ABSTRACT
Klebsiella pneumoniae (KP), with production of abundant capsular polysaccharide (CPS), is capable of causing invasive syndrome. Environmental glucose stimuli may increase CPS biosynthesis. We aimed to investigate the relationship between glycemic control and KP-mediated invasive syndrome in diabetic patients and the effect of glucose on CPS biosynthesis. Diabetic patients with community-acquired KP bacteremia were included to study the risk factors of invasive syndrome. KP-M1, a serotype-K1 KP clinical isolate, was used to examine the CPS biosynthesis and cps gene expression, and the effect of exogenous glucose on bacterial phagocytosis and killing. We found that invasive syndrome was significantly more common in diabetic patients who were infected with strains expressing the K1 serotype (adjusted odds ratio [AOR], 8.32; 95% confidence interval [CI], 1.56−44.24; p=0.01), and had poor glycemic control (HbA1c ≥9%; AOR, 5.66; 95% CI, 2.01−15.92; p<0.01). Pre-incubation of KP-M1 in media containing different gradient glucose concentrations enhanced CPS biosynthesis and cps gene expression in high glucose (0.5%) concentration, which leads to increasing bacterial resistance to phagocytosis and killing. High glucose levels reflected by poor glycemic control may stimulate CPS biosynthesis and cps gene expression of highly virulent KP, which increase resistance to phagocytosis and contribute to development of invasive syndrome.
Introduction
Klebsiella pneumoniae (KP) has been identified as the predominant bacteria responsible for pyogenic liver abscess worldwide.Citation1-3 Several bacterial virulence factors, including capsular polysaccharide (CPS), have been identified as determinants of invasive infections.Citation4 CPS may help KP evade phagocytosis and impede bacterial clearance from the host.Citation5 Moreover, it has been used to develop a sero-typing system for KP isolates, and currently 77 capsular serotypes have been identified. Compared to those belonging to non-K1/K2 serotypes, isolates of the serotype K1/K2 are significantly more virulent and more likely to cause invasive syndrome.Citation2 Apart from the bacterial factor, the incidence of pyogenic liver abscess due to KP has been documented to be frequently associated with type 2 diabetes mellitus (DM).Citation2, 3, 6 In the study by Chen et al,Citation7 DM was shown to be associated with a 7.7-fold (95% confidence interval [CI], 2.1–29) increased risk for developing invasive syndrome due to KP. An animal study also suggests that DM might provide a unique environment that allows KP strains to disseminate from the intestines into the blood.Citation8
It has been well known that the neutrophil is involved in the innate immunity against bacterial infection.Citation9 Diabetic patients often have abnormalities in both cell-mediated and humoral immune responses, which result in increased susceptibility to infectious diseases.Citation10 Previous studies have shown that uncontrolled glycemia plays a role in impairing neutrophil phagocytosis of KP of K1/K2 serotype.Citation6 However, data on the impact of glycemic control on the biosynthesis of KP CPS are scanty. We hypothesized that a high glucose environment may inhibit the phagocytosis of KP by neutrophils as a result of increased CPS biosynthesis. In this study, we aimed to examine the effect of exogenous graded glucose on CPS biosynthesis, cps gene expression, phagocytosis and bactericidal activity of KP in in-vitro assays. Furthermore, we evaluated the relationship between glycemic control and invasive syndrome due to KP among diabetic patients with community-acquired KP bacteremia in a prospective observational study.
Results
Risk factors for K. pneumoniae-mediated invasive syndrome
During the 3-year study period, 175 diabetic patients were hospitalized with community-acquired KP bacteremia and 41 (23.4%) had invasive syndrome. Capsular serotype K1 was the most commonly encountered serotype (n = 14, 8.0%) followed by K2 (n = 8, 4.5%), K54 (n = 3, 1.7%), K20 (n = 2, 1.1%), K57 (n = 2, 1.1%), and K5 (n = 1, 0.6 %). Compared with age-matched control group (), diabetic patients with KP-mediated invasive syndrome were more likely to be infected with strains expressing the K1 serotype (p <0.01) and have poor glycemic control (p <0.01). On the contrary, community-acquired bacteremic diabetic patients with good glycemic control (HbA1c ≤7%) were less likely to have invasive syndrome (p = 0.02). As demonstrated in , KP-mediated invasive syndrome was significantly more likely to occur in the patients infected with strains expressing the K1 serotype than those infected with non-K1 serotypes (AOR, 8.32; 95% CI, 1.56−44.24; p = 0.01); and in those with poor glycemic control (Hb1Ac ≥9%) than those with suboptimal or good, glycemic control (AOR, 5.66; 95% CI, 2.01−15.92; p <0.01) in multivariate analysis.
Table 1. Comparisons of characteristics between diabetic patients with community-acquired K. pneumoniae bacteremia who developed invasive syndrome and those who did not develop invasive syndrome.
