacity to attach to and form a multilayered mature and three-dimensional organized biofilm on the analyzed surface including at the liquid–air interface (micrograph b, ). MAR002 also showed multilayered mature biofilm on both above and below areas of the liquid-air interface (data not shown). However, the Δ11085 strain formed significantly simpler and fewer cell aggregates at the liquid-air interface. Mostly, this mutant strain forms unorganized single layers of adherent cells on the analyzed surfaces (micrograph c, ). The complementation of the mutant strain with a plasmid copy of the parental allele showed the formation of biofilm with a three-dimensional structure similar to that displayed by MAR002 parental strain (micrograph d, ). These results are in agreement with the data obtained with crystal violet biofilm assays ().
Effect of LH92_11085 gene inactivation on attachment to eukaryotic cells
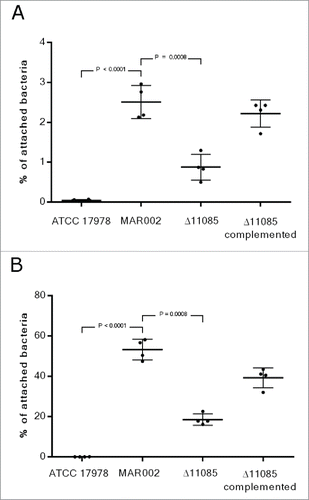
The interaction of MAR002 and its derivative strains with a biotic surface was also examined. To address this issue, A549 human alveolar epithelial cells were infected with ATCC 17978, MAR002, Δ11085 or the Δ11085 complemented strain to determine their ability to attach to eukaryotic cells and thus, to investigate the role of the LH92_11085 gene in adherence. A549 alveolar cells were infected for 3 and 24 h showing that MAR002 adhered to A549 cells ca. 60-fold and 1000-fold more than ATCC 17978, respectively (P value < 0.0001), which was used as a non-adherent control strain (). After 3 h of incubation 2.5% of MAR002 cells attached to A549 cells. also shows how the lack of LH92_11085 gene significantly reduced the attachment of MAR002 to A549 cells, resulting in only 0.9% of bacteria attached (P value 0.0008). After 24 h of incubation the number of attached MAR002 cells reached the 53% and the Δ11085 derivative strain showed a reduced adherence phenotype (18%, P value 0.0008) (). When the mutant strain was complemented the phenotype was restored, with 2.20% and 39% of bacteria attached to the epithelial cells, at 3 and 24 h respectively. Further, invasiveness experiments performed after 24 h of incubation showed that just 0.5 % and 0.2 % of MAR002 and Δ11085 mutant cells, respectively, were able to invade the eukaryotic cells (data not shown).
Figure 4. Quantification of bacterial adhesion to A549 human alveolar epithelial cells by A. baumannii ATCC 17978, MAR002, MAR002Δ11085 (Δ11085) and MAR002Δ11085 complemented (Δ11085 complemented). A) Percentage of attached bacteria after 3 h of infection. B) Percentage of attached bacteria after 24 h of infection. Four independent replicates were performed. T-student test were done and bars indicate the standard deviation.
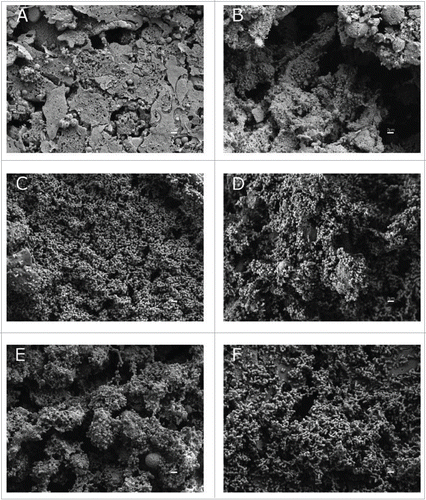
SEM analysis confirmed that the ability of the LH92_11085-deficient strain to attach to A549 cells was reduced (). Microscopy observations and attachment assays showed that parental and knockout complemented strains were able to attach to A549 cells at a similar level, while the Δ11085 mutant strain showed a reduced attachment phenotype (). Only the parental strain was able to form three-dimensional biofilm structures on the top of the polarized A549 cells (micrograph C, ). Damage of A549 cells can be assessed by the amount of surfactant present on top of the polarized A549 cells. The MAR002 strain and its derivative mutant caused serious damage on A549 polarized cells as compared with the ATCC 17978 strain (micrographs D and E, ). Micrograph A () shows uninfected polarized A549 cells covered by surfactant, which represents a healthy monolayer. The A549 cells still show surfactant after 72 h from infection with the ATCC 17978 strain (micrograph B, ) while surfactant has gone in the case of the A549 cells infected with the MAR002 strain under the same conditions (micrograph D, ). Although the Δ11085 mutant strain showed a considerable reduction in the amount of adhered bacteria, the lack of surfactant indicated that the polarized cells were damaged (micrograph E, ). In the case of the complemented strain (micrograph F, ), surfactant can still be seen underneath the A549 cells, which indicates that this strain produces less damage than the parental strain, but also partially restores the attachment phenotype.
Figure 5. SEM analysis of bacterial attachment to A549 human alveolar epithelial cells. Uninfected and healthy A549 cells covered by surfactant are shown in micrograph A as a negative control. A549 cells were infected with A. baumannii ATCC 17978 strain (B), with the MAR002 parental strain (C) and (D), with the MAR002 mutant strain lacking LH92_11085 (Δ11085) (E) or the mutant strain (Δ11085) complemented (F). All micrographs were taken at 5,000x magnification. Bars indicate the scale (2 µm).
