ABSTRACT
Antimicrobial resistance in bacterial pathogens is a worldwide challenge leading high morbidity and mortality in clinical settings. Multidrug resistant patterns in gram-positive and –negative bacteria have resulted in difficult-to-treat or even untreatable infections with conventional antimicrobials. Since the early identification of causative microorganisms and their antimicrobial susceptibility patterns in patients with bacteremia and other serious infections is lacking in many healthcare institutions, broad spectrum antibiotics are liberally and mostly unnecessarily used. Such practice has, in turn, caused dramatic increases in emerging resistance and when coupled with poor practice of infection control, resistant bacteria can easily be disseminated to the other patients and the environment. Thus, availability of updated epidemiological data on antimicrobial resistance in frequently encountered bacterial pathogens will be useful not only for deciding on empirical treatment strategies, but also devising an effective antimicrobial stewardship program in hospitals.
Introduction
Multidrug resistance (MDR) in various bacterial pathogens has reached to a pandemic level during the last 2 decades. The Centers for Disease Control and Prevention (CDC) estimates that in the US more than 2 million people are infected every year with antibiotic resistant microbes and at least 23.000 die due to these infections.Citation1 The calculated price tag is $20 billion in direct healthcare costs, with far more costs in lost productivity. A similar report from Britain predicts that the toll of global antimicrobial resistance will be 10 million deaths per year and up to $100 trillion lost to the global economy by 2050.Citation2 A recent study revealed that 30% reduction of efficacy of antibiotics for antibacterial prophylaxis in surgery and cancer chemotherapy may result in 120.000 additional surgical site- and post-chemotherapy-infections per year in the US and 6300 infection-related deaths.Citation3
Any bacteria can develop antimicrobial resistance (AMR), but still maintain its susceptibility to many others, allowing successful treatment in clinical settings. Recently, a selected group of bacteria has been described by the acronym of ESKAPE and they predominantly cause most of the nosocomial infections in healthcare settings.Citation4 The term refers Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species. However, selection of these bacteria and the acronym itself have been criticized by others since it excludes other enteric gram-negative pathogens including Escherichia coli which is one of the most frequent bacterial agents causing severe infections with significant MDR mechanisms. Thus, a more appropriate term ESCAPE has been proposed where “C” refers to Clostridium difficile, an important nosocomial pathogen that may easily acquire an MDR phenotype and “E” refers Enterobacteriaceae covering all gram-negative enteric bacteria including E. coli, K. pneumoniae, Proteus spp. and Enterobacter spp.Citation5
Various risk factors have been described leading increased rates of AMR among which inappropriate and widespread use of antibiotics is the most significant one.Citation6 Poor governance and corruption have also been suggested to contribute to the levels of AMR in a given country.Citation7
Epidemiology of global antimicrobial resistance
In its global report on surveillance in AMR, The World Health Organization (WHO) declared that AMR in wide range of infectious agents has become a serious public health problem and a post-antibiotic era is a real possibility for the 21st century.Citation8 Although, there was significant gaps in surveillance and lack of standards for methodology in many countries worldwide, WHO reported very high rates of resistance both for health-care associated (HCA) and community-acquired (CA) infections. The data pointed out that fluoroquinolone resistance in E. coli has been reported in 92 member states out of 194 and 5 out of 6 WHO global regions. Similarly, the 3rd generation cephalosporin resistance (most probably due to an extended-spectrum cephalosporinase) was recorded in 86 member states and 5 regions. Comparable figures were noted for the 3rd generation cephalosporin or carbapenem resistance in K. pneumoniae and for methicillin resistance in S. aureus.Citation8
Other major epidemiological surveillance networks including those in Europe (i.e., European Antimicrobial Resistance Surveillance Network -EARS-Net and Central Asian and Eastern European Surveillance of Antimicrobial Resistance-CAESAR) and in the US (The National Healthcare Safety Network-NHSN at the CDC) also documented that the antibiotic resistant bacteria have become much more prevalent during the last decade.Citation9-11 The details of their data will be summarized below for different types of bacteria which may cause bacteremia in different healthcare settings. An online tool is also available showing the current resistance rates and antibiotic use in various countries on an interactive map and data are updated as the new information becomes available (http://resistancemap.cddep.org).
Epidemiology of antimicrobial resistance for selected, important human pathogens causing bacteremia
Gram-positive bacteria
Staphylococcus aureus (SA) and coagulase-negative staphylococci (CNS)
Staphylococcus aureus is the most significant cause of gram-positive bacteremia in the developed world for which incidence varies 10 to 30 per 100.000 person years.Citation12 Methicillin resistance is the hallmark of antimicrobial resistance in both SA and CNS which can be regarded an indicator for multidrug resistance. During the last decade, the rates of nosocomial methicillin-resistant S. aureus (MRSA) bacteremia has been either stabilized or declined in many geographic regions of the world ().Citation13-16 Although this reduction was attributed to improved infection control practices in the West, the decline was also noted from the developing countries where infection control remains to be an unsolved problem, although the figures reported from these countries may be less reliable.Citation12 The most recent data available from ECDC in 2013 indicate that in Europe, Romania has the highest rates of MRSA (>50%) isolated from cerebrospinal fluid (CSF) or blood and additional 5 countries in the EU region (Cyprus, Greece, Hungary, Italy and Spain) have rates between 25% and 50%.Citation17 The lowest figures are from the Scandinavia and the Netherlands (<5%).
Figure 1. Data shown on the graph are obtained from the following sources: Australia: Australian Group on Antimicrobial Resistance (AGAR). Available at http://www.agargroup.org, France, Germany, Greece, UK: European Antimicrobial Resistance Surveillance Network (EARS Net). Available at http://www.ersnet.org, Turkey: The data for the period of 2003–2008 are derived from EARSS European Antimicrobial Resistance Surveillance System (EARSS) available at http://www.rivm.nl. For 2009–2010; personal communication with Dolunay Gulmez, M.D. For 2011–2013; from National Antimicrobial Resistance Surveillance System Yearly Reports 2011–2013. Turkish Public Health Insititution. Available at http://uamdss.thsk.gov.tr, USA: Resistance Map-The Center for Disease Dynamics Economics & Policy (CDDEP) Citation2015. Available at: http://resistancemap.cddep.org.
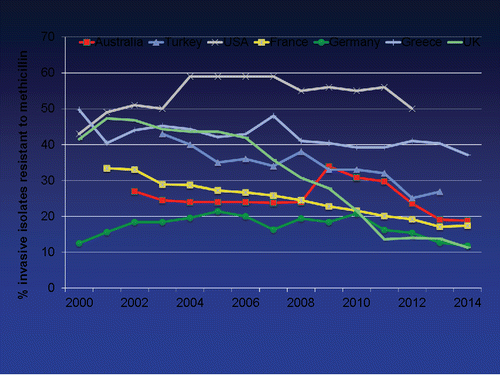
Methicilin sensitive S. aureus (MSSA) strains are generally sensitive to clindamycin, however resistance can be selected during treatment. Particulary in those strains initially resistant to erythromycin, if treated with clindamycin, resistance can rapidly be selected during therapy.Citation18
Trimethoprim-sulfamethoxazole (TMP-SMX) is highly active against MSSA and community-acquired MRSA (CA-MRSA) isolates. Sensitivity varies among MRSA strains, ranging 26–100% in US and 0–92% worlwide.Citation19
Reduced susceptibility to glycopeptides in S. aureus has emerged during the last 2 decades and may have significant implications for those receiving these antibiotics (i.e., vancomycin and teicoplainin). Vancomycin-intermediate S. aureus (VISA, vancomycin MIC 4–8 mg/L) and heteroresistant (hVISA) strains with vancomycin MICs within the susceptible range but with suppopulations that are able to grow in the presence of vancomycin were reported worldwide. VISA isolates exhibit decreased susceptibility to teicoplainin as well. The true incidence of hVISA are difficult to establish since the laboratory detection is too cumbersome. A 2009 report from the US indicated that the prevalence was 0.4% in MRSA isolates. This rate was increased as the MIC of vancomycin escalated (i.e. 10.5% in those with 2 mg/L vancomycin MIC vs 0.1% in those with 1 mg/L).Citation20 The strains with a high-vancomycin MIC, but still within susceptible range to vancomycin (i.e., . 1–2 mg/L) may cause treatment failure and constitute a public health problem. The rise of vancomycin MICs over time was described as ‘MIC creep’ and mainly reported in centers where large amounts of vancomycin are consumed.Citation21 However, recent data indicated that the ‘MIC creep’ was not translated into increased mortality rates.Citation22,23
Daptomycin is active against staphylococci with decreased glycopeptide activity. Development of resistance to this lipopetide antibiotic has been rarely reported.Citation24 However, in a study with S. aureus bacteremia with or without endocarditis, 6 of 120 patients treated with daptomycin had microbiological failure and daptomycin MICs increased from 0.25 mg/L baseline to >2 mg/L during therapy.Citation25
The new lipogylcopeptide telavancin has potent bactericidal activity against all S. aurues isolates including those non-susceptible to daptomycin.Citation26 No clinically-relevant resistant isolates have been described for this agent, so far.
