ABSTRACT
Secretory aspartyl proteinases (Saps) of Candida albicans are key virulence traits which cause inflammasome-dependent, aseptic inflammation in a mouse model of vaginitis. In this paper, neutrophil migration in response to Sap2, Sap6 and chemo-attractive products released from Sap-treated vaginal epithelium was measured in vitro, ex vivo and in vivo. Our results show that Sap2 and Sap6 induce neutrophil migration and production of potent chemoattractive chemokines such as IL-8 and MIP-2 by vaginal epithelial cells. Our data suggest that at least part of MIP-2 production depends upon IL-1β activity. The vaginal fluid of Candida-infected mice contained a heat-labile inhibitor of neutrophil candidacidal activity that was absent from the vaginal fluid of Sap-treated mice. Overall, our data provide additional information on the capacity of C. albicans Saps to cause aseptic vaginal inflammation and highlight the potential role of some chemokines released from vaginal epithelial cells in this phenomenon.
Introduction
Extracellular proteolytic activity by secretory aspartyl proteinases (Saps) has long been suggested as important virulence trait of the pathogenic yeast Candida albicans. C. albicans utilizes Saps, a family of 10 proteins,Citation1 as active enzymes to favor adherence to, and damage of, epithelial cells. Saps secretion is also associated with hyphal formation and immune-escape activities.Citation2,3
The role of Saps in disease has been extensively studied for mucosal infections, particularly vaginal candidiasis.Citation4 Recent data suggest that Saps are able to trigger a pathogenic inflammatory immune response likely occurring through activation of the NLRP3 inflammasome and that this inflammation is the critical determinant of vaginal candidiasis.Citation5-7 Neutrophils are actively recruited into the vaginal cavity during Sap-induced inflammatory response,Citation6 suggesting that Saps have direct neutrophil chemotactic activity. Moreover, recent data suggest a non-protective role for neutrophils during vaginal infection that might even exacerbate disease in humans.Citation8
Expanding on initial observations in different disease models,Citation9,10 here we verified that 2 Saps, Sap2 and Sap6, could induce neutrophil migration in in vitro, ex vivo and in vivo mouse model of Sap-induced vaginitis without infection (aseptic vaginitis). Moreover, a possible mechanism of immune-pathogenesis of vaginal candidiasis was hypothesized.
Results and discussion
Because some C. albicans Saps can cause a massive neutrophil influx, in particular into the mouse vagina,Citation6 we firstly analyzed whether 2 selected Saps (Sap2 and Sap6) can act as chemoattractants for human neutrophils in vitro. IL-8 was used as positive control in these experiments. We focused on Sap2 and Sap6, which are well-studied members of the Sap1-3 and Sap4-6 sub-families,Citation4 to follow-up our previous work where we showed that Sap2 and Sap6 were more consistent than other used Saps in inducing monocyte inflammatory responses in vitro Citation11,12 and vaginal inflammation in a mouse model.Citation6 Nevertheless, data from different experimental models using a C. albicans sap9Δ mutant, suggested that Sap9 is required for the induction of neutrophil chemotaxis toward C. albicans filaments.Citation10
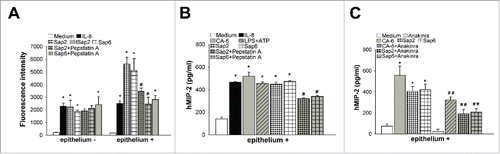
Dose-dependent analysis showed that a concentration of around 0.5 µg/ml was optimal for both Saps to stimulate human neutrophil migration (not shown). This dose was therefore used for all experiments. To verify if enzymatic activity was necessary for the chemotactic effect, Sap2 and Sap6 were pre-incubated in the presence or absence of Pepstatin A at a dose of 1 µg/ml, that was previously shown to strongly inhibit Sap activity.Citation6 An enzymatically-inactive recombinant Sap2 (tSap2 Citation6) was also used to provide further evidence that Sap2-induced migration did not require enzymatic activity. Sap2 and Sap6 were direct chemoattractants in vitro and their enzymatic activity was not necessary for Saps-induced migration (). Next, vaginal epithelium was left unstimulated or was stimulated for 24 h with IL-8, Sap2 or Sap6, in the presence or absence of Pepstatin A. Then, neutrophil migration was monitored. Results in (right) show that vaginal epithelium, stimulated with IL-8, Sap2 or Sap6, had a significant potential for neutrophil chemotactic activity. Saps-induced neutrophil migration was significantly higher as compared to experiments in the absence of epithelial cells. Pretreatment of Saps with Pepstatin A significantly reduced, but did not abolish the neutrophil migration. In line with previous evidence of vaginal inflammation in the mouse modelCitation6 the differences from data above, obtained in the presence or absence of epithelium, are likely indicative of the need of Sap enzymatic activity to favor Saps entering the epithelial cells and activate the cytokine-chemokine response (see below). This additional internal activation adds to the direct external stimulation by Saps, which does not require enzymatic activity.
