Abstract
Aspergillus fumigatus is the most prevalent airborne fungal pathogen causing invasive fungal infections in immunosuppressed individuals. The histidine biosynthetic pathway is found in bacteria, archaebacteria, lower eukaryotes, and plants, but is absent in mammals.
Here we demonstrate that deletion of the gene encoding imidazoleglycerol-phosphate dehydratase (HisB) in A. fumigatus causes (i) histidine auxotrophy, (ii) decreased resistance to both starvation and excess of various heavy metals, including iron, copper and zinc, which play a pivotal role in antimicrobial host defense, (iii) attenuation of pathogenicity in 4 virulence models: murine pulmonary infection, murine systemic infection, murine corneal infection, and wax moth larvae. In agreement with the in vivo importance of histidine biosynthesis, the HisB inhibitor 3-amino-1,2,4-triazole reduced the virulence of the A. fumigatus wild type and histidine supplementation partially rescued virulence of the histidine-auxotrophic mutant in the wax moth model. Taken together, this study reveals limited histidine availability in diverse A. fumigatus host niches, a crucial role for histidine in metal homeostasis, and the histidine biosynthetic pathway as being an attractive target for development of novel antifungal therapy approaches.
Introduction
Aspergillus fumigatus is the most common air-borne human fungal pathogen, causing the life-threatening disease invasive aspergillosis, particularly in immunocompromised patients.Citation1 Depending on the immune status and other factors in the patients, mortality rates in connection with invasive aspergillosis range from 60–90%, largely due to poor therapeutic interventions and only limited specific diagnostic methods available.Citation2 Novel antifungal therapy approaches aim to target fungal-specific pathways that are essential for virulence. One such potential pathway is the biosynthesis of histidine.
L-histidine is one of the 21 proteinogenic amino acids and an essential nutrient for animals but is synthesized de novo by plants and microorganisms. In lower eukaryotes and prokaryotes the highly conserved biosynthesis of histidine comprises 10 enzymatic reactions encoded in A. fumigatus by 7 genes, summarized in Fig. S1.Citation3 The first committed step, feedback-inhibited by histidine, is the condensation of 5-phosphoribosyl 1-pyrophosphate (PRPP) and ATP.Citation3 The first 4 enzymes are not only essential for biosynthesis of histidine but are also involved in de novo biosynthesis of purines (Fig. S1). The first enzyme exclusively dedicated to histidine biosynthesis in A. fumigatus is imidazoleglycerol-phosphate dehydratase, (IGPD), catalyzing the sixth step in histidine biosynthesis. In Aspergillus nidulans, deletion of the IGPD encoding gene, termed hisB, was shown to cause histidine auxotrophy.Citation4 In Archaea, Eukarya, and most bacteria, the IGPD activity-comprising protein is monofunctional, while in Enterobacteriaceae and some other bacteria, IGPD and histidinol-phosphate phosphatase domains have fused to form a bifunctional protein.Citation5 A competitive inhibitor of IGPD and consequently histidine biosynthesis, 3-amino-1,2,4 triazole (3-AT or amitrole), was used as an herbicide, but is likely to be a human carcinogen.Citation6,7 Nevertheless, these reports indicate that IGPD is a druggable target.Citation6 Moreover, histidine has a high affinity for binding metals, both as free amino acid and as metal-coordinating residues in proteins.Citation8 In agreement with a role in metal homeostasis, iron starvation was found to result in a fold9- increase in the cellular histidine content in A. fumigatus.Citation9 The crucial role of metal homeostasis in fungal virulence makes histidine biosynthesis particularly interesting.Citation10
In this study, we characterized the role of IGPD and consequently histidine biosynthesis in metal homeostasis and virulence of A. fumigatus via deletion of the IGPD-encoding hisB gene and treatment with the IGPD inhibitor 3-AT.
Results
Deletion of the hisB gene in A. fumigatus causes histidine auxotrophy
To analyze the role of histidine biosynthesis in A. fumigatus, we generated a mutant strain lacking HisB, termed ΔhisB, by replacement of the encoding gene hisB with the hygromycin resistance hph cassette as described in Material and Methods and Fig. S2. As recipient, the A. fumigatus akuA::loxP strain derived from ATCC46645, (AfS77, termed wt here), largely lacking non-homologous recombination, was used.Citation11,12 To ascertain hisB-specific effects in the deletion mutant ΔhisB, the hisB gene was reinserted in single copy at the hisB locus, yielding strain hisBC (see Material and Methods and Fig. S2). Correct genetic manipulation was confirmed by PCR (data not shown) and Southern blot analysis (Fig. S2). Growth analyses of wt, ΔhisB, and hisBC strains in liquid media (data not shown) and on plates () demonstrated that HisB-deficiency causes histidine auxotrophy in A. fumigatus as previously shown in A. nidulans.Citation4,13 On minimal medium, histidine concentrations of ≥0 .5 mM were required to support growth of ΔhisB, while supplementation of ≥1 mM histidine fully restored growth. Similarly, ΔhisB also required histidine supplementation for growth on blood agar, indicating a blood histidine content that is too low to support growth of the mutant. In agreement, the serum histidine concentration is about 0.09 mM in mice and 0.1 mM in adult humans.Citation14,15 Similar to the A. fumigatus arginine auxotrophic ΔargB mutant,Citation17 the ΔhisB mutant strain was unable to grow on minimal medium containing 1% bovin serum albumin (BSA) as nitrogen source (Fig. S3). Moreover, the ΔhisB mutant was incapable to grow on BSA that was hydrolyzed with A. fumigatus proteases, which contrasts the ΔargB mutant strain (Fig. S3). In contrast, complete medium, which contains the histidine sources yeast extract and peptone, allowed limited growth ().
Figure 1. Inactivation of HisB in A. fumigatus results in histidine auxotrophy (A) and growth inhibition of A. fumigatus by 3-AT is neutralized by histidine supplementation (B). Fungal strains were point-inoculated on Aspergillus minimal medium, complex medium and blood agar containing the indicated histidine concentrations and incubated at 37°C. Growth on minimal medium and blood agar was scored after 48 h, on CM after 24 h. For investigation of 3-AT-activity (B) minimal medium was used.
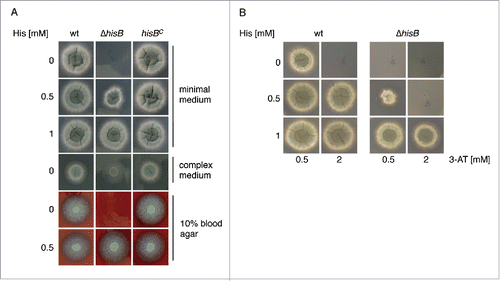
Taken together, these data demonstrate that genetic abrogation of HisB blocks histidine biosynthesis in A. fumigatus, and that growth of the mutant can be rescued by an external histidine supply.