Staphylococcus aureus is the second most common pathogen only after E. coli causing community-acquired bloodstream infections (CA-BSIs).Citation28 It has been estimated that in the Western countries, the incidence of CA-BSIs due to SA is approximately 15 per 100.000 and a mortality rate of 3 per 100.000.Citation29 Community-acquired MRSA infections have emerged as a global problem since the turn of the 21st century.Citation30-32 Five major clones are found to be associated with most of the CA-MRSA infections worldwide including multilocus sequence type 1 (ST-1)/USA400 and ST-8/USA300 dominantly found in North America, ST-59 observed in South East Asia, ST-80 observed in Europe and ST-30 distributed worldwideCitation28. The clonal structure remains to be determined in CAESAR countries.Citation10 Although CA-MRSA strains initially caused mainly skin and soft tissue infections in healthy individuals and in homeless and imprisoned population, recently increased rates of bacteremia and other invasive infections with CA-MRSA strains have been reported. Moreover, these strains moving into the hospital settings increasingly caused HCA infections including ventilator associated pneumonia, surgical site infections and bacteremia.Citation33,34 They usually remain susceptible to many non-beta-lactam antibiotics including clindamycin and TMP-SMX.Citation27
Coagulase-negative streptococci are the most common cause nosocomial BSIs and are responsible almost one third of all healthcare associated bacteremia. The incidence is highest in those with cancer and neutropenia and those with catheter- and/or prosthetic-device related infections.Citation35,36 Multiple antibiotic resistance is highly encountered among hospital isolates and usually related with methicillin resistance.Citation37
Resistance rates up to 90% for methicillin, 78.6% for levofloxacin, 68% for ciprofloxacin, and 48.5% for clindamycin have been reported in US.Citation38 Multidrug resistancein CNS was found to be related with levofloxacin prescribing patterns.Citation38
Resistance to vancomycin is very rare, however a 20.8% resistance to teicoplanin was reported from UK, particulary in S. haemolyticus.Citation37 Although the reported rates are usually <1 .5%, linezolid resistance is emerging and might be a future concern in CNS.Citation39,40 At present, the resistance is negligible for daptomycin, tigecycline and newer agents such as televancin, oritavancin and dalbavancin.Citation41 Ceftobiprole, a new anti-MRSA cephalosporin, is highly active to both MRSA and methicillin-resistant CNS, but not approved for the treatment of bacteremia caused by any of these pathogens.Citation42 Another anti-MRSA cephalosporin, ceftaroline, although not approved for the treatment of bacteremia, has been used off-label for S. aureus bacteremia frequently caused by MRSA and successful results were reported.Citation43 The results of a currently completeed prospective, non-comparative study for the treatment of S. aureus bacteremia is yet to be released.Citation44
Streptococcus spp. including S. pneumonia and viridans streptococci
Streptococcus pneumonia is the third most common cause of CA bacteremia. Its prevalence in the Western World ranges between 10 and 20 per 100.000 population.Citation28 Only 10% of cases are related with healthcare associated infections.Citation45 One of the important aspects of invasive pneumococcal infections related with bacteremia is that the universal use of conjugate pneumococcal vaccine in infants and more recently in the adult population: Decreased exposure from infants led the adult population have a lower incidence of invasive pneumococcal disease which is also described as herd protection.Citation46
In the US, >90% of all pneumococci causing infections other than meningitis are sensitive (<0.06 mg/L) and only 2% are resistant (>2 mg/L) to parenteral penicillin or oral amoxicillin. Only 1% of isolates are resistant to ceftriaxone.Citation47 In Europe, higher rates of resistance to penicillin (>0.06 mg/L) was reported in several countries in 2013: 54% in Turkey, between 25 to <50% in Spain, Croatia, Cyprus and Romania, 10 to 25% in Bulgaria, Latvia and Lithuania.Citation10,17 The remaining countries has lower rates (<10%) and France, UK, Norway, Belgium, Netherlands, Austria, Czech Republic, Slovenia and Estonia has the lowest rates (<1%).Citation17 It should be noted that European isolates are from blood or CSF origin and those of US are from non-meningitis isolates and not necessarily obtained from invasive infections. Much higher rates are reported from Asian countries.Citation48,49 Penicillin resistant pneumococci are more likely to show higher resistance to other classes of antimicrobials. Current figures of resistance in US include 35% to macrolides, 10% to clindamycin, 30% to trimethoprim sulfamehoxazole, 18% to doxycycline and 2% to respiratory quinolones.Citation47 Higher rates of macrolide resistance are reported from Europe.Citation17
Viridans streptococci can cause infective endocarditis particularly in patients with compromised heart valves, although its prevalence has decreased particularly in the Western World.Citation50 They can also produce bacteremia and septic shock particularly in neutropenic patients.Citation51 Clinically significant bacteremia are attributed to viridans streptococci in 0.5% of all blood cultures received in clinical microbiology laboratory.Citation52 Although these bacteria are susceptible to most antimicrobials, β-lactam resistance has emerged and may cause a significant problem especially in patients with immunosuppression and bacteremia.Citation51,53 In the latter group, penicillin resistance rates >50% is reported.Citation54 These bacteria do not produce a β-lactamase, the β-lactam resistance is due to altered penicillin binding proteins. Ceftriaxone and cefepime resistance has been reported up to 23 and 25%, respectively in strains isolated from hospitalized or cancer patients.Citation54,55 Viridans streptococci may cause bacteremia and septic shock in patients with neutropenia and with certain risk factors. Since the latter 2 cephalosporins are frequently used for initial empirical therapy, such resistance may have serious clinical implicationsCitation53. Vancomycin is higly effective on such strains and should be preferred as the empirical agent of choice in this type of neutropenic patients.
Enterococci
Among all enterococci, E. faecium is the most challenging bacterium in terms of antibacterial resistance and therapy. In the US, enterococci are the second most common bacteria isolated from catheter related (CR)-BSIs.Citation11 Enterococci are intrinsically resistant to many antimicrobials, but also easily acquire mutations and exogenous genes to develop further resistance.Citation56 While aminopenicillin resistance is rare (<1%) to low (up to 25%) in E. faecalis, it is encountered around 90 % of nosocomial E. faecium isolates.Citation17,56 Beta-lactamase production is infrequently associated with resistance and can be overcome with the use of β-lactamase inhibitor compounds. The production of PBP5 with low affinity to penicillins is the major culprit for β-lactam resistance.Citation56
High-level resistance to all aminoglycosides eliminates the synergistic activity of penicillins and vancomycin both of which can enhance activity of aminoglycosides in enterococci with low-to-moderate resistance. High-level aminoglycoside resistance has increased in both E. faecalis and E. faecium during the last 3 decades and current figures from Europe indicate between 5–>50% in E. faecalis and 25–>50% in E. faecium.Citation17
Glycopeptide resistance in enterococci is a much bigger problem in the US than Europe and elsewhere. By 2007, >80% of E. faecium isolates in the US hospitals were reported to be resistant to vancomycin whereas in Europe only Ireland reported a resistance rate of >50%.Citation17,56,57 Similarly, MDR enterococci is much more prevalent in the US than elsewhere.Citation56
Enterococci are the third most frequent agents of bacteremia in hematological cancer patients and stem cell transplant recipients and may affect up to 12% of all transplant patients.Citation51 In these patient groups, a shift from E. faecalis to to E. faecium has resulted in higher rates of vancomycin resistant enterococcus (VRE) infections.Citation51 However, similar to the general epidemiology, VRE infections are less of a problem in Western European transplant centers with <5 % of enterococci being to resistant to vancomycin.355 Resistance to linezolid and daptomycin is rarely reported.Citation58
Clostridium difficile
Clostridium difficile very rarely causes bacteremia and usually constitutes one of the offending microorganisms of a polymicrobial etiology in bacteremic patients.Citation59 However, it is a major cause of antibiotic associated diarrhea and its prevalence has siginificantly increased during the last decade particulary in North America and Europe and recently in Israel.Citation60 This increase was associated with the emergence of hypervirulent C. difficile strains.Citation61 The most prominent hypervirulent strain is PCR-ribotype (RT) 027 which is responsible serious and frequently recurring infections and many of these strains carry an MDR phenotype. A European survey in 2005 indicated that 55% of 148 isolates with resistance to at least one antibiotic was multidrug resistant.Citation62 In the US, among 508 toxigenic C. difficile isolates collected between 2001 and 2013, 28.1% was RT027 strain.Citation63 Additionally, other hypervirulent strains not necessarily related with RT027 were identified. Among these RT078 cause Clostridium difficile disease both in community and hospital settings, and also in animals.Citation61
Gram-negative bacteria
Escherichia coli
Production of one or more extended spectrum β-lactamases (ESBLs) is the main resistance mechanism to broad-spectrum penicillins and cephalosporins in enteric gram-negative pathogens. Among the numerous ESBLs, CTX-M type of enzymes have become clinically the most important β-lactamases and are highly prevalent in E. coli.Citation64,65