Effect of exogenous gradient glucose on K. pneumoniae CPS biosynthesis
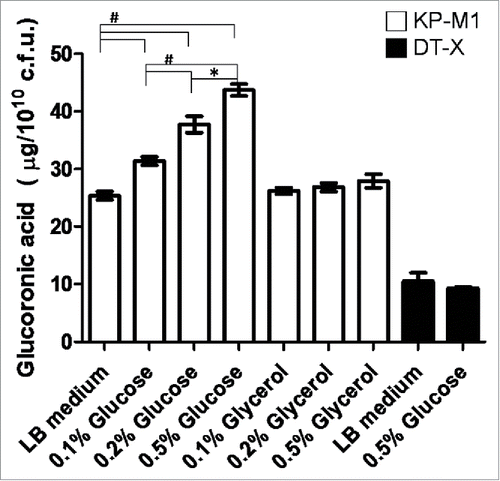
To analyze if exogenous glucose or glycerol affects KP CPS biosynthesis, KP-M1 and DT-X bacteria were grown in Luria-Bertani (LB) broth supplemented with increasing amount of glucose or glycerol (0.1%, 0.2% and 0.5%) for quantification of CPS biosynthesis. As shown in , the addition of exogenous glucose but not glycerol concentration increased CPS biosynthesis of KP-M1 in a dose-dependent manner. The addition of exogenous glucose concentration did not influence CPS biosynthesis of DT-X.
Figure 1. Exogenous glucose affects the capsular polysaccharide (CPS) biosynthesis of KP-M1 or DT-X. Bacterial strains were grown in Luria-Bertani (LB) broth supplemented glucose or glycerol as indicated at 37°C agitation. After 6 h of growth, the glucoronic acid content was determined. The addition of exogenous glucose enhanced CPS biosynthesis of KP-M1 in dose-dependent manner. *, P < 0.05; #, P < 0.01 compared to the indicated group.
Effect of exogenous glucose on K. pneumoniae cps transcription
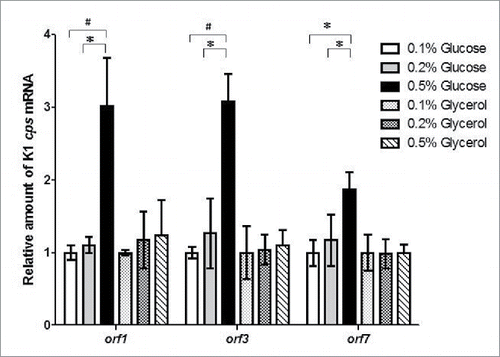
KP-M1 strains were cultured in LB broth supplemented with 0.1%, 0.2% and 0.5% glucose or glycerol and the mRNA levels of orf1, orf3, and orf7 were measured by qRT-PCR. No statistical difference in transcription of orf1, orf3, and orf7 was observed in these growth conditions of different glycerol concentration. However, growth in 0.5% glucose increased transcription of orf1, orf3, and orf7 in comparison with growth in 0.1%, or 0.2% glucose ().
Figure 2. Exogenous glucose up-regulated cps transcription. Quantitative reverse-transcription polymerase-chain-reaction (qRT-PCR) assays were performed to investigate the expression of K1 cps gene (orf1, orf3, and orf7) in KP-M1 in Luria-Bertani (LB) broth supplemented with glucose or glycerol as indicated at 37°C 6 h agitation. *, P < 0.05; #, P < 0.01 compared to the indicated group.
Effects of exogenous glucose on neutrophil phagocytosis and leukocyte bactericidal activity against K. pneumoniae
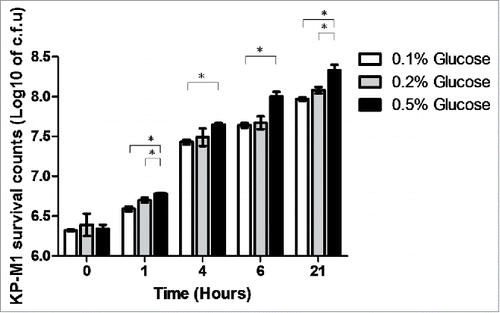
Growth in LB broth with 0.5% glucose significantly inhibited the percentage of phagocytosis of KP-M1 in comparison with growth in LB broth with 0.1% or 0.2% glucose (; p <0.01). The rate of KP-M1 survive was also significant increased for bacteria growth in LB broth with 0.5% glucose in comparison with which growth in LB broth with 0.1% or 0.2% glucose at checking time (p <0.05; ). The results indicated that KP-M1 in high sugar condition was able to diminish significantly the neutrophils phagocytosis and whole blood leukocyte killing against this culprit.
Figure 3. (A) Exogenous glucose affects the neutrophil phagocytosis of KP-M1. Neutrophils from 5 healthy individuals were tested against the bacteria that had been previously opsonized with pool human serum separately. The percentage of ingested bacteria was counted at 60 min. (A) Trypan blue quenching of the extracellular fluorescence of bound bacteria and phagocytosed bacteria by neutrophils after incubation at 37°C for 60 min were evaluated by flow cytometry. (B) Comparisons of neutrophil phagocytosis of KP-M1 organisms pre-incubated in Luria-Bertani (LB) broth supplemented with different concentrations of glucose were made, which revealed that 0.5% glucose in LB broth significantly inhibited neutrophil phagocytosis of KP-M1 than 0.1% and 0.2% glucose in LB broth (P < 0.01).
