Apoptosis and autophagy do not merely mediate cell death, but have the additional function of fighting against microbial invaders. If initiated at the proper time, apoptosis can limit viral infection by killing virus-infected cells. Autophagy (the “recycling factory” of the cell) can dismantle the pathogen. In response to these pressures, many viruses have co-evolved to hijack or manipulate these processes to escape being killed by the host.
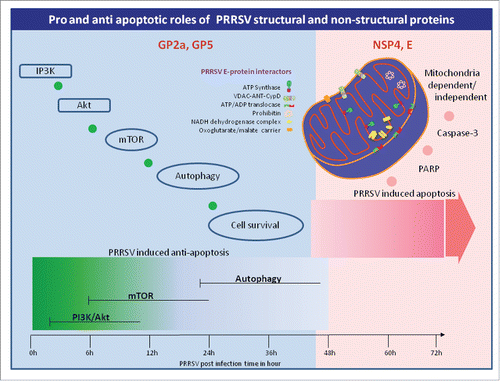
Anti-apoptotic activity has been described for Porcine Respiratory and Reproductive Syndrome Virus (PRRSV) in its initial phase of infection. Suppression of the host apoptotic pathway gives the virus an additional window of opportunity for replication, assembly and maturation. Substantial progress has been made in understanding the lifecycle of PRRSV. A synchronized activation of anti-apoptotic, autophagic and pro-apoptotic events shape the infection process of PRRSV (Figure). In the eclipse phase of viral infection, when replication and synthesis of viral proteins are at their peak, PRRSV is able to block the cellular antiviral defense mechanisms and hijack host apoptosis for dissemination and infection. However, the mechanism of PRRSV regulation of the anti-apoptosis event is unclear.
Zhou et al describe an important link in the regulation of anti-apoptotic events by autophagy for PRRSV infection in MARC-145 cells.Citation1 They found that in PRRSV infected cells, there was a parallel up-regulation of the apoptotic BAD and autophagic Beclin 1 proteins (also implicated in autophagic programmed cell death). The pro-apoptotic BAD can also be pro-survival upon phosphorylation. PRRSV infection also activates the IP3K/Akt pathway to phosphorylate BAD. BAD interacts with Beclin 1 (which is localized on the autophagic vacuole) as evident from the focal cytoplasmic distribution in PRRSV infected cells. This is in contrast to uninfected cells that had a diffused cytoplasmic distribution. This host protein interaction is linked to the PRRSV induced apoptosis delay. However, it is interesting to determine how this process is initiated and which viral protein(s) is responsible for this.
Authors have previously worked on the structural proteins of PRRSV and its interactions with host proteins to understand more about PRRSV pathogenesis. The ORF2 encodes two different proteins (Gp2a and E) that have differential expression patterns and have anti-apoptotic and pro-apoptotic properties respectively.Citation2,3 GP2a modulates the NFkB and AP1 transcription factors to delay the process of apoptosis. On the other hand, the E protein physically interacts with several mitochondrial proteins to initiate apoptosis.Citation4
Additionally, it is worth discussing recent findings on GP5. Cell lines expressing GP5 deletion mutants lacking amino acids 97 to 119 have arrested cell cycle at the G2/M phase whereas deletion of amino acids 84–96 inhibits the replication of PRRSV.Citation5 It opens further avenues to explore and assign functional significance of these proteins in vivo. It is interesting to note that G2 phase cells have high protein synthesis and cell growth and cells that arrested in mitosis tend to adhere less to the neighboring cells than cells in other stages of the cell cycle. Protein synthesis and cell growth phase could be used for viral maturation and higher surface area during mitotic arrest for virus dissemination. HIV-infected T lymphocytes isolated from patients are arrested in G2 to limit the host immune response and can possibly be linked to understand the PRRSV life cycle and pathogenesis.
In conclusion, the role of BAD-Becelin-1 interaction could be the missing link in the anti-apoptotic event of PRRSV. Future research should be prioritized to see how viral protein(s) modulate these host protein interactions.
Figure 1. Survival and apoptotic signaling kinetics in PRRSV infection. Pro-survival signaling cascade initiates within the first couple of hours of PRRSV post infection. Activation of IP3K/Akt follows mTOR and autophagy signaling. Literature demonstrates the possible role of GP2a and GP5 in pro-survival signaling. Apoptosis process starts somewhere between 36 h to 48 h post infection activating both mitochondrial dependent and mitochondrial independent pathways. NSP4 and E proteins trigger the apoptotic event. How NSP4 triggers the signaling cascade needs to be investigated, however E protein directly interacts with mitochondrial proteins as pictured above that alters the cellular ATP status and triggers apoptosis.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed
Funding
This research was in part supported by NIH/NIAID grant R01AI116636 to JLR.
References
- Zhou A, Li S, Khan FA, Zhang S. Autophagy postpones apoptotic cell death in PRRSV infection through Bad-Beclin1 interaction. Virulence 2016:7(2):98–109; PMID:26670824; http://dx.doi.org/10.1080/21505594.2015.1131381
- Pujhari S, Kryworuchko M, Zakhartchouk AN. Role of phosphatidylinositol-3-kinase (PI3K) and the mammalian target of rapamycin (mTOR) signalling pathways in porcine reproductive and respiratory syndrome virus (PRRSV) replication. Virus Res 2014; 194:138-44; PMID:25304692; http://dx.doi.org/10.1016/j.virusres.2014.09.017
- Pujhari S, Baig TT, Zakhartchouk AN. Potential role of porcine reproductive and respiratory syndrome virus structural protein GP2 in apoptosis inhibition. Biomed Res Int 2014; 2014:160505; PMID:24511529; http://dx.doi.org/10.1155/2014/160505
- Pujhari S, Zakhartchouk AN. Porcine Reproductive and Respiratory Syndrome Virus Envelope (E) protein interacts with mitochondrial proteins and induces apoptosis. Archives of virology (In press)
- Mu Y, Li L, Zhang B, Huang B, Gao J, Wang X, Wang C, Xiao S, Zhao Q, Sun Y, et al. Glycoprotein 5 of porcine reproductive and respiratory syndrome virus strain SD16 inhibits viral replication and causes G2/M cell cycle arrest, but does not induce cellular apoptosis in Marc-145 cells. Virology 2015; 484:136-45; PMID:26093497; http://dx.doi.org/10.1016/j.virol.2015.05.019
