ABSTRACT
Prompt administration of anti-toxin reduces mortality following Corynebacterium diphtheriae infection. Current treatment relies upon equine diphtheria anti-toxin (DAT), with a 10% risk of serum sickness and rarely anaphylaxis. The global DAT supply is extremely limited; most manufacturers have ceased production. S315 is a neutralizing human IgG1 monoclonal antibody to diphtheria toxin that may provide a safe and effective alternative to equine DAT and address critical supply issues. To guide dose selection for IND-enabling pharmacology and toxicology studies, we dose-ranged S315 and DAT in a guinea pig model of diphtheria intoxication based on the NIH Minimum Requirements potency assay. Animals received a single injection of antibody premixed with toxin, were monitored for 30 days, and assigned a numeric score for clinical signs of disease. Animals receiving ≥ 27.5 µg of S315 or ≥ 1.75 IU of DAT survived whereas animals receiving ≤ 22.5 µg of S315 or ≤ 1.25 IU of DAT died, yielding a potency estimate of 17 µg S315/IU DAT (95% CI 16–21) for an endpoint of survival. Because some surviving animals exhibited transient limb weakness, likely a systemic sign of toxicity, DAT and S315 doses required to prevent hind limb paralysis were also determined, yielding a relative potency of 48 µg/IU (95% CI 38–59) for this alternate endpoint. To support advancement of S315 into clinical trials, potency estimates will be used to evaluate the efficacy of S315 versus DAT in an animal model with antibody administration after toxin exposure, more closely modeling anti-toxin therapy in humans.
Introduction
Diphtheria is a potentially fatal illness caused by infection with Corynebacterium diphtheriae and subsequent elaboration of a potent exotoxin. Diphtheria toxin causes tissue death at the site of production, mostly commonly the respiratory tract, leading to the development of a pathognomonic pharyngeal pseudomembrane and local edema which can compromise the airway.Citation1,2 Hematogenous toxin dissemination can cause cranial nerve dysfunction, peripheral neuropathy and cardiotoxicity, which is responsible for 50–75% of diphtheria deaths.Citation3,4
Expanded access to vaccination with diphtheria toxoid has decreased the incidence of diphtheria cases dramatically, though diphtheria remains endemic in several countries.Citation5-7 Over the last decade, 4,000–12,000 diphtheria cases have been reported to the World Health Organization annually, although the number of cases is likely underestimated and deaths underreported.Citation8 In addition, periodic outbreaks continue with recent epidemics in Haiti, Nigeria, South Africa, Indonesia, and Laos and are often associated with high case fatality rates (>10 %) in resource-limited countries.Citation9-14 Fatal cases can also occur in developed countries among unvaccinated or undervaccinated populations, as illustrated with a recent case from Spain.Citation15 Sporadic epidemics will likely continue to occur due to disruption in national or regional vaccination programs from political instability, natural disasters, or emerging infectious diseases; international travel and relocation of susceptible persons also contribute to the global risk of disease.
Morbidity and mortality due to diphtheria are greatly reduced by prompt administration of anti-toxin antibodies to neutralize diphtheria toxin and prevent further tissue damage, in conjunction with antibiotic therapy to eliminate C. diphtheriae and stop toxin production.Citation16 Current treatment relies upon equine-derived antibodies to diphtheria toxin (Diphtheria Anti-Toxin, DAT) that carry the risk of severe allergic reactions. The global supply of DAT is extremely limited as many manufacturers have ceased production.Citation15,17-19 In the United States, the standard of care for suspected diphtheria is receipt of an unlicensed DAT product provided under the federal expanded access to investigational drugs program through a protocol sponsored by the Centers for Disease Control and Prevention.Citation20
To address this unmet medical need, we are developing a human monoclonal antibody (mAb) to diphtheria toxin to replace equine DAT for the treatment of diphtheria. We previously identified a human IgG1 mAb (designated S315) that binds to the toxin's receptor binding domain and blocks the interaction with its putative receptor, heparin-binding epidermal growth factor-like growth factor.Citation21 In preliminary animal experiments, S315 improved the survival of animals exposed to diphtheria toxin.Citation21 In this report, we establish the relative potency of the human anti-toxin mAb by determining its neutralizing capacity relative to equine polyclonal DAT standard in a guinea pig model of disease. Although guinea pigs do not exhibit respiratory symptoms when subcutaneously challenged with diphtheria toxin, they do express the cell surface receptor for the toxin and are susceptible to the end organ effects of systemic toxin (peripheral neuropathy, cardiomyopathy).Citation22 A modified version of the National Institutes of Health (NIH) Minimum Requirements assay designed for determination of potency of polyclonal DAT was utilized to estimate the concentration of S315 mAb that provides equivalent protection to DAT in guinea pigs subcutaneously injected with diphtheria toxin.Citation23 We used this model to evaluate the relative potency of a novel human mAb to equine polyclonal DAT utilizing 2 different endpoints: overall survival at 30 d post-toxin exposure and survival without hind limb paralysis.