Escherichia coli is one of the most frequent pathogens causing bacteremia both in CA and in HCA bacteremia including those in patients with cancer and neutropenia.Citation35,51,66 Escherichia coli sequence type (ST)131 with CTX-M-15 ESBL production has a high epidemic potential and spread worldwide.Citation64,65,67 EARS-Net data indicated that in the EU mean resistance rate to the 3rd generation cephalosporins was 11.9%, ranging 4.4% in Sweden, 38.1% in Bulgaria.Citation9 The ESBL-positive strains were reported between 71.5% and 100% in different European countries. The CAESAR data noted that resistance rates varied between 7.0% in Switzerland and 44% in Turkey among all invasive strains.Citation10 Higher rates of ESBL production were reported from India (>80%), and China (>60%), however these isolates were mainly from intraabdominal infections, but in some cases there were secondary bacteremia.Citation68,69 Current prevalence of ESBL production in bacteremic isolates in US varies between 8.1% to 13.7%.Citation70,71 Isolates from ICU patients and patients with hematological malignancies showed higher resistance rates.Citation66,71 In those countries where antimicrobial use is not strictly controlled, high prevalence of ESBL production has been reported in community-settings as well. Examples including countries in Asia including IndiaCitation69, CambodiaCitation72 ChinaCitation73, South KoreaCitation74, ThailandCitation75, and in Europe including TurkeyCitation76 and RomaniaCitation77 provide information about prevalence of ESBLs for both CA and HCA isolates of E. coli in various countries. Many ESBL-producing E. coli are also resistant to non-β-lactam antibiotics including aminoglycosides and quinolones with different resistance mechanisms.Citation66,78 ESBL-encoding plasmids may also encode resistance to aminoglycosides, tetracyclines, sulphonamides and trimethoprim.Citation67 These plasmids frequently encode an inhibitor-resistant β-lactamase, namely OXA-1 which confers resistance to β-lactamase inhibitors including amoxicillin-clavulanate and piperacillin-tazobactam.Citation67,79
Quinolone resistance has been reported 41.8% in US in CR-BSIs and between 11 to 52 percent in invasive isolates from EU countries;Citation11,17 whereas CAESAR data indicated 8% and 41% resistance among isolates from blood and CSF in Switzerland and Turkey, respectively.Citation10
Aminoglycoside resistance among E. coli and other enteric pathogens is determined by aminoglycoside-modifying enzymes which can be encoded on the same plasmid with ESBLs. This type of resistance in E. coli varied between 5–25% in Europe excluding Bulgaria where higher rates of resistance (32%) was recorded in isolated from HCA infections.Citation10,17
Carbapenem resistance in E. coli in Europe is rarely reported, the highest rates are found in Bulgaria (2.6%) and Turkey (4.0%).Citation10,17 Esherichia coli was the second most frequent carbapenem-resistant Enterobacteriaceae (CRE) after K. pneumoniae in a recent US survey where the incidence of CRE was determined as 2.93 per 100.000 population.Citation80 A large surveillance study from China reported the prevalence of carbapenem-resistant E. coli is 1.0% and of K. pneumoniae 5.5%.Citation81 One of the most significant carbapenemases described in Enterobacteriaceae is New Delhi metallo-β-lactamase-1 (NDM-1). This enzyme is prevalent in the Indian subcontinent, but also frequently reported in Balkans and in the Middle East.Citation82 The bacteria harboring this enzyme have spread worldwide and are usually only susceptible to colistin, tigecycline and fosfomycin, although susceptibility is not universal.Citation83 So far, the resistant strains are endemic only in India, Pakistan and Sri Lanka where environmental strains carrying this enzyme were also found. The estimated prevalance of carriage among public in India is between 5 to 15%.Citation82 Outbreaks have been described in UK, France, Italy, Greece, China, Australia and elsewhere in Africa and South America.Citation82 Since E. coli infections are very frequent in the outpatient settings, it is feared that a progressive increase in the prevalence of NDM-1 producing E. coli may occur and this pattern finally may replace the CTX-M producing strains in the community setting.Citation82
Plasmid-mediated colistin resistance (via mcr-1 colistin resistance gene) has recently described in E. coli isolates worldwide from mainly livestock and less frequently in human samples.Citation84-89 The implications of this finding may be horrendous since the offending plasmid can easily be transferred between E. coli strains including those with epidemic potential (e.g. ST131) and to K. pneumoniae and P. aeruginosa.Citation90 As a matter of fact, recent reports already noted the presence of this gene from plasmids in SalmonellaeCitation91,92 and K. pneumoniae.Citation93,94 Recovery of an E. coli strain from chicken meal in China co-producing plasmid-mediated MCR-1, NDM-9, CTX-M-65 and FosA3 (accounted for fosfomycin resistance) proteins is highly concerning that such strains can colonize the human intestine and transfer resistance plasmids to other gram-negatives. Citation95
Klebsiella pneumoniae
The third generation cephalosporin resistance in K. pneumoniae in Europe ranged between 2.7% in Finland to 70.1% in Greece and 88% in Serbia; the highest resistance rates were reported from Central, Eastern and South of Europe.Citation10,17 The ESBL positivity in these isolates were between 65% and 100%.Citation4 The data from the NHSN indicated that 28.8% of K. pneumoniae causing CR-BSIs in participating centers in the US were resistant to the 3rd generation cephalosporins.Citation11 In Asia, China and Thailand were reported to have the highest prevalence of ESBL producers (33.7% and 40.7%, respectively).Citation96
Carbapenem resistance has become the most important epidemiologic and therapeutic challenge in K. pneumoniae.Citation82 There are mainly 3 classes of carbapenemases involved including KPC (Class A), OXA-48 (Class D) and NDM (Class B) for which different epidemiological reservoirs exist. For KPC, the highest-prevalence countries are Greece and Italy in Europe and US and Israel.Citation82,97 OXA-48 are most prevalent in Turkey, North Africa and India.Citation82 The main reservoirs for NDM are Indian subcontinent, Middle East and Balkan countries.Citation83
KPC-producing isolates are also endemically reported in various Latin American countriesCitation82, ChinaCitation98,99 and TaiwanCitation100. A specific KPC-2 or KPC-3-producing clone (sequence type 258) has been widely disseminated worldwide contributing the spread of resistance.Citation82
OXA-48-producing isolates were first described in and then spread from Turkey where frequent nosocomial outbreaks were reported.Citation101-105 These isolates have now been disseminated to many European countries.Citation82 OXA-48 producing K. pneumoniae strains have been reported in many Middle Eastern and African countries, but rarely from North and South Americas.Citation82
Similar to E. coli isolates described above, NDM-producing K. pneumoniae and other enteric gram-negatives are extensively isolated from out- and in-patient settings and also from the environment in Indian subcontinent.Citation106
EARS-Net database in 2013 indicated that Greece (59.4%), Italy ( 34.3%) and Romania (20.5% ) have the highest rates of carbapenem resistance in K. pneumoniae.Citation17 The remaining countries in the EU have a prevalence <2%. However, trend analyses showed a significantly increasing pattern in carbapenem resistance.Citation17 In the CAESAR program, resistance rates were reported from Serbia (36%), Turkey (11%), and Switzerland (1%).Citation10 In the US, a recent epidemiological survey from 7 different geographic areas identified 599 CRE isolates in 481 patients of which 58.6% were K. pneumonia. The only carbapenemase identified in all isolates was KPC.Citation57 The frequency of CRE infections in children are increasing, but still low as compared with ESBL-producing strains.Citation107
Carbapenem resistant isolates usually show a MDR pattern and are susceptible only to colistin, fosfomycin and tigecycline. However, there is also emergence of resistance against these antibiotics. A multicenter survey from Italy indicated that 43% of KPC- producing K. pneumoniae are already resistant to colistin.Citation108 A very recent multicenter study from the same country reported that during a 4.5 y period, the colistin resistance increased >3-fold in participating centers and related with a mortality rate of 51%.Citation109
Pseudomonas aeruginosa
In Europe, P. aeruginosa strains with high resistance rates to aminoglycosides, ceftazidime, quinolones, piperacillin-tazobactam and carbapenems are usually reported from Southern and Eastern part of the continent.Citation9 However, ECDC reported that a trend analysis for 2009 to 2012 indicated a stable resistance pattern for these antimicrobials.
In the US, 10% aminoglycoside resistance in P. aeruginosa isolates from catheter-releted blood stream infections was reported 10% during 2009–2010.Citation11 In Europe, 9 out of 29 EU member countries reported >20% aminoglycoside resistance from invasive isolates in 2014.Citation17 Serbia and Romania have the highest (>50%) resistance rate to aminoglycosides in invasive isolates.
Carbapenem resistance was described in >50% of isolates in 3 countries, between 25 to <50% in 7 countries and 10 to <25% in 11 out of 29 reporting countries.Citation17 However, a trend analysis indicated that the rate of resistance have been stable in invasive isolates between 2009 and 2012, no significant increase was detected.Citation9 In CAESAR database, resistance to amikacin, quinolones, piperacillin-tazobactam, ceftazidime and carbapenems in Switzerland was reported as 1%, 10%, 7%, 6% and 9%, respectively; whereas the same rates were 11%, 22%, 27%, 26% and 33% in Turkey.Citation10 Resistance is high in South America and Southeast Asia and intermediate in the US.Citation11,110 Several β-lactamases have been described for causing resistance and these include AmpC, ESBL (particularly PER-1) and metallo-β-lactamases.Citation67 PER-1 producing P. aeruginosa which shows high-level resistance to ceftazidime, but susceptible to clavulanate and tazobactam was widely detected in Turkey and less frequently in several European and Asian countries.Citation111 Carbapenem resistance in P. aeruginosa is mostly due to porin deficiencies and rarely caused by carbapenemase production. A detailed resistance mechanisms of antibiotics and their epidemiology in Pseudomonas aeruginosa have recently been reviewed.Citation112 Emergence of colistin resistance in P. aeruginosa has also been reported worldwide.Citation113
Acinetobacter baumannii
In EARS-Net database, in 18 countries in the EU from where susceptibility rates for A. baumannii were reported >50% of all isolates were resistant to carbapenems, quinolones and aminoglycosides.Citation9 Carbapenem resistance was reported in >50% of isolates in Portugal, Greece, Italy, Cyprus, Romania and Bulgaria. Lower rates were detected in other countries (e.g., France, UK, and Germany). These isolates are usually co-resistant to aminoglycosides and quinolones. In CAESAR surveillance, Serbia reported 91% quinolone and 93% carbapenem resistance, whereas Switzerland's figures for both antibiotics were 11%. In the US, with CR-BSI, the resistance to carbapenems was detected in 62.6% and an MDR phenotype in 67.6% of isolates.Citation11
The most frequent Class A ESBLs found in A. baumanni are PER-, GES- and VEB-type enzymes. These β-lactamases confer resistance to extended-spectrum cephalosporins, but inhibited by tazobactam and clavulanic acid.Citation112 PER-1 producing A. baumannii are prevalent in Turkey, but also disseminated in several Eastern European countries including Russia, Hungary, Romania, East and Southeast Asia including China and Korea and finally detected in the US.Citation112 TEM, SHV and CTX-M-type ESBLs are rarely found in A. baumannii.