Figure 1. Effect of Saps on neutrophil migration and on MIP-2 production. Human fluorescent neutrophils were incubated for 2 h to the upper compartment of transwell filters containing Medium, IL-8 (100 ng/ml), Sap2, tSap2 or Sap6 (all 0.5 µg/ml) in the presence or absence of Pepstatin A (1 µg/ml) (A, left) or vaginal epithelium, stimulated for 24 h in the presence or absence (Medium) of IL-8 (100 ng/ml), Sap2, tSap2 or Sap6 (all 0.5 µg/ml), pretreated or not with Pepstatin A (1 µg/ml) (A, right), in the lower compartment. The number of migrating neutrophils into the lower compartment was measured by using fluorescence signal. Data are expressed as fluorescence intensity of migrated neutrophils (triplicates samples of 5 different experiments, mean ± SEM). Human MIP-2 was assessed on cultures supernatants of vaginal epithelium stimulated for 24 h in the presence or absence (Medium) of IL-8 (100 ng/ml), C. albicans (CA-6) (1 × 107/ml), LPS (10 µg/ml) plus ATP (5 mM), Sap2 or Sap6 (both 0.5 µg/ml), pretreated or not with Pepstatin A (1 µg/ml), by specific Elisa assay (triplicates samples of 5 different experiments, mean ± SEM) (B). Human MIP-2 was also assessed on cultures supernatants of vaginal epithelium unstimulated (Medium) or stimulated for 24 h with C. albicans (CA-6) (1 × 107/ml), Sap2 or Sap6 (both 0.5µg/ml) in the presence or absence of Anakinra (10 µM) by specific Elisa assay (triplicates samples of 3 different experiments, mean ± SEM) (C). *, p < 0.05 IL-8, CA-6, LPS plus ATP or Saps treated vs Medium treated. #, p < 0.05 Saps + Pepstatin A treated vs Saps treated. # #, p < 0.05 CA-6 or Saps + Anakinra treated vs CA-6 or Saps treated.
Experiments were then done to detect chemo-attractive products released from the epithelial cells upon Sap stimulation. We focused on well known chemokines such as IL-8 and MIP-2.
We found that Sap2- or Sap6-stimulated vaginal epithelium produced consistent amounts of MIP-2, a known strong chemotactic factor. Sap pretreatment with Pepstatin A significantly reduced, but did not abolish, MIP-2 production. IL-8, whole Candida cells or LPS plus ATP were used as positive controls ().