Cell surface structures in MAR002 and its derivative strains
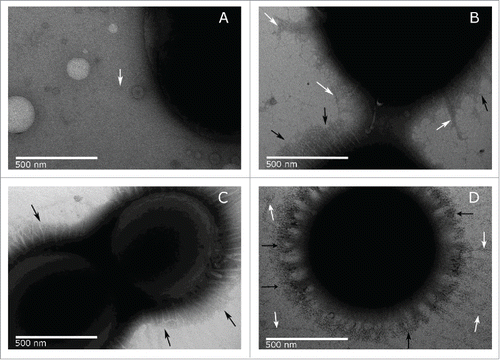
Bacteria produce numerous cell surface structures that can be used in cell adhesion and attachment to surfaces. We investigated the production of pili in A. baumannii MAR002 and derivative strains, under the same culture conditions used in the biofilm assays previously described, using negative staining and transmission electron microscopy (TEM). Examination of the surface appendages produced by these strains revealed quantitative and qualitative differences among the examined strains. Pili-like structures around ATCC 17978 cells are very scarce as shown in micrograph A () where only one pilus-like formation is marked with a white arrow. In contrast, TEM showed abundant pili-like structures around the parental MAR002 cells (micrograph B, ). The MAR002 strain possesses at least two distinct pili-like structures, (i) shorter bundle-forming pili with a length of 200-300 nm (marked with black arrows) very abundant and distributed all around the cell surface and (ii) longer thin type of pili (marked with white arrows) less abundant and located in certain points around the cell surface. However, only the shorter pili formations (marked with black arrows) could be found in the LH92_11085 null cells (micrograph C, ). Finally, the complemented knockout strain displayed an increased amount of the longer type of pili (marked with white arrows) as shown in micrograph D ().
Figure 6. TEM images of A. baumannii strains ATCC 17978 (A), MAR002 (B), MAR002Δ11085 (C) and complemented MAR002Δ11085 (D). Images were taken at 50,000x magnification. The longer thin pili present in small amounts are pointed out by white arrows. The shorter thick pili that form a dense halo around the surface are pointed by black arrows. Bars indicate the scale (500 nm).
Role of LH92_11085 gene in virulence of MAR002
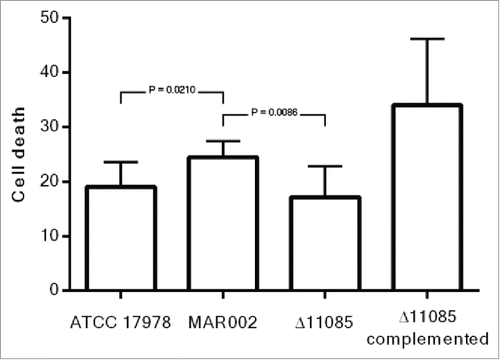
The ability of A. baumannii MA002 and its derivative mutant Δ11085 strain to reduce the viability of A549 human alveolar epithelial cells was assessed using LIVE/DEAD staining and fluorescence microscopy. The percentage of dead A549 cells was measure after 24 h of infection. As shown in , the MAR002 strain showed an increased virulence (24 % of A549 cell death) compared to A. baumannii ATCC 17978 (19% of cell death), being the difference statistically significant with a P value of 0.0210. When the LH92_11085 gene was deleted, the virulence was significantly reduced (P value 0.0086), resulting in 18% of dead cells. The Δ11085 complemented strain restored the phenotype giving a 34% of cell death.
Discussion
Multiple factors seem to contribute to the pathogenicity of Acinetobacter baumannii and its success as an infective agent. The capacity of A. baumannii to persist in a medical environment could be partly attributed to its ability to adhere to human cells and to form biofilm on abiotic surfaces. Previous reports have described the ability of certain clinical strains of A. baumannii to attach to and to form biofilm, both of them considered as important virulence factors.Citation10,14,31 Bacteria forming a biofilm are enclosed in a polymeric matrix constituting a protective mechanism to bacterial survival.Citation8,14,32-34 The MAR002 strain has been assigned to the ST271 genotype which has not been clustered into any International Clonal Lineage Citation35 or into any emerging group previously classified as higher biofilm producers.Citation36 However, during the present work MAR002 was identified as an isolate with a high ability to form biofilm on abiotic surfaces and a remarkable capacity to attach to A549 human alveolar cells when compared to ATCC 17978. These observations make MAR002 a good model strain to identify potential targets involved in the pathogenicity of A. baumannii. There are many studies that correlate the ability to form biofilm with the adhesion to eukaryotic cells. For example, Lee et al.Citation31 found a correlation between the ability to form large amounts of biofilm and epithelial cell adherence after a screening of a collection of A. baumannii multidrug-resistant isolates. Our electron microscopy data indicate that MAR002 produces at least two types of pili-like structures all around its surface: (i) thick, bundle-forming pili of 200-300 nm that form a dense halo around the bacterial cell surface and (ii) thin, longer pili present in smaller amounts. It was hypothesized that the ability of MAR002 to form biofilm and adhere to A549 human alveolar cells could be due to the presence of this large amount of pili. Thus, based on this observation, a study of genes that could somehow be involved in pili production was performed in order to explain the hyperpiliation phenotype as well as the high biofilm and attachment abilities of this strain. The expression level analysis of some of the pili genes identified in the newly sequenced genome of A. baumannii MAR002 strain Citation27 gave us three candidate genes, LH92_11085, LH92_07670 and LH92_08970, with a potential role in biofilm formation. LH92_11085 and LH92_07670 corresponded with the A1S_2091 and A1S_1510 genes in ATCC 17978 strain whereas LH92_08970 was not found in the ATCC 17978 genome. In a recent work, the pellicle matrix of three representative clinical strains of A. baumannii was analyzed.Citation26 Associated with this pellicle matrix the authors described three subunits of pili assembled by chaperon-usher systems: CsuA/B and the products of genes A1S_1510 and A1S_2091. Our findings agree with Chabane et al.Citation26 since we have detected an over-expression of genes A1S_1510 (LH92_07670) and A1S_2091 (LH92_11085) in biofilm-associated cells when compared to planktonic MAR002 bacteria. The csuA/B gene (LH92_11710) was also found to have higher expression levels in both exponential phase and biofilm associated cells compared with other genes