Class B β-lactamases (metalloenzymes) are also reported in A. baumannii and include IMP-, VIM- and NDM-type enzymes. These β-lactamases provide activity against not only to carbapenemes, but also to broad-spectrum cephalosporins and penicillins. The majority of NDM-producing Acinetobacter spp are reported from China and the Middle-East.Citation106 Species identification may be important within the A. baumannii group since carbapenem resistance is more frequently reported in A. baumannii and mortality is much higher in patients with bacteremia casused by these strains as compared with other species such as A. pittii and A. nosocomialis which are the other clinically important members of the A. baumannii group.Citation114 Various carbapenemases including (NDM-type and Class D enzymes) are also described in A. pittii, recently.Citation115,116
Class D, OXA-type carbapenemases are responsible for most carbapenem resistance in A. baumannii.Citation67 OXA-51 is a naturally occurring β-lactamase in A. baumannii and normally has weak carbapenemase activity.Citation112 However, several additionally acquired class D enzymes are described and include OXA-23, −40, −58, −143, and −235. These enzymes cause weak resistance to carbapenems, but are not active against extended-spectrum cephalosporins. Thus, high-level resistance usually require other mechanisms involved such efflux and porin loss.Citation112 OXA-23 enzymes are the most prevalent β-lactamases in A. baumannii and detected worldwide.Citation117
The ArmA enzyme is the most frequently found methylase which is responsible for high-level resistance to all aminoglycosides in A. baumannii. The gene responsible for this enzyme is often identified among OXA-23 producing A. baumannii strains. Other methylases are also described.Citation112
Overexpression of efflux pumps can provide resistance to quinolones. These pumps also use aminoglycosides, tetracyclines, chloramphenicol and trimethoprim as substrates, thus the quinolone resistance can be selected by non-quinolone antibiotics as well. Usually several of these mechanisms are present in MDR Acinetobacter isolates.
Tigecycline, although not approved for the treatment of Acinetobacter infections, has been frequently prescribed for various nosocomial infections caused by MDR A. baumanni. Efflux pumps were described to cause resistance in clinical A. baumanni isolates. A brief exposure to the drug during therapy may trigger resistance and hamper the efficacy of tigecycline.Citation118
Colistin is one of the most important therapeutic alternatives for treating A. baumanni infections. Resistance to this agent in Acinetobacter is rarely reported.Citation112 Laboratory errors are frequent and most laboratories do not use a microdilution method which should be the standart test for evaluating colistin resistance. Most of the resistant isolates are described from South Korea, Spain, the US and Iran.Citation112
A concise summary of main resistance mechanisms in clinically important gram-negative bacteria is given in .
Table 1 Frequent resistance mechanisms for selected antibacterial antibiotics in clinically important gram-negative bacteria (47,48,50,55,58,59,61,63,70,76,84,91) Main resistance determinants.
Reversing the tide of emerging antimicrobial resistance
The main drive behing emerging and spreading resistance is antibiotic consumption both in humans and animals, in the latter for promoting growth and prophylaxis.Citation84,119 Environmental contamination with antibiotics may lead emergence of antibiotic resistance and in turn creates a reservoire for antibiotic resistance genes.Citation120 On the other hand, it has been consistently shown that those areas where the usage is high, the resistance is widespread both in community and hospital settings.Citation7,121,122,123 Since the problem of emerging resistance is multifaceted, its prevention and control will require multiple, coordinated interventions by various parties.Citation119 At the hospital level; education and promotion of prudent use of antimicrobials, development and effective use of rapid and point of care diagnostic tests, devising effective surveillance systems for monitoring resistance and implementation of effective infection control programmes are essential parts of an antibiotic stewardship program. These programmes are desparetely needed and may be lacking even in hospitals in the developed part of the world.Citation124,125
Conclusions
Antimicrobial resistance, particularly MDR phenotype in clinically important community and nosocomial pathogens are prevalent and its incidence is increasing. As the rate of resistance escalates in a given environment, antimicrobials with broader-spectrum of activity are more frequently used in empirical fashion fearing that a delay in initiating effective antimicrobial therapy may result in higher mortality especially in patients with severe underlying diseases and immunosuppression. Such practice may cause a vicious circle leading further increase in resistant isolates. A thorough knowledge about local epidemiology of resistance may contribute to limiting resistance and may have a significant role in designing effective antimicrobial stewardship policies.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Centers for Disease Control and Prevention (CDC). Antibiotic resistance threats in the United States. 2013. Available at http://www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013-508
- O'Neill J (chair). Review on antimicrobial resistance: tackling a crisis for the health and wealth of nations. 2014. Available at http://amr-review.org/
- Teillant A, Gandra S, Barter D, Morgan DJ, Laxminarayan R. Potential burden of antibiotic resistance on surgery and cancer chemotherapy antibiotic prophylaxis in the USA: a literature review and modelling study. Lancet Infect Dis 2015; 15:1429-37; PMID:26482597; http://dx.doi.org/10.1016/S1473-3099(15)00270-4
- Rice LB. Federal funding for the study of antimicrobial resistance in nosocomial pathogens: no ESKAPE. J Infect Dis 2008; 197:1079-81; PMID:18419525; http://dx.doi.org/10.1086/533452
- Peterson LR. Bad bugs, no drugs: no ESCAPE revisited. Clin Infect Dis 2009; 49:992; PMID:19694542; http://dx.doi.org/10.1086/605539
- Paphitou NI. Antimicrobial resistance: action to combat the rising microbial challenges. Int J Antimicrob Agents 2013; 42 Suppl: S25-8; PMID:23684003; http://dx.doi.org/10.1016/j.ijantimicag.2013.04.007
- Collignon P, Athukorala PC, Seanayake S, Khan F. Antimicrobial resistance: the major contribution of poor governance and corruption to this growing problem. PLoS One 2015; 10:e0116746; PMID:25786027; http://dx.doi.org/10.1371/journal.pone.0116746
- World Health Organization. Antimicrobial Resistance. Global Report on Surveillance 2014. Available at http://www.who.int/drugresistance/documents/surveillancereport/en/
- European Centre for Disease Prevention and Control (ECDC). Annual epidemiological report 2014. Antimicrobial resistance and healthcare-associated infections. 2015. Available at http://ecdc.europa.eu/en/publications/Publications/antimicrobial-resistance-annual-epidemiological-report.pdf.
- WHO Regional Office for Europe. Central Asian and Eastern European surveillance of antimicrobial resistance. Ann Rep 2014. Available at http://www.euro.who.int/en/health-topics/disease-prevention/antimicrobial-resistance/antimicrobial-resistance/central-asian-and-eastern-european-surveillance-of-antimicrobial-resistance-caesar
- Dawn M., Sievert, Philip Ricks, Jonathan R, Edwards MS, Schneider A, Patel J, Srinivasan A, Kallen A, Limbago B, Fridkin S for the National Healthcare Safety Network (NHSN) Team and Participating NHSN Facilities. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009–2010. Infect Control Hosp Epidemiol 2013; 34:1-14; PMID:23221186; http://dx.doi.org/10.1086/668770
- Tong SYC, Davis JS, Eichenberger E, Holland TL, Fowler VG Jr. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev 2015; 28:603-61; PMID:26016486; http://dx.doi.org/10.1128/CMR.00134-14
- de Kraker ME, Davey PG, Grundmann H. Mortality and hospital stay associated with resistant Staphylococcus aureus and Escherichia coli bacteremia: estimating the burden of antibiotic resistance in Europe. PLoS Medicine 2011, 8: e1001104; PMID:22022233; http://dx.doi.org/10.1371/journal.pmed.1001104
- Johnson AP, Davies J, Guy R, Abernethy J, Sheridan E, Pearson A, Duckworth G. Mandatory surveillance of methicillin-resistant Staphylococcus aureus (MRSA) bacteraemia in England: the first 10 years. J Antimicrob Chemother 2012; 67:802-9; PMID:22223229; http://dx.doi.org/10.1093/jac/dkr561
- Kallen AJ, Mu Y, Bulens S, Reingold A, Petit S, Gershman K, Ray SM, Harrison LH, Lynfield R, Dumyati G, et al. Health care-associated invasive MRSA infections, 2005–2008. JAMA 2010; 304:641-8; PMID:20699455; http://dx.doi.org/10.1001/jama.2010.1115
- Jarlier V, Trystram D, Brun-Buisson C, Fournier S, Carbonne A, Marty L, Andremont A, Arlet G, Buu-Hoi A, Carlet J, et al. Curbing methicillin-resistant Staphylococcus aureus in 38 French hospitals through a 15-year institutional control program. Arch Intern Med 2010; 170:552-9; PMID:20308642; http://dx.doi.org/10.1001/archinternmed.2010.32
- EARS-Net: European Centre for Disease Prevention and Control (ECDC), Antimicrobial resistance interactive database (Internet). Stockholm (Sweden): ECDC (cited 2015 Oct 22). Available from http://ecdc.europa.eu/en/healthtopics/antimicrobial_resistance/database/Pages/database.aspx.