Results
Overall survival from a lethal diphtheria toxin challenge using DAT or S315
For the endpoint of survival following a lethal toxin challenge, a dose-dependent response was observed for both the DAT and S315 mAb cohorts (). Among the DAT-treated cohorts, all animals receiving ≤ 1.25 IU of DAT died, with animals in the 2 lowest dose cohorts dying earlier than those who received higher doses (). All animals receiving ≥ 1.75 IU of DAT survived. Variable survival was observed in cohorts receiving 1.5 IU – 1.6 IU DAT; in the first experiment, 5 of 5 animals receiving 1.5 IU DAT survived while in the second experiment 3 of 5 animals receiving 1.5 IU of DAT survived. All 5 animals treated with 1.55 IU of DAT survived, while one of the 5 animals treated with 1.6 IU of DAT died. For the S315 cohorts, no animals receiving doses ≤ 22.5 µg survived, and a dose-dependent effect on time of death was observed (). All animals receiving ≥ 27.5 µg of mAb survived. Variable survival was observed for the 25 µg dose; 1 of 5 animals receiving 25 µg of mAb survived in the first experiment while in the second experiment, 4 of 5 animals receiving 25 µg of mAb survived.
Figure 1. Percent survival following receipt of fixed diphtheria toxin dose pre-mixed with varying amount of antibody by outcome for DAT and mAb treated cohorts. The proportion of animals surviving at each antibody dose is shown in the solid bars and the proportion surviving without symptoms of hind limb paralysis is shown in the hatched bars. The number of animals (n) evaluated in each cohort is noted on the graph. The results for cohorts receiving equine diphtheria anti-toxin (DAT) are shown in panel A and those for cohorts given S315 human monoclonal antibody to diphtheria toxin are shown in panel B.
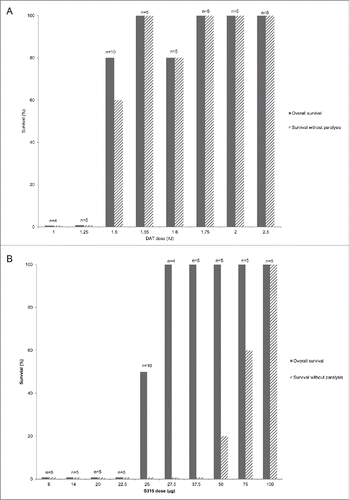
Figure 2. Survival over time by antibody cohort following diphtheria toxin challenge. The proportion of animals surviving over the 30-day study period by study day following receipt of toxin-antibody mixture is shown for equine diphtheria anti-toxin (DAT) dosing cohorts in panel A and for S315 human monoclonal antibody dosing cohorts in panel B.
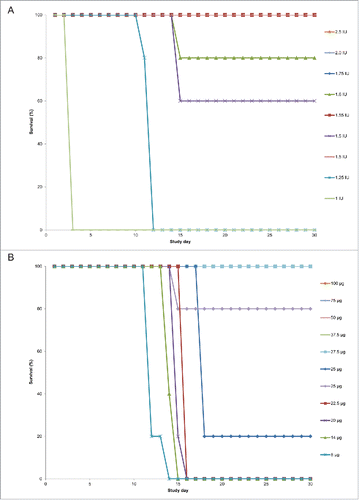
Using a logistic model, the calculated half maximal effective dose (ED50) for overall survival was 25.0 µg for mAb (95% CI: 23.4, 26.5) and 1.45 IU for DAT (95% CI: 1.21, 1.50), yielding a relative potency estimate of 17 µg mAb/IU DAT (95% CI: 16, 21).