A.fumigatus histidine biosynthesis can be inhibited by 3-amino-1,2,4-triazole (3-AT)
Three-AT is a commercially available herbicide, targeting IGPD (HisB) activity and consequently biosynthesis of histidine.Citation6,16 Here we show that 3-AT is also active against A. fumigatus, i.e. the presence of 2 mM 3-AT inhibited growth of the wt on minimal medium completely (). Histidine supplementation rescued growth in the presence of 3-AT, whereby histidine supplementation was less effective for ΔhisB compared to the wt (). Similarly, cultivation of the wt in liquid minimal medium in the presence of 1 mM 3-AT reduced the biomass production to 18% compared to growth without 3-AT (data not shown). Taken together, these data demonstrate that 3-AT blocks growth of A. fumigatus by inhibiting histidine biosynthesis.
Decreased histidine availability reduces heavy metal resistance and adaptation to metal starvation of A. fumigatus
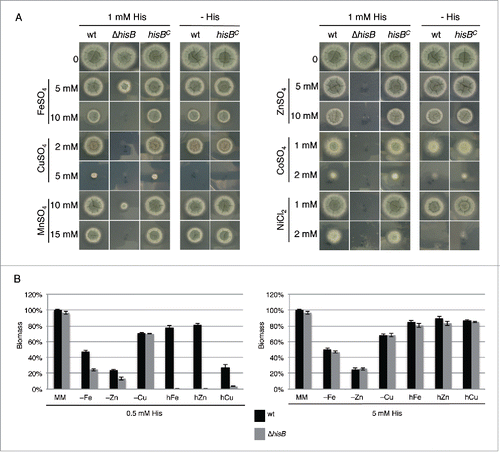
The metal chelating property of histidine and the fold9- increase of the cellular histidine content of A. fumigatus during iron starvation suggested a role of this amino acid in metal homeostasis.Citation9 In a first step, we analyzed the role of histidine biosynthesis in heavy metal resistance by comparing the growth of wt, ΔhisB and hisBC strains on plates with 1 mM histidine supplementation (). This histidine concentration allows approximately wt-like growth of the histidine auxotrophic ΔhisB mutant. In line with the crucial role of histidine in cellular management of heavy metals, HisB-deficiency decreased the resistance of A. fumigatus to iron, copper, manganese, zinc, cobalt and nickel (). Interestingly, HisB-deficiency did not affect resistance to cadmium (data not shown). Furthermore, the comparison of the growth of the histidine prototrophic strains (wt and hisBC) with and without 1 mM histidine supplementation revealed that histidine supplementation increased the resistance to copper, cobalt and nickel. In contrast, arginine auxotrophy, using the ΔargB mutantCitation17 or arginine supplementation did not affect heavy metal resistance of A. fumigatus (data not shown) indicating that the observed effects are histidine specific. The effects of 3 of these metals, iron copper and zinc, which play important roles in virulence (see Discussion), were further analyzed in liquid growth assays (). These tests confirmed the role of histidine availability in resistance against excessive concentrations of these metals, i.e., supplementation with 0.5 mM histidine allowed wt-like growth of the ΔhisB mutant in minimal medium but did not support growth in the presence of high metal concentrations. wt-like growth of ΔhisB was rescued by supplementation with 5 mM histidine underlining that histidine shortage was the reason for the dramatically reduced metal resistance. Furthermore, the biomass production of ΔhisB was significantly decreased during starvation for iron or zinc with 0.5 mM but not 5 mM histidine supplementation, which demonstrates that histidine availability also affects adaptation to metal starvation. Taken together, these data clearly demonstrate a crucial role of histidine in metal homeostasis of A. fumigatus.
Figure 2. Histidine plays a crucial role in metal homeostasis of A. fumigatus. (A) Fungal strains were point-inoculated on minimal medium (MM) supplemented with the given concentrations of heavy metal salts, either with or without 1 mM externally added histidine. Photographs were taken after 48 h of incubation at 37°C. (B) Biomass production (dry weight) of wt and ΔhisB was quantified after growth for 24 h in liquid minimal medium with low contents of iron (-Fe), zinc (-zinc) or copper (-Cu) (see Material and Methods) as well as minimal medium containing additionally 5 mM FeSO4 (hFe), 4 mM ZnSO4 (hZn) or 1.5 mM CuSO4 (hCu). All cultivation conditions were performed with supplementation of either 0.5 mM or 5 mM histidine. Data represent the mean of 3 biological replicates ± standard deviation normalized to the biomass of the wt grown in minimal medium with the same histidine supplementation. The biomass of the wt in minimal medium was 0.78 ± 0.05 g at both 0.5 mM and 5 mM histidine concentrations supplemented. The decreased biomass production during metal starvation and excess underline the shortage and toxicity, respectively, of the metal concentrations.
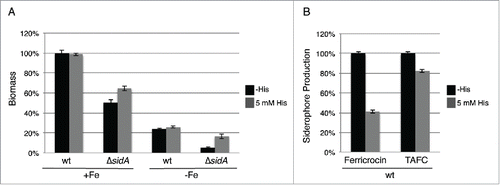
A. fumigatus employs 2 high-affinity iron uptake systems, reductive iron assimilation and siderophore-mediated iron acquisition, and uses intracellular siderophores for transport and storage of iron.Citation18,19 To further characterize the role of histidine in iron metabolism, we compared the effect of histidine supplementation on the growth of wt and a mutant lacking siderophore biosynthesis, ΔsidA, during iron-replete and deplete conditions. As shown previously,Citation20 deficiency in siderophore biosynthesis (ΔsidA) decreased biomass production: during iron sufficiency to 50% and during iron starvation to 21% of wt grown under the same conditions (). Histidine supplementation did not affect biomass production of the wt but increased that of the ΔsidA mutant by 22% and 70% during iron sufficiency and starvation conditions, respectively. Moreover, in the wt histidine supplementation decreased production of the intracellular siderophore ferricrocin and of the extracellular siderophore triacetylfusarinine C (TAFC) by 59% and 18%, respectively, compared to conditions without histidine supplementation (). Taken together, these data indicate a particular beneficial role of histidine during iron starvation.
Figure 3. Histidine supplementation improves growth of the siderophore-deficient ΔsidA mutant (A) and decreases extra- and intracellular siderophore production (B). (A) Biomass production (dry weight) of wt and ΔsidA was quantified after growth for 21 h in liquid minimal medium under iron-replete (+Fe, 30 µM FeSO4) and iron depleted (-Fe) conditions with and without 5 mM histidine supplementation. Data represent the mean of 3 biological replicates ± standard deviation normalized to the biomass of the wt grown in +Fe conditions. The biomass of the wt in minimal medium +Fe was 0.63 g and 0.62 g with and without 5 mM histidine supplementation, respectively. (B) Intracellular (ferricrocin) and extracellular (TAFC) production of siderophores was quantified from the iron starvation conditions with and without 5 mM histidine supplementation (A) and normalized to the biomass and the wt grown without histidine supplementation.