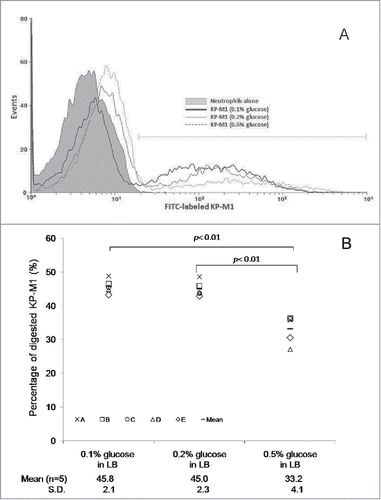
Figure 4. Human leukocyte bactericidal activity assay. Human peripheral white blood cells from 5 healthy males were collected and incubated with the KP-M1 that had been previously cultured in Luria-Bertani (LB) broth with different exogenous glucose concentrations then washed and opsonized with pool human serum. The rate of survival assay was significant increased for KP-M1 growth in LB broth with 0.5% glucose in comparison with which growth in LB broth with 0.1% or 0.2% glucose at checking time. *, P < 0.05.
Discussion
The CPS surrounding KP bacteria enables the bacteria to evade phagocytosis,Citation11 and increased production of CPS by exogenous graded glucose may therefore intensify the pathogenicity of KP strains. Indeed, LB broth is almost glucose-free. The presence of glucose in these experiments is supposed to be similar to the human bloodstream concentrations; the comparison is between a healthy subject, who would have 0.1% glucose blood concentration, and diabetic one who would have 0.2–0.5% glucose blood concentrations. Hence, our study showed that addition of exogenous glucose in LB broth enhanced CPS biosynthesis and mRNA of cps gene expression in high glucose (0.5%) concentration. The KP-M1 co-incubated in LB broth with a 0.5% glucose concentration inhibited neutrophil phagocytosis and leukocyte killing of this pathogen to a significantly greater extent than those in LB broth at 0.1% or 0.2% glucose concentration. Furthermore, our prospective cohort analysis of community-acquired bacteremia due to KP also indicated that poor glycemic control in diabetic patients was a risk for developing invasive syndrome.
The serotypes K1 and K2 of KP have been shown to be the major strains that cause KP -mediated invasive syndrome in East Asian countries, especially in Taiwan.Citation2 A study that collected KP colonizing the intestinal tract of healthy Chinese and overseas Chinese adults in Asian countries showed that serotypes K1/K2 accounted for the majority of KP isolates from stool samples.Citation12 In addition to infection with serotypes K1 KP, our findings also suggest that diabetic patients with uncontrolled glycemia are at increased risks for KP-mediated invasive syndrome, which is similar to other reports.Citation2, 4 DM is a documented risk factor for Gram-negative bacteremia, including the episodes derived from abdominal foci of infection,Citation13 to which impaired neutrophil function associated with DM has been shown to be contributory.Citation14 However, comparisons of neutrophils isolated from normal healthy subjects and those from diabetic patients with good glycemic control did not show a significant reduction of phagocytosis of serotypes K1/K2 KP.Citation6 In contrast, our study showed that neutrophils collected from healthy male volunteers demonstrated markedly reduced phagocytosis and killing of virulent K1 KP pre-incubated in LB broth with a 0.5% glucose concentration, which suggests that an environmental factor such as high glucose levels might be one important factor affecting the phagocytosis and bactericidal activity. Epidemiological studies have demonstrated that the majority of KP liver abscess are preceded by the colonization of the gastrointestinal tract.Citation15 The poor glycemic control in DM patients causes tissue hyperglycemia, which may enhance the CPS production of KP strains that colonize the gastrointestinal tract followed by resistance to neutrophil phagocytosis and leukocyte killing. Our clinical findings also support the notion that poor glycemic control plays a role in diabetic patients with community-acquired bacteremia due to KP to develop invasive syndrome.
The biosynthesis of CPS in KP is regulated by multiple environmental stimuli and protein regulators. The complex network of multiple regulators such as Rcs system, RmpA/A2, KvgA, KvhA, and Fur has been even reported.Citation16-19 The mechanism of increasing KP CPS biosynthesis in the presence of increased exogenous glucose concentrations was proposed to be related to the regulation of cAMP-dependent carbon catabolite repression (CCR).Citation20 The cAMP-CCR signaling has been shown to regulate the expression of various genes encoding carbon metabolism enzymes and virulence factors, such as flagella, fimbriae, protease, exotoxin, and secretion systems.Citation21-25 These studies indicated that, in response to specific environmental signals, KP bacteria may express genes encoding virulence factors that help to establish a successful infection. To investigate how glucose level affects KP CPS biosynthesis, we performed qRT-PCR analysis for the transcriptional level of orf1, orf3, orf7 and found that increase of CPS biosynthesis was regulated by glucose concentration as stimulus at 0.5% glucose (). Therefore, a high exogenous glucose concentration stimulates KP CPS biosynthesis to protect the bacteria from phagocytosis/leukocyte killing, and the poor glycemic control reflected by high glucose levels in the bloodstream might have a major impact on the bacterial virulence.