studied; however, this gene was not overexpressed in biofilm cells compared to planktonic exponential cells. The A1S_1510 gene, which codes for a predicted fimbrial protein in A. baumannii ATCC 17978, and the ATCC 19606T csuA/B gene both have been reported as involved in biofilm formation.Citation19,21 However, the LH92_11070-11085 homolog operon previously described in ATCC 17978 (A1S_2088-2091) has not been functionally characterized although Giles et al. suggested its potential role in biofilm formation.Citation37 Since LH92_11085 gene showed the highest expression level among the analyzed encoding pili genes, we selected it to investigate whether this gene, integrated into the operon LH92_11070-11085, plays a role in the biofilm hyper-producing phenotype shown by MAR002. SEM analysis showed that the absence of the LH92_11085 gene reduced but did not abolish biofilm mass in MAR002. The ability of the LH92_11085 isogenic-deficient derivative to attach to the plastic surface has been demonstrated although it was not able to form a mature multilayered biofilm. Similar results were found with a homologue of a staphylococcal biofilm-associated protein (Bap), which was shown to be involved in maintaining the mature biofilm architecture in A. baumannii 307-0294 strain.Citation22 Bap protein is involved in the adherence of the A. baumannii Ab307 strain to both normal human bronchial epithelial cells and normal human neonatal keratinocytes.Citation38 Similarly, the BLP1 and BLP2 mutant derivative strains of A. baumannii AYE, showed a decrease in biofilm formation and adhesion to A549 cells abilities.Citation23 In agreement with the results obtained with abiotic surfaces, the LH92_11085 gene proved to be important for the ability of MAR002 to attach to biotic surfaces, specifically to A549 human alveolar epithelial cells. Data indicated that the LH92_11085 gene plays an important role in biofilm formation and attachment to A549 polarized cells. TEM analysis was performed in order to detect any change on the pili structures produced by MAR002 and we observed that the longer pili detected in the wild-type strain were more abundant in the complemented strain and did not appear in the knockout strain. This observation led us to correlate the expression of the LH92_11085 gene with the presence of the longer pili. The greater abundance of the longer pili in the complemented strain compared to the wild-type could be explained by the higher expression level of the LH92_11085 gene in the complemented strain than in the wild-type, as assessed by qRT-PCR, which is probably due to the control of a strong promoter harbored in the pWH1266 plasmid as well as the copy number of this shuttle plasmid vector.
The MAR002 LH92_11070-11085 operon described in the present work belongs to the type I pili systems, previously associated with biofilm formation.Citation19,21 Using bioinformatic tools this operon was found to have similar structure to the Fim and Pap systems previously described in E. coli Citation25 or to the CsuA/BABCDE pilus chaperone-usher assembly system described in A. baumannii strain ATCC 19606T.Citation21 However, when comparing the genetic structures of pili systems it seems that the CsuA/BABCDE system possesses 2 fimbrial subunits more in the pilus tip than the pili system here described. In A. baumannii ATCC 19606T, the CsuA/BABCDE was characterized as a chaperone-usher pili assembly system involved in adhesion to abiotic surfaces and biofilm formation,Citation21 while CsuA/BABCDE-independent short pili were shown to be involved in adherence of bacteria to biotic surfaces.Citation39 However, the LH92_11085 gene, belonging to the MAR002 LH92_11070-11085 operon described here, plays a role in the interaction of MAR002 cells with both abiotic and biotic surfaces. The MAR002 pili system seems to be structurally and functionally different from the PilA-based system previously described in A. baumannii M2 strain, which belongs to the type IV pili systems and that has been related with twitching motility.Citation40 Genetic analysis showed that the MAR002 pili system is composed by four genes coding for a fimbrial protein subunit (LH92_11070), an usher (LH92_11075), a chaperone (LH92_11080) and a pilus rod (LH92_11085). The predicted structure of the protein encoded by the LH92_11085 gene showed to be similar to FimA of E.coli,Citation29 that belongs to the Fim system, which corresponds to the protein encoded by the A1S_2091 gene in the ATCC 17978 A. baumannii strain. Moreover, its important role in pili subunits formation agrees with those found for FimA and PapA from E. coli Citation25 and also with the CsuA/B described in ATCC 19606T strain.Citation21 Finally, since the lack of the LH92_11085 gene had an effect on the viability of A549 cells as assessed by live/dead assays as well as on adherence and biofilm formation, we suggest that this gene could be partly responsible for the virulence of the A. baumannii MAR002 strain.
In summary, the results presented in this work show the remarkable ability of the A. baumannii MAR002 clinical strain to attach to A549 human alveolar epithelial cells and to form a robust multilayer mature biofilm on plastic surfaces. The LH92_11085 gene predicted to encode for a pili subunit belongs to a gene operon (LH92_11070-11085) coding for a functionally uncharacterized A. baumannii MAR002 pili assembly and secretion system. Data presented in this report indicate this LH92_11085 gene is required for the development of a mature biofilm on plastic as well as for the attachment of bacteria to A549 human alveolar cells and the ability to cause eukaryotic cell death. Taken together, these observations indicate the potential role this LH92_11085 gene could play in the pathobiology of A. baumannii and its value as a potential target to impair host pathogen interactions that are critical in the pathogenesis and virulence of this pathogen.
