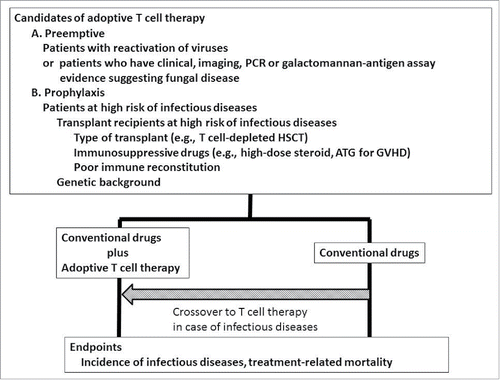
ABSTRACT
The outcome after allogeneic haematopoietic stem cell transplantation (allo-HSCT) has significantly improved during the last decades. However, opportunistic infections such as viral and mold infections are still a major obstacle for cure. Within this field, adoptive T cell therapy against pathogens is a promising treatment approach. Recently, the techniques to develop T cell products including pathogen-specific T cells have been sophisticated and are now available in accordance to good manufacturing practice (GMP). Here, we aim to summarize current knowledge about adoptive T cell therapy against viral and mold infections.
Introduction
Allogeneic haematopoietic stem cell transplantation (allo-HSCT) is one of the standard treatment modalities for patients with hematological diseases. The clinical outcomes after allo-HSCT have been significantly improved, and the number of allo-HSCT recipients is increasing all over the world because the development of lower intensity conditioning regimen enables allo-HSCT for older patients or patients with pre-existing comorbidities.Citation1 However, treatment-related morbidity and mortality related to infectious diseases such as viral infections, invasive fungal infections, acute/chronic graft-versus-host disease (GVHD) and organ failure are still major obstacles.Citation2-4 In the era of allo-HSCT from an alternative donor including unrelated donor, haploidentical related donor and cord blood transplant, management of infectious diseases is still a highly important topic.Citation5,6
Regarding viral and invasive fungal infections, various drugs have been developed.Citation7-9 However, the clinical outcome is still unsatisfactory once overt infections occur after allo-HSCT.Citation8,10,11 In addition, drugs against viral and fungal infections are rather expensive and often associated with significant toxicity. Although primary prophylaxis using drugs might be efficient while drugs are used, it cannot efficiently reduce the risk of late-onset diseases.Citation12,13 Furthermore, resistance against anti-viral or anti-fungal drugs is an emerging problem and could be associated with a high probability of treatment failure.Citation14-20 Therefore, it is attractive if we could be able to induce specific anti-pathogen specific immunity to control the infections after allo-HSCT. In terms of viral infections, it is well established that T cells play an important role.Citation21-30 In terms of mold infections, innate immunity alone was considered important historically in the control of mold infections. However, recent studies found that adaptive immunity, in particular Th1 cells, was relevant in the control of aspergillus infections.Citation31 Thus, adoptive immunotherapy of Th1 cells has a potential to play a major role to prevent/treat them, although clinical development of adoptive immunotherapy against aspergillus infections is still underway in contrast to the remarkable progress in adoptive immunotherapy against viral infections. Here, we aim to summarize current knowledge about adoptive immunotherapy against viral and invasive aspergillus infections.
Immunotherapy against viral infections
Importance of T cells against viral infections
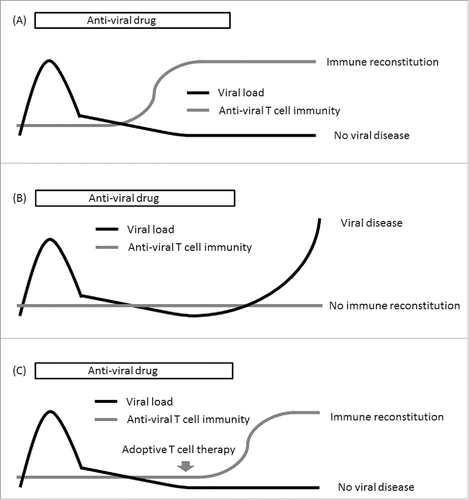
The reconstitution of functional virus-specific T cells and the importance of these T cells in the control of viral diseases following allo-HSCT has been extensively investigated.Citation32 This led to a successful transfer into the clinical setting within adoptive immunotherapeutic approaches.Citation33 In terms of T cell immunity against viruses, most studies were related to cytomegalovirus (CMV), EB virus (EBV) and adenovirus (ADV). In these viruses, CMV is the most intensively studied pathogen.Citation34-36 Lack of T cell immunity against CMV is associated with an increased risk of late onset/recurrent CMV antigenemia and CMV disease.Citation21,23-29 In EBV and ADV infections, importance of T cell immunity against was also reported.Citation37-39 Therefore, if T cell immunity is found to be insufficient in patients with viral reactivation, adoptive immunotherapy to enhance T cell reconstitution could be an option to consider in order to treat or prevent the development of end-organ disease ().
Figure 1. Possible strategy to reduce the risk of recurrent/late-onset viral disease using adoptive T cell therapy. (A) Patients with sufficient immune reconstitution are at low risk of recurrent/late-onset viral disease. (B) Patients with insufficient immune reconstitution are at high risk of recurrent/late-onset viral disease. (C) In patients with insufficient immune reconstitution, adoptive T cell therapy might reduce the risk of recurrent/late-onset viral disease.
Adoptive immunotherapy against viral infections
Adoptive T cell immunotherapy against viral infections has been already extensively studied, and now several methods which are fully adapted to good manufacturing practice (GMP) conditions are available.