This study has several limitations. The patients included in the cohort study were those who were hospitalized in the tertiary care center, and, therefore, our findings may not be generalizable to the patients who sought care in primary care facilities or those who were treated as outpatients. Only one strain (KP-M1; serotype K1) was assessed in this study. However, epidemiological reports have indicated that capsular serotype K1 is the most prevalent in KP-mediated invasive syndrome.Citation2 As non-K1 strains did not increase CPS biosynthesis significantly in terms of different exogenous glucose stimulus (data not shown), our results derived from the representative serotype K1 strain may be generalized to the majority of KP strains that cause invasive syndrome. Last, the degree of hypermucoviscosity has been shown to positively correlate with KP-mediated invasive syndromeCitation3 and KP-M1 strain also cause hypermucoviscosity. However, the degree of hypermucoviscosity in KP-M1 strains affected by exogenous glucose stimuli cannot be measured quantitatively by string test.Citation26 Despite these limitations, our results provide evidence to support the role of glucose level as an environmental factor in the pathogenesis of KP-mediated invasive syndrome.
In summary, our study demonstrates that type 2 DM with poor glycemic control is an independent factor for developing KP-mediated invasive syndrome in diabetic patients with KP community-acquired bacteremia. Exogenous high glucose concentrations that reflect the poor glycemic control in diabetic patients may stimulate the increase of CPS biosynthesis and mRNA of cps gene expression of virulent KP, which become resistant to neutrophil phagocytosis/leukocyte killing and enable the evasion of host immune system.
Materials and methods
Study population
All the protocols used in the present study had been approved by the Institutional Review Board at Kaohsiung Chang Gung Memorial Hospital (KCGMH approval 103-7336C). Informed consent was waived for the observational study to evaluate the relationship between glycemic control and KP-mediated invasive syndrome among diabetic patients with community-acquired KP bacteremia. In addition, 5 healthy male volunteers provided their written informed consent to participate in the study.
Study design
In this prospective cohort study, diabetic patients who were admitted to the KCGMH between January 1, 2008, and December 31, 2010, with community-acquired mono-microbial bacteremia caused by KP were enrolled for the investigation of risk factors for KP-mediated invasive syndrome. Only the KP isolates from the participants' first blood samples were used in the in-vitro study. Diabetic patients were defined as those with a history of type 2 DM and/or those receiving either insulin replacement therapy and/or oral hypoglycemic agents. Cases of newly diagnosed DM were recorded. To assess the glycemic control,Citation27 the glycated hemoglobin (HbA1c) was determined when patients were admitted to the hospital. The impact of glycemic control on various outcomes of interest was evaluated by categorizing all the patients into three categories on the basis of HbA1c levels: HbA1c ≤7% (good glycemic control), HbA1c 7–9% (suboptimal glycemic control) and HbA1c ≥9% (poor glycemic control).Citation28 High-dose steroid use was defined as ≥20 mg prednisolone daily for more than 3 weeks.
The diagnosis of KP-mediated invasive syndrome was made when the criteria for sepsis were metCitation29 plus the presence of at least one of the following complications: pyogenic liver abscess, meningitis, empyema, mycotic aneurysm, necrotizing fasciitis or endophthalmitis.Citation30 To investigate the risk factors of KP-mediated invasive syndrome in diabetic patients, the following clinical variables were assessed: age, sex, and comorbidities (including liver cirrhosis, malignancy, chronic renal failure, and biliary tract disease). Subsequently, each diabetic patient with community-acquired KP bacteremia who developed invasive syndrome (invasive syndrome group) was age-matched to 1 diabetic patient with community-acquired KP bacteremia who did not develop invasive syndrome (non-invasive syndrome group). Peripheral blood samples were collected from 5 healthy male volunteers aged between 25 and 40 years for experiments of neutrophil phagocytosis and leukocyte bactericidal activity assay as described below.
Bacterial isolates and serotype determination
All KP isolates were identified using standard methods. Capsular genotyping of seven clinically significant capsular types (K1, K2, K5, K20, K54, K57, and KN1) was performed with the use of polymerase-chain-reaction (PCR) assay. Briefly, PCR was performed using primers designed for the cps variable region. Genomic DNA was extracted by boiling selected colonies in distilled water for 10 minutes. The PCR conditions were 96°C for 3 min, followed by 30 temperature cycles of 96°C for 30s, 53°C for 15s, and 72°C for 30s.Citation31 A KP-M1 (serotype K1) strain that was isolated from a patient with invasive syndrome presenting as pyogenic liver abscess and endophthalmitis and a acapsular K. pneumoniae mutant of DT-X was isolated by subculture of strain DT-S (biotype edwardsii, capsular serotype K1)Citation32 were used as the representing strain in the following experiments. The lack of capsular in DT-X was confirmed by staining with Indian ink. Bacteria were routinely cultured at 37°C in LB medium.