Survival without hind limb paralysis using DAT or S315
Some of the surviving animals in the DAT or S315 dosed cohorts developed transient limb weakness, described historically in this model and attributed to toxin-mediated peripheral neuropathy.Citation24 Therefore, we sought to determine the doses of DAT and mAb required to prevent a systemic sign of toxicity, using hind limb paralysis (clinical score = 4) as a marker of end-organ damage.
Among the DAT-treated cohorts, a similar dose-dependent response was observed (). At DAT doses equal or above 1.75 IU, all animals survived without hind limb paralysis. Variability was observed again for the animals dosed with 1.5 IU of DAT; 2 of the 5 surviving animals in the first experiment exhibited transient hind limb weakness, while in the second experiment, none of the surviving animals in the 1.5 IU cohort exhibited hind limb paralysis (). The proportion of animals surviving without hind limb paralysis was identical to the overall survival percentage for the 1.55 IU cohort (100%) and the 1.6 IU cohort (80%).
Figure 3. Survival over time without hind limb paralysis by antibody cohort following diphtheria toxin challenge. The proportion of animals surviving without hind limb paralysis over the 30-day study period by study day following receipt of toxin-antibody mixture is shown for equine diphtheria anti-toxin (DAT) dosing cohorts in panel A and S315 human monoclonal antibody dosing cohorts in panel B.
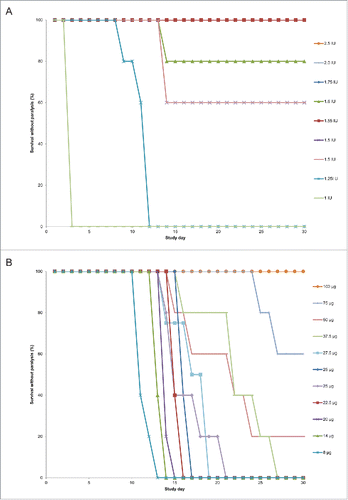
For the mAb-treated animals, a higher dose was required to observe survival without hind limb paralysis (). All five animals in the 27.5 µg and 37.5 µg cohorts developed transient limb weakness but survived (). As the dose of S315 increased, the onset of paralysis was delayed and a smaller proportion of surviving animals in each cohort exhibited limb weakness. All five animals in the 100 µg mAb group survived without hind limb paralysis.
The calculated ED50 for survival without hind paralysis was 67.4 µg for mAb (95% CI 53.4, 80.2) and 1.42 IU for DAT (95% CI 1.25, 1.52). The relative potency of S315 to DAT using this endpoint was 48 µg mAb/IU DAT (95% CI: 38, 58).
Weight gain following diphtheria toxin challenge
Average baseline weights for each dosing cohort ranged from 249 to 258 g in the DAT group and from 253 to 272 g in the mAb group and were not significantly different between dosing cohorts (ANOVA test, p = 0.87 for the DAT group and p = 0.34 for the mAb group).
For the DAT treatment groups, mean weight gains at Day 7 (Week 1) ranged from 39 to 51 g and were not significantly different among dose levels (p = 0.54, Chi-square test using GEE model). The mean weight in the dynamic cohorts of survivors continued to increase (ranged from 31.0 to 43.9 g per week) for all but the 1.25 IU group, whose mean weight dropped 10.6 g during Week 2, with all animals subsequently euthanized for being moribund (clinical score = 5) by Day 12. At Day 28 (Week 4), estimated mean weight gains from baseline were similar among the 1.5 IU, 1.55 IU, 1.75 IU and 2.0 IU dosing cohorts (152–163 g, p = 0.44), but it was significantly higher for the 2.5 IU cohort (183 g, p = 0.01) and significantly lower for the 1.6 IU cohort (133 g, p = 0.01).