Hypoxia decreases histidine requirement of ΔhisB
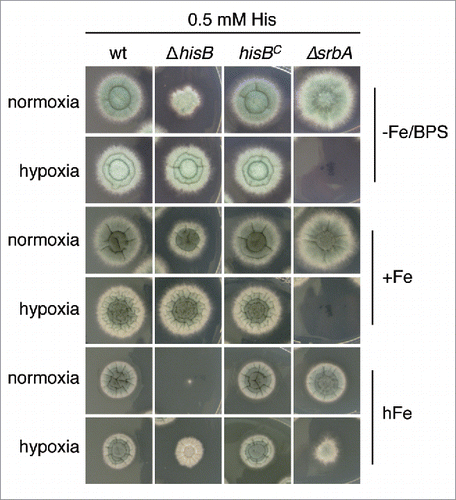
A. fumigatus can adapt to extremely low oxygen availability, which is important in certain microenvironments including inflamed and necrotic tissue during the infection process.Citation21 Interestingly, the growth defect of ΔhisB with 0.5 mM histidine supplementation was less pronounced under hypoxic conditions compared to normoxic conditions on plates reflecting iron starvation, iron sufficiency and particularly iron excess, where ΔhisB displayed the most extreme phenotype (). Iron excess was included as one example of metals, the toxicity of which increased during decreased histidine availability (see above). These data might be explained by reduced histidine requirement during hypoxic conditions, which appears unlikely. More likely, hypoxia might increase histidine uptake, which is supported by the finding that hypoxia leads to transcriptional upregulation of genes encoding amino acid transporters.Citation22
Figure 4. Hypoxia decreases histidine requirement of ΔhisB. Fungal strains were point-inoculated on minimal medium reflecting iron starvation (-Fe/BPS, containing 100 µM of the ferrous iron-specific chelator bathophenanthroline disulfonate), iron sufficiency (+Fe, 30 µM FeSO4), and iron excess (hFe, 5 mM FeSO4) and incubated at 37°C for 48 h in normoxic and hypoxic conditions, respectively. As a control for hypoxia, we included a mutant deficient in the transcription factor SrbA, ΔsrbA, which is essential for growth during hypoxic conditions unless supplemented with high iron concentrations. Citation50 Supplementation with 5 mM histidine resulted in wt-like growth of ΔhisB and did not affect the growth of ΔsrbA (data not shown).
HisB-deficiency results in attenuation of A. fumigatus virulence in the insect host model Galleria mellonella
To assess the role of histidine biosynthesis in the pathogenicity of A. fumigatus, we compared wt, ΔhisB and hisBC in the G. mellonella infection model (). Histidine auxotrophy resulted in a significant higher survival rate (p < 0.0001) of G. mellonella larvae compared to the wt and hisBC over a period of 6 d (). Already 72 h after infection the attenuating effect of hisB disruption resulted in a survival rate of 80%, whereas none of the larvae infected with the wt or hisBC survived. The strongly attenuated virulence of the histidine auxotrophic mutant ΔhisB indicates that histidine biosynthesis plays an essential role for virulence of A. fumigatus in the insect model.
Figure 5. Histidine biosynthesis plays a crucial role in A. fumigatus virulence in G. mellonella. Larvae of the greater wax moth G. mellonella (n= 20 larvae per group) were infected with conidia of the respective strains and survival was monitored over a period of 6 d Control cohorts received either no injection (untreated) or were injected with the conidial solution buffer (IPS control). (A) HisB-deficiency (ΔhisB) attenuates virulence of A. fumigatus compared to the wt and hisBC strain (p <0.0001). (B) Co-injection of histidine increases virulence of ΔhisB (p<0.0018). (C) Co-injection of the histidine biosynthesis inhibitor 3-AT decreases virulence of the wt (p<0.0098).
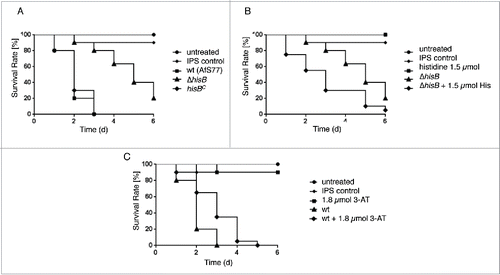
Co-injection of 1.5 µmol histidine with the ΔhisB inoculum led to partial reconstitution of A. fumigatus virulence in the G. mellonella infection model (p < 0.0018, ). 72 h after infection, 80% of larvae infected with the ΔhisB mutant remained alive, while only 30% survived infection with ΔhisB combined with histidine co-injection. Larvae injected with Insect Physiological Saline (IPS) or 1.5 µmol histidine as well as untreated larvae were used as controls and showed 90100%– survival rates after 6 d
Co-injection of 1.8 µmol 3-AT (equals 6 µmol/g larvae) with the wt inoculum significantly increased survival rates of larvae compared to larvae infected with the wt strain alone (p < 0.0098, ). 48 h post infection, larvae infected with the wt plus 3-AT displayed a survival rate of 65% in contrast to the 20% of larvae remaining alive when infected with the wt without the histidine biosynthesis inhibitor, suggesting that 3-AT attenuates virulence of A. fumigatus in the G. mellonella infection model. Injection of 1.8 µmol 3-AT without conidia as a control did not affect mortality rates compared to the IPS control, revealing no cytotoxic effect of 3-AT at this concentrations.
Taken together, these data demonstrate the importance of histidine biosynthesis in virulence of A. fumigatus in the G. mellonella infection model.
HisB-deficiency attenuates virulence of A. fumigatus in murine intranasal and intravenous infection models
To analyze the role of histidine biosynthesis in murine bronchopulmonary aspergillosis, 12 to 15 BALB/c mice per group were immunosuppressed with cortisone acetate and cyclophosphamide, rendering them neutropenic, and intranasally infected with 2 × 105 conidiospores of wt, ΔhisB, or hisBC. Survival was monitored daily over a period of 12 d (). Survival curves demonstrate that both the wt and the complemented strains caused high mortality rates. In contrast, the ΔhisB strain showed significantly reduced virulence (p<0.001) in infected mice with a survival rate of 90% 6 d post infection compared to the wt and hisBC strains that resulted in survival rates of only 20% and 0%, respectively. Control infection with saline (PBS) resulted in zero mortality.
Figure 6. HisB-deficiency attenuates virulence of A. fumigatus in murine intranasal and intravenous infection models. (A) Survival curve for mice infected intranasally with 2 × 105 conidia (p <0,001). (B) Survival curve for mice infected intravenously with 1 × 105 conidia (p<0,001). (C) Histological analyses of mice infected intranasally with 2 × 105 A. fumigatus conidia demonstrated that in contrast to ΔhisB, the wt and hisBC strains cause significantly increased cellular infiltration leading to major tissue damage. Murine lungs were embedded in Tissue-Tek O.C.T. (Sakura) and cryosections were co-stained with hemotoxylin-eosin and Grocott's Methenamine Silver according to standard protocols.