Quantification of CPS biosynthesis
The CPS concentration was determined with the use of a modified carbazole assay for glucoronic acid.Citation33 Total CPS was measured after quantitative extraction of whole bacterial cultures with zwitterionic detergent in citrate buffer.Citation34 A 0.5-mL sample was mixed with 3 mL of 0.025 M sodium tetraborate (VWR, Radnor, PA) in sulfuric acid, and heated at 100°C for 10 min. After cooling, 0.1 mL of 0.125% carbazole (Sigma-Aldrich, St. Louis, MO) in absolute ethanol was added, and the samples were heated for another 15 min. The absorbance of the sample at 530 nm wave length was measured, and the concentration of glucoronic acid was extrapolated from a standard curve that was constructed using glucuronolactone standards (Sigma-Aldrich, St. Louis, MO). To quantify CPS after exogenous glucose treatment, KP bacteria were cultured in LB broth containing 0.1%, 0.2%, 0.5% glucose or glycerol and incubated 6 h at 37°C. The glycerol was a negative control for the glucose supplementation. All samples were assayed at least 3 times. The quantity of CPS detected was expressed as micrograms of glucoronic acid per 1010 colony-forming units (μg glucoronic acid/1010 cfu).
Quantitative Reverse-Transcription Polymerase-Chain-Reaction (qRT-PCR) assay
The biosynthesis of KP K1 CPS is controlled by 20 genes and the gene clusters responsible for biosynthesis of KP K1 CPS contain 3 transcriptional units: orf1, orf3, and orf7.Citation35 Total RNAs were isolated from early exponential-phase grown bacterial cells with the use of the RNeasy midi-column (QIAGEN) according to the manufacturer's instructions. RNA was DNase-treated with RNase-free DNase I (QIAGEN) to eliminate DNA contamination. RNA of 100 ng was reverse-transcribed with the Transcriptor First Strand cDNA Synthesis Kit (Epicentre) using random primers. qRT-PCR was performed in a Applied Biosystems 7500 Instrument using TaqMan Universal Master Mix II (Applied Biosystems). Primers (forward: 5′-CGTCATCCAGACCAAAGAGC-3′ for orf1, 5′-TACCGGGACAGAGAATGAGC-3′ for orf3, 5′-CGTTTTATGGTAATGTTCTCCTCA’ for orf7) and probes were designed for orf1, orf3, and orf7 in K1 cps gene cluster using Custom TaqMan Gene Expression Assays (Applied Biosystems). Data were analyzed using the real-time PCR software of AB 7500 Instrument. To quantify the relative gene expressions after exogenous glucose treatment, KP-M1 bacteria were cultured in LB broth containing 0.1%, 0.2%, 0.5% glucose or glycerol and incubated 6 h at 37°C. All samples were assayed using the comparative threshold cycle 2−ΔΔCT method with 23 S rRNA as the endogenous reference at least 3 times.
Flow cytometric analysis of neutrophil phagocytosis
A standard density gradient separation method to isolate human neutrophils from these 5 healthy subjects' whole blood using commercially available separation media that is a mixture of sodium metrizoate and Dextran 500. This method has been shown to yield samples of >95% neutrophils with >95% viability. Pooled serum was obtained from these five volunteers and stored in aliquots at −20°C until required. The KP-M1 bacteria were cultured 6 h at 37°C on LB medium containing 0.1%, 0.2%, or 0.5% glucose. Capsule of KP was disrupted by heat but not by ultraviolet light treatment.Citation36 The bacterial colonies were irradiated with UV light for bacterial inactivation, followed by sub-culture in plates to confirm sterility after overnight incubation. The purpose of inactive bacteria colonies is keeping consistent MOI (10:1) for the phagocytosis assay. The irradiated cultures were resuspended in carbonate buffer containing 0.1% fluorescein isothiocyanate (FITC). The FITC-stained KP (FITC-KP) cells were counted using a bacterial cytometer and a fluorescence microscope. The FITC-KP cells were analyzed using a FACS Calibur flow cytometer (BD Biosciences, San Jose, CA) to verify that bacterial staining was uniform throughout each sample. Phagocytosis was measured using a standard assay.Citation37 Briefly, 10 μL of FITC-KP (representing 5 ×108cfu/mL) was added to each 990 μL volume containing a mixture of 100 μL of a neutrophil suspension (representing 5 × 106 cells/mL), 100 μl of pooled normal human serum (10% v/v for opsonization), and 790 μL of PBS. The final volume was 1.0 mL and the multiplicity of infection was 10:1. Each tube was incubated in a shaking water bath at 37°C and transferred to an ice bath at a designated time point. A 600μl of suspension was transferred into a new tube, and trypan blue was added to a final concentration of 50 mg/L before measurement. The trypan blue does not penetrate into the cells, ingested bacteria retain their green fluorescence, while membrane-bound particles display a red fluorescence to differentiate adherent bacteria of internalized bacteria.Citation38 A FACScan emitting an argon laser beam at 488 nm was used to detect FITC fluorescence. A total of 20,000 cells were processed using Cellquest version 1.0 software (Becton, Dickinson and Company). Green fluorescence (FL1-H) intensity data (collected using a logarithmic amplifier) were displayed as single histograms. With the technique, FITC-unstained (adherent) and FITC-stained (intracellular) bacteria can be quantitated. The percentage of ingested bacteria was counted at 60 min.