For the mAb treatment groups, mean weight gains at Day 7 (Week 1) were similar among all but the 27.5 µg cohort (31–49 g, p = 0.15); it was significantly lower for the 27.5 µg cohort (23.5 g, p = 0.03). The mean weight in the dynamic cohorts of survivors continued to increase (ranged from 10.7 to 43.8 g per week) for all except for the 8 µg cohort, whose mean weight dropped 2.5 g during Week 2, with all animals euthanized by Day 14 for being moribund, and the 14 µg cohort whose mean weight at Week 2 was not estimable. At Day 28 (Week 4), estimated mean weight gains from baseline significantly differed among the 25 to 100 µg dosing cohorts, but were similar between the 25 and 27.5 µg cohorts (106–117 g, p = 0.2), between the 37.5 and 75 µg cohorts (140–143 g, p = 0.74) and between the 50 and 100 µg cohorts (161–176 g, p = 0.06)
Discussion
We evaluated the potency of a human mAb to diphtheria toxin (S315) relative to a standard preparation of equine polyclonal DAT in order to quantify the biologic activity of the mAb in an animal model of diphtheria intoxication. We used an assay that is employed to assign potency of diphtheria anti-toxin preparations, modified with an extended period of observation to allow for greater differentiation of dose effects and evaluation of delayed effects of diphtheria toxin.
Relative potency is the biologic activity of a substance compared to a standard. Equine DAT is a polyclonal antibody preparation derived from horses hyperimmunized with toxin. Similar to other polyclonal antibody products used clinically, DAT contains a variety of immunoglobulins, most of which are not specific for diphtheria toxin. In addition, the amount and composition of antibodies which are specific to diphtheria toxin will vary among product lots as the antibody repertoire differs both between blood donors as well as between different sampling timepoints in the same donor. Therefore, clinical grade polyclonal antibody products, such as DAT, hepatitis B immunoglobulin, and human rabies immunoglobulin, are quantified by an assignment of units of neutralizing activity per volume of serum or plasma (IU/mL) relative to a standard preparation rather than by protein concentration. In contrast, a monoclonal antibody product consists of one antibody molecule with singular specificity whose protein concentration can be directly measured by ultraviolet light absorbance. The neutralizing activity contained in one microgram of purified mAb can therefore be determined by evaluating the mAb relative to a standard. The FDA diphtheria anti-toxin standard is a polyclonal preparation quantified in international units (IUs); therefore, the relative potency of the S315 mAb is expressed as micrograms of the monoclonal antibody per one unit of the polyclonal standard (µg/IU).
When overall survival was used as the clinical endpoint of the potency assessment, a dose response with regard to time of death was observed with more rapid onset in the cohorts receiving lower doses of DAT or mAb, though there was insufficient power for a formal time-to-event analysis. Interestingly, a steep threshold effect was observed with progression from 0% survival to 100% survival over a narrow dose range: 22.5 µg-27.5 µg for S315 mAb and 1.25–1.75 IU for DAT.
Given that some of the surviving animals in both the mAb and the DAT groups exhibited transient hind limb weakness, a likely manifestation of diphtheria toxin's effects on peripheral nerves,Citation25,26 we performed a second analysis with an endpoint of survival without hind limb paralysis. For the DAT group, the dose-response curve looked similar to the overall survival endpoint; however, some surviving animals in the 1.5 IU DAT cohort developed transient paralysis. There was a pronounced threshold effect, with all animals receiving ≥ 1.75 IU DAT surviving without paralysis. For the mAb-treated group, a more linear dose response was observed with this endpoint. Transient paralysis was observed in some animals in the cohorts with 100% overall survival, with the proportion of animals exhibiting hind limb weakness decreasing with higher mAb doses. All animals receiving 100 µg of mAb survived without developing paralysis. The doses observed for full protection from toxin-induced symptoms in this study are higher than those reported previously.Citation21 Smaller sample size and more limited daily observation in the preliminary studies may explain these differences.