First, multimers such as tetramer and pentamer can be used to detect and isolate virus-specific T cells. However, they bind to T cells irreversibly, which makes it difficult to dissociate from T cells. On the other hand, streptamer can be easily dissociated from the isolated T cells.Citation40,41 Streptamer was reported to detect as accurate as other multimers.Citation42,43 The purity of CMV-specific T cells using streptamer was reported to be high. Odendahl et al. reported that the median purity of CMV-specific T cells was 90.2% in 22 processes.Citation44 High purity is the strength of this technique considering the risk of GVHD in adoptive T cell therapy. Schmitt et al. reported 2 cases who received streptamer-isolated CMV-specific T cells.Citation45 It was striking that patient 1 received only 2.2 × 105 cells/kg and the frequency of CMV-specificT cells increased from 0% to a maximum of 27.1% of all T cells. Patient 2 received much less dose of cells (0.37 × 105/kg CMV-specific cells) and experienced less expansion of CMV-specific T cells, but successfully achieved the persistent clearance of CMV antigenemia. Another report also showed that streptamer-isolated CMV-specific T cells successfully controlled CMV antigenemia without the development of severe GVHD. The limitation of this technique is that it can be applied to only common HLA types with appropriate virus-derived peptide. Therefore, this technique cannot be useful in patients with rare HLA types. In terms of other virus-specific T cells, it is still under development.Citation46 We need more data about the virus-derived peptides with high avidity for each HLA type which is essential to produce streptamer with high efficacy.
Second, IFN-γ capture uses the system that enrichment of IFN-γ-secreting cells after the stimulation using virus-derived antigens was performed by using the Cytokine Secretion System and the CliniMACS device for immunomagnetic separation (both Miltenyi Biotec, Bergisch Gladbach, Germany). The strength of this method is that it is possible to isolate both CD4+ and CD8+ T cells, and a detailed information about HLA restriction of peptides is not necessary.Citation47,48 Peggs et al. reported their experiences using IFN-γ secretion assay to isolate CMV-specific T cells.Citation49 The isolated products contained a median of 2840 CMV-specific CD4+ T cells/kg and 630 CMV-specific CD8+ T cells/kg. A median purity of CMV-specific T cells was 43.9%. Expansions of both CD4+ and CD8+ CMV-specific T cells were observed in vivo within days of adoptive transfer. The expansion of CD8+ T cells was more robust than that of CD4+ T cells. Feuchtinger et al. also reported the efficacy of CMV-specific T cells which were isolated using IFN-γ secretion assay.Citation50 In this study, adoptive T cell therapy was used in patients with CMV disease or viremia refractory to antiviral chemotherapy or both. A mean dose of CMV-specific T cell was 2.1 × 104/kg. In 83% of cases, CMV infection was improved after adoptive T cell therapy. The purity of the product was around 10%, containing more CD4 T cells. As shown in these 2 reports, the limitation of this method is low purity compared with that using multimer, which might be associated with an increased risk of GVHD after adoptive T cell therapy. The efficacy and safety using this method has been already reported in CMV, ADV and EBV.Citation49-56 As reported using streptamer-isolated T cells, even low-dose of virus-specific T cells isolated by IFN-γ capture worked efficiently to eradicate viral infections in these reports. These findings suggest that virus-specific T cells have a high potential to expand in vivo. It can be because these T cell products contain T memory stem cell subset, although the proportion was not necessarily high.Citation57
Third, of short-term culture, GMP-compliant technique using G-Rex device reported promising results.Citation58,59 The technique used clinical grade peptide mixes for the stimulation and culture for around 10 d.Citation60 Although the purity of virus-specific T cells is expected to be low in this technique, multi-virus specific-T cells can be expanded using various combinations of peptide mix.
Fourth, there is also a possibility to use genetically introduced T cell receptor technique. We are able to prepare for variety of TCRs with high avidity against respective HLA molecules with virus-derived peptide.Citation61 Techniques to isolate each peptide-specific T cells might be useful to facilitate the identification and isolation of antigen-specific T cells.Citation62 T cells with genetically engineered TCR would contribute to break a major obstacle in a patient or donor with low frequency of specific T cells.
In summary, now fully GMP-compliant techniques to obtain virus-specific T cells are available. Further clinical studies to confirm the safety and efficacy of adoptive T cell therapy against viral infection are warranted.
Immunotherapy against aspergillus infections
Importance of immunity against aspergillus infections
Although it is well-established that innate immunity plays an important role to prevent and to treat aspergillus infections, recent research showed the importance of adaptive immune responses against aspergillus infections.Citation63 Dendritic cells (DCs) bridge innate and adaptive immunity with DCs having a primary role in surveillance for pathogens. Once DCs acquire Aspergillus antigen and mature, DCs present fungal peptides to CD4+ T cells. There is a paucity of data about presentation to CD8+ T cells. Adaptive immunity against Aspergillus is mainly mediated by Th1 response. Th1 cells augment the effector functions of innate immune cells via secretion of inflammatory cytokines such as IFN-γ and GM-CSF. The importance of immune response to Aspergillus is also supported by the strong association of genetic polymorphisms with the risk of invasive aspergillus infection.Citation64 For instance, patients with genetic variants of Dectin-1 or DC-SIGN were reported to have a significantly increased risk of invasive fungal infections.Citation65,66
Several studies using mouse models support the idea that Th1 cells play an important role in the control of Aspergillus. Censi et al. reported that treatment of immunocompetent mice with Aspergillus crude culture filtrate antigens resulted in the development of protective Th1 memory responses, mediated by antigen-specific CD4+ T cells producing IFN-γ and IL-2.Citation67 Protective Th1 responses were not observed in mice deficient of IFN-γ or IL-12, suggesting the importance of these cytokines. Ito et al. also reported that prior infection or Aspergillus fumigatus sonicate vaccine protected mice against subsequent lethal invasive pulmonary aspergillosis using a model of invasive pulmonary aspergillosis in corticosteroid-treated mice.Citation68 The group subsequently reported that sera from the vaccinated animals contained antibodies against Asp f3.Citation69 The vaccination of Asp f3 was effective to prevent the subsequent Aspergillus infection in corticosteroid-treated mice. CD4+ T cell depletion abrogated the protective effects of vaccination to levels comparable to non-vaccinated mice, which supports the idea that CD4+ T cell is critical for the effectiveness of vaccination to prevent Aspergillus infection.Citation70 Bozza et al. reported that vaccination with DCs pulsed with Asp f16 (Crf1) in combination with adjuvant CpG oligodeoxynucleotides for Toll-like receptor priming induced Th1 priming and resistance to Aspergillus infection.Citation71 Later on, the group reported that other proteins such as Pep1, Gel1, Crf1 induced protection against the subsequent Aspergillus infection in similar to the protection by conidia.Citation72 They found that these antigens can activate human Th1 cells.