Material and methods
Bacterial strains and culture conditions
A total of 172 strains collected during the 2nd Spanish study of colonizations/infections caused by A. baumannii (GEIH/REIPI-Ab2010)Citation41 were used for phenotypic analysis, being 25 representative strains further selected for biofilm formation assays. One of them was Acinetobacter baumannii strain MAR002, isolated from a wound sample collected from a patient in the Hospital del Mar (Barcelona, Spain). A. baumannii and Escherichia coli strains were routinely grown in Luria-Bertani (LB) broth. Agar at 2% was added when necessary. All strains were grown at 37°C with shaking (180 rpm), and stored at −80°C in LB broth containing 10 % glycerol. The concentration of kanamycin (Sigma-Aldrich) used for selection of transformant strains was 50 μg/mL. Swimming broth medium (SB), containing 10 g tryptone/L and 5 g NaCl/L, was used for biofilm analysis. For obtaining planktonic cells, a single colony of both A. baumannii MAR002 and ATCC 17978 strains was taken from LB agar plates and grown in 5 mL of LB broth overnight. One mL of the resulting culture was used to inoculate 100 mL of LB broth. Optical density (OD600nm) was evaluated each 30 min to determine bacterial growth. Cells were harvested during the exponential phase (OD600nm = 0.6) and resuspended in RNA later reagent (Sigma-Aldrich), frozen using liquid nitrogen and stored at −80°C.
Biofilm generation for qRT-PCR assays
A. baumannii MAR002 biofilms used to isolate total RNA for qRT-PCR studies were obtained in the Fermentation Laboratory of the Agrobiotechnology Institute (Navarra, Spain). A sample from an overnight culture of A. baumannii grown in LB broth was used to inoculate 60-mL microfermentors (Institute Pasteur, Paris, France), which were then maintained at 37°C for 24 h. The bacterium was grown in LB broth medium under a continuous-flow culture system and continuous aeration consisting of 40 mL of compressed, sterile air/h. Biofilms that formed on the slides were removed with a cell scraper and frozen in liquid nitrogen at −80°C.
Bioinformatic analysis
Multiple-genome alignment tool from MAUVE software (version 2.3.1) was used for comparative genomics.Citation42 The 3-dimensional structure of the protein studied was predicted using the Phyre2 server.Citation28 The Interpro program was used for the functional analysis of proteins.Citation43 MLST Pasteur database (http://pubmlst.org/abaumannii/) was used to determine the sequence type (ST) of A. baumannii MAR002 strain.
RNA extraction and real-time RT-PCR
High Pure RNA Isolation Kit (Roche) was used to isolate RNA from planktonic exponential growth phase cells and sessile biofilm-associated cells. RNA samples were treated with DNAse I and purified with RNeasy MinElute Cleanup Kit (Qiagen). The samples were quantified using a BioDrop µLITE (Isogen Life Science). Real-time reverse transcription-PCR (qRT-PCR) was carried out to determine the expression level of the genes of interest using UPL probes (Roche). The LightCycler 480 RNA Master hydrolysis probes kit and a LightCycler 480 RNA instrument (Roche) were used following the program: initial incubation of 65°C, 3 min, followed by a denaturation step at 95°C for 30 s, 45 cycles at 95°C, 15 s and 60°C, 45 s, and a final elongation step at 40°C, 30 s. Three independent biological replicates were done. The expression level was standardized relative to the transcription level of the housekeeping gene gyrB. In order to check whether genes are cotranscribed and belong to a polycistronic operon, the cDNA was obtained from RNA samples using the iScrip™ cDNA Synthesis Kit (Bio-Rad) following the manufacturer's recommendations. The cDNA of MAR002 strain was amplified with GoTaq DNA Polymerase (Promega). Pairs of primers were designed to be complementary to the 3′ end of every gene and the 5′ end of the next one. Genomic DNA of A. baumannii MAR002 was used as template for positive control and total RNA was used as template for the negative control of the amplification. These analyses were done in triplicate. The primers and probes used were those listed in Table S1.
Quantitative biofilm assay
Biofilm formation was quantified following the procedure described by Tomaras et al,Citation21 with some modifications. A. baumannii was grown on LB agar for 18 h at 37°C and used to inoculate 5 mL of LB broth. Cultures were grown at 37°C with shaking. Overnight cultures were pelleted, washed and resuspended in 5 mL of SB. A 1:100 dilution of each strain was incubated at 37°C for 48 h under static conditions. Growth culture was measured at OD600 to estimate total cell biomass. Biofilm formation was quantified by staining with crystal violet and solubilisation with ethanol-acetone. The OD580/OD600 ratio was used to normalize the amount of biofilm formed to the total cell content of each sample tested to avoid variations due to differences in bacterial growth under different experimental conditions. Eight independent replicates were performed. Student's t-test was performed to evaluate the statistical significance of observed differences between MAR002 strain and its derivative strains.
Constructions of knockout strain
Plasmid pMo130 [Genbank: EU862243], a suicide vector containing the genes xylE, sacB and a kanamycin resistance marker, was used as described by Hamad et al.Citation30 Briefly, 900-1000 bp upstream and downstream regions flanking the gene selected for deletion in A. baumannii MAR002 were cloned into the pMo130 vector using primers listed in Table S1. The resulting plasmid was used to transform A. baumannii by electroporation. Recombinant colonies representing the first crossover event were obtained using a combination of kanamycin selection and visual detection of XylE activity following the catechol-based method. Bright yellow kanamycin resistant colonies were grown overnight in LB supplemented with 15 % sucrose and then plated on the same agar medium. Second crossover events were then confirmed by PCR using primers listed in Table S1.