While analysis of hind limb paralysis in survivors appears to offer additional insight into the neutralization of toxin by different doses of anti-toxin antibody, there was no differential effect on overall health with respect to weight gain except in animals progressing rapidly toward death. For the cohorts with surviving animals, the mean weight gain was similar to a reference set of data (n = 1641) for the same strain animals receiving irrelevant protein only (tetanus toxoid) housed under equivalent conditions, that showed mean weekly weight gains of 46.2 g [SD 13.2] (unpublished data). Thus, analyses of weight or growth appear likely to be less informative than careful observation of locomotor function in guinea pig acute diphtheria intoxication models.
The DAT ED50 for an endpoint of survival without paralysis (1.42 IU) was similar to the ED50 for the overall survival endpoint (1.45 IU). For the mAb-treated animals, a higher dose was required to protect from hind limb weakness, with the ED50 increasing from 25.0 µg for the overall survival endpoint to 67.4 µg using survival without paralysis as the endpoint. The greater magnitude of change for the mAb compared to DAT for these 2 endpoints is challenging to interpret, as dosing for the mAb was based on measured protein concentrations (µg/mL) whereas DAT doses were unit measures of functional activity (IU/mL). The dose-response curves of an anti-diphtheria toxin monoclonal antibody compared to polyclonal antibody were not parallel under these experimental conditions. The use of a guinea pig model with a lower toxin dose may allow a more linear dose response for survival endpoints to be established for both types of antibody preparations.
These potency estimates for 2 different clinical endpoints provide a foundation for further development of S315. Since the treatment of diphtheria is empirical dosing of equine DAT based on the clinical condition of the patient and there are no human data correlating serum concentrations of diphtheria anti-toxin with clinical efficacy, dose selection of a monoclonal antibody to diphtheria toxin for evaluation in clinical trials will be facilitated by the relative potency estimates established in animal models. Given the variation in the point estimates of relative potency depending on the endpoint analyzed in a modified NIH Minimum Requirements assay, we plan to establish a guinea pig model of anti-toxin administration after diphtheria toxin exposure to rigorously evaluate the potency estimates. This model will more closely mimic the timing of anti-toxin treatment in humans and will enable use of a lower dose of toxin, potentially allowing a more linear dose-response curve to be established for DAT. Results from this post-exposure treatment model will assist us in determining a protective therapeutic dose of S315 relative to DAT and allow us to investigate the correlation between serum neutralizing titer and protection in the toxin challenge model. These data, in conjunction with ongoing efforts to quantify the serum neutralizing activity achieved following DAT dosing in patients with suspected diphtheria, will further refine an appropriate range for dosing S315 in a Phase 1 clinical trial.
Materials and methods
Laboratory animals
All experiments were performed with the approval of the University of Massachusetts Medical School Institutional Animal Care and Use Committee and in accordance with National Institute of Health Guidelines. Female Hartley guinea pigs (weight 240–280 g) were obtained from Charles River Laboratories (Wilmington, MA) and acclimated to our facility for 1 week prior to study intervention.
Diphtheria toxin
K561, a standardized diphtheria toxin reagent prepared from a crude filtrate of vaccine strain C. diphtheriae culture (MassBiologics, Boston, MA), was utilized in these studies. Qualification was performed to determine the dilution of toxin K561 that, when pre-mixed with 1 IU of DAT (Food and Drug Administration DAT standard, lot #F4509), caused death of a guinea pig between 40 and 96 hours. This dose, defined as the “L+ dose” of diphtheria toxin in the NIH Minimum Requirements,Citation23 contained 2 Lf of toxin (an Lf or “limit of flocculation” unit is defined by immunoprecipitation of antibody and antigen)Citation27 or 102 minimum lethal doses (MLD, defined as the lowest dose of toxin that is lethal to a 200–300 g guinea pig between 72 and 96 hours after sub-cutaneous administration). A control group of 2 guinea pigs receiving 1 IU of DAT pre-mixed with toxin was included in each experiment to confirm toxin activity.