In summary, Th1 cells in the orchestration of other immune cells play critical roles in the prevention of Aspergillus infection in mice models.
Presence of T-cell responses against Aspergillus in humans
Reports which assessed the importance of T cell responses against Aspergillus in human beings are still limited. One reason is that the frequencies of fungus-specific T cells were significantly lower than those of virus-specific T cells.Citation73 However, in patients with clinical evidence of invasive Aspergillus infections and the regression of invasive aspergillosis (IA), Aspergillus-specific T cells could be detected. Chaudhary et al. also found that Aspergillus antigen could induce T cell responses.Citation74 In their study, Asp f3, Asp f9/16 (Crf1), Asp f11 and Asp f22 elicited relatively high IFN-γ production.
Hebart et al. reported that water-soluble cellular extract of Aspergillus fumigatus induced a positive lymphoproliferative response using PBMCs from healthy individuals.Citation75 IFN-γ was released after the stimulation with Aspergillus fumigatus antigens, indicating a Th1 response. Ramadan et al. found that peptides derived from Crf1 could induce Th1 cell responses in healthy donors.Citation76 Jolink et al. analyzed the T-cell immune responses against Aspergillus fumigatus proteins Crf1 and Catalase 1 in healthy individuals.Citation77 They identified novel peptides derived from Crf1 and Catalase 1, although the frequency of specific T cells was low in Elispot or flow cytometric analysis. In their study, they used CD137 to isolate T cells which were demonstrated to be IFN-γ-producing T cells. Stuehler et al. also demonstrated that the Aspergillus fumigatus proteins Crf1, Gel1, and Pmp20 induced Th1 responses in healthy individuals, and interestingly generated T-cell lines against Aspergillus fumigatus cross-reacted to other Aspergillus species, Mucorales species and Candida albicans.Citation78
In patients with a hematological malignancy who developed invasive Aspergillus infection, there was a favorable response to anti-fungal therapy in patients with a higher IFN-γ/interleukin-10 ratio in culture supernatants which suggested the significance of Th1 cells.Citation75 In addition, steroid treatment suppressed the lymphoproliferation and IFN-γ secretion after the stimulation with aspergillus antigen. Stuehler et al. also found that a patient with well-controlled IA experienced expansion of Crf1-specific T cells analyzed HLA-DRB1*04 tetramer.Citation78 Jolink et al. also found that Crf1 and Catalase1-specific T cells were induced after Aspergillus infection improved, but no such specific T cells were detected in patients who died of Aspergillus infection.Citation79 Potenza et al. also demonstrated that the presence of Aspergillus-specific T cells in patients who suffered from IA and the increase of such protective T cells might be associated with a better clinical outcome.Citation80
In summary, aspergillus-specific T cells can be detected in healthy individuals, and in patients with a hematological malignancy, the presence of aspergillus-specific T cells seems to be associated with a favorable clinical outcome. These data supports the idea that adoptive immunotherapy using aspergillus-specific T cells could be a promising option in patients with IA as described below.
Adoptive immunotherapy against aspergillus infections
In comparison to adoptive T cell therapy against viral infections, there is still a paucity of clinical experiences of adoptive T cell therapy against mold infections. As described above, Th1 cells play an important role in the control of mold infections, although the data is still limited in non-Aspergillus mold infections. Therefore, it would be reasonable to try adoptive T cell therapy against mold infections. To introduce the adoptive immunotherapy against mold infections, it is important to develop technique of clinical-scale generation of human anti-Aspergillus T cells. Such technique should be non-labor intensive and quickly prepared. Beck et al. reported the generation of anti-Aspergillus Th1 cells using the combination of interferon-γ secretion assay and short term culture (14 days). PBMCs from healthy donors were isolated and coincubated with Aspergillus antigens for 12 hours and Aspergillus-specific T cells were enriched using IFN-γ secretion assay. Isolated cells were cultured for up to 14 d. Using this method, it was possible to obtain the enriched T cells with purity around 15% of Aspergillus-specific T cells. Tramsen et al. also reported that the similar technique incorporating IFN-γ secretion assay was able to generate Aspergillus-specific T cells according to GMP conditions, although the purity with a median of 5.1% of IFN-γ producing cells after the restimulation with Aspergillus antigen could be still unsatisfactory, considering the possible risk of GVHD.Citation81 The low purity reflects the difficulty to use IFN-γ secretion assay to isolate T cells with low precursor frequency in healthy donors like Aspergillus-specific T cells. Their group also reported that they isolated multi-specific T cells against Aspergillus fumigatus, Candida albicans and Rhozpus oryzae.Citation82 Such multi-specific T cells are practically attractive as, in many cases, it is difficult to determine the fungal pathogen which caused the infectious diseases. In particular, it is often difficult to distinguish between aspergillus infection and mucor infection. Another method to isolate fungus-specific Th1 cells is the isolation based on activation-dependent expression of CD154.Citation73 CD154 is transiently expressed on CD4 T cells after activation but much less on CD8 T cells. Khanna et al. reported the generation and expansion of Aspergillus Crf1 peptide-specific T cells using this method.Citation73 PBMCs from healthy donors were stimulated with Aspergillus fumigatus Crf1 peptide p41, and antigen-specific T cells were enriched by the selection of CD154+ cells via magnetic cell separation. Isolated CD154+ cells were cultured for 14 d. Tetramer-staining showed 40% (range, 25-63) of Aspergillus fumigatus p41 peptide-specific CD4+ T cells. If DCs can be used in accordance to GMP, it might help to further expand specific T cells.Citation83
Perrucio et al. conducted a study using expanded Aspergillus-specific T cells after haploidentical transplantation in patients with a history of IA.Citation84 In this study, control transplant recipients who did not receive such adoptive transfer tended to suffer from IA and had no detectable reconstitution of Aspergillus-specific T cells after transplantation. On the other hand, patients who received adoptive T cell therapy had significantly higher number of specific T cells against Aspergillus.