Since the inflammasome activation in Sap2-stimulated vaginal epithelium leads to IL-1β production Citation6 and MIP-2 secretion is enhanced by several inflammatory stimuli, including IL-1β,Citation13 we wondered whether IL-1β could play a role in MIP-2 production. Results in showed that the blockade of IL-1β receptor by using Anakinra significantly inhibited Sap-induced MIP-2 production by vaginal epithelium. Anakinra also inhibited Candida cell-stimulated chemokine production () and this is in agreement with our previous results that the blockade of IL-1β receptor inhibited the neutrophil influx in Candida-infected mouse vagina.Citation6 While these studies show a likely role of IL-1β and Sap-induced production of chemokines for neutrophil migration, further studies are needed to obtain more direct insight into this phenomenon.
To investigate the in vivo significance of our findings, we used our mouse model of Sap-induced vaginitis without infection, that closely mimics the vaginal inflammation induced by the fungus.Citation6 Vaginal washes, obtained from mice vaginally injected with Saline, LPS, Sap2 or Sap6, were used as potential chemoattractants. The migration of neutrophils is shown in . Maximal migration was induced by vaginal washes obtained from mice injected with Sap2, but a significant migration was also induced by vaginal washes of mice injected with Sap6 or LPS. Moreover, we tested whether classical chemoattractants such as IL-8 or MIP-2 were induced by Sap- or LPS-exposed mouse vaginal epithelial cells and thus can contribute to neutrophil chemotaxis. As shown in , a cytofluorimetric analysis revealed a significant increase of intracellular IL-8 in vaginal epithelial cells of mice treated with Sap2 or Sap6. Moreover, IL-8 () and MIP-2 () were produced in vaginal fluid of mice treated with Sap2 or Sap6. MIP-2 production was significantly decreased (by 73.6%) in vaginal fluid of mice intravaginally injected with Sap2 in the presence of anti-Sap2 Fab capable of binding Sap2 and inhibiting its enzymatic activity. These results closely correlate with a marked inhibition (by 85.5%) of neutrophil recruitment in the same vaginal fluid. Treatment with Pepstatin A had the same inhibitory effect as the Fab in decreasing Sap2-induced MIP-2 production and neutrophil influx (by 74.4% and 82.7%, respectively). The specificity of these effects was evidenced by the inability of Fab and Pepstatin A to inhibit LPS-induced MIP-2 production and neutrophil influx (not shown).
Figure 2. Neutrophil influx and activity during vaginal candidiasis. Human fluorescent neutrophils were incubated for 2 h to the upper compartment of transwell filters containing, in the lower compartment, vaginal washes of mice injected for 24 h with Saline, LPS (50 µg/10 µl/mouse), Sap2 or Sap6 (both 0.5 µg/10 µl/mouse). The number of migrating neutrophils into the lower compartment was measured by using fluorescence signal. Data are expressed as fluorescence intensity of migrated neutrophils (triplicates samples of 3 different experiments, mean ± SEM) (A). Vaginal washes of mice injected as above described were centrifuged, cellular fraction was microscopically analyzed to evaluate neutrophil recruitment (arrow) (representative images of 3 separate experiments with similar results, magnification 10 x, Bar = 50 µm) (B) or the % of double positive CD326+/IL-8+ cells (triplicates samples of 3 different experiments, mean ± SEM) (C) and supernatants were collected and tested for mouse IL-8 (D) and mouse MIP-2 (E) production by specific Elisa assays (triplicates samples of 3 different experiments, mean ± SEM). Killing activity against C. albicans of human neutrophils mixed with Medium or vaginal washes of mice injected for 24 h with Saline, Sap2 or Sap6 (both 0.5 µg/10 µl/mouse), C. albicans (CA-6) (2 × 107/10µl/mouse) or H. I. vaginal washes of mice injected for 24 h with C. albicans (CA-6) (2 × 107/10µl/mouse) was shown (triplicates samples of 5 different experiments, mean ± SEM) (F). *, p < 0.05 LPS or Saps treated vs Saline treated mice. #, differences between LPS, Sap2 or Sap6 treated animals resulted not significant. †, p < 0.05 CA-6 treated vs Medium treated mice.