Antibodies
Equine diphtheria anti-toxin standard was obtained from the FDA (lot # F4509). The human mAb S315 is an IgG1 antibody originally cloned from a human B cell. Details of the isolation, production and preparation of the recombinant molecule were previously described in detail.Citation21
Animal model of lethal challenge with diphtheria toxin
After acclimation, animals were divided into dosing cohorts of 5 animals each. A protocol-specified dose of DAT or S315 was pre-mixed with the fixed dose of K561 toxin and incubated at room temperature for at least one hour prior to injection. Each animal received a single 3.0 mL subcutaneous injection of toxin/anti-toxin mixture on Day 0. This approach was based on the NIH Minimum Requirements for determination of potency of diphtheria anti-toxin preparations.Citation23 The NIH Bureau of Biologic Standards was previously the agency responsible for regulating biologics; it has now become the Center for Biologics Evaluation and Research (CBER) which is part of the FDA. The NIH Minimum Requirements are standards issued by this agency to govern the measurement of diphtheria toxin neutralizing activity and remain in use today. The assay was modified to extend the period of observation to 30 d (from the standard 96 hours) to allow for greater differentiation of dose effects and for evaluation of signs of toxin-induced end-organ damage occurring up to 30 d after toxin exposure. Animals were observed daily for signs of diphtheria intoxication and weights were obtained weekly as a measure of overall health status. A numeric scoring system of clinical signs was assigned a priori: No symptoms = 0; Scruffy fur = 1; Lethargic = 2; Dehydrated = 3; Dragging one or both hind legs = 4; Moribund = 5; Death = 6. Moribund animals or those with > 20% weight loss from baseline were euthanized.
In order to generate a dose-response curve which could be utilized to estimate relative potency, DAT and monoclonal antibody doses were selected that were likely to produce 0–20% protection in the lowest dosed cohort to 80–100% protection in the highest dosed cohort. As the dose of toxin utilized in this animal model consistently causes death between 40 hrs and 96 hrs when pre-mixed with 1 IU of DAT, DAT doses of 1.25 IU to 1.5 IU were evaluated in an initial experiment. Initial doses of S315 ranging from 8 µg to 25 µg were evaluated based on previous study results.Citation21 A second experiment was performed with a higher dose range for DAT (1.5 IU to 2.5 IU) and for S315 (25 µg to 100 µg) to determine relative potency for an endpoint of survival without hind limb paralysis. The 1.5 IU DAT dose and the 25 µg S315 dose were repeated in the second study to bridge results between the 2 experiments.
Statistical analyses
The percentage of animals in each cohort that survived for 30 d post-toxin exposure or survived for 30 d without signs of hind limb paralysis was calculated. Euthanized animals were counted as deaths in the survival analyses. Logistic analyses were performed in SAS IML to calculate the half maximal effective dose (ED50) for DAT and S315; the SAS IML code was validated against the original publication.Citation28 The ED50 values were used to estimate the relative potency of S315 (in µg) to DAT (in units). Fieller's Theorem was used to calculate the 95% fiducial limits around the ED50 values and relative potency.Citation29 Baseline weights were examined among the dosing cohorts using ANOVA tests for the 2 antibody treatments. Partially conditional regression models were used to estimate weekly weight changes from the baseline at different time points up to 28 d among the surviving subjects.Citation30 For each antibody group, the model assumed an initial weight change at Day 7 and a linear weight change slope in the dynamic cohorts of survivors for each dose level and the parameters were estimated by generalized estimating equation (GEE).
Abbreviations
| (CHO) | = | Chinese hamster ovary |
| (DAT) | = | diphtheria anti-toxin |
| (FDA) | = | Food and Drug Administration |
| (GEE) | = | generalized estimating equation |
| (IU) | = | international unit |
| (mAb) | = | monoclonal antibody |
| (NIH) | = | National Institutes of Health |
Disclosure of potential conflicts of interest
HLS, PC, ML, and DCM are employees of MassBiologics of the University of Massachusetts Medical School where the S315 monoclonal antibody was discovered.
Acknowledgments
The authors wish to thank Erica Messick and Jennifer Brennan for their technical expertise, Larry Allen for assistance with data preparation, and Su-Chun Cheng for additional statistical analyses.
Funding
These studies were funded by MassBiologics of the University of Massachusetts Medical School.