Human leukocyte bactericidal activity assay
Bactericidal activity was measured using a standard assay method.Citation39 The KP-M1 organisms were cultured in LB broth containing 0.1%, 0.2%, or 0.5% glucose, respectively, and incubated 6 h at 37°C. The KP-M1 organisms were washed then opsonized by the addition of 10% pooled human serum. The suspension was mixed at a multiplicity of infection of 10:1 to whole blood leukocytes. Samples were collected immediately and at 1h, 4h, 6h, and 21 h separately then diluted in H2O (pH 11.0, adjusted by NaOH) to lyse the leukocytes and disperse the bacteria for the power-plate colony assay.Citation40 All tests were performed in triplicate to ensure reproducibility.
Statistical analysis
The categorical variables were compared using the chi-square test or the Fisher exact test, as appropriate. A multivariate logistic regression model was used to evaluate risk factors for invasive syndrome by calculating the adjusted odds ratio (AOR) and 95% confidence interval (95% CI) of each clinical variable. All experimental data were expressed as the mean ± standard deviation. Quantitative data was analyzed by ANOVA followed by Tukey's posttest. All statistical analyses were two-sided, and values of p less than 0.05 were considered significant. Goodness-of-fit was assessed by the Hosmer and Lemeshow statistic. A receiver-operating-characteristic (ROC) curve analysis was used to evaluate the predictive performance of the logistic regression model.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We are grateful to Leung Kei Siu, PhD, National Health Research Institutes, for kindly providing the K. pneumoniae strain DT-X. Dr. Chien-Ching Hung at the Department of Internal Medicine, National Taiwan University Hospital is acknowledged for his critical review of this manuscript.
Funding
This work was funded by grants from the Ministry of Science and Technology of Taiwan (MOST 101-2314-B-182A-061-MY3 and MOST 104-2314-B-182A-051-MY2) and Chang Gung Memorial Hospital, Taiwan (CMRPG8D1211).
References
- Wang JH, Liu YC, Lee SS, Yen MY, Chen YS, Wann SR, Lin HH. Primary liver abscess due to Klebsiella pneumoniae in Taiwan. Clin Infect Dis 1998; 26:1434-8; PMID:9636876; http://dx.doi.org/10.1086/516369
- Ko WC, Paterson DL, Sagnimeni AJ, Hansen DS, Von Gottberg A, Mohapatra S, Casellas JM, Goossens H, Mulazimoglu L, Trenholme G, et al. Community-acquired Klebsiella pneumoniae bacteremia: global differences in clinical patterns. Emerg Infect Dis 2002; 8:160-6; PMID:11897067; http://dx.doi.org/10.3201/eid0802.010025
- Lee CH, Liu JW, Su LH, Chien CC, Li CC, Yang KD. Hypermucoviscosity associated with Klebsiella pneumoniae-mediated invasive syndrome: a prospective cross-sectional study in Taiwan. Int J Infect Dis 2010; 14:e688-92; PMID:20547084; http://dx.doi.org/10.1016/j.ijid.2010.01.007
- Fang CT, Lai SY, Yi WC, Hsueh PR, Liu KL, Chang SC. Klebsiella pneumoniae genotype K1: an emerging pathogen that causes septic ocular or central nervous system complications from pyogenic liver abscess. Clin Infect Dis 2007; 45:284-93; PMID:17599305; http://dx.doi.org/10.1086/519262
- Cortés G, Borrell N, de Astorza B, Gómez C, Sauleda J, Alberti S. Molecular analysis of the contribution of the capsular polysaccharide and the lipopolysaccharide O side chain to the virulence of Klebsiella pneumoniae in a murine model of pneumonia. Infect Immun 2002; 70:2583-90; http://dx.doi.org/10.1128/IAI.70.5.2583-2590.2002
- Lin JC, Siu LK, Fung CP, Tsou HH, Wang JJ, Chen CT, Wang SC, Chang FY. Impaired phagocytosis of capsular serotype K1 or K2 Klebsiella pneumoniae in type 2 diabetic mellitus patients with poor glycemic control. J Clin Endocrinol Metab 2006; 91:3084-7; PMID:16720670; http://dx.doi.org/10.1210/jc.2005-2749
- Chen SC, Lee YT, Lai KC, Cheng KS, Jeng LB, Wu WY, Chen CC, Lee MC. Risk factors for developing metastatic infection from pyogenic liver abscesses. Swiss Med Wkly 2006; 136:119-26; PMID:16633956