Complementation of stable knockout mutant
To complement the Δ11085 knockout strain, the target gene was amplified from A. baumannii MAR002 genomic DNA using the primers listed in Table S1 and then cloned into the EcoRV and BamHI restriction sites of the pWH1266 plasmid Citation44 in which a kanamycin resistance cassette was cloned previously using primers listed in Table S1. The construction was used to transform the mutant strain. Transformants were selected on kanamycin-containing plates and confirmed by PCR using primers listed in Table S1.
Adhesion and invasiveness to A549 human alveolar epithelial cells
Adhesion and invasion abilities were determined following the procedure described by Gaddy et al. with some modifications.Citation24 Briefly, A549 human alveolar epithelial cells were grown in 5 % CO2 at 37°C in Dulbecco's Modified Eagle Medium (DMEM) (Sigma-Aldrich) supplemented with 10 % heat-inactivated fetal bovine serum (FBS) and 1 % of penicillin-streptomycin (Gibco). Confluent monolayers were washed twice with saline solution and once with modified Hank's balanced salt solution (mHBSS, same as HBSS but without glucose). Then, A549 cells were infected with 105 bacteria per well and incubated for 3 and 24 h in mHBSS at 37°C. To determine bacterial adhesion, the infected monolayers were washed three times with saline solution and then lysed in 500 µL of 0.5 % sodium deoxycholate. To determine bacterial invasion A549 cells were infected as described above for 24 h and each well was treated for 2 h with gentamicin (256 µg/mL) before washing. Dilutions of the lysates were plated onto LB agar and incubated at 37°C for 24 h. Colony forming units were counted to determine the % of bacteria that had attached to or invaded A549 cells at 24 h compared to the growth control, this being static conditions and same medium without cells. For 3 h attachment assays, the percentage of bacteria attach to A549 cells was compared with the total amount of infecting bacteria. Four independent replicates were done. T-student tests were performed to evaluate the statistical significance of the observed differences between MAR002 and its derivative strains.
Transmission electron microscopy (TEM)
Bacterial cells were grown on polystyrene coverslips in SB for 48 h at 37°C without shaking. Coverslips were removed from the culture media, and placed wet into new petridishes. Freshly prepared carbon coated, nitrocellulose substrated TEM grids were placed substrate side down, directly on top of the bacterial cells adherent to the plastic at air-liquid interface and allowed to sit covered for 5 min. Grids were removed and negatively stained with 5 µL of 1.5% (wt/vol) ammonium molybdate for 5 min. The grids were then wicked dry with filter paper and allowed to dry. Images were captured at 120kv with a JEOL 1200-EX II TEM.
Scanning electron microscopy (SEM)
Overnight cultures of A. baumannii were used to inoculate 5 ml of LB in 50-ml conical tubes at a 1:100 dilution. Sterile polystyrene coverslips were placed in the inoculated 50-mL conical tubes and the tubes were incubated for 48 h at 37°C without shaking as previously described.Citation24 Coverslips were washed, dehydrated in ethanol, processed with a critical point drier, and sputter coated as described previously.Citation21 Biofilms formed above, at and below of the liquid-air interface were viewed using a Zeiss Supra Gemini Series 35V scanning electron microscope as described previously.Citation21
Polarization of A549 human alveolar epithelial cells and infection with A. baumannii for SEM
A549 human alveolar epithelial cells were routinely maintained in 25 cm2 tissue culture flasks (BioLite 25 cm2 Flask, Thermo Fisher Scientific, Rochester, New York) in DMEM medium (Cellgro Mediatech Inc., Manassas, Virginia) supplemented with 10% heat-inactivated fetal bovine serum (Cellgro Mediatech Inc., Manassas, Virginia) and 1% of 100 μg/mL penicillin-streptomycin (Cellgro Mediatech Inc., Manassas, Virginia) (DMEM). Costar transwell permeable support polycarbonate membrane 24 well plates (Costar Transwell Polyester Supports, Corning Inc., Corning, New York) were preconditioned 24 h prior to seeding with DMEM on both sides of the membrane and incubated at 37°C and 5 % CO2. DMEM was removed from the conditioned transwell plates and the membranes were seeded with 105 A549 cells per membrane. A549 cells were maintained submerged (DMEM on top and bottom) on the transwell membranes for one week. Following the initial week of submerged growth, DMEM was removed from the top of the membrane to allow the A549 cells to polarize and begin secreting surfactant. Cells were polarized for 2 weeks to create a water-tight seal around the membrane as measured by resistance. One day prior to and for the duration of infection, A549 cells were fed DMEM supplemented with 10% heat inactivated FBS without penicillin-streptomycin (DMEM-). Bacteria, previously grown in LB at 37°C for 24 h in a shaking incubator at 180 rpm, were washed and resuspended in Hank's Buffered Salt Solution (HBSS) (Hyclone Laboratories, Inc., Logan, Utah). A concentration of 102 bacteria was applied to the apical surface of A549 cells by pipetting 1 μL of suspension onto the center of each membrane. The transwell plate was then incubated and maintained for 72 h at 37°C and 5% CO2. After 72 h, the membranes were washed with HBSS to remove secretions and unattached bacterial cells. The membranes were then fixed for 24 h in 4 % formaldehyde-HBSS at 4°C. The membranes were then prepared for SEM using the previously described.Citation21