- McGehee R, Smith CB, Wilcox C, Finland M. Comparative studies of antibacterial activity in vitro and absorption and excretion of lincomycin and clindamycin. Am J Med Sci 1968; 256:279-92; PMID:4386950; http://dx.doi.org/10.1097/00000441-196811000-00002
- Grim SA, Rapp RP, Martin CA, Evans ME. Trimethoprim-sulfamethoxazole as a viable treatment option for infections caused by methicillin-resistant Staphylococcus aureus. Pharmacotherapy 2005; 25:253-64; PMID:15767239; http://dx.doi.org/10.1592/phco.25.2.253.56956
- Richter SS, Satola SW, Crispell EK, et al. Detection of Staphylococcus aureus isolates with heterogeneous intermediate-level resistance to vancomycin in the United States. J Clin Microbiol 2011; 49:4203-7; PMID:21976769; http://dx.doi.org/10.1128/JCM.01152-11
- Jones RN. Microbiological features of vancomycin in the 21st century: minimum inhibitory concentration creep, bactericidal/static activity, and applied breakpoints to predict clinical outcomes or detect resistant strains. Clin Infect Dis 2006; 42 (suppl 1):S13-S24; PMID:16323115; http://dx.doi.org/10.1086/491710
- Kalil AC, Van Schooneveld TC, Fey PD, Rupp ME. Association between vancomycin minimum inhibitory concentration and mortality among patients with Staphylococcus aureus bloodstream infections: a systematic review and meta-analysis. JAMA 2014; 312:1552-64; PMID:25321910; http://dx.doi.org/10.1001/jama.2014.6364
- Chong YP, Park KH, Kim ES, Kim MN, Kim SH, Lee SO, Choi SH, Jeong JY, Woo JH, Kim YS. Clinical and microbiologic analysis of the risk factors for mortality in patients with heterogeneous vancomycin-intermediate Staphylococcus aureus bacteremia. Antimicrob Agents Chemother 2015; 59:3541-7; PMID:25845875; http://dx.doi.org/10.1128/AAC.04765-14
- Sader HS, Jones RN. The activity of daptomycin against wild-type Staphylococcus aureus and strains with reduced susceptibility to vancomycin. Clin Infect Dis 2006; 43:798-9; PMID:16912964; http://dx.doi.org/10.1086/507109
- Fowler VG Jr, Boucher HW, Corey GR, Abrutyn E, Karchmer AW, Rupp ME, Levine DP, Chambers HF, Tally FP, Vigliani GA, et al. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N Engl J Med 2006; 355:653-65; PMID:16914701; http://dx.doi.org/10.1056/NEJMoa053783
- Steed ME, Vidaillac C, Rybak MJ. Evaluation of telavancin activity versus daptomycin and vancomycin against daptomycin-nonsusceptible Staphylococcus aureus in an in vitro pharmacokinetic/pharmacodynamic model. Antimicrob Agents Chemother 2012; 56:955-9; PMID:22123693; http://dx.doi.org/10.1128/AAC.05849-11
- Stryjewski ME, Corey GR. Methicillin-resistant Staphylococcus aureus: an evolving pathogen. Clin Infect Dis 2014; 58 (suppl 1):S10-S19; PMID:24343827; http://dx.doi.org/10.1093/cid/cit613
- Laupland KB, Church DL. Population-based epidemiology and microbiology of community-onset bloodstream infections. Clin Microbiol Rev 2014; 27:647-4; PMID:25278570; http://dx.doi.org/10.1128/CMR.00002-14
- van Hal SJ, Jensen SO, Vaska VL, Espedido BA, Paterson DL, Gosbell IB. Predictors of mortality in Staphylococcus aureus bacteremia. Clin Microbiol Rev 2012; 25:362-86; PMID:22491776; http://dx.doi.org/10.1128/CMR.05022-11
- Chuang YY, Huang YC. Molecular epidemiology of community associated meticillin-resistant Staphylococcus aureus in Asia. Lancet Infect Dis 2013; 13:698-708; PMID:23827369; http://dx.doi.org/10.1016/S1473-3099(13)70136-1
- David MZ, Daum RS. Community-associated methicillin resistant Staphylococcus aureus: epidemiology and clinical consequences of an emerging epidemic. Clin Microbiol Rev 2010; 23:616-87; PMID:20610826; http://dx.doi.org/10.1128/CMR.00081-09
- Laupland KB, Lyytikainen O, Sogaard M, Kennedy KJ, Knudsen JD, Ostergaard C, Galbraith JC, Valiquette L, Jacobsson G, Collignon P, et al. The changing epidemiology of Staphylococcus aureus bloodstream infection: a multinational population-based surveillance study. Clin Microbiol Infect 2013; 19:465-71; PMID:22616816; http://dx.doi.org/10.1111/j.1469-0691.2012.03903.x
- Skov RL, Jensen KS. Community-associated meticillin-resistant Staphylococcus aureus as a cause of hospital-acquired infections. J Hosp Infect 2009; 73:364-70; PMID:19786313; http://dx.doi.org/10.1016/j.jhin.2009.07.004
- Rhee Y, Aroutcheva A, Hota B, Weinstein RA, Popovich KJ. Evolving epidemiology of Staphylococcus aureus bacteremia. Infect Control Hosp Epidemiol 2015; 36:1417-22; PMID:26372679; http://dx.doi.org/10.1017/ice.2015.213
- Mikulska M, Viscoli C, Orasch C, Livermore DM, Averbuch D, Cordonnier C, Akova M, on behalf of the Fourth European Conference on Infections in Leukemia Group (ECIL-4), a joint venture of EBMT, EORTC, ICHS, ELN and ESGICH/ESCMID. Aetiology and resistance in bacteraemias among adult and paediatric haematology and cancer patients. J Infect 2014 68(4):321-31; PMID:24370562; http://dx.doi.org/10.1016/j.jinf.2013.12.006
- Mermel LA, Allon M, Bouza E, Craven DE; Flynn P, O'Grady NP, Raad II, Rijnders BJ, Shereretz RJ, Warren DK. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis 2009; 49:1-45; PMID:19489710; http://dx.doi.org/10.1086/599376
- Hope R, Livermore DM, Brick G, Lillie M, Reynolds R; BSAC Working Parties on Resistance Surveillance. Non-susceptibility trends among staphylococci from bacteraemias in the UK and Ireland, 2001-06. J Antimicrob Chemother 2008; 62 Suppl 2:ii65-74; PMID:18819981
- May L, Klein EY, Rothman RE, Laxminarayan R. Trends in antibiotic resistance in coagulase negative staphylococci in the United States, 1999 to 2012. Antimicrob Agents Chemother 2014; 58:1404-9; PMID:24342646; http://dx.doi.org/10.1128/AAC.01908-13
- Gu B1, Kelesidis T, Tsiodras S, Hindler J, Humphries RM. The emerging problem of linezolid-resistant Staphylococcus. J Antimicrob Chemother 2013; 68:4-11; PMID:22949625; http://dx.doi.org/10.1093/jac/dks354
- Decousser JW, Desroches M, Bourgeois-Nicolaos N, Potier J, Jehl F, Lina G, Cattoir V, Vandenesh F, Doucet-Populaire F; Microbs Study Group. Susceptibility trends including emergence of linezolid resistance among coagulase-negative staphylococci and meticillin-resistant Staphylococcus aureus from invasive infections. Int J Antimicrob Agents 2015; 46:622-30; PMID:26453147; http://dx.doi.org/10.1016/j.ijantimicag.2015.07.022
- Stuart JI, John MA, Milburn S, Diagre D, Wilson B, Hussain Z. Susceptibility patterns of coagulase negative staphylococci to several newer antimicrobial agents in comparison with vancomycin and oxacillin. Int J Antimicrob Agents 2011; 37:248-252; PMID:21295951; http://dx.doi.org/10.1016/j.ijantimicag.2010.11.020
- El Solh A. Ceftobiprole: a new broad spectrum cephalosporin. Expert Opin Pharmacother 2009; 10:1675-86; PMID:19527192; http://dx.doi.org/10.1517/14656560903048967
- Kullar R, Sakoulas G, Deresinski S, van Hal SJ. When sepsis persists: a review of MRSA bacteraemia salvage therapy. J Antimicrob Chemother 2016; 71:576-86; PMID:26565015; http://dx.doi.org/10.1093/jac/dkv368
- Forest Laboratories. Safety and efficacy study of ceftaroline in subjects with Staphylococcus aureus bacteremia or with persistent methicillin-resistant Staphylococcus aureus bacteremia. ClinicalTrials.gov Identifier: NCT01701219. https://clinicaltrials.gov/ct2/show/NCT01701219
- Lyytikainen O, Klemets P, Ruutu P, Kaijalainen T, Rantala M, Ollgren J, Nuorti JP. Defining the population-based burden of nosocomial pneumococcal bacteremia. Arch Intern Med 2007; 167:1635-40; PMID:17698686; http://dx.doi.org/10.1001/archinte.167.15.1635]
- Haber M, Barskey A, Baughman W, Barker L, Whitney CG, Shaw KM, Orenstein W, Stephens DS. Herd immunity and pneumococcal conjugate vaccine: a quantitative model. Vaccine 2007; 25:5390-8; PMID:17583392; http://dx.doi.org/10.1016/j.vaccine.2007.04.088
- Bennett JE, Dolin R, Blaser MJ. Mandell, Douglas, and Bennett's Principle and Practice of Infectious Diseases. 8th ed. Philadelphia (USA): Elsevier Saunders; c2015. Chapter 201, Streptococcus pneumonia; p.2310-27.