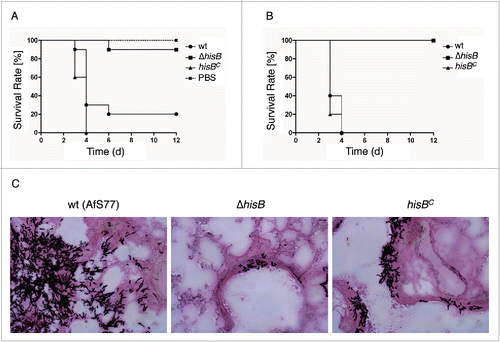
Lungs were surgically removed for histological analysis (). The A. fumigatus ΔhisB mutant did not germinate within the lung tissue in contrast to invasive tissue penetration of the wt and the hisBC strain. Invasive growth of hyphal elements, characterized by extensive penetration of surrounding tissues, was evident for mice infected with the wt and hisBC strains. In complete contrast, mice infected with ΔhisB displayed only very few discrete foci of pulmonary infection in vivo and barely grew in infected mice. These data demonstrate that A. fumigatus requires biosynthesis of histidine for germination and penetration into the lung tissue.
To model systemic infection, the virulence of wt, ΔhisB, and hisBC A. fumigatus strains was compared after intravenous infection of neutropenic BALB/c mice with 1 × 105 conidia (). This model revealed avirulence of the ΔhisB strain (p<0.001) whereas all mice infected with the wt and hisBC strain died 4 d post infection. These results indicate that de novo synthesis of histidine is absolutely required for A. fumigatus virulence and suggest that blood does not contain sufficient amounts of histidine to support systemic dissemination of the histidine auxotrophic mutant. This is supported by the lack of growth of the ΔhisB mutant on blood agar ().
HisB-deficiency results in attenuation of A. fumigatus virulence in a murine keratitis model
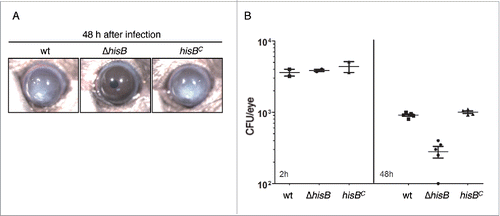
Infection and inflammation of the cornea caused by A. fumigatus is a common cause of visual impairment and blindness in immunocompetent patients.Citation23 To test whether disruption of histidine biosynthesis attenuates A. fumigatus virulence in the cornea, we used a well established murine keratitis model.Citation24 Therefore, 4 × 104 A. fumigatus conidia of the wt, ΔhisB and hisBC strains were injected into the corneal stroma of animals without immunosuppression. Corneal opacity is a result of inflammatory cell infiltration into the cornea during fungal infection, which was tracked for 48 h. As seen in , mice infected with the wt and hisBC strain developed significant corneal opacity at 48 h post infection while the ΔhisB mutant did not induce opacification. In agreement, corneas infected with the ΔhisB mutant displayed a significantly decreased fungal load compared to infection with wt and hisBC strains (). These findings indicate that histidine biosynthesis is essential for growth of A. fumigatus in the cornea and development of corneal disease.
Figure 7. HisB-deficiency attenuates virulence of A. fumigatus in a murine model of fungal keratitis (A,B) and 3-AT treatment does not decrease the fungal burden (B). (A) 4 × 104 A. fumigatus wt, ΔhisB or hisBC conidia were injected into the corneal stroma and corneal opacity and (B) CFU were measured at 48 h after infection (p <0.0).
Discussion
The histidine biosynthetic pathway is found in bacteria, archaebacteria, lower eukaryotes, and plants but is absent in mammals, making the involved enzymes highly attractive targets for the design of new antifungal compounds with selective toxicity. A blast search confirmed that humans lack homologs of all 7 histidine biosynthetic enzymes (HisG, HisI/D, HisA, HisF/H, HisB, HisC, HisJ) of A. fumigatus (Table S1). Only, HisC (histidinol-phosphate aminotransferase) displays significant (E = 1e-09) similarity to a human protein, NP_001027025.2. Here we show that inactivation of histidine biosynthesis by deletion of the IGPD-encoding hisB gene causes histidine auxotrophy, which attenuates virulence in 2 infection models involving immunosuppression, murine systemic and pulmonary infection, and in 2 infection models without immunosuppression, a murine model of corneal infection and Galleria mellonella. The crucial role of histidine biosynthesis in pathogenicity is further supported by the previously identified transcriptional upregulation of 2 histidine biosynthetic genes encoding HisB and HisG during A. fumigatus lung infection of leukopenic mice.Citation25
Mutant virulence studies serve to identify virulence determinants but at the same time allow the characterization of the host niche. In this respect, the presented data demonstrate insufficient histidine availability for the growth of histidine auxotrophic A. fumigatus in several host niches. In other words, the histidine uptake mechanisms of A. fumigatus are not efficient enough to support growth in these host niches. Previously, lysine auxotrophy was found to strongly attenuate virulence in murine pulmonary aspergillosis, which requires tissue penetration, but did not affect murine systemic infection, in which conidia are directly injected into the bloodstream.Citation26 The branched chain amino acid biosynthetic pathway (isoleucine, leucine, valine) was reported to be crucial for both pulmonary and systemic aspergillosis.Citation27 In contrast, arginine biosynthesis was found to be dispensable for virulence of A. fumigatus in G. mellonella.Citation28 Taken together, these data display significant differences in availability of different amino acids for A. fumigatus in the host. Previously,Citation29 virulence of the intracellular bacterial pathogen Brucella suis was found to be attenuated by transposon-mediated inactivation of the histidine biosynthetic genes encoding histidinol dehydrogenase (HisD) or glutamine amidotransferase/cyclase (HisF/H), underlining the crucial role of histidine biosynthesis for pathogenicity. In contrast, persistence of Candida glabrata in mice was found to be unaffected by histidine auxotrophy.Citation30 Moreover, histidine auxotrophy does not alter virulence of Candida albicans in a Caenorhabditis elegans infection model.Citation31 Consequently, A. fumigatus and Candida spp. appear to significantly differ with regard to the impact of histidine biosynthesis in virulence. The differences might be explained by the degree of histidine requirement, the efficiency of histidine uptake or by different host niches offering different histidine availability.