A recent topic in the field of adoptive T cell therapy is genetically modified T cells expressing chimeric antigen receptors (CAR). To further enhance the effectiveness of adoptive T cell therapy against Aspergillus infection, a study group in the United States developed a system of adoptive transfer of CAR T cells that possess the pattern-recognition properties of Dectin-1.Citation85 T cells are electroporated with DNA plasmids from the Sleeping Beauty transposon/transposase system to express this CAR, and expanded with artificial antigen presenting cells in the presence of cytokines.Citation86 Dectin-1 specifically recognizes β-glucans which are expressed on the cell wall of fungi. Their approach is clinically attractive, as T cells can be long-lived as they contained central memory T cells, and can be expanded ex vivo in compliance with GMP for clinical trials. Targeting of other antigens by CAR modified T cells can be applicable to any antigens on the cell surface of fungus for which monoclonal antibody can be generated. The potential benefits of CAR modified T cells could be the high efficiency as demonstrated in the treatment of acute lymphoblastic leukemia. The potential drawbacks could be the life-threatening complications. In clinical trials of CAR modified T cells, on target/off target toxicity have to be carefully monitored.Citation87 It is well known that CAR T cells targeting CD19 caused cytokine release syndrome (CRS).Citation88 Higher disease burden before CAR T therapy was reported to be a risk factor for CRS.Citation89,90 Therefore, when this technique is applied in the treatment of infectious disease, we also have to pay much attention to the disease burden. In the setting of allo-HSCT, if CAR T cells work efficiently, accompanying spread of pathogen-associated molecular patterns (PAMPs) from fungus could aggravate GVHD through the interaction between PAMPs and pathogen recognition receptors [PRRs] such as NOD-like receptors (NLRs) and Toll-like receptors (TLRs).Citation91 The more infection progressed, the more severe the toxicity associated with CAR T would be. In order to prevent the lethal toxicity, the introduction of an inducible “suicide gene” that can be electively activated in the event of severe toxicity is ideal. Techniques used in the setting of adoptive T cell therapy such as herpes simplex virus thymidine kinase (HSV-TK) and human inducible caspase 9 (iCasp9) might be combined in the system of CAR modified T cells.Citation92 We need to investigate the way with which we can improve safety without compromising the potential efficacy of CAR modified T cells.Citation93 If the safety issue is solved, adoptive T cell therapy as prevention of fungal infections might become a realistic treatment strategy in patients at very high risk of fungal infections.Citation94 We could expect that non-modified donor lymphocyte infusion would disappear in the near future, considering the low specificity against the target and substantial risk of GVHD.
As adjunctive therapy to target fungal infections as well as augment the effectiveness of adoptive T cell therapy, selection of anti-fungal drugs might be important as these drugs were reported to have impact on the host immune response in vitro.Citation95 For instance, caspofungin can unmask β-glucan residues in the cell wall of fungi which might enhance the effectiveness of administered T cells.Citation96 Fluconazole was reported to induce a protective Th1 response against candidiasis in mice model.Citation97 Such immunomodulatory effects of anti-fungal drugs should be assessed in humans. Drugs which can enhance the effects of administered T cells are to be chosen as a combinatory therapy of adoptive T cell therapy against fungal diseases.
In summary, now it is possible to purify and expand Aspergillus-specific T cells according to GMP conditions. The safety and effectiveness of Aspergillus-specific T cells should be tested in clinical trials. Unfortunately, currently there is a paucity of clinical data about adoptive T cell therapy using Aspergillus-specific T cells. Therefore, at first, we need trials of adoptive T cell therapy against refractory fungal infections. If the efficacy and safety are confirmed in the setting, there is a possibility to apply such cell therapy in the setting of prophylaxis of fungal infections.
Conclusion
In terms of adoptive T cell therapy against viral infections, promising clinical results have been already reported from various teams. Confirmatory studies to establish the benefit of adoptive T cell therapy are warranted. In terms of adoptive T cell therapy against invasive aspergillus infections, basic information relating to T cell immunity against Aspergillus has been clarified over the last decades. The safety and effectiveness of adoptive T cell therapy against Aspergillus should be assessed in future clinical trials. If the safety and efficacy of adoptive T cell therapy are demonstrated in the study for patients with refractory infectious diseases, there is a possibility that we could apply such adoptive T cell therapy for prophylaxis of infectious diseases in patients with high risk at infectious diseases ().