- Hung IF1, Tantawichien T, Tsai YH, Patil S, Zotomayor R. Regional epidemiology of invasive pneumococcal disease in Asian adults: epidemiology, disease burden, serotype distribution, and antimicrobial resistance patterns and prevention. Int J Infect Dis 2013; 17:e364-73; PMID:23416209; http://dx.doi.org/10.1016/j.ijid.2013.01.004
- Mamishi S, Moradkhani S, Mahmoudi S, Hosseinpour-Sadeghi R, Pourakbari B. Penicillin-resistant trend of Streptococcus pneumoniae in Asia: a systematic review. Iran J Microbiol 2014; 6:198-210; PMID:25802701
- Slipczuk L, Codolosa JN, Davila CD, Romero-Corral A, Yun J, Pressman GS, Figueredo VMl. Infective endocarditis epidemiology over five decades: a systematic review. PLoS One 2013; 8:e82665; PMID:24349331; http://dx.doi.org/10.1371/journal.pone.0082665
- Balletto E, Mikulska M. Bacterial infections in hematopoietic stem cell transplant recipients. Mediterr J Hematol Infect Dis 2015; 7:e2015045; PMID:26185610; http://dx.doi.org/10.4084/mjhid.2015.045
- Swenson FJ, Rubin SJ. Clinical significance of viridans streptococci isolated from blood cultures. J Clin Microbiol 1982; 15:725-7; PMID:7068840
- Cordonnier C, Buzyn A, Leverger G, Herbrecht R, Hunault M, Leclercq R, Bastuji-Garin S; Club de Réflexion sur les Infections en Onco-Hématologie. Epidemiology and risk factors for gram-positive coccal infections in neutropenia: toward a more targeted antibiotic strategy. Clin Infect Dis 2003; 36:149-58; PMID:12522746; http://dx.doi.org/10.1086/345435
- Pfaller MA, Jones RN, Marshall SA, Edmond MB, Wenzel RP. Nosocomial streptococcal bloodstream infections in the SCOPE program: species occurrence and antimicrobial susceptibility. Diagn Microbiol Infect Dis 1997; 29:259-63; PMID:9458983; http://dx.doi.org/10.1016/S0732-8893(97)00159-4
- Pfaller MA, Marshall SA, Jones RN. In vitro activity of cefepime and ceftazidime against 197 nosocomial blood stream isolates of streptococci: a multicenter study. Diagn Microbiol Infect Dis 1997; 29:273-6; PMID:9458985; http://dx.doi.org/10.1016/S0732-8893(97)00139-9
- Arias CA, Murray BE. The rise of the Enterococcus: beyond vancomycin resistance. Nat Rev Microbiol 2012; 10:266-78; PMID:22421879; http://dx.doi.org/10.1038/nrmicro2761
- O'Driscoll T, Crank CW. Vancomycin-resistant enterococcal infections: epidemiology, clinical manifestations, and optimal management. Infection Drug Res 2015; 8:217-30
- Cattoir V, Leclercq R. Twenty-five years of shared life with vancomycin-resistant enterococci: is it time to divorce? J Antimicrob Chemother 2013; 68:731-42; PMID:23208830; http://dx.doi.org/10.1093/jac/dks469
- Kazanji N, Gjeorgjievski M, Yadav S, Mertens AN, Lauter C. Monomicrobial vs polymicrobial Clostridium difficile bacteremia: a case report and review of the literature. Am J Med 2015; 128:e19-26; PMID:26071832; http://dx.doi.org/10.1016/j.amjmed.2015.05.014
- Adler A, Miller-Roll T, Bradenstein R, Block C, Mendelson B, Parizade M, Paitan Y, Schwartz D, Peled N, Carmeli Y, Schwaber MJ. A national survey of the molecular epidemiology of Clostridium difficile in Israel: the dissemination of the ribotype 027 strain with reduced susceptibility to vancomycin and metronidazole. Diagn Microbiol Infect Dis 2015; 83:21-4; PMID:26116225; http://dx.doi.org/10.1016/j.diagmicrobio.2015.05.015
- Spigaglia P. Recent advances in the understanding of antibiotic resistance in Clostridium difficile infection. Ther Adv Infect Dis 2016; 3:23-42; PMID:26862400
- Spigaglia P, Barbanti F, Mastrantonio P; European Study Group on Clostridium difficile (ESGCD). Multidrug resistance in European Clostridium difficile clinical isolates. J Antimicrob Chemother 2011; 66:2227-34; PMID:21771851; http://dx.doi.org/10.1093/jac/dkr292
- Tickler IA, Goering RV, Whitmore JD, Lynn AN, Persing DH, Tenover FC; Healthcare Associated Infection Consortium. Strain types and antimicrobial resistance patterns of Clostridium difficile isolates from the United States, 2011 to 2013. Antimicrob Agents Chemother 2014; 58:4214-8; PMID:24752264; http://dx.doi.org/10.1128/AAC.02775-13
- D'Andrea MM, Arena F, Pallecchi L, Rossolini GM. CTX-M-type β-lactamases: a successful story of antibiotic resistance. Int J Med Microbiol 2013; 303:305-17; PMID:23490927; http://dx.doi.org/10.1016/j.ijmm.2013.02.008
- Rossolini GM, D'Andrea MM, Mugnaioli C. The spread of CTX-M-type extended-spectrum beta-lactamases. Clin Microbiol Infect 2008; 14 Suppl 1:33-41; PMID:18154526; http://dx.doi.org/10.1111/j.1469-0691.2007.01867.x
- Kara Ö, Zarakolu P, Aşçioğlu S, Etgül S, Uz B, Büyükaşik Y, Akova M. Epidemiology and emerging resistance in bacterial bloodstream infections in patients with hematologic malignancies. Infect Dis (Lond) 2015; 47:686-93; PMID:26024284; http://dx.doi.org/10.3109/23744235.2015.1051105
- Livermore DM. Current epidemiology and growing resistance of gram-negative pathogens. Korean J Intern Med 2012; 27:128-42; PMID:22707882; http://dx.doi.org/10.3904/kjim.2012.27.2.128
- Hsueh PR, Badal RE, Hawser SP, Hoban DJ, Bouchillon SK, Ni Y, Paterson DL; 2008 Asia–Pacific SMART Group. Epidemiology and antimicrobial susceptibility profiles of aerobic and facultative Gram-negative bacilli isolated from patients with intra- abdominal infections in the Asia-Pacific region: 2008 results from SMART (Study for Monitoring Antimicrobial Resistance Trends). Int J Antimicrob Agents 2010; 36:408-14; PMID:20728316; http://dx.doi.org/10.1016/j.ijantimicag.2010.07.002
- Chaudhuri BN, Rodrigues C, Balaji V, Iyer R, Sekar U, Wattal C, Chitnis DS, Dhole TN, Joshi S. Incidence of ESBL producers amongst Gram-negative bacilli isolated from intra-abdominal infections across India (based on SMART study, 2007 data). J Assoc Physicians India 2011; 59:287-92; PMID:21751604
- Sader HS, Flamm RK, Jones RN. Frequency of occurrence and antimicrobial susceptibility of Gram-negative bacteremia isolates in patients with urinary tract infection: results from United States and European hospitals (2009-2011). J Chemother 2014; 26:133-8; PMID:24091000; http://dx.doi.org/10.1179/1973947813Y.0000000121
- Sader HS, Farrell DJ, Flamm RK, Jones RN. Antimicrobial susceptibility of Gram-negative organisms isolated from patients hospitalized in intensive care units in United States and European hospitals (2009-2011). Diagn Microbiol Infect Dis 2014; 78:443-8; PMID:24492025; http://dx.doi.org/10.1016/j.diagmicrobio.2013.11.025
- Vlieghe ER1, Huang TD, Phe T, Bogaerts P, Berhin C, De Smet B, Peetermans WE, Jacobs JA, Glupczynski Y. Prevalence and distribution of beta-lactamase coding genes in third-generation cephalosporin-resistant Enterobacteriaceae from bloodstream infections in Cambodia. Eur J Clin Microbiol Infect Dis 2015; 34:1223-9; PMID:25717021; http://dx.doi.org/10.1007/s10096-015-2350-9
- Zhang J, Zheng B, Zhao L, Wei Z, Ji J, Li L, Xiao Y. Nationwide high prevalence of CTX-M and an increase of CTX-M-55 in Escherichia coli isolated from patients with community-onset infections in Chinese county hospitals. BMC Infect Dis 2014; 14:659; PMID:25466590; http://dx.doi.org/10.1186/s12879-014-0659-0
- Park YS, Bae IK, Kim J, Jeong SH, Hwang SS, Seo YH, Cho YK, Lee K, Kim JM. Risk factors and molecular epidemiology of community-onset extended-spectrum β-lactamase-producing Escherichia coli bacteremia. Yonsei Med J 2014; 55:467-75; PMID:24532519; http://dx.doi.org/10.3349/ymj.2014.55.2.467