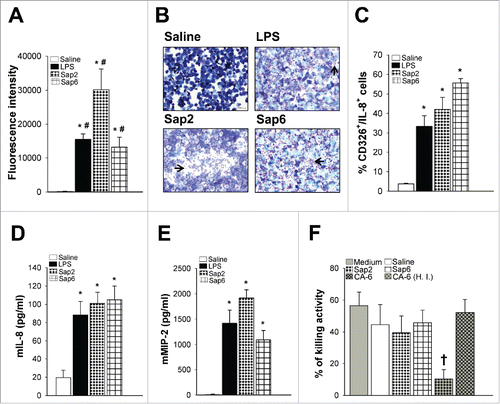
Our recent findings showed that purified Saps and C. albicans cells are able to activate caspase-1 in murine vaginal epithelial cells leading to IL-1β production and that blockade of the IL-1β receptor caused a strong inhibition of neutrophil influx.Citation6 Moreover, other research groups have shown that MIP-2 secretion by epithelial cells is enhanced by IL-1β.Citation13 Hence, the high level of MIP-2 in vaginal washes of Sap-exposed mice could be ascribed, at least in part, to the elevated levels of Sap-induced IL-1β.
It has been suggested that neutrophils, which massively infiltrate the epithelial tissues during candidal vaginitis, do not play a protective role, but are rather the cause of the disease.Citation14 Since neutrophils are very efficacious Candida killersCitation15 it is possible that vaginal neutrophils have reduced anti-Candida activities due to factors released by the vaginal tissues or Candida cells during infection. Thus, we examined whether cell-free vaginal fluids from Saps or Candida exposed vaginal epithelial tissue could modulate the anticandidal activity of neutrophils. Neutrophils were mixed with medium or vaginal washes obtained from mice injected with Saline, Sap2, Sap6 or C. albicans cells or were left untreated. Next, the killing activity was monitored. Results in show that neutrophils alone display the expected remarkable candidacidal activity, that was not significantly changed when neutrophils were mixed with vaginal washes obtained from mice injected with Saline, Sap2 or Sap6. On the other end, the candidacidal activity was significantly impaired when neutrophils were mixed with vaginal washes obtained from mice injected with C. albicans cells (), suggesting that soluble factor(s), able to dampen neutrophil candidacidal activity, were released during vaginal Candida infection. These soluble factors were not found during Sap-induced inflammation. When neutrophils were mixed with heat inactivated (H. I.) vaginal washes obtained from C. albicans-injected mice, the killing activity was restored. Besides being heat-labile, we have no precise data about the nature of this factor(s). Notably, hyphae-associated glycoproteins of C. albicans have already been reported to be inhibitory for neutrophils functions Citation16 with, however, no proof they are active in vaginal candidiasis. In addition, the inhibitor could well be of host origin.
Concluding remarks
Previous studies have shown that Saps contribute to an inflammatory mucosal response by the activation of IL-1β.Citation6 Also on the basis of observations made in other models Citation9,10 we predicted that Saps may have a role in the induction of chemoattractive cytokines. Our results clearly indicate that: i) Saps directly induce neutrophil migration independently on their enzymatic activity in an in vitro experimental model; ii) even higher migration is obtained in the presence of epithelial cells. In this case, the surplus of Sap-induction requires enzymatic activity, coherently with previous in vivo data.Citation6 This is possibly due to the need of degrading ectocellular proteinaceous material in order to gain intracellular access, as discussed elsewhere Citation6; iii) Saps induce production of MIP-2 and IL-8 by vaginal epithelium. These potent chemokines could therefore mediate or contribute together with other factors to Sap-induced chemotaxis in vivo; iv) Saps do not modulate the candidacidal activity of neutrophils. However, we observed here that candidacidal activity of neutrophils can be inhibited by other factors produced by C. albicans cells or by the host during experimental vaginal infection. While this last observation warrants a future specific investigation, our present data further highlight the crucial role of Sap-induced local inflammation during vaginal candidiasis and suggest that an anti-Sap based therapy may help to control fungal virulence and excess inflammation during this common type of mucosal infection. They also provide a preliminary glimpse about the mechanism of immune-pathogenesis of vaginal candidiasis: the release of Saps during vaginal C. albicans infection triggers the recruitment of neutrophils whose activity may not control the infection because of the presence of inhibitors released by Candida itself or the host.