- Lin YC, Lu MC, Tang HL, Liu HC, Chen CH, Liu KS, Lin C, Chiou CS, Chiang MK, Chen CM, et al. Assessment of hypermucoviscosity as a virulence factor for experimental Klebsiella pneumoniae infections: comparative virulence analysis with hypermucoviscosity-negative strain. BMC Microbiol 2011; 11:50; PMID:21385400; http://dx.doi.org/10.1186/1471-2180-11-50
- Lin JC, Chang FY, Fung CP, Yeh KM, Chen CT, Tasi YK, Siu LK. Do neutrophils play a role in establishing liver abscesses and distant metastases caused by Klebsiella pneumoniae. PloS One 2010; 5:e15005; PMID:21151499; http://dx.doi.org/10.1371/journal.pone.0015005
- Gallcher S, Thomson G, Fraser WD, Fisher BM, Gemmell CG, MacCuish AC. Neutrophils bactericidal function in diabetes mellitus: evidence for association with blood glucose control. Diabet Med 1995; 12:916-20; PMID:8846684; http://dx.doi.org/10.1111/j.1464-5491.1995.tb00396.x
- Lee CH, Su LH, Liu JW, Chang CC, Chen RF, Yang KD. Aspirin enhances opsonophagocytosis and is associated to a lower risk for Klebsiella pneumoniae invasive syndrome. BMC Infect Dis 2014; 14:47; PMID:24476545; http://dx.doi.org/10.1186/1471-2334-14-47
- Lin YT, Siu LK, Lin JC, Chen TL, Tseng CP, Yeh KM, Chang FY, Fung CP. Seroepidemiology of Klebsiella pneumoniae colonizing the intestinal tract of healthy Chinese and overseas Chinese adults in Asian countries. BMC Microbiol 2012; 12:13; PMID:22260182; http://dx.doi.org/10.1186/1471-2180-12-13
- Thomsen RW, Hundborg HH, Lervang HH, Johnsen SP, Schønheyder HC, Sørensen HT. Diabetes mellitus as a risk and prognostic factor for community-acquired bacteremia due to enterobacteria: a 10-year, population-based study among adults. Clin Infect Dis 2005; 40:628-31; PMID:15712091; http://dx.doi.org/10.1086/427699
- Delamire M, Maugendre D, Moreno M, Le Goff MC, Allannic H, Genetet B. Impaired leucocyte function in diabetic patients. Diabet Med 1997; 14:29-34; PMID:9017350; http://dx.doi.org/10.1002/(SICI)1096-9136(199701)14:1%3c29::AID-DIA300%3e3.0.CO;2-V
- Fung CP, Lin YT, Lin JC, Chen TL, Yeh KM, Chang FY, Chuang HC, Wu HS, Tseng CP, Siu LK. Klebsiella pneumoniae in gastrointestinal tract and pyogenic liver abscess. Emerg Infect Dis 2012; 18:1322-5; PMID:22840473; http://dx.doi.org/10.3201/eid1808.111053
- Lin CT, Huang TY, Liang WC, Peng HL. Homologous response regulators KvgA, KvhA and KvhR regulate the synthesis of capsular polysaccharide in Klebsiella pneumoniae CG43 in a coordinated manner. J Bacteriol 2006; 140:429-38.
- Majdalani N, Gottesman S. The Rcs phosphorelay: a complex signal transduction system. Annu Rev Microbiol 2005; 59:379-405; PMID:16153174; http://dx.doi.org/10.1146/annurev.micro.59.050405.101230
- Gottesman S, Stout V. Regulation of capsular polysaccharide synthesis in Escherichia coli K12. Mol Microbiol 1991; 5:1599-606; PMID:1943696; http://dx.doi.org/10.1111/j.1365-2958.1991.tb01906.x
- Stout V. Regulation of capsule synthesis includes interactions of the RcsC/RcsB regulatory pair. Res Microbiol 1994; 145:389-92; PMID:7855424; http://dx.doi.org/10.1016/0923-2508(94)90086-8
- Lin CT, Chen YC, Jinn TR, Wu CC, Hong YM, Wu WH. Role of the cAMP-dependent carbon catabolite repression in capsular polysaccharide biosynthesis in Klebsiella pneumoniae. PLoS One 2013; 8:e54430; PMID:23408939; http://dx.doi.org/10.1371/journal.pone.0054430
- Mendez M, Huang IH, Ohtani K, Grau R, Shimizu T, Sarker MR. Carbon catabolite repression of type IV pilus-dependent gliding motility in the anaerobic pathogen Clostridium perfringens. J Bacteriol 2008; 190:48-60; PMID:17981974; http://dx.doi.org/10.1128/JB.01407-07
- Muller CM, Aberg A, Straseviciene J, Emody L, Uhlin BE, Balsalobre C. Type 1 fimbriae, a colonization factor of uropathogenic Escherichia coli, are controlled by the metabolic sensor CRP-cAMP. PLoS Pathog 2009; 5:1000303; http://dx.doi.org/10.1371/journal.ppat.1000303
- Stella NA, Kalivoda EJ, O'Dee DM, Nau GJ, Shanks RM. Catabolite repression control of flagellum production by Serratia marcescens. Res Microbiol 2008; 159:562-8; PMID:18718529; http://dx.doi.org/10.1016/j.resmic.2008.07.003
- Kalivoda EJ, Stella NA, O'Dee DM, Nau GJ, Shanks RM. The cyclic AMP-dependent catabolite repression system of Serratia marcescens mediates biofilm formation through regulation of type 1 fimbriae. Appl Environ Microbiol 2008; 74:3461-70; PMID:18424546; http://dx.doi.org/10.1128/AEM.02733-07