Histidine biosynthesis, particularly IGPD, is the target of the herbicide 3-AT, which on the one hand unfortunately proved to be potentially carcinogenic, but on the other hand underlines that IGPD is a druggable target.Citation6 In agreement, we found that HisB-deficiency decreases resistance to 3-AT in A. fumigatus, which strongly indicates that HisB is the 3-AT target, and demonstrated that 3-AT treatment increases the survival in the wax moth model. The crystal structure of IGPD from Cryptococcus neoformans, which displays about 51 % identity at the amino acid level to A. fumigatus HisB (Fig. S4) has already been resolvedCitation32 and might guide the way for development of inhibitors with higher specificity. Apart from HisB, the A. fumigatus histidine biosynthetic pathway encompasses 6 additional proteins, of which only one shows limited similarity to a human protein (Table S1); i.e. the histidine biosynthetic pathway provides several attractive targets for development of specific inhibitors. The expression of hisB is possibly subject to regulation by the cross pathway control (CPC) system,Citation4 which generally antagonizes amino acid starvation.Citation33 To avoid induction of compensating CPC mechanism, it will be worthwhile to take CPC-independence into account for choosing the right drug target.
The virulence attenuation of A. fumigatus caused by histidine auxotrophy might be solely based on the requirement of this amino acid for protein biosynthesis. Additionally or alternatively, the virulence attenuation might be due to the role of histidine in metal management, indicated by its crucial role in adaptation to metal excess as well as metal starvation, particularly for iron, copper and zinc. The lines of evidence indicating a crucial role of histidine during iron starvation are: (i) iron starvation significantly increases the cellular histidine content of A. fumigatus,Citation9 (ii) histidine auxotrophy was more detrimental during iron starvation compared to iron sufficiency, (iii) histidine supplementation partially cured the growth defect caused by siderophore deficiency (ΔsidA mutation), and (iv) histidine supplementation decreased the production of extra-and intracellular siderophores. As siderophore biosynthesis is induced by iron starvation,Citation18,19 the latter might indicate alleviation of cellular iron starvation by histidine supplementation, most likely by the metal-chelating capacity of this amino acid. On the other hand, we demonstrate that histidine biosynthesis plays an important role in metal resistance, including zinc, copper cobalt and nickel. Previously, histidine was found to play a role in resistance to cobalt, copper and nickel in Saccharomyces cerevisiae,Citation34 as well as to cobalt in Schizosaccharomyces pombe.Citation35 Moreover, an increased cellular histidine content was found to confer tolerance and hyperaccumulation of nickel in plants.Citation36,37 Taken together, these data indicate that the role of histidine in metal management is a widespread phenomenon, most likely due to its high metal binding capacity. Histidine has a high affinity for binding metals, both as the free amino acid and as metal-coordinating residues in proteinsCitation8 exemplified by its use in 6xHis-tagging combined with Ni-NTA chromatography for purification of recombinant proteins as well as its use in various zinc fingers for metal coordination. Citation38
The role of histidine in metal homeostasis might be particularly relevant during the infection process as vertebrates have evolved sophisticated mechanisms to limit the availability of some crucial metals, e.g. iron, copper and zinc, a concept termed “nutritional immunity,” while concurrently flooding sites of infection such as the phagolysosome with antimicrobial concentrations of metals.Citation39 In response, pathogens developed a series of metal regulatory, acquisition, and detoxification systems to compete for limited metals within the host while simultaneously preventing metal toxicity. In agreement, high-affinity uptake systems for iron and zinc as well as regulatory circuits for adaptation to metal starvation have been shown to be essential for virulence of A. fumigatus as well as for other pathogens.Citation18,19 On the other hand, copper detoxification is an essential fungal virulence determinant.Citation40 Similarly, the host defense against Mycobacterium tuberculosis was shown to take advantage of copper and zinc toxicity.Citation41 Taken together, it appears likely that during the infection process histidine is important not only for protein biosynthesis but also for metal homeostasis in A. fumigatus to combat metal-based host defense mechanisms.
Materials and methods
Strains, media and growth conditions
A. fumigatus strains were cultivated at 37°C on complete medium (2 g peptone and 1 g yeast extract per l) or on/in minimal medium according to Pontecorvo et al.,Citation42 containing 1% glucose as carbon source, 20 mM glutamine as nitrogen source, 30 µM FeSO4, 30 µM ZnSO4, and 1.6 µM CuSO4. To starve A. fumigatus for iron or copper, addition of FeSO4 or CuSO4 was omitted. For zinc starvation, the ZnSO4 content was decreased to 0.1 µM as lack of zinc addition eliminated growth of A. fumigatus almost completely. Supplements are described in the respective experiments. The blood agar medium was performed with 0.5% sodium chloride and 10% (vol/vol) blood. For hypoxic conditions, the gas concentrations in the hypoxic chamber (C-Chamber and Pro-Ox, Pro-CO2 controller; Biosherics) were set to 1% O2, 5% CO2 and 94% N2. All experiments were done in parallels under normoxic conditions. Liquid cultures were inoculated with 106 spores/ml medium. For plate assays, 104 conidia were point-inoculated. As recipient strain for genetic manipulation of A. fumigatus, the akuA-deficient derivative of ATCC46645, AfS77, termed wild type (wt), was used.Citation11 Primers used in this study are listed in Table S2.
Deletion of hisB (AFUA_6G04700) and reconstitution of the ΔhisB strain
For generating the hisB-deletion strain, the bipartite marker technique was used.Citation43 Therefore, the A. fumigatus strain AfS77 was co-transformed with 2 DNA constructs containing 5´- and 3´- incomplete but overlapping fragments of the hygromycin resistance cassette (hph) fused to 0,8 kb and 0,9 kb of hisB flanking sequences, respectively (Fig. S2). During transformation, hph is complemented by homologous recombination of the fragments via the 447 bp overlap. The hisB 5′- and 3´- flanking regions were PCR-amplified from genomic AfS77 DNA using the primer pairs oAfIpd1–1/oAfIpd1–2 and oAfIpd1–4/oAfIpd1–5, respectively. Subsequent to gel-purification, the 5´- and 3´- flanking fragments were digested with AvrII and XbaI, respectively. The hph selection marker was released from the plasmid pAN7.1Citation44 by digestion with AvrII and XbaI and ligated with the 5´- and 3´- flanking region, respectively. For transformation, the 2 overlapping fragments were amplified from the ligation products using primers oAfIpd1–3/ohph14 for the 5´- flanking region (1.9 kb), and oAfIpd1–6/ohph15 for the 3´- flanking region (2.3 kb). AfS77 protoplasts were transformed with both fragments. ΔhisB strains were selected with 0.1 mg·ml−1 hygromycin B (Calbiochem) on minimal medium plates.