Materials and methods
Aspartyl proteinases and Fab format monoclonal antibody (mAb) production
Endotoxin-free recombinant C. albicans aspartyl proteinases 2 and 6 (Sap2 and Sap6) were expressed as recombinant proteins using, respectively, Escherichia coli BL21 (pLysS) or Pichia pastoris clones as previously described.Citation6,17 An E. coli recombinant truncated Sap2 (tSap2 preparation) has been previously described.Citation6 Fab format of mAb capable of binding Sap2 and strongly inhibiting its enzymatic activity was produced by AbD-Serotec (Germany) as previously described.Citation6
Candida albicans strain
Candida albicans strain (CA-6) used in this study has previously been described.Citation18
Ethic statement
Blood samples were collected from subjects after obtaining informed consent, in accordance with the Declaration of Helsinki, and approval was obtained from the ethical review board.
All animal experiments were performed in agreement with the EU Directive 2010/63 and the National Law 116/92. The protocol was approved by Perugia University Ethics Committee (permit number 149/2009-B). All animals were housed in the animal facility of the University of Perugia (Authorization number 34/2003A).
Preparation of polymorphonuclear cells
Human peripheral blood neutrophils, obtained from healthy donors, were separated by density gradient centrifugation on Ficoll-Hypaque (Euroclone, Milan, Italy), followed by the hypotonic lysis of erythrocytes. Neutrophils were then washed twice with phosphate buffered saline (PBS) (Sigma Aldrich) and then resuspended in 5 ml RPMI-1640 without phenol red (BioWhittaker, Walkersville, MD) containing 10% heat-treated fetal calf serum (RPMI-FCS). Calcein AM, fluorescent cell permeable derivative of calcein (Sigma Aldrich) (5 µg/ml) was added to the 5 ml suspension of cells in RPMI-FCS and the cells were incubated for 30 min at 37°C plus 5% CO2. Neutrophils were washed twice with PBS, counted and resuspended in RPMI-FCS at 5 × 106/ml.Citation19
Vaginal epithelium
Human vaginal epithelium was obtained as previously described.Citation20
Vaginal washes
Female CD1 mice (Envigo, Milan, Italy) were purchased at 4 to 5 weeks of age and maintained under pseudoestrus condition by subcutaneous (s. c.) injection of 0.2 mg of estradiol valerate in 100 μl of sesame oil (both from Sigma-Aldrich) 2 d prior to infection. Mice, anaesthetized with 2.5–3.5 (v/v) isofluorane gas, were injected into the vaginal lumen with Saline, LPS (50 μg/10 μl/mouse) (Sigma Aldrich), Sap2 or Sap6 (both 0.5 μg/10 μl/mouse) or C. albicans (2 × 107/10 µl/mouse). In selected experiments, mice, under pseudoestrus condition, were injected 30 min before and again 30 min after treatment with LPS (50 μg/10 μl/mouse) or Sap2 (0.5 μg/10 μl/mouse) with anti-Sap2 Fab (0.36 ng/10 µl/mouse) or Pepstatin A (1 µg/10 µl/mouse). Twenty four h post-infection, the vaginal lumen was thoroughly washed with 150 μl of Saline, given in three separate 50 µl volumes.Citation6 The washes were centrifuged at 3000 rpm for 5 min, the supernatants were collected and centrifuged at 3000 rpm for 5 min. Cell-free supernatants were tested for modulation of neutrophil killing activity and migration, IL-8 and MIP-2 production by specific Elisa assays (BMASSAY, BeiJing, China; RayBiotech, Norcross, GA,respectively).