LIVE/DEAD fluorescence microscopy on infected A549 monolayers
A549 human alveolar epithelial cells were grown in DMEM supplemented with 10% fetal bovine serum, 100 mg/L of penicillin, and 100 mg/L streptomycin at 37°C in the presence of 10% CO2. For cell viability assays, monolayers of A549 cells were grown in 24-well plates to a density of 1 × 105 cells per well. Later, the cells were infected with 2 × 104 CFU/well of A. baumannii in new DMEM without antibiotics and grown for 24 h at 37°C.Citation45 A Live/Dead Cell Double Staining Kit (Sigma-Aldrich) was used according to the manufacturer's instructions to measure cell viability post-infection. Briefly, at 24 h post-infection, the A549 cells were incubated for 15 min at 37°C with phosphate-buffered saline (PBS) containing a mixture of the two fluorescent molecules to obtain simultaneous fluorescent staining; calcein-AM, is able to stain viable cells (green), while propidium iodide, a nucleus-staining dye that is unable to penetrate the cell membranes of viable cells, can stain only dead cells (red). Microscopic images of the stained cells (alive and dead) were obtained using an inverted fluorescence microscope (Nikon Eclipse Ti) and analyzed with the NIS Elements Br software package. The excitation absorbance of calcein-AM was 490 nm, and emission was at 515 nm, while the excitation range of propidium iodide was 535 nm and emission was 617 nm. At least four replicates of each assay were analyzed, and the statistical significance was determined using Student's t test.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
KVIR_A_S1145335.docx
Download MS Word (15.1 KB)Funding
The results of this work have been funded by the Projects PI11/01034 to MP and PI14/00059 to MP and AB and PI12/00552 and PI15/00860 to GB, integrated in the National Plan for Scientific Research, Development and Technological Innovation 2013-2016 and funded by the ISCIII- General Subdirection of Assessment and Promotion of the Research – European Regional Development Fund (FEDER) “A way of making Europe” and Miami University (Ohio, USA) Research Funds. We also thank Spanish Network for Research in Infectious Diseases (REIPI RD12/0015/0014 to GB), cofinanced by the European Development Regional Fund (EDRF) “A Way to Achieve Europe, Instituto de Salud Carlos III, Subdirección General de Redes y Centros de Investigación Cooperativa, Ministerio de Economía y Competitividad. A.P. was financially supported by the Galician Plan for Research, Innovation and Growth (I2C Plan 2012-2016). J.A. Vallejo was financially supported by the Sara Borrell Program (ISCIII, Spain CD13/00373). We wish to thank grant GEIH/REIPI-Ab2010 from Instituto de Salud Carlos III - Ministerio Economía y Competitividad for providing MAR002 strain and M.I. Voskuil for pMo130.
References
- Peleg AY, Seifert H, Paterson DL. Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev 2008; 21:538–82; PMID:18625687; http://dx.doi.org/10.1128/CMR.00058-07
- Dijkshoorn L, Nemec A, Seifert H. An increasing threat in hospitals: multidrug-resistant Acinetobacter baumannii. Nat Rev Microbiol 2007; 5:939–51; PMID:18007677; http://dx.doi.org/10.1038/nrmicro1789
- Ong CW, Lye DC, Khoo KL, Chua GS, Yeoh SF, Leo YS, Tambyah PA, Chua AC. Severe community-acquired Acinetobacter baumannii pneumonia: an emerging highly lethal infectious disease in the Asia-Pacific. Respirology 2009; 14:1200–5; PMID:19909464; http://dx.doi.org/10.1111/j.1440-1843.2009.01630.x
- Merino M, Alvarez-Fraga L, Gomez MJ, Aransay AM, Lavin JL, Chaves F, Bou G, Poza M. Complete Genome Sequence of the Multiresistant Acinetobacter baumannii Strain AbH12O-A2, Isolated during a Large Outbreak in Spain. Genome Announc 2014; 2:e01182-14; PMID:25395646; http://dx.doi.org/10.1128/genomeA.01182-14
- del Mar Tomas M, Cartelle M, Pertega S, Beceiro A, Llinares P, Canle D, Molina F, Villanueva R, Cisneros JM, Bou G. Hospital outbreak caused by a carbapenem-resistant strain of Acinetobacter baumannii: patient prognosis and risk-factors for colonisation and infection. Clin Microbiol Infect 2005; 11:540–6; PMID:15966971; http://dx.doi.org/10.1111/j.1469-0691.2005.01184.x
- Corbella X, Montero A, Pujol M, Dominguez MA, Ayats J, Argerich MJ, Garrigosa F, Ariza J, Gudiol F. Emergence and rapid spread of carbapenem resistance during a large and sustained hospital outbreak of multiresistant Acinetobacter baumannii. J Clin Microbiol 2000; 38:4086–95; PMID:11060073