For reconstitution of hisB in ΔhisB, a 2.7 kb fragment containing a complete hisB copy, was amplified from genomic DNA using primers oAfIpd1–1 and oAfIpd1–5. This PCR fragment was subcloned into the pGEM-T Easy plasmid (Promega). The resulting phisB plasmid was linearized with SmaI to foster homologous recombination in the 3´-hisB flanking region and introduced into the ΔhisB strain by protoplast transformation with selection for histidine prototrophy. Colonies from single homokaryotic spores were picked to obtain homokaryotic transformants. Screening for transformants with the correct genotype was performed by Southern blot analysis. For extraction of genomic DNA, mycelia were homogenized and DNA isolated according to Sambrook et al.Citation45
Quantification of siderophore production
Extra- and intracellular siderophores were isolated from culture supernatants and lyophilized mycelia, respectively, and analyzed as described previously.Citation9
Galleria mellonella infection studies
Virulence assays in G. mellonella were carried out according to Fallon et al.Citation46 G. mellonella larvae (K.Pechmann, Biologische Wurmzucht, Langenzersdorf, Austria) were kept in the dark at 18°C before use. One × 107 A. fumigatus conidia in 20 µl insect physiological saline (IPS) were injected into the hemocoel via one of the hind pro-legs of larvae weighing between 0.3 and 0.4 g. Untreated larvae and larvae injected with 20 µl of IPS served as controls. Larvae were incubated at 30°C in the dark and monitored daily up to 6 d Incubation at 30°C was favored to avoid temperature-triggered effects on the larval immune response.Citation47 Significance of survival data was evaluated by using Kaplan-Meier survival curves, analyzed with the log-rank (Mantel Cox) test utilizing GraphPad Prism software. Differences were considered significant at p-values ≤0 .05.
Virulence assay of pulmonary and systemic mouse models
Female inbred BALB/c mice (Charles Rivers Breeders) were used for infection experiments. Immunosuppression was carried out by subcutaneous injection of 112 mg·kg−1 body weight (BW) hydrocortisone acetate and intraperitoneal injection of 150 mg·kg−1 BW cyclophosphamide following a sequential protocol as previously described,Citation48 with the modification that 2 doses of cortisone on days −3 and −1 were applied.Citation49 Bacterial infections were prevented by adding 2 g·l−1 neomycin to the drinking water. Inocula were prepared by harvesting conidia from 5-day-old slants of solid medium followed by filtration through Miracloth tissue and washing with saline. Mice were anesthetized with an intraperitoneally injected mixture of ketamine (50 μg·g−1 BW) and xylazine (5 μg·g−1 BW) in phosphate buffered saline (PBS) in a total volume of 10 μl·g−1 BW. Infection was carried out either by intranasal instillation of 2 × 105 conidiospores suspended in 50 µl of saline or intravenous injection of a 50 µl suspension of 1 × 105 conidia into the retro-orbital venous plexus. Disease progression was followed twice daily by tabulating weight profiles and following the animals' behavior. Signs of respiratory distress, hunched posture or poor mobility, as well as severe weight loss of more than 20% determined the experimental end point for each animal.
Virulence assay of keratitis mouse models
Aspergillus strains were cultured on Vogel´s minimal media +2% agar for 2–3 d Fresh conidia were dispersed with a bacterial L-loop, harvested in 5 ml PBS, and filtered through sterile PBS-soaked cotton gauze in a 10 ml syringe to obtain pure conidial suspensions. Conidia were quantified using a hemocytometer and adjusted in PBS to a final stock solution of 15–20,000 conidia/µl. Mice were anaesthetized with 1.25% 2, 2, 2-tri-bromoethanol in PBS. The corneal epithelium was abraded using a 30-gauge needle, through which a 2 µl injection containing conidia was released into the corneal stroma using a 33-gauge Hamilton syringe. Mice were examined daily under a stereomicroscope for corneal opacification. At 48 h, animals were euthanized by CO2 asphyxiation, and eyes were placed in 1 ml of sterile PBS, homogenized and colony forming units (CFU) were quantified by manual count.
Ethics statement of mouse models
For pulmonary and systemic infection, mice were cared for in accordance with the principles outlined by the European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes (European Treaty Series, no. 123; http://conventions.coe.int/Treaty/en/Treaties/Html/123.htm). All infection experiments were in compliance with the German animal protection law in a protocol approved by the Government of Lower Franconia (file number: 55.2–2531.01–12/13).
For the keratitis model, animals were treated in accordance with the guidelines provided in the Association for Research in Vision and Ophthalmology ARVO statement for the Use of Animals in Ophthalmic and Vision Research, and were approved by Case Western Reserve University IACUC. C57BL/6 mice (6–12 week old) mice were obtained from the Jackson Laboratory (Bar Harbor, ME).
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
KVIR_Supp_1146848.docx
Download MS Word (3.8 MB)Funding
This work was supported by the Austrian Science Fund/Infect-ERA program (FWF grant I1616/Infect-ERA project AspMetNet to HH), the Medical University of Innsbruck (MUI START grant 19970 to UB), the Deutsche Forschungsgemeinschaft (DFG, SFB Transregio FungiNet A3 to AB) and NIH grants (F31 EY019841 to SML, RO1 EY018612 to EP, and P30 EY011373 to EP). These studies were also supported by a Research to Prevent Blindness Medical Student Fellowship (SML), unrestricted grants from the Research to Prevent Blindness Foundation (EP), and the Ohio Lions Eye Research Foundation (EP). A.-M.D. is an associate student of the HOROS doctoral program.