- Kanoksil M, Jatapai A, Peacock SJ, Limmathurotsakul D. Epidemiology, microbiology and mortality associated with community-acquired bacteremia in northeast Thailand: a multicenter surveillance study. PLoS One 2013; 8:e54714; PMID:23349954; http://dx.doi.org/10.1371/journal.pone.0054714
- Saltoglu N, Karali R, Yemisen M, Ozaras R, Balkan II, Mete B, Tabak F, Mert A, Hondur N, Ozturk R. Comparison of community-onset healthcare-associated and hospital-acquired urinary infections caused by extended-spectrum beta-lactamase-producing Escherichia coli and antimicrobial activities. Int J Clin Pract 2015; 69:766-70; PMID:25683907; http://dx.doi.org/10.1111/ijcp.12608
- Hristea A, Olaru ID, Adams-Sapper S, Riley LW. Characterization of ESBL-producing Escherichia coli and Klebsiella pneumoniae from bloodstream infections in three hospitals in Bucharest, Romania: a preliminary study. Infect Dis (Lond) 2015; 47:46-51; PMID:25365029; http://dx.doi.org/10.3109/00365548.2014.959043
- Paterson DL. Resistance in gram-negative bacteria: enterobacteriaceae. Am J Infect Control 2006; 34 (5 Suppl 1):S20-S28; PMID:16813978; http://dx.doi.org/10.1016/j.ajic.2006.05.238
- Livermore DM, Hope R, Mushtaq S, Warner M. Orthodox and unorthodox clavulanate combinations against extended-spectrum beta-lactamase producers. Clin Microbiol Infect 2008; 14 Suppl 1:189-93; PMID:18154546; http://dx.doi.org/10.1111/j.1469-0691.2007.01858.x
- Guh AY, Bulens SN, Mu Y, Jacob JT, Reno J, Scott J, Wilson LE, Vaeth E, Lynfield R, Shaw KM, et al. Epidemiology of carbapenem-resistant enterobacteriaceae in 7 US communities, 2012-2013. JAMA 2015; 314:1479-87; PMID:26436831; http://dx.doi.org/10.1001/jama.2015.12480
- Xu A, Zheng B, Xu YC, Huang ZG, Zhong NS, Zhuo C. National epidemiology of carbapenem-resistant and extensively drug-resistant Gram-negative bacteria isolated from blood samples in China in 2013. Clin Microbiol Infect 2016 Feb 1. pii: S1198-743X(15)00870-8. http://dx.doi.org/10.1016/j.cmi.2015.09.015. [Epub ahead of print
- Nordmann P, Poirel L. The difficult-to-control spread of carbapenemase producers among Enterobacteriaceae worldwide. Clin Microbiol Infect 2014; 20:821-30; PMID:24930781; http://dx.doi.org/10.1111/1469-0691.12719
- Cornaglia G, Giamarellou H, Rossolini GM. Metallo-beta-lactamases: a last frontier for beta-lactams? Lancet Infect Dis 2011; 11:381-93; PMID:21530894; http://dx.doi.org/10.1016/S1473-3099(11)70056-1
- Liu YY, Wang Y, Walsh TR, Yi LX, Zhang R, Spencer J, Doi Y, Tian G, Dong B, Huang X, et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect Dis 2016; 16:161-8; PMID:26603172; http://dx.doi.org/10.1016/S1473-3099(15)00424-7
- Hasman H, Hammerum AM, Hansen F, Hendriksen RS, Olesen B, Agersø Y, Zankari E, Leekitcharoenphon P, Stegger M, Kaas RS, et al. Detection of mcr-1 encoding plasmid-mediated colistin-resistant Escherichia coli isolates from human bloodstream infection and imported chicken meat, Denmark 2015. Euro Surveill 2015 Dec 10; 20(49); http://dx.doi.org/10.2807/1560-7917.ES.2015.20.49.30085
- Malhotra-Kumar S, Xavier BB, Das AJ, Lammens C, Hoang HT, Pham NT, Goossens H. Colistin-resistant Escherichia coli harbouring mcr-1 isolated from food animals in Hanoi, Vietnam. Lancet Infect Dis 2016 Jan 7. pii: S1473-3099(16)00014-1; http://dx.doi.org/10.1016/S1473-3099(16)00014-1
- Malhotra-Kumar S, Xavier BB, Das AJ, Lammens C, Butaye P, Goossens H. Colistin resistance gene mcr-1 harboured on a multidrug resistant plasmid. Lancet Infect Dis 2016 Jan 7. pii: S1473-3099(16)00012-8. http://dx.doi.org/10.1016/S1473-3099(16)00012-8
- Falgenhauer L, Waezsada SE, Yao Y, Imirzalioglu C, Käsbohrer A, Roesler U, Michael GB, Schwarz S, Werner G, Kreienbrock L, Chakraborty T; RESET consortium. Colistin resistance gene mcr-1 in extended-spectrum β-lactamase-producing and carbapenemase-producing Gram-negative bacteria in Germany. Lancet Infect Dis 2016 Jan 7. pii: S1473-3099(16)00009-8; http://dx.doi.org/10.1016/S1473-3099(16)00009-8
- Perrin-Guyomard A, Bruneau M, Houée P, Deleurme K, Legrandois P, Poirier C, Soumet C, Sanders P. Prevalence of mcr-1 in commensal Escherichia coli from French livestock, 2007 to 2014. Euro Surveill 2016; 21: pii=30135; http://dx.doi.org/10.2807/1560-7917.ES.2016.21.6.30135
- Paterson DL, Harris PN. Colistin resistance: a major breach in our last line of defence. Lancet Infect Dis 2016; 16:132-3; PMID:26603171; http://dx.doi.org/10.1016/S1473-3099(15)00463-6
- Tse H, Yuen KY. Dissemination of the mcr-1 colistin resistance gene. Lancet Infect Dis 2016; 16:145-6; PMID:26711362; http://dx.doi.org/10.1016/S1473-3099(15)00532-0
- Webb HE, Granier SA, Marault M, Millemann Y, den Bakker HC, Nightingale KK, Bugarel M, Ison SA, Scott HM, Loneragan GH. Dissemination of the mcr-1 colistin resistance gene. Lancet Infect Dis 2016; 16:144-5; PMID:26711363; http://dx.doi.org/10.1016/S1473-3099(15)00538-1
- Du H, Chen L, Tang YW, Kreiswirth BN. Emergence of the mcr-1 colistin resistance gene in carbapenem-resistant Enterobacteriaceae. Lancet Infect Dis 2016 Jan 29. pii: S1473-3099(16)00056-6; http://dx.doi.org/10.1016/S1473-3099(16)00056-6
- Stoesser N, Mathers AJ, Moore CE, Day NP, Crook DW. Colistin resistance gene mcr-1 and pHNSHP45 plasmid in human isolates of Escherichia coli and Klebsiella pneumoniae. Lancet Infect Dis 2016 Jan 7. pii: S1473-3099(16)00010-4. http://dx.doi.org/10.1016/S1473-3099(16)00010-4
- Yao X, Doi Y, Zeng L, Lv L, Liu JH. Carbapenem-resistant and colistin-resistant Escherichia coli co-producing NDM-9 and MCR-1. Lancet Infect Dis 2016 Jan 29. pii: S1473-3099(16)00057-8. http://dx.doi.org/10.1016/S1473-3099(16)00057-8
- Huang CC, Chen YS, Toh HS, Lee YL, Liu YM, Ho CM, Lu PL, Liu CE, Chen YH, Wang JH, et al. Impact of revised CLSI breakpoints for susceptibility to third-generation cephalosporins and carbapenems among Enterobacteriaceae isolates in the Asia-Pacific region: results from the Study for Monitoring Antimicrobial Resistance Trends (SMART), 2002-2010. Int J Antimicrob Agents 2012; 40 Suppl: S4-10; PMID:22749058; http://dx.doi.org/10.1016/S0924-8579(12)70003-1
- Cantón R, Akova M, Carmeli Y, Giske CG, Glupczynski Y, Gniadkowski M, Livermore DM, Miriagou V, Naas T, Rossolini GM, et al. Rapid evolution and spread of carbapenemases among Enterobacteriaceae in Europe. Clin Microbiol Infect 2012; 18:413-31; http://dx.doi.org/10.1111/j.1469-0691.2012.03821.x
- Hu L, Zhong Q, Shang Y, Wang H, Ning C, Li Y, Hang Y, Xiong J, Wang X, Xu Y, et al. The prevalence of carbapenemase genes and plasmid-mediated quinolone resistance determinants in carbapenem-resistant Enterobacteriaceae from five teaching hospitals in central China. Epidemiol Infect 2014; 142:1972-7; PMID:24252194; http://dx.doi.org/10.1017/S0950268813002975
- Munoz-Price LS, Poirel L, Bonomo RA, Schwaber MJ, Daikos GL, Cormican M, Cornaglia G, Garau J, Gniadkowski M, Hayden MK, et al. Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. Lancet Infect Dis 2013; 13:785-96; PMID:23969216; http://dx.doi.org/10.1016/S1473-3099(13)70190-7
- Tseng IL, Liu YM, Wang SJ, Yeh HY, Hsieh CL, Lu HL, Tseng YC, Mu JJ, et al. Emergence of carbapenemase producing Klebsiella pneumonia and spread of KPC-2 and KPC-17 in Taiwan: a nationwide study from 2011 to 2013. PLoS One 2015; 10: e0138471; PMID:26384242; http://dx.doi.org/10.1371/journal.pone.0138471