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Gooley TA, Chien JW, Pergam SA, Hingorani S, Sorror ML, Boeckh M, Martin PJ, Sandmaier BM, Marr KA, Appelbaum FR, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med 2010; 363:2091-101; PMID:21105791; http://dx.doi.org/10.1056/NEJMoa1004383
- Afessa B, Peters SG. Major complications following hematopoietic stem cell transplantation. Semin Respir Crit Care Med 2006; 27:297-309; PMID:16791762; http://dx.doi.org/10.1055/s-2006-945530
- Gratwohl A, Brand R, Frassoni F, Rocha V, Niederwieser D, Reusser P, et al. Cause of death after allogeneic haematopoietic stem cell transplantation (HSCT) in early leukaemias: an EBMT analysis of lethal infectious complications and changes over calendar time. Bone Marrow Transplant 2005; 36:757-69; PMID:16151426; http://dx.doi.org/10.1038/sj.bmt.1705140
- Styczynski J, Czyzewski K, Wysocki M, Gryniewicz-Kwiatkowska O, Kolodziejczyk-Gietka A, Salamonowicz M, Hutnik L, Zajac-Spychala O, Zaucha-Prazmo A, Chelmecka-Wiktorczyk L, et al. Increased risk of infections and infection-related mortality in children undergoing haematopoietic stem cell transplantation compared to conventional anticancer therapy: a multicentre nationwide study. Clin Microbiol Infect 2016; 22:179 e1-e10; PMID:26493843; http://dx.doi.org/10.1016/j.cmi.2015.10.017
- Crocchiolo R, Bramanti S, Vai A, Sarina B, Mineri R, Casari E, Tordato F, Mauro E, Timofeeva I, Lugli E, et al. Infections after T-replete haploidentical transplantation and high-dose cyclophosphamide as graft-vs.-host disease prophylaxis. Transpl Infect Dis 2015; 17:242-9; PMID:25648539; http://dx.doi.org/10.1111/tid.12365
- Park M, Lee YH, Lee SH, Yoo KH, Sung KW, Koo HH, Lee JW, Kang HJ, Park KD, Shin HY, et al. Cytomegalovirus infection in seropositive unrelated cord blood recipients: a study of 349 Korean patients. Ann Hematol 2015; 94:481-9; PMID:25417830; http://dx.doi.org/10.1007/s00277-014-2222-x
- Nucci M, Anaissie E. How we treat invasive fungal diseases in patients with acute leukemia: the importance of an individualized approach. Blood 2014; 124:3858-69; PMID:25339358; http://dx.doi.org/10.1182/blood-2014-04-516211
- Lindemans CA, Leen AM, Boelens JJ. How I treat adenovirus in hematopoietic stem cell transplant recipients. Blood 2010; 116:5476-85; PMID:20837781; http://dx.doi.org/10.1182/blood-2010-04-259291
- Boeckh M, Murphy WJ, Peggs KS. Recent advances in cytomegalovirus: an update on pharmacologic and cellular therapies. Biol Blood Marrow Transplant 2015; 21:24-9; PMID:25452035; http://dx.doi.org/10.1016/j.bbmt.2014.11.002
- Kontoyiannis DP, Marr KA, Park BJ, Alexander BD, Anaissie EJ, Walsh TJ, Ito J, Andes DR, Baddley JW, Brown JM, et al. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001-2006: overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) Database. Clin Infect Dis 2010; 50:1091-100; PMID:20218877; http://dx.doi.org/10.1086/651263
- Corzo-Leon DE, Satlin MJ, Soave R, Shore TB, Schuetz AN, Jacobs SE, Walsh TJ. Epidemiology and outcomes of invasive fungal infections in allogeneic haematopoietic stem cell transplant recipients in the era of antifungal prophylaxis: a single-centre study with focus on emerging pathogens. Mycoses 2015; 58:325-36; PMID:25808822; http://dx.doi.org/10.1111/myc.12318
- Marr KA. Delayed opportunistic infections in hematopoietic stem cell transplantation patients: a surmountable challenge. Hematology Am Soc Hematol Edu Program 2012; 2012:265-70; PMID:23233590
- Boeckh M, Nichols WG, Chemaly RF, Papanicolaou GA, Wingard JR, Xie H, Syrjala KL, Flowers ME, Stevens-Ayers T, Jerome KR, et al. Valganciclovir for the prevention of complications of late cytomegalovirus infection after allogeneic hematopoietic cell transplantation: a randomized trial. Annals Intern Med 2015; 162:1-10; http://dx.doi.org/10.7326/M13-2729
- Seyedmousavi S, Mouton JW, Melchers WJ, Bruggemann RJ, Verweij PE. The role of azoles in the management of azole-resistant aspergillosis: from the bench to the bedside. Drug Resist Updat 2014; 17:37-50; PMID:25066814; http://dx.doi.org/10.1016/j.drup.2014.06.001
- van der Linden JW, Camps SM, Kampinga GA, Arends JP, Debets-Ossenkopp YJ, Haas PJ, Rijnders BJ, Kuijper EJ, van Tiel FH, Varga J, et al. Aspergillosis due to voriconazole highly resistant Aspergillus fumigatus and recovery of genetically related resistant isolates from domiciles. Clin Infect Dis 2013; 57:513-20; PMID:23667263; http://dx.doi.org/10.1093/cid/cit320
- Steinmann J, Hamprecht A, Vehreschild MJ, Cornely OA, Buchheidt D, Spiess B, Koldehoff M, Buer J, Meis JF, Rath PM. Emergence of azole-resistant invasive aspergillosis in HSCT recipients in Germany. J Antimicrob Chemother 2015; 70:1522-6; PMID:25630644; http://dx.doi.org/10.1093/jac/dku566