References
- Tekaia F, Latge JP. Aspergillus fumigatus: saprophyte or pathogen? Curr Opin Microbiol 2005; 8:385-92; PMID: 16019255; http://dx.doi.org/10.1016/j.mib.2005.06.017
- Brakhage AA, Langfelder K. Menacing mold: the molecular biology of Aspergillus fumigatus. Annu Rev Microbiol 2002; 56:433-55; PMID:12142473; http://dx.doi.org/10.1146/annurev.micro.56.012302.160625
- Alifano P, Fani R, Lio P, Lazcano A, Bazzicalupo M, Carlomagno MS, Bruni CB. Histidine biosynthetic pathway and genes: structure, regulation, and evolution. Microbiol Rev 1996; 60:44-69; PMID:8852895
- Busch S, Hoffmann B, Valerius O, Starke K, Duvel K, Braus GH. Regulation of the Aspergillus nidulans hisB gene by histidine starvation. Curr Genet 2001; 38:314-22; PMID:11270573; http://dx.doi.org/10.1007/s002940000171
- Brilli M, Fani R. Molecular evolution of hisB genes. J Mol Evol 2004; 58:225-37; PMID:15042344; http://dx.doi.org/10.1007/s00239-003-2547-x
- Ahangar MS, Vyas R, Nasir N, Biswal BK. Structures of native, substrate-bound and inhibited forms of Mycobacterium tuberculosis imidazoleglycerol-phosphate dehydratase. Acta Crystallogr D Biol Crystallogr 2013; 69:2461-7; PMID:24311587; http://dx.doi.org/10.1107/S0907444913022579
- Furukawa A, Oikawa S, Harada K, Sugiyama H, Hiraku Y, Murata M, Shimada A, Kawanishi S. Oxidatively generated DNA damage induced by 3-amino-5-mercapto-1,2,4-triazole, a metabolite of carcinogenic amitrole. Mutat Res 2010; 694:7-12; PMID:20732334; http://dx.doi.org/10.1016/j.mrfmmm.2010.08.004
- Haydon MJ, Cobbett CS. Transporters of ligands for essential metal ions in plants. New Phytol 2007; 174:499-506; PMID:17447906; http://dx.doi.org/10.1111/j.1469-8137.2007.02051.x
- Schrettl M, Beckmann N, Varga J, Heinekamp T, Jacobsen ID, Jochl C, Moussa TA, Wang S, Gsaller F, Blatzer M, et al. HapX-mediated adaption to iron starvation is crucial for virulence of Aspergillus fumigatus. PLoS Pathog 2010; 6:e1001124; PMID:20941352; http://dx.doi.org/10.1371/journal.ppat.1001124
- Ding C, Festa RA, Sun TS, Wang ZY. Iron and copper as virulence modulators in human fungal pathogens. Mol Microbiol 2014; 93:10-23; PMID:24851950; http://dx.doi.org/10.1111/mmi.12653
- Krappmann S, Sasse C, Braus GH. Gene targeting in Aspergillus fumigatus by homologous recombination is facilitated in a nonhomologous end- joining-deficient genetic background. Eukaryot Cell 2006; 5:212-5; PMID:16400185; http://dx.doi.org/10.1128/EC.5.1.212-215.2006
- Hartmann T, Dumig M, Jaber BM, Szewczyk E, Olbermann P, Morschhauser J, Krappmann S. Validation of a self-excising marker in the human pathogen Aspergillus fumigatus by employing the beta-rec/six site-specific recombination system. Appl Environ Microbiol 2010; 76:6313-7; PMID:20656854; http://dx.doi.org/10.1128/AEM.00882-10
- Millington Ward AM, Reuser JA, Scheele JY, van Lohuizen EJ, van Gorkum van Diepen IR, Klasen EA, Bresser M. Bifunctionality and polarized infidelity at the hisB locus of Aspergillus nidulans. Mol Gen Genet 1984; 193:332-9; PMID:6363882; http://dx.doi.org/10.1007/BF00330690
- Rivera S, Lopez-Soriano FJ, Azcon-Bieto J, Argiles JM. Blood amino acid compartmentation in mice bearing Lewis lung carcinoma. Cancer Res 1987; 47:5644-6; PMID:3664471
- Pitkanen H, Mero A, Oja SS, Komi PV, Pontinen PJ, Saransaari P, Takala T. Serum amino acid responses to three different exercise sessions in male power athletes. J Sports Med Phys Fitness 2002; 42:472-80; PMID:12391443
- Mori I, Fonne-Pfister R, Matsunaga S, Tada S, Kimura Y, Iwasaki G, Mano J, Hatano M, Nakano T, Koizumi S, et al. A Novel Class of Herbicides (Specific Inhibitors of Imidazoleglycerol Phosphate Dehydratase). Plant Physiol 1995; 107:719-23; PMID:12228396
- Jadoun J, Shadkchan Y, Osherov N. Disruption of the Aspergillus fumigatus argB gene using a novel in vitro transposon-based mutagenesis approach. Curr Genet 2004; 45:235-41; PMID:14727059; http://dx.doi.org/10.1007/s00294-003-0480-6
- Haas H. Iron - A Key Nexus in the Virulence of Aspergillus fumigatus. Front Microbiol 2012; 3:28; PMID:22347220; http://dx.doi.org/10.3389/fmicb.2012.00028
- Amich J, Calera JA. Zinc acquisition: a key aspect in Aspergillus fumigatus virulence. Mycopathologia 2014; 178:379-85; PMID:24947168; http://dx.doi.org/10.1007/s11046-014-9764-2
- Schrettl M, Bignell E, Kragl C, Joechl C, Rogers T, Arst HN, Jr., Haynes K, Haas H. Siderophore biosynthesis but not reductive iron assimilation is essential for Aspergillus fumigatus virulence. J Exp Med 2004; 200:1213-9; PMID:15504822; http://dx.doi.org/10.1084/jem.20041242
- Grahl N, Shepardson KM, Chung D, Cramer RA. Hypoxia and fungal pathogenesis: to air or not to air? Eukaryot Cell 2012; 11:560-70; PMID:22447924; http://dx.doi.org/10.1128/EC.00031-12
- Kroll K, Pahtz V, Hillmann F, Vaknin Y, Schmidt-Heck W, Roth M, Jacobsen ID, Osherov N, Brakhage AA, Kniemeyer O. Identification of hypoxia-inducible target genes of Aspergillus fumigatus by transcriptome analysis reveals cellular respiration as an important contributor to hypoxic survival. Eukaryot Cell 2014; 13:1241-53; PMID:25084861; http://dx.doi.org/10.1128/EC.00084-14
- Thomas PA, Kaliamurthy J. Mycotic keratitis: epidemiology, diagnosis and management. Clin Microbiol Infect 2013; 19:210-20; PMID:23398543; http://dx.doi.org/10.1111/1469-0691.12126
- Leal SM, Jr., Cowden S, Hsia YC, Ghannoum MA, Momany M, Pearlman E. Distinct roles for Dectin-1 and TLR4 in the pathogenesis of Aspergillus fumigatus keratitis. PLoS Pathog 2010; 6:e1000976; PMID:20617171; http://dx.doi.org/10.1371/journal.ppat.1000976
- Bertuzzi M, Schrettl M, Alcazar-Fuoli L, Cairns TC, Munoz A, Walker LA, Herbst S, Safari M, Cheverton AM, Chen D, et al. The pH-responsive PacC transcription factor of Aspergillus fumigatus governs epithelial entry and tissue invasion during pulmonary aspergillosis. PLoS Pathog 2014; 10:e1004413; PMID:25329394; http://dx.doi.org/10.1371/journal.ppat.1004413