- Fuchs EL, Brutinel ED, Klem ER, Fehr AR, Yahr TL, Wolfgang MC. In vitro and in vivo characterization of the Pseudomonas aeruginosa cyclic AMP (cAMP) phosphodiesterase CpdA, required for cAMP homeostasis and virulence factor regulation. J Bacteriol 2010; 192:2779-90; PMID:20348254; http://dx.doi.org/10.1128/JB.00168-10
- Lee HC, Chuang YC, Yu WL, Lee NY, Chang CM, Ko NY, Wang LR, Ko WC. Clinical implications of hypermucoviscosity phenotype in Klebsiella pneumoniae isolates: association with invasive syndrome in patients with community-acquired bacteremia. J Intern Med 2006; 259:606-14; PMID:16704562; http://dx.doi.org/10.1111/j.1365-2796.2006.01641.x
- American Diabetes Association. Standards of medical care in diabetes-2009. Diabetes Care 2009; 32:S13-61; PMID:19118286; http://dx.doi.org/10.2337/dc09-S013
- Selvin E, Wattanakit K, Steffens MW, Coresh J, Sharrett AR. HbA1c and peripheral arterial disease in diabetes: the atherosclerosis risk in communities study. Diabetes Care 2006; 29:877-82; PMID:16567831; http://dx.doi.org/10.2337/diacare.29.04.06.dc05-2018
- American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med 1992; 20:864-74; PMID:1597042; http://dx.doi.org/10.1097/00003246-199206000-00025
- Yu VL, Hansen DS, Ko WC, Sagnimeni A, Klugman KP, von Gottberg A, Goossens H, Wagener MM, Benedi VJ, International Klebseilla Study Group. Virulence characteristics of Klebsiella and clinical manifestations of K. pneumoniae bloodstream infections. Emerg Infect Dis 2007; 13:986-93; PMID:18214169; http://dx.doi.org/10.3201/eid1307.070187
- Tenover FC, Arbeit RD, Goering RV, Mickelsen PA, Murray BE, Persing DH, Swaminathan B. Capsular polysaccharide synthesis regions in Klebsiella pneumoniae serotype K57 and a new capsular serotype. J Clin Microbiol 2008; 46:2231-40; PMID:18508935; http://dx.doi.org/10.1128/JCM.00480-08
- Yoshida K, Matsumoto T, Tateda K, Uchida K, Tsujimoto S, Yamaguchi K. Induction of interleukin-10 and down-regulation of cytokine production by Klebsiella pneumoniae capsule in mice with pulmonary infection. J Med Microbiol 2001; 50:456-61; PMID:11339254; http://dx.doi.org/10.1099/0022-1317-50-5-456
- Bitter T, Muir HM. A modified uronic acid carbazole reaction. Anal Biochem 1962; 4:330-4; PMID:13971270; http://dx.doi.org/10.1016/0003-2697(62)90095-7
- Domenico P, Diedrich DL, Cunha BA. Quantitative extraction and purification of exopolysaccharides from Klebsiella pneumoniae. J Microbiol Methods 1989; 9:211-9; http://dx.doi.org/10.1016/0167-7012(89)90038-9
- Wu KM, Li LH, Yan JJ, Tsao N, Liao TL, Tsai HC, Fung CP, Chen HJ, Liu YM, Wang JT, et al. Genome sequencing and comparative analysis of Klebsiella pneumoniae NTUH-K2044, a strain causing liver abscess and meningitis. J Bacteriol 2009; 191:4492-501; PMID:19447910; http://dx.doi.org/10.1128/JB.00315-09
- Lee CH, Chang CC, Liu JW, Chen RF, Yang KD. Sialic acid involved in hypermucoviscosity phenotype of Klebsiella pneumoniae and associated with resistance to neutrophil phagocytosis. Virulence 2014; 5:673-9; PMID:25098744; http://dx.doi.org/10.4161/viru.32076
- Heinzelmann M, Gardner SA, Mercer-Jones M, Roll AJ, Polk HC, Jr. Quantification of phagocytosis in human neutrophils by flow cytometry. Microbiol Immunol 1999; 43:505-12; PMID:10480545; http://dx.doi.org/10.1111/j.1348-0421.1999.tb02435.x
- Busetto S, Trevisan E, Patriarca P, Menegazzi R. A single-step, sensitive flow cytofluorometric assay for the simultaneous assessment of membrane-bound and ingested Candida albicans in phagocytosing neutrophils. Cytometry A. 2004; 58:201-6; PMID:15057974; http://dx.doi.org/10.1002/cyto.a.20014
- Hampton MB, Vissers MC, Winterbourn CC. A single assay for measuring the rates of phagocytosis and bacterial killing by neutrophils. J Leukoc Biol 1994; 55:147-52; PMID:8301210
- Green JN, Winterbourn CC, Hampton MB. Analysis of neutrophil bactericidal activity. Methods Mol Biol 2007; 412:319-32; PMID:18453121; http://dx.doi.org/10.1007/978-1-59745-467-4_21