- Poirel L, Héritier C, Tolün V, Nordmann P. Emergence of oxacillinase-mediated resistance to imipenem in Klebsiella pneumoniae. Antimicrob Agents Chemother 2004; 48:15-22; PMID:14693513; http://dx.doi.org/10.1128/AAC.48.1.15-22.2004
- Carrer A, Poirel L, Eraksoy H, Cagatay AA, Badur S, Nordmann P. Spread of OXA-48-positive carbapenem-resistant Klebsiella pneumoniae isolates in Istanbul, Turkey. Antimicrob Agents Chemother 2008; 52:2950-54; PMID:18519712; http://dx.doi.org/10.1128/AAC.01672-07
- Gulmez D, Woodford N, Palepou MF, Mushtaq S, Metan G, Yakupogullari Y, Kocagoz S, Uzun O, Hascelik G, Livermore DM, et al. Carbapenem-resistant Escherichia coli and Klebsiella pneumoniae isolates from Turkey with OXA-48-like carbapenemases and outer membrane protein loss. Int J Antimicrob Agents 2008; 31:523-6; PMID:18339523; http://dx.doi.org/10.1016/j.ijantimicag.2008.01.017
- Alp E, Perçin D, Colakoğlu S, Durmaz S, Kürkcü CA, Ekincioğlu P, Güneş T, et al. Molecular characterization of carbapenem-resistant Klebsiella pneumoniae in a tertiary university hospital in Turkey. J Hosp Infect 2013; 84:178-80; PMID:23623803; http://dx.doi.org/10.1016/j.jhin.2013.03.002
- Aktas Z, Kayacan CB, Schneider I, Can B, Midilli K, Bauernfeind A. Carbapenem-hydrolyzing oxacillinase OXA-48 persists in Klebsiella pneumoniae in Istanbul, Turkey. Chemotherapy 2008; 54:101-6; PMID:18303258; http://dx.doi.org/10.1159/000118661
- Dortet L, Poirel L, Nordmann P. Worldwide dissemination of the NDM-type carbapenemases in Gram-negative bacteria. Biomed Res Int 2014; 249856; PMID:24790993
- Logan LK, Renschler JP, Gandra S, Weinstein RA, Laxminarayan R, for the Centers for Disease Control and Prevention Epicenters Program. Carbapenem-resistant Enterobacteriaceae in children, United States, 1999–2012. Emerging Infect Dis 2015; 21:2014-21; PMID:26486124; http://dx.doi.org/10.3201/eid2111.150548
- Monaco M, Giani T, Raffone M, Arena F, Garcia-Fernandez A, Pollini S; Network EuSCAPE-Italy, Grundmann H, Pantosti A, Rossolini GM, et al. Colistin resistance superimposed to endemic carbapenem-resistant Klebsiella pneumoniae: a rapidly evolving problem in Italy, November 2013 to April 2014. Euro Surveill 2014; 19:pii: 20939; PMID:25358041; http://dx.doi.org/10.2807/1560-7917.ES2014.19.42.20939
- Giacobbe DR, Del Bono V, Trecarichi EM, De Rosa FG, Giannella M, Bassetti M, Bartoloni A, Losito AR, Corcione S, Bartoletti M, et al. Risk factors for bloodstream infections due to colistin-resistant KPC-producing Klebsiella pneumoniae: results from a multicenter case-control-control study. Clin Microbiol Infect 2015; 21:1106.e1-8; PMID:26278669
- Bertrand X, Dowzicky MJ. Antimicrobial susceptibility among gram-negative isolates collected from intensive care units in North America, Europe, the Asia-Pacific Rim, Latin America, the Middle East, and Africa between 2004 and 2009 as part of the Tigecycline Evaluation and Surveillance Trial. Clin Ther 2012; 34:124-37; PMID:22154196; http://dx.doi.org/10.1016/j.clinthera.2011.11.023
- Kolayli F, Gacar G, Karadenizli A, Sanic A, Vahaboglu H. PER-1 is still widespread in Turkish hospitals among Pseudomonas aeruginosa and Acinetobacter spp. FEMS Microbiol Lett 2005; 15:241-5; http://dx.doi.org/10.1016/j.femsle.2005.06.012
- Potron A, Poirel L, Nordmann P. Emerging broad-spectrum resistance in Pseudomonas aeruginosa and Acinetobacter baumannii: mechanisms and epidemiology. Int J Antimicrob Agents 2015; 45:568-85; PMID:25857949; http://dx.doi.org/10.1016/j.ijantimicag.2015.03.001
- Jean SS, Lee WS, Yu KW, Liao CH, Hsu CW, Chang FY, Ko WC, Chen RJ, Wu JJ, Chen YH, et al. Rates of susceptibility of carbapenems, ceftobiprole, and colistin against clinically important bacteria collected from intensive care units in 2007: results from the Surveillance of Multicenter Antimicrobial Resistance in Taiwan (SMART). J Microbiol Immunol Infect 2015 Jan 10. pii: S1684-1182(15)00021-3. [Epub ahead of print]
- Garnacho-Montero J, Dimopoulos G, Poulakou G, Akova M, Cisneros JM, De Waele J, Petrosillo N, Seifert H, Timsit JF, Vila J, et al. Task force on management and prevention of Acinetobacter baumannii infections in the ICU. Intensive Care Med 2015; 41:2057-75; PMID:26438224; http://dx.doi.org/10.1007/s00134-015-4079-4
- Roca I, Mosqueda N, Altun B, Espinal P, Akova M, Vila J. Molecular characterization of NDM-1-producing Acinetobacter pittii isolated from Turkey in 2006. J Antimicrob Chemother 2014; 69:3437-8; PMID:25096072; http://dx.doi.org/10.1093/jac/dku306
- Zander E, Fernandez-Gonzalez A, Schleicher X, Dammhayn C, Kamolvit W, Seifert H, Higgins PG. Worldwide dissemination of acquired carbapenem-hydrolysing class D beta-lactamases in Acinetobacter spp. other than Acinetobacter baumannii. Int J Antimicrob Agents 2014; 43:375-7; PMID:24612983; http://dx.doi.org/10.1016/j.ijantimicag.2014.01.012
- Poirel L, Nordmann P. Carbapenem resistance in Acinetobacter baumannii: mechanisms and epidemiology. Clin Microbiol Infect 2006; 12:826-36; PMID:16882287; http://dx.doi.org/10.1111/j.1469-0691.2006.01456.x
- Reid GE, Grim SA, Aldeza CA, Janda WM, Clark NM. Rapid development of Acinetobacter baumannii resistance to tigecycline. Pharmacotherapy 2007; 27:1198-201; PMID:17655518; http://dx.doi.org/10.1592/phco.27.8.1198
- Roca I, Akova M, Baquero F, Carlet J, Cavaleri M, Coenen S, Cohen J, Findlay D, Gyssens I, Heure OE, et al. The global threat of antimicrobial resistance: science for intervention. New Microbes New Infect 2015; 6:22-9; PMID:26029375; http://dx.doi.org/10.1016/j.nmni.2015.02.007
- Wellington EM, Boxall AB, Cross P, Feil EJ, Gaze WH, Hawkey PM, et al. The role of the natural environment in the emergence of antibiotic resistance in Gram-negative bacteria. Lancet Infect Dis 2013; 13:155-65; PMID:23347633; http://dx.doi.org/10.1016/S1473-3099(12)70317-1
- Van Boeckel TP, Gandra S, Ashok A, Caudron Q, Grenfell BT, Levin SA, Laxminarayan R. Global antibiotic consumption 2000 to 2010: an analysis of national pharmaceutical sales data. Lancet Infect Dis 2014; 14:742-50; PMID:25022435; http://dx.doi.org/10.1016/S1473-3099(14)70780-7
- Versporten A, Bolokhovets G, Ghazaryan L, Abilova V, Pyshnik G, Spasojevic T, Korinteli I, Raka L, Kambaralieva B, Cizmovic L, et al; WHO/Europe-ESAC Project Group. Antibiotic use in eastern Europe: a cross-national database study in coordination with the WHO Regional Office for Europe. Lancet Infect Dis 2014; 14:381-7; PMID:24657114; http://dx.doi.org/10.1016/S1473-3099(14)70071-4
- Center for Disease Dynamics, Economics & Policy. State of the World's Antibiotics, 2015. CDDEP: Washington, D.C., 2015
- Bruce J, MacKenzie FM, Cookson B, Mollison J, van der Meer JW, Krcmery V, Gould IM; ARPAC Steering Group. Antibiotic stewardship and consumption: findings from a pan-European hospital study. J Antimicrob Chemother 2009; 64:853-60; PMID:19675012; http://dx.doi.org/10.1093/jac/dkp268
- Hansen S, Zingg W, Ahmad R, Kyratsis Y, Behnke M, Schwab F, Pittet D, Gastmeier P; PROHIBIT study group. Organization of infection control in European hospitals. J Hosp Infect 2015; 91:338-45; PMID:26542950; http://dx.doi.org/10.1016/j.jhin.2015.07.011