References
- Beh P, Byard RW. Diphtheria and lethal upper airway obstruction. Forensic Sci Med Pathol 2015; 11:133-5; PMID:25332174; http://dx.doi.org/10.1007/s12024-014-9623-y
- Kole AK, Roy R. Images in clinical medicine. Respiratory diphtheria. N Engl J Med 2013; 369:1544; PMID:24131179; http://dx.doi.org/10.1056/NEJMicm1309222
- Logina I, Donaghy M. Diphtheritic polyneuropathy: a clinical study and comparison with Guillain-Barré syndrome. J Neurol Neurosurg Psychiatry 1999; 67:433-8; PMID:10486387; http://dx.doi.org/10.1136/jnnp.67.4.433
- Varghese MJ, Ramakrishnan S, Kothari SS, Parashar A, Juneja R, Saxena A. Complete heart block due to diphtheritic myocarditis in the present era. Ann Pediatr Cardiol 2013; 6:34-8; PMID:23626433; http://dx.doi.org/10.4103/0974-2069.107231
- Bhagat S, Grover SS, Gupta N, Roy RD, Khare S. Persistence of Corynebacterium diphtheriae in Delhi & National Capital Region (NCR). Indian J Med Res 2015; 142:459-61; PMID:26609038
- Santos LS, Sant'anna LO, Ramos JN, Ladeira EM, Stavracakis-Peixoto R, Borges LLG, Santos CS, Napoleão F, Camello TCF, Pereira GA, et al. Diphtheria outbreak in Maranhão, Brazil: microbiological, clinical and epidemiological aspects. Epidemiol Infect 2015; 143:791-8; PMID:25703400; http://dx.doi.org/10.1017/S0950268814001241
- Bitragunta S, Murhekar MV, Hutin YJ, Penumur PP, Gupte MD. Persistence of diphtheria, Hyderabad, India, 2003–2006. Emerg Infect Dis 2008; 14:1144-6; PMID:18598644; http://dx.doi.org/10.3201/eid1407.071167
- WHO | Diphtheria [Internet]. WHO [cited 2015 Dec 11]; Available from: http://www.who.int/immunization/monitoring_surveillance/burden/diphtheria/en/
- Centers for Disease Control and Prevention. Diphtheria: Haiti Pre-decision Brief for Public Health Action [Internet]. [cited 2013 Aug 28]; Available from: http://emergency.cdc.gov/disasters/earthquakes/haiti/diphtheria_pre-decision_brief.asp
- Besa NC, Coldiron ME, Bakri A, Raji A, Nsuami MJ, Rousseau C, Hurtado N, Porten K. Diphtheria outbreak with high mortality in northeastern Nigeria. Epidemiol Infect 2013; 142(4):1-6
- National Institute for Communicable Diseases. Update on the diphtheria outbreak in KwaZulu-Natal [Internet]. 2015 [cited 2015 Dec 11]; Available from: http://www.nicd.ac.za/?page=alerts&id=5&rid=572
- Hughes GJ, Mikhail AFW, Husada D, Irawan E, Kafatos G, Bracebridge S, Pebody R, Efstratiou A. Seroprevalence and Determinants of Immunity to Diphtheria for Children Living in Two Districts of Contrasting Incidence During an Outbreak in East Java, Indonesia. Pediatr Infect Dis J 2015; 34:1152-6; PMID:26226444; http://dx.doi.org/10.1097/INF.0000000000000846
- Nanthavong N, Black AP, Nouanthong P, Souvannaso C, Vilivong K, Muller CP, Goossens S, Quet F, Buisson Y. Diphtheria in Lao PDR: insufficient coverage or ineffective vaccine? PLoS ONE 2015; 10:e0121749; PMID:25909365; http://dx.doi.org/10.1371/journal.pone.0121749
- Jhancy M, Satyavani A, Manikyamba D, Tulasi Deepthi K. Re-emergence of diphtheria – An outbreak from east Godavari District, Andhra Pradesh. Pediatr Infect Dis 2015; 7(2):33-35; Available online 12 September 2015
- European Centre for Disease Prevention and Control. Rapid risk assessment: a case of diphtheria in Spain, 15 June 2015 [Internet]. Stockholm: ECDC; 2015 [cited 2015 Jul 23]. Available from: http://ecdc.europa.eu/en/publications/Publications/diphtheria-spain-rapid-risk-assessment-june-2015.pdf
- Tiwari T. Diphtheria. In: Manual for the surveillance of vaccine-preventable diseases. Atlanta, GA: Centers for Disease Control and Prevention; 2011.