Fluorescence-based determination of neutrophil migration and cytokine production
Human neutrophil migration was measured by a 96-well chemotaxis chamber with 3.2 mm in diameter yielding a filter with membrane porosity of 5 µm (Neuro Probe). Fluorescent neutrophils (5 × 106/ml) were transferred into ChemoTx filters placed in 96-well plates containing medium, recombinant IL-8 (100 ng/ml) (ImmunoTools GmbH, Germany), tSap2, Sap2 or Sap6 (all 0.5 µg/ml) pretreated or not with Pepstatin A (1 µg/ml) (Sigma Aldrich) for 30 min at 37°C plus 5% CO2. Vaginal washes from mice treated as above described or vaginal epithelium, stimulated for 24 h at 37°C plus 5% CO2 in the presence or absence (medium) of IL-8 (100 ng/ml), tSap2, Sap2 or Sap6 (all 0.5 µg/ml), pretreated or not with Pepstatin A (1 µg/ml) (Sigma Aldrich) for 30 min at 37°C plus 5% CO2, were also used as chemoattractants. The chamber was incubated for 2 h at 37°C plus 5% CO2. After incubation, the non-migrating cells on the origin side (top) of the filter were removed by gently wiping the filter and the cells that are migrated into the bottom chamber were measured by using fluorescence signal (excitation, 485 nm; emission, 530 nm).
For human MIP-2 production, vaginal epithelium was also stimulated for 24 h at 37°C plus 5% CO2 in the presence or absence (medium) of IL-8 (100 ng/ml), C. albicans (1 × 107/ml), LPS (10 µg/ml) plus ATP (5 mM), Sap2 or Sap6 (both 0.5 µg/ml), pretreated or not with Pepstatin A (1 µg/ml) (Sigma Aldrich) for 30 min at 37°C plus 5% CO2. Human MIP-2 production was also tested in supernatants of vaginal epithelium unstimulated (medium) or stimulated for 24 h at 37°C plus 5% CO2 with C. albicans (1 × 107/ml), Sap2 or Sap6 (both 0.5 µg/ml) in the presence or absence of an antagonist of IL-1β receptor: Anakinra, (10 µM).Citation6 Human MIP-2 production was evaluated by specific Elisa assay (abcam, Cambridge, UK).
Flow cytometry analysis
Total cellular fractions obtained from vaginal washes were fixed with 1.5% formalin for 10 min at room temperature (RT), washed and incubated with APC-labeled mAb to mouse CD326, expressed on epithelial cells, (rat IgG2b,kisotype; 0.05 μg/tube; eBioscience) for 20 min at RT in the dark. After incubation, cells were washed twice with fluorescent buffer (FB), permeabilized with absolute methanol (500 μl/106 cells) for 10 min on ice and incubated with purified mAb to IL-8 (rabbit IgG isotype; dilution 1/250; GeneTex Inc., CA) for 20 min at RT followed by Cy3-labeled conjugated affinity purified secondary Ab (dilution 1/100; Chemicon Int., Temecula, CA). In selected experiments, fixed total cellular fraction, obtained from vaginal washes, were stained with FITC-conjugated mAb to mouse Ly-6G (GR-1) (rat IgG2b,k isotype; 0.05 μg/tube; eBioscience) per 20 min at RT in the dark. After incubation, cells were washed with FB and resuspended in 0.5 ml of FB. Cells were then analyzed by flow cytometry using FACSCalibur (BD Biosciences). Autofluorescence was assessed by using untreated cells. Data are expressed as percentage of double positive cells. Control staining of cells with irrelevant Abs was used to obtain background fluorescence values.
Microscopical analysis
Vaginal samples of mice injected as above described were centrifuged by cytospin (700 rpm for 7 min), stained with Haemacolor stain and examined by light microscopy (Olympus). The scale bars are in µm.