- Hadrich I, Makni F, Neji S, Abbes S, Cheikhrouhou F, Trabelsi H, Sellami H, Ayadi A. Invasive aspergillosis: resistance to antifungal drugs. Mycopathologia 2012; 174:131-41; PMID:22327841; http://dx.doi.org/10.1007/s11046-012-9526-y
- Komatsu TE, Pikis A, Naeger LK, Harrington PR. Resistance of human cytomegalovirus to ganciclovir/valganciclovir: a comprehensive review of putative resistance pathways. Antiviral Res 2014; 101:12-25; PMID:24184129; http://dx.doi.org/10.1016/j.antiviral.2013.10.011
- Campos AB, Ribeiro J, Boutolleau D, Sousa H. Human cytomegalovirus antiviral drug resistance in hematopoietic stem cell transplantation: current state of the art. Rev Med Virol 2016; 26(3):161-82; PMID:26990717
- Shmueli E, Or R, Shapira MY, Resnick IB, Caplan O, Bdolah-Abram T, Wolf DG. High rate of cytomegalovirus drug resistance among patients receiving preemptive antiviral treatment after haploidentical stem cell transplantation. J Infect Dis 2014; 209:557-61; PMID:23983215; http://dx.doi.org/10.1093/infdis/jit475
- Boeckh M, Leisenring W, Riddell SR, Bowden RA, Huang ML, Myerson D, Stevens-Ayers T, Flowers ME, Cunningham T, Corey L. Late cytomegalovirus disease and mortality in recipients of allogeneic hematopoietic stem cell transplants: importance of viral load and T-cell immunity. Blood 2003; 101:407-14; PMID:12393659; http://dx.doi.org/10.1182/blood-2002-03-0993
- Heemskerk B, Lankester AC, van Vreeswijk T, Beersma MF, Claas EC, Veltrop-Duits LA, Kroes AC, Vossen JM, Schilham MW, van Tol MJ. Immune reconstitution and clearance of human adenovirus viremia in pediatric stem-cell recipients. J Infect Dis 2005; 191:520-30; PMID:15655775; http://dx.doi.org/10.1086/427513
- Tormo N, Solano C, Benet I, Clari MA, Nieto J, de la Camara R, López J, López-Aldeguer N, Hernández-Boluda JC, Remigia MJ, et al. Lack of prompt expansion of cytomegalovirus pp65 and IE-1-specific IFNgamma CD8+ and CD4+ T cells is associated with rising levels of pp65 antigenemia and DNAemia during pre-emptive therapy in allogeneic hematopoietic stem cell transplant recipients. Bone Marrow Transplant 2010; 45:543-9; PMID:19617905; http://dx.doi.org/10.1038/bmt.2009.172
- Tormo N, Solano C, Benet I, Nieto J, de la Camara R, Lopez J, Garcia-Noblejas A, Muñoz-Cobo B, Costa E, Clari MA, et al. Reconstitution of CMV pp65 and IE-1-specific IFN-gamma CD8(+) and CD4(+) T-cell responses affording protection from CMV DNAemia following allogeneic hematopoietic SCT. Bone Marrow Transplant 2011; 46:1437-43; PMID:21243030; http://dx.doi.org/10.1038/bmt.2010.330
- Gratama JW, Boeckh M, Nakamura R, Cornelissen JJ, Brooimans RA, Zaia JA, Forman SJ, Gaal K, Bray KR, Gasior GH, et al. Immune monitoring with iTAg MHC Tetramers for prediction of recurrent or persistent cytomegalovirus infection or disease in allogeneic hematopoietic stem cell transplant recipients: a prospective multicenter study. Blood 2010; 116:1655-62; PMID:20508161; http://dx.doi.org/10.1182/blood-2010-03-273508
- Gratama JW, Brooimans RA, van der Holt B, Sintnicolaas K, van Doornum G, Niesters HG, Löwenberg B, Cornelissen JJ. Monitoring cytomegalovirus IE-1 and pp65-specific CD4+ and CD8+ T-cell responses after allogeneic stem cell transplantation may identify patients at risk for recurrent CMV reactivations. Cytometry B Clin Cytom 2008; 74:211-20; PMID:18454493; http://dx.doi.org/10.1002/cyto.b.20420
- Borchers S, Luther S, Lips U, Hahn N, Kontsendorn J, Stadler M, Buchholz S, Diedrich H, Eder M, Koehl U, et al. Tetramer monitoring to assess risk factors for recurrent cytomegalovirus reactivation and reconstitution of antiviral immunity post allogeneic hematopoietic stem cell transplantation. Transpl Infect Dis 2011; 13:222-36; PMID:21585633; http://dx.doi.org/10.1111/j.1399-3062.2011.00626.x
- Gratama JW, van Esser JW, Lamers CH, Tournay C, Lowenberg B, Bolhuis RL, Cornelissen JJ. Tetramer-based quantification of cytomegalovirus (CMV)-specific CD8+ T lymphocytes in T-cell-depleted stem cell grafts and after transplantation may identify patients at risk for progressive CMV infection. Blood 2001; 98:1358-64; PMID:11520783; http://dx.doi.org/10.1182/blood.V98.5.1358
- Ganepola S, Gentilini C, Hilbers U, Lange T, Rieger K, Hofmann J, Maier M, Liebert UG, Niederwieser D, Engelmann E, et al. Patients at high risk for CMV infection and disease show delayed CD8+ T-cell immune recovery after allogeneic stem cell transplantation. Bone Marrow Transplant 2007; 39:293-9; PMID:17262060; http://dx.doi.org/10.1038/sj.bmt.1705585
- Heslop HE. How I treat EBV lymphoproliferation. Blood 2009; 114:4002-8; PMID:19724053; http://dx.doi.org/10.1182/blood-2009-07-143545