- Jones ME, Draghi DC, Thornsberry C, Karlowsky JA, Sahm DF, Wenzel RP. Emerging resistance among bacterial pathogens in the intensive care unit–a European and North American Surveillance study (2000-2002). Ann Clin Microbiol Antimicrob 2004; 3:14; PMID:15283864; http://dx.doi.org/10.1186/1476-0711-3-14
- Raad, II, Mohamed JA, Reitzel RA, Jiang Y, Dvorak TL, Ghannoum MA, Hachem RY, Chaftari AM. The prevention of biofilm colonization by multidrug-resistant pathogens that cause ventilator-associated pneumonia with antimicrobial-coated endotracheal tubes. Biomaterials 2011; 32:2689–94; PMID:21295343; http://dx.doi.org/10.1016/j.biomaterials.2010.12.015
- Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science 1999; 284:1318–22; PMID:10334980; http://dx.doi.org/10.1126/science.284.5418.1318
- Vidal R, Dominguez M, Urrutia H, Bello H, Gonzalez G, Garcia A, Zemelman R. Biofilm formation by Acinetobacter baumannii. Microbios 1996; 86:49–58; PMID:8771775
- Hall-Stoodley L, Costerton JW, Stoodley P. Bacterial biofilms: from the natural environment to infectious diseases. Nat Rev Microbiol 2004; 2:95–108; PMID:15040259; http://dx.doi.org/10.1038/nrmicro821
- Donlan RM. Biofilms: microbial life on surfaces. Emerg Infect Dis 2002; 8:881–90; PMID:12194761; http://dx.doi.org/10.3201/eid0809.020063
- Donlan RM, Costerton JW. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev 2002; 15:167–93; PMID:11932229; http://dx.doi.org/10.1128/CMR.15.2.167-193.2002
- Rodriguez-Bano J, Marti S, Soto S, Fernandez-Cuenca F, Cisneros JM, Pachon J, Pascual A, Martinez-Martinez L, McQueary C, Actis LA, et al. Biofilm formation in Acinetobacter baumannii: associated features and clinical implications. Clin Microbiol Infect 2008; 14:276–8; PMID:18190568; http://dx.doi.org/10.1111/j.1469-0691.2007.01916.x
- Gonzalez RH, Nusblat A, Nudel BC. Detection and characterization of quorum sensing signal molecules in Acinetobacter strains. Microbiol Res 2001; 155:271–7; PMID:11297357; http://dx.doi.org/10.1016/S0944-5013(01)80004-5
- Gonzalez RH, Dijkshoorn L, Van den Barselaar M, Nudel C. Quorum sensing signal profile of Acinetobacter strains from nosocomial and environmental sources. Rev Argent Microbiol 2009; 41:73–8; PMID:19623895
- Niu C, Clemmer KM, Bonomo RA, Rather PN. Isolation and characterization of an autoinducer synthase from Acinetobacter baumannii. J Bacteriol 2008; 190:3386–92; PMID:18281398; http://dx.doi.org/10.1128/JB.01929-07
- Cabral MP, Soares NC, Aranda J, Parreira JR, Rumbo C, Poza M, Valle J, Calamia V, Lasa I, Bou G. Proteomic and functional analyses reveal a unique lifestyle for Acinetobacter baumannii biofilms and a key role for histidine metabolism. J Proteome Res 2011; 10:3399–417; PMID:21612302; http://dx.doi.org/10.1021/pr101299j
- Rumbo-Feal S, Gomez MJ, Gayoso C, Alvarez-Fraga L, Cabral MP, Aransay AM, Rodriguez-Ezpeleta N, Fullaondo A, Valle J, Tomas M, et al. Whole transcriptome analysis of Acinetobacter baumannii assessed by RNA-sequencing reveals different mRNA expression profiles in biofilm compared to planktonic cells. PLoS One 2013; 8:e72968; PMID:24023660; http://dx.doi.org/10.1371/journal.pone.0072968
- Tomaras AP, Flagler MJ, Dorsey CW, Gaddy JA, Actis LA. Characterization of a two-component regulatory system from Acinetobacter baumannii that controls biofilm formation and cellular morphology. Microbiology 2008; 154:3398–409; PMID:18957593; http://dx.doi.org/10.1099/mic.0.2008/019471-0
- Tomaras AP, Dorsey CW, Edelmann RE, Actis LA. Attachment to and biofilm formation on abiotic surfaces by Acinetobacter baumannii: involvement of a novel chaperone-usher pili assembly system. Microbiology 2003; 149:3473–84; PMID:14663080; http://dx.doi.org/10.1099/mic.0.26541-0
- Loehfelm TW, Luke NR, Campagnari AA. Identification and characterization of an Acinetobacter baumannii biofilm-associated protein. J Bacteriol 2008; 190:1036–44; PMID:18024522; http://dx.doi.org/10.1128/JB.01416-07
- De Gregorio E, Del Franco M, Martinucci M, Roscetto E, Zarrilli R, Di Nocera PP. Biofilm-associated proteins: news from Acinetobacter. BMC Genomics 2015; 16:933; PMID:26572057; http://dx.doi.org/10.1186/s12864-015-2136-6
- Gaddy JA, Tomaras AP, Actis LA. The Acinetobacter baumannii 19606 OmpA protein plays a role in biofilm formation on abiotic surfaces and in the interaction of this pathogen with eukaryotic cells. Infect Immun 2009; 77:3150–60; PMID:19470746; http://dx.doi.org/10.1128/IAI.00096-09
- Busch A, Waksman G. Chaperone-usher pathways: diversity and pilus assembly mechanism. Philos Trans R Soc Lond B Biol Sci 2012; 367:1112–22; PMID:22411982; http://dx.doi.org/10.1098/rstb.2011.0206
- Nait Chabane Y, Marti S, Rihouey C, Alexandre S, Hardouin J, Lesouhaitier O, Vila J, Kaplan JB, Jouenne T, De E. Characterisation of pellicles formed by Acinetobacter baumannii at the air-liquid interface. PLoS One 2014; 9:e111660; PMID:25360550; http://dx.doi.org/10.1371/journal.pone.0111660
- Álvarez-Fraga L, López M, Merino M, Rumbo-Feal S, Tomás M, Bou G, Poza M. Draft Genome Sequence of the Biofilm Hyperproducing Acinetobacter baumannii Clinical Strain MAR002. Genome Announc 2015; 3:e00824-15; PMID:26205868