- Schobel F, Jacobsen ID, Brock M. Evaluation of lysine biosynthesis as an antifungal drug target: biochemical characterization of Aspergillus fumigatus homocitrate synthase and virulence studies. Eukaryot Cell 2010; 9:878-93; PMID:20363898; http://dx.doi.org/10.1128/EC.00020-10
- Oliver JD, Kaye SJ, Tuckwell D, Johns AE, Macdonald DA, Livermore J, Warn PA, Birch M, Bromley MJ. The Aspergillus fumigatus dihydroxyacid dehydratase Ilv3A/IlvC is required for full virulence. PLoS One 2012; 7:e43559; PMID:23028460; http://dx.doi.org/10.1371/journal.pone.0043559
- Beckmann N, Schafferer L, Schrettl M, Binder U, Talasz H, Lindner H, Haas H. Characterization of the Link between Ornithine, Arginine, Polyamine and Siderophore Metabolism in Aspergillus fumigatus. PLoS One 2013; 8:e67426; PMID:23825660; http://dx.doi.org/10.1371/journal.pone.0067426
- Kohler S, Foulongne V, Ouahrani-Bettache S, Bourg G, Teyssier J, Ramuz M, Liautard JP. The analysis of the intramacrophagic virulome of Brucella suis deciphers the environment encountered by the pathogen inside the macrophage host cell. Proc Natl Acad Sci U S A 2002; 99:15711-6; PMID:12438693; http://dx.doi.org/10.1073/pnas.232454299
- Jacobsen ID, Brunke S, Seider K, Schwarzmuller T, Firon A, d'Enfert C, Kuchler K, Hube B. Candida glabrata persistence in mice does not depend on host immunosuppression and is unaffected by fungal amino acid auxotrophy. Infect Immun 2010; 78:1066-77; PMID:20008535; http://dx.doi.org/10.1128/IAI.01244-09
- Pukkila-Worley R, Peleg AY, Tampakakis E, Mylonakis E. Candida albicans hyphal formation and virulence assessed using a Caenorhabditis elegans infection model. Eukaryot Cell 2009; 8:1750-8; PMID:19666778; http://dx.doi.org/10.1128/EC.00163-09
- Sinha SC, Chaudhuri BN, Burgner JW, Yakovleva G, Davisson VJ, Smith JL. Crystal structure of imidazole glycerol-phosphate dehydratase: duplication of an unusual fold. J Biol Chem 2004; 279:15491-8; PMID:14724278; http://dx.doi.org/10.1074/jbc.M312733200
- Krappmann S, Bignell EM, Reichard U, Rogers T, Haynes K, Braus GH. The Aspergillus fumigatus transcriptional activator CpcA contributes significantly to the virulence of this fungal pathogen. Mol Microbiol 2004; 52:785-99; PMID:15101984; http://dx.doi.org/10.1111/j.1365-2958.2004.04015.x
- Pearce DA, Sherman F. Toxicity of copper, cobalt, and nickel salts is dependent on histidine metabolism in the yeast Saccharomyces cerevisiae. J Bacteriol 1999; 181:4774-9; PMID:10438744
- Ryuko S, Ma Y, Ma N, Sakaue M, Kuno T. Genome-wide screen reveals novel mechanisms for regulating cobalt uptake and detoxification in fission yeast. Mol Genet Genomics 2012; 287:651-62; PMID:22806344; http://dx.doi.org/10.1007/s00438-012-0705-9
- Kramer U, CotterHowells JD, Charnock JM, Baker AJM, Smith JAC. Free histidine as a metal chelator in plants that accumulate nickel. Nature 1996; 379:635-8; PMID:NOT_FOUND; http://dx.doi.org/10.1038/379635a0
- Callahan DL, Baker AJ, Kolev SD, Wedd AG. Metal ion ligands in hyperaccumulating plants. J Biol Inorg Chem 2006; 11:2-12; PMID:16328457; http://dx.doi.org/10.1007/s00775-005-0056-7
- Crowe J, Masone BS, Ribbe J. One-step purification of recombinant proteins with the 6xHis tag and Ni-NTA resin. Mol Biotechnol 1995; 4:247-58; PMID:8680931; http://dx.doi.org/10.1007/BF02779018
- Becker KW, Skaar EP. Metal limitation and toxicity at the interface between host and pathogen. FEMS Microbiol Rev 2014; 38:1235-49; PMID:25211180; http://dx.doi.org/10.1111/1574-6976.12087
- Samanovic MI, Ding C, Thiele DJ, Darwin KH. Copper in microbial pathogenesis: meddling with the metal. Cell Host Microbe 2012; 11:106-15; PMID:22341460; http://dx.doi.org/10.1016/j.chom.2012.01.009
- Neyrolles O, Mintz E, Catty P. Zinc and copper toxicity in host defense against pathogens: Mycobacterium tuberculosis as a model example of an emerging paradigm. Front Cell Infect Microbiol 2013; 3:89; PMID:24350063; http://dx.doi.org/10.3389/fcimb.2013.00089
- Pontecorvo G, Roper JA, Hemmons LM, Macdonald KD, Bufton AW. The genetics of Aspergillus nidulans. Adv Genet 1953; 5:141-238; PMID:13040135; http://dx.doi.org/10.1016/S0065-2660(08)60408-3
- Nielsen ML, Albertsen L, Lettier G, Nielsen JB, Mortensen UH. Efficient PCR-based gene targeting with a recyclable marker for Aspergillus nidulans. Fungal Genet Biol 2006; 43:54-64; PMID:16289954; http://dx.doi.org/10.1016/j.fgb.2005.09.005
- Punt PJ, Oliver RP, Dingemanse MA, Pouwels PH, van den Hondel CA. Transformation of Aspergillus based on the hygromycin B resistance marker from Escherichia coli. Gene 1987; 56:117-24; PMID:2824287; http://dx.doi.org/10.1016/0378-1119(87)90164-8
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. New York: Cold Spring Harbor Laboratory Press 1989; 2nd ed.
- Fallon JP, Troy N, Kavanagh K. Pre-exposure of Galleria mellonella larvae to different doses of Aspergillus fumigatus conidia causes differential activation of cellular and humoral immune responses. Virulence 2011; 2:413-21; PMID:21921688; http://dx.doi.org/10.4161/viru.2.5.17811
- Mowlds P, Kavanagh K. Effect of pre-incubation temperature on susceptibility of Galleria mellonella larvae to infection by Candida albicans. Mycopathologia 2008; 165:5-12; PMID:17922218; http://dx.doi.org/10.1007/s11046-007-9069-9
- Smith JM, Tang CM, Van Noorden S, Holden DW. Virulence of Aspergillus fumigatus double mutants lacking restriction and an alkaline protease in a low-dose model of invasive pulmonary aspergillosis. Infect Immun 1994; 62:5247-54; PMID:7960101
- Amich J, Schafferer L, Haas H, Krappmann S. Regulation of sulphur assimilation is essential for virulence and affects iron homeostasis of the human-pathogenic mould Aspergillus fumigatus. PLoS Pathog 2013; 9:e1003573; PMID:24009505; http://dx.doi.org/10.1371/journal.ppat.1003573
- Blatzer M, Barker BM, Willger SD, Beckmann N, Blosser SJ, Cornish EJ, Mazurie A, Grahl N, Haas H, Cramer RA. SREBP coordinates iron and ergosterol homeostasis to mediate triazole drug and hypoxia responses in the human fungal pathogen Aspergillus fumigatus. PLoS Genet 2011; 7:e1002374; PMID:22144905; http://dx.doi.org/10.1371/journal.pgen.1002374