- Romani L. Immunity to fungal infections. Nat Rev Immunol 2011; 11:275-88; PMID:21394104; http://dx.doi.org/10.1038/nri2939
- Fuji S, Kapp M, Einsele H. Monitoring of pathogen-specific T-cell immune reconstitution after allogeneic hematopoietic stem cell transplantation. Front Immunol 2013; 4:276; PMID:24062744
- Fuji S, Kapp M, Grigoleit GU, Einsele H. Adoptive immunotherapy with virus-specific T cells. Best Pract Res Clin Haematol 2011; 24:413-9; PMID:21925094; http://dx.doi.org/10.1016/j.beha.2011.06.003
- Einsele H, Hamprecht K. Immunotherapy of cytomegalovirus infection after stem-cell transplantation: a new option? Lancet 2003; 362:1343-4; PMID:14585632; http://dx.doi.org/10.1016/S0140-6736(03)14673-9
- Einsele H, Mielke S, Grigoleit GU. Diagnosis and treatment of cytomegalovirus 2013. Curr Opin Hematol 2014; 21:470-5; PMID:25295744; http://dx.doi.org/10.1097/MOH.0000000000000090
- Einsele H, Roosnek E, Rufer N, Sinzger C, Riegler S, Loffler J, Grigoleit U, Moris A, Rammensee HG, Kanz L, et al. Infusion of cytomegalovirus (CMV)-specific T cells for the treatment of CMV infection not responding to antiviral chemotherapy. Blood 2002; 99:3916-22; PMID:12010789; http://dx.doi.org/10.1182/blood.V99.11.3916
- Hiwarkar P, Gaspar HB, Gilmour K, Jagani M, Chiesa R, Bennett-Rees N, Breuer J, Rao K, Cale C, Goulden N, et al. Impact of viral reactivations in the era of pre-emptive antiviral drug therapy following allogeneic haematopoietic SCT in paediatric recipients. Bone Marrow Transplant 2013; 48:803-8; PMID:23178547; http://dx.doi.org/10.1038/bmt.2012.221
- Kampmann B, Cubitt D, Walls T, Naik P, Depala M, Samarasinghe S, Robson D, Hassan A, Rao K, Gaspar H, et al. Improved outcome for children with disseminated adenoviral infection following allogeneic stem cell transplantation. Br J Haematol 2005; 130:595-603; PMID:16098075; http://dx.doi.org/10.1111/j.1365-2141.2005.05649.x
- Hislop AD, Taylor GS. T-Cell Responses to EBV. Curr Top Microbiol Immunol 2015; 391:325-53; PMID:26428380
- Knabel M, Franz TJ, Schiemann M, Wulf A, Villmow B, Schmidt B, Bernhard H, Wagner H, Busch DH. Reversible MHC multimer staining for functional isolation of T-cell populations and effective adoptive transfer. Nat Med 2002; 8:631-7; PMID:12042816; http://dx.doi.org/10.1038/nm0602-631
- Neudorfer J, Schmidt B, Huster KM, Anderl F, Schiemann M, Holzapfel G, Schmidt T, Germeroth L, Wagner H, Peschel C, et al. Reversible HLA multimers (Streptamers) for the isolation of human cytotoxic T lymphocytes functionally active against tumor- and virus-derived antigens. J Immunol Methods 2007; 320:119-31; PMID:17306825; http://dx.doi.org/10.1016/j.jim.2007.01.001
- Ciaurriz M, Beloki L, Bandres E, Mansilla C, Zabalza A, Perez-Valderrama E, Lachén M, Ibáñez B, Olavarría E, Ramírez N. Streptamer technology allows accurate and specific detection of CMV-specific HLA-A*02 CD8 T cells by flow cytometry. Cytometry B Clin Cytom 2016; [epub ahead of print] PMID:26918565 http://dx.doi.org/10.1002/cyto.b.21367
- Wang XC, Pang H, Xu X, Schmitt A, Freund M, Schmitt M, Chen BA. Streptamer versus tetramer-based selection of functional cytomegalovirus-specific T cells. J Formos Med Assoc 2013; 112:338-45; PMID:23787011; http://dx.doi.org/10.1016/j.jfma.2012.02.020
- Odendahl M, Grigoleit GU, Bonig H, Neuenhahn M, Albrecht J, Anderl F, Germeroth L, Schmitz M, Bornhäuser M, Einsele H, et al. Clinical-scale isolation of “minimally manipulated” cytomegalovirus-specific donor lymphocytes for the treatment of refractory cytomegalovirus disease. Cytotherapy 2014; 16:1245-56; PMID:25108651; http://dx.doi.org/10.1016/j.jcyt.2014.05.023
- Schmitt A, Tonn T, Busch DH, Grigoleit GU, Einsele H, Odendahl M, Germeroth L, Ringhoffer M, Ringhoffer S, Wiesneth M, et al. Adoptive transfer and selective reconstitution of streptamer-selected cytomegalovirus-specific CD8+ T cells leads to virus clearance in patients after allogeneic peripheral blood stem cell transplantation. Transfusion 2011; 51:591-9; PMID:21133926; http://dx.doi.org/10.1111/j.1537-2995.2010.02940.x
- Freimuller C, Stemberger J, Artwohl M, Germeroth L, Witt V, Fischer G, Tischer S, Eiz-Vesper B, Knippertz I, Dörrie J, et al. Selection of adenovirus-specific and Epstein-Barr virus-specific T cells with major histocompatibility class I streptamers under Good Manufacturing Practice (GMP)-compliant conditions. Cytotherapy 2015; 17:989-1007; PMID:25866178; http://dx.doi.org/10.1016/j.jcyt.2015.03.613
- Feucht J, Joachim L, Lang P, Feuchtinger T. Adoptive T-cell transfer for refractory viral infections with cytomegalovirus, Epstein-Barr virus or adenovirus after allogeneic stem cell transplantation. Klinische Padiatrie 2013; 225:164-9; PMID:23700092; http://dx.doi.org/10.1055/s-0033-1333749