Killing activity
Human neutrophils (1 × 105/50 µl) were mixed with 50 µl of medium or 50 µl of vaginal washes of mice injected with Saline, Sap2, Sap6, C. albicans cells, or H. I. (for 30 min at 60°C) vaginal washes of mice injected with C. albicans, then incubated with C. albicans (1 × 104/100 µl) for 2 h at 37°C plus 5 % CO2. After incubation, killing activity was evaluated as previously described.Citation21
Statistical analysis
The results reported in the bar graphs are the mean ± SEM from triplicate samples of 3–5 different experiments. Quantitative variables were tested for normal distribution and compared by means of Student's t test. A value of p < 0.05 was considered significant.
Abbreviations
| ATP | = | adenosine triphosphate |
| H. I. | = | heat inactivated |
| Sap | = | Secretory aspartyl proteinase |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We kindly thank Mario Amacker, Christian Moser and Matthias Kaeser for providing recombinant, enzymatically-active Sap2 and tSap2.
Funding
This work was supported by “FondazioneCassa di Risparmio 2012.0128.021.” The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
References
- Tavares AH, Burgel PH, Bocca AL. Turning Up the Heat: Inflammasome Activation by Fungal Pathogens. PLoS Pathog 2015; 11:e1004948; PMID:26204108; http://dx.doi.org/10.1371/journal.ppat.1004948
- Hube B, Monod M, Schofield DA, Brown AJ, Gow NA. Expression of seven members of the gene family encoding secretory aspartyl proteinases in Candida albicans. Mol Microbiol 1994; 14:87-99; PMID:7830564; http://dx.doi.org/10.1111/j.1365-2958.1994.tb01269.x
- Monod M, Borg-von Zepelin M. Secreted proteinases and other virulence mechanisms of Candida albicans. Chem Immunol 2002; 81:114-28; PMID:12101999; http://dx.doi.org/10.1159/000058865
- Naglik JR, Challacombe SJ, Hube B. Candida albicans secreted aspartyl proteinases in virulence and pathogenesis. Microbiol Mol Biol Rev 2003; 67:400-28, table of contents; PMID:12966142; http://dx.doi.org/10.1128/MMBR.67.3.400-428.2003
- Gabrielli E, Pericolini E, Luciano E, Sabbatini S, Roselletti E, Perito S, Kasper L, Hube B, Vecchiarelli A. Induction of Caspase-11 by Aspartyl Proteinases of Candida albicans and Implication in Promoting Inflammatory Response. Infect Immun 2015; 83:1940-8; PMID:25712931; http://dx.doi.org/10.1128/IAI.02895-14
- Pericolini E, Gabrielli E, Amacker M, Kasper L, Roselletti E, Luciano E, Sabbatini S, Kaeser M, Moser C, Hube B, et al. Secretory aspartyl proteinases cause vaginitis and can mediate vaginitis caused by candida albicans in mice. MBio 2015; 6:e00724; PMID:26037125; http://dx.doi.org/10.1128/mBio.00724-15
- Bruno VM, Shetty AC, Yano J, Fidel PL, Jr., Noverr MC, Peters BM. Transcriptomic analysis of vulvovaginal candidiasis identifies a role for the NLRP3 inflammasome. MBio 2015; 6:e00182-15; PMID:25900651; http://dx.doi.org/10.1128/mBio.00182-15
- Vecchiarelli A, Gabrielli E, Pericolini E. Experimental models of vaginal candidiasis and inflammation. Future Microbiol 2015; 10:1265-8; PMID:26225494; http://dx.doi.org/10.2217/FMB.15.52