- Kelley LA, Mezulis S, Yates CM, Wass MN, Sternberg MJ. The Phyre2 web portal for protein modeling, prediction and analysis. Nat Protoc 2015; 10:845–58; PMID:25950237; http://dx.doi.org/10.1038/nprot.2015.053
- Puorger C, Vetsch M, Wider G, Glockshuber R. Structure, folding and stability of FimA, the main structural subunit of type 1 pili from uropathogenic Escherichia coli strains. J Mol Biol 2011; 412:520–35; PMID:21816158; http://dx.doi.org/10.1016/j.jmb.2011.07.044
- Hamad MA, Zajdowicz SL, Holmes RK, Voskuil MI. An allelic exchange system for compliant genetic manipulation of the select agents Burkholderia pseudomallei and Burkholderia mallei. Gene 2009; 430:123–31; PMID:19010402; http://dx.doi.org/10.1016/j.gene.2008.10.011
- Lee HW, Koh YM, Kim J, Lee JC, Lee YC, Seol SY, Cho DT, Kim J. Capacity of multidrug-resistant clinical isolates of Acinetobacter baumannii to form biofilm and adhere to epithelial cell surfaces. Clin Microbiol Infect 2008; 14:49–54; PMID:18005176; http://dx.doi.org/10.1111/j.1469-0691.2007.01842.x
- Espinal P, Marti S, Vila J. Effect of biofilm formation on the survival of Acinetobacter baumannii on dry surfaces. J Hosp Infect 2012; 80:56–60; PMID:21975219; http://dx.doi.org/10.1016/j.jhin.2011.08.013
- Wroblewska MM, Sawicka-Grzelak A, Marchel H, Luczak M, Sivan A. Biofilm production by clinical strains of Acinetobacter baumannii isolated from patients hospitalized in two tertiary care hospitals. FEMS Immunol Med Microbiol 2008; 53:140–4; PMID:18400015; http://dx.doi.org/10.1111/j.1574-695X.2008.00403.x
- Otto K, Silhavy TJ. Surface sensing and adhesion of Escherichia coli controlled by the Cpx-signaling pathway. Proc Natl Acad Sci U S A 2002; 99:2287–92; PMID:11830644; http://dx.doi.org/10.1073/pnas.042521699
- Higgins PG, Janssen K, Fresen MM, Wisplinghoff H, Seifert H. Molecular epidemiology of Acinetobacter baumannii bloodstream isolates obtained in the United States from 1995 to 2004 using rep-PCR and multilocus sequence typing. J Clin Microbiol 2012; 50:3493–500; PMID:22895032; http://dx.doi.org/10.1128/JCM.01759-12
- Giannouli M, Antunes LC, Marchetti V, Triassi M, Visca P, Zarrilli R. Virulence-related traits of epidemic Acinetobacter baumannii strains belonging to the international clonal lineages I-III and to the emerging genotypes ST25 and ST78. BMC Infect Dis 2013; 13:282; PMID:23786621; http://dx.doi.org/10.1186/1471-2334-13-282
- Giles SK, Stroeher UH, Eijkelkamp BA, Brown MH. Identification of genes essential for pellicle formation in Acinetobacter baumannii. BMC Microbiol 2015; 15:116; PMID:26047954; http://dx.doi.org/10.1186/s12866-015-0440-6
- Brossard KA, Campagnari AA. The Acinetobacter baumannii biofilm-associated protein plays a role in adherence to human epithelial cells. Infect Immun 2012; 80:228–33; PMID:22083703; http://dx.doi.org/10.1128/IAI.05913-11
- de Breij A, Gaddy J, van der Meer J, Koning R, Koster A, van den Broek P, Actis L, Nibbering P, Dijkshoorn L. CsuA/BABCDE-dependent pili are not involved in the adherence of acinetobacter baumannii ATCC19606(T) to human airway epithelial cells and their inflammatory response. Res Microbiol 2009; 160:213–8; PMID:19530313; http://dx.doi.org/10.1016/j.resmic.2009.01.002
- Harding CM, Tracy EN, Carruthers MD, Rather PN, Actis LA, Munson RS, Jr. Acinetobacter baumannii strain M2 produces type IV pili which play a role in natural transformation and twitching motility but not surface-associated motility. MBio 2013; 4:e00360-13; PMID:23919995; http://dx.doi.org/10.1128/mBio.00360-13
- Rumbo C, Gato E, Lopez M, Ruiz de Alegria C, Fernandez-Cuenca F, Martinez-Martinez L, Vila J, Pachon J, Cisneros JM, Rodriguez-Bano J, et al. Contribution of efflux pumps, porins, and β-lactamases to multidrug resistance in clinical isolates of Acinetobacter baumannii. Antimicrob Agents Chemother 2013; 57:5247–57; PMID:23939894; http://dx.doi.org/10.1128/AAC.00730-13
- Darling AC, Mau B, Blattner FR, Perna NT. Mauve: multiple alignment of conserved genomic sequence with rearrangements. Genome Res 2004; 14:1394–403; PMID:15231754; http://dx.doi.org/10.1101/gr.2289704
- Jones P, Binns D, Chang HY, Fraser M, Li W, McAnulla C, McWilliam H, Maslen J, Mitchell A, Nuka G, et al. InterProScan 5: genome-scale protein function classification. Bioinformatics 2014; 30:1236–40; PMID:24451626; http://dx.doi.org/10.1093/bioinformatics/btu031
- Hunger M, Schmucker R, Kishan V, Hillen W. Analysis and nucleotide sequence of an origin of DNA replication in Acinetobacter calcoaceticus and its use for Escherichia coli shuttle plasmids. Gene 1990; 87:45–51; PMID:2185139; http://dx.doi.org/10.1016/0378-1119(90)90494-C
- Beceiro A, Moreno A, Fernandez N, Vallejo JA, Aranda J, Adler B, Harper M, Boyce JD, Bou G. Biological cost of different mechanisms of colistin resistance and their impact on virulence in Acinetobacter baumannii. Antimicrob Agents Chemother 2014; 58:518–26; PMID:24189257; http://dx.doi.org/10.1128/AAC.01597-13