- European Centre for Disease Prevention and Control. Rapid risk assessment: cutaneous diphtheria among recently arrived refugees and asylum seekers in the EU [Internet]. 2015 [cited 2015 Dec 11]; Available from: http://ecdc.europa.eu/en/publications/Publications/Diphtheria-cutaneous-EU-July-2015.pdf
- Both L, White J, Mandal S, Efstratiou A. Access to diphtheria antitoxin for therapy and diagnostics. Euro Surveill 2014; 19:pii: 20830; http://dx.doi.org/10.2807/1560-7917.ES2014.19.24.20830
- Wagner KS, Stickings P, White JM, Neal S, Crowcroft NS, Sesardic D, Efstratiou A. A review of the international issues surrounding the availability and demand for diphtheria antitoxin for therapeutic use. Vaccine 2009; 28:14-20; PMID:19818425; http://dx.doi.org/10.1016/j.vaccine.2009.09.094
- Centers for Disease Control and Prevention (CDC). Use of Diphtheria Antitoxin (DAT) for Suspected Diphtheria Cases BB IND 11184 [Internet]. [cited 2015 Dec 11]; Available from: http://www.cdc.gov/diphtheria/downloads/protocol.pdf
- Sevigny LM, Booth BJ, Rowley KJ, Leav BA, Cheslock PS, Garrity KA, Sloan SE, Thomas W Jr, Babcock GJ, Wang Y. Identification of a human monoclonal antibody to replace equine diphtheria antitoxin for treatment of diphtheria intoxication. Infect Immun 2013; 81:3992-4000; PMID:23940209; http://dx.doi.org/10.1128/IAI.00462-13
- Tasman A, Minkenhof JE, Vink HH, Brandwijk AC, Smith L. Importance of intravenous injection of diphtheria antiserum. Lancet 1958; 1:1299-304; PMID:13550990; http://dx.doi.org/10.1016/S0140-6736(58)92061-0
- US Department of Health, Education and Welfare Public Health Service, National Institute of Health. Minimum Requirements: Diphtheria Toxoid. Fourth revision. 1947;
- Tasman A, Minkenhof JE, Vink HH, Brandwijk AC, Smith L. On the pathogenesis of diphtheria. V. Importance of intravenous injection of diphtheria serum. Antonie Van Leeuwenhoek 1958; 24:161-86; PMID:13595643; http://dx.doi.org/10.1007/BF02548444
- Pleasure DE, Feldman B, Prockop DJ. Diphtheria toxin inhibits the synthesis of myelin proteolipid and basic proteins by peripheral nerve in vitro. J Neurochem 1973; 20:81-90; PMID:4687209; http://dx.doi.org/10.1111/j.1471-4159.1973.tb12106.x
- Pullen AH. Neurofilament reorganisation and neurofilament antigen redistribution in spinal motoneurones following retrograde axonal transport of diphtheria toxin. Acta Neuropathol (Berl) 1994; 87:32-46; http://dx.doi.org/10.1007/BF00386252
- Ramon G. Sur une technique de titrage in vitro du sérum antidiphthérique. CRSocBiol 1923; 86:711-2
- Stokes ME, Davis CS, Koch GG. Categorical Data Analysis using the SAS System. 1995; Pp. 287-292.
- Zerbe G. On Fieller's theorem and the general linear model. Am Stat 1978; 32:102-5
- Kurland BF, Johnson LL, Egleston BL, Diehr PH. Longitudinal Data with Follow-up Truncated by Death: Match the Analysis Method to Research Aims. Stat Sci Rev J Inst Math Stat 2009; 24:211