- Ran Y, Iwabuchi K, Yamazaki M, Tsuboi R, Ogawa H. Secreted aspartic proteinase from Candida albicans acts as a chemoattractant for peripheral neutrophils. J Dermatol Sci 2013; 72:191-3; PMID:23849944; http://dx.doi.org/10.1016/j.jdermsci.2013.06.006
- Hornbach A, Heyken A, Schild L, Hube B, Loffler J, Kurzai O. The glycosylphosphatidylinositol-anchored protease Sap9 modulates the interaction of Candida albicans with human neutrophils. Infect Immun 2009; 77:5216-24; PMID:19805528; http://dx.doi.org/10.1128/IAI.00723-09
- Pietrella D, Rachini A, Pandey N, Schild L, Netea M, Bistoni F, Hube B, Vecchiarelli A. The Inflammatory response induced by aspartic proteases of Candida albicans is independent of proteolytic activity. Infect Immun 2010; 78:4754-62; PMID:20713630; http://dx.doi.org/10.1128/IAI.00789-10
- Pietrella D, Pandey N, Gabrielli E, Pericolini E, Perito S, Kasper L, Bistoni F, Cassone A, Hube B, Vecchiarelli A. Secreted aspartic proteases of Candida albicans activate the NLRP3 inflammasome. Eur J Immunol 2013; 43:679-92; PMID:23280543; http://dx.doi.org/10.1002/eji.201242691
- Ohtsuka Y, Lee J, Stamm DS, Sanderson IR. MIP-2 secreted by epithelial cells increases neutrophil and lymphocyte recruitment in the mouse intestine. Gut 2001; 49:526-33; PMID:11559650; http://dx.doi.org/10.1136/gut.49.4.526
- Yano J, Lilly E, Barousse M, Fidel PL, Jr. Epithelial cell-derived S100 calcium-binding proteins as key mediators in the hallmark acute neutrophil response during Candida vaginitis. Infection and immunity 2010; 78:5126-37; PMID:20823201; http://dx.doi.org/10.1128/IAI.00388-10
- Naglik JR, Moyes DL, Wachtler B, Hube B. Candida albicans interactions with epithelial cells and mucosal immunity. Microbes Infect 2011; 13:963-76; PMID:21801848; http://dx.doi.org/10.1016/j.micinf.2011.06.009
- Cheng SC, Joosten LA, Kullberg BJ, Netea MG. Interplay between Candida albicans and the mammalian innate host defense. Infect Immun 2012; 80:1304-13; PMID:22252867; http://dx.doi.org/10.1128/IAI.06146-11
- Borg-von Zepelin M, Beggah S, Boggian K, Sanglard D, Monod M. The expression of the secreted aspartyl proteinases Sap4 to Sap6 from Candida albicans in murine macrophages. Mol Microbiol 1998; 28:543-54; PMID:9632257; http://dx.doi.org/10.1046/j.1365-2958.1998.00815.x
- Cenci E, Romani L, Vecchiarelli A, Puccetti P, Bistoni F. Role of L3T4+ lymphocytes in protective immunity to systemic Candida albicans infection in mice. Infect Immun 1989; 57:3581-7; PMID:2572556
- Frevert CW, Wong VA, Goodman RB, Goodwin R, Martin TR. Rapid fluorescence-based measurement of neutrophil migration in vitro. J Immunol Methods 1998; 213:41-52; PMID:9671124; http://dx.doi.org/10.1016/S0022-1759(98)00016-7
- Ridge J, Muller J, Noguchi P, Chang EH. Dynamics of differentiation in human epidermoid squamous carcinoma cells (A431) with continuous, long-term gamma-IFN treatment. In Vitro Cell Dev Biol 1991; 27A:417-24; PMID:1712768; http://dx.doi.org/10.1007/BF02630962
- Mosci P, Gabrielli E, Luciano E, Perito S, Cassone A, Pericolini E, Vecchiarelli A. Involvement of IL-17A in preventing the development of deep-seated candidiasis from oropharyngeal infection. Microbes and infection / Institut Pasteur 2014; 16:678-89; PMID:24980544