- Feuchtinger T, Lang P, Hamprecht K, Schumm M, Greil J, Jahn G, Niethammer D, Einsele H. Isolation and expansion of human adenovirus-specific CD4+ and CD8+ T cells according to IFN-gamma secretion for adjuvant immunotherapy. Exp Hematol 2004; 32:282-9; PMID:15003314; http://dx.doi.org/10.1016/j.exphem.2003.12.009
- Peggs KS, Thomson K, Samuel E, Dyer G, Armoogum J, Chakraverty R, Pang K, Mackinnon S, Lowdell MW. Directly selected cytomegalovirus-reactive donor T cells confer rapid and safe systemic reconstitution of virus-specific immunity following stem cell transplantation. Clin Infect Dis 2011; 52:49-57; PMID:21148519; http://dx.doi.org/10.1093/cid/ciq042
- Feuchtinger T, Opherk K, Bethge WA, Topp MS, Schuster FR, Weissinger EM, Mohty M, Or R, Maschan M, Schumm M, et al. Adoptive transfer of pp65-specific T cells for the treatment of chemorefractory cytomegalovirus disease or reactivation after haploidentical and matched unrelated stem cell transplantation. Blood 2010; 116:4360-7; PMID:20625005; http://dx.doi.org/10.1182/blood-2010-01-262089
- Feucht J, Opherk K, Lang P, Kayser S, Hartl L, Bethge W, Matthes-Martin S, Bader P, Albert MH, Maecker-Kolhoff B, et al. Adoptive T-cell therapy with hexon-specific Th1 cells as a treatment of refractory adenovirus infection after HSCT. Blood 2015; 125:1986-94; PMID:25617426; http://dx.doi.org/10.1182/blood-2014-06-573725
- Feuchtinger T, Matthes-Martin S, Richard C, Lion T, Fuhrer M, Hamprecht K, Handgretinger R, Peters C, Schuster FR, Beck R, et al. Safe adoptive transfer of virus-specific T-cell immunity for the treatment of systemic adenovirus infection after allogeneic stem cell transplantation. Br J Haematol 2006; 134:64-76; PMID:16803570; http://dx.doi.org/10.1111/j.1365-2141.2006.06108.x
- Feuchtinger T, Richard C, Joachim S, Scheible MH, Schumm M, Hamprecht K, Martin D, Jahn G, Handgretinger R, Lang P. Clinical grade generation of hexon-specific T cells for adoptive T-cell transfer as a treatment of adenovirus infection after allogeneic stem cell transplantation. J Immunother 2008; 31:199-206; PMID:18481389; http://dx.doi.org/10.1097/CJI.0b013e31815ef862
- Qasim W, Gilmour K, Zhan H, Derniame S, McNicol AM, Ip W, Hiwarkar P, Veys P, Gaspar HB. Interferon-gamma capture T cell therapy for persistent Adenoviraemia following allogeneic haematopoietic stem cell transplantation. Br J Haematol 2013; 161:449-52; PMID:23432400; http://dx.doi.org/10.1111/bjh.12251
- Moosmann A, Bigalke I, Tischer J, Schirrmann L, Kasten J, Tippmer S, Leeping M, Prevalsek D, Jaeger G, Ledderose G, et al. Effective and long-term control of EBV PTLD after transfer of peptide-selected T cells. Blood 2010; 115:2960-70; PMID:20103780; http://dx.doi.org/10.1182/blood-2009-08-236356
- Icheva V, Kayser S, Wolff D, Tuve S, Kyzirakos C, Bethge W, Greil J, Albert MH, Schwinger W, Nathrath M, et al. Adoptive transfer of epstein-barr virus (EBV) nuclear antigen 1-specific t cells as treatment for EBV reactivation and lymphoproliferative disorders after allogeneic stem-cell transplantation. J Clin Oncol 2013; 31:39-48; PMID:23169501; http://dx.doi.org/10.1200/JCO.2011.39.8495
- Qian C, Wang Y, Cai H, Laroye C, De Carvalho Bittencourt M, Clement L, Stoltz JF, Decot V, Reppel L, Bensoussan D. Adenovirus-specific T-cell Subsets in Human Peripheral Blood and After IFN-gamma Immunomagnetic Selection. J Immunother 2016; 39:27-35; PMID:26641259
- Papadopoulou A, Gerdemann U, Katari UL, Tzannou I, Liu H, Martinez C, Leung K, Carrum G, Gee AP, Vera JF, et al. Activity of broad-spectrum T cells as treatment for AdV, EBV, CMV, BKV, and HHV6 infections after HSCT. Sci Transl Med 2014; 6:242ra83; PMID:24964991; http://dx.doi.org/10.1126/scitranslmed.3008825
- Geyeregger R, Freimuller C, Stemberger J, Artwohl M, Witt V, Lion T, Fischer G, Lawitschka A, Ritter J, Hummel M, et al. First-in-man clinical results with good manufacturing practice (GMP)-compliant polypeptide-expanded adenovirus-specific T cells after haploidentical hematopoietic stem cell transplantation. J Immunother 2014; 37:245-9; PMID:24714358
- Vera JF, Brenner LJ, Gerdemann U, Ngo MC, Sili U, Liu H, Wilson J, Dotti G, Heslop HE, Leen AM, et al. Accelerated production of antigen-specific T cells for preclinical and clinical applications using gas-permeable rapid expansion cultureware (G-Rex). J Immunother 2010; 33:305-15; PMID:20445351; http://dx.doi.org/10.1097/CJI.0b013e3181c0c3cb
- Morris EC, Stauss HJ. Optimizing T cell receptor gene therapy for hematologic malignancies. Blood 2016; 127(26):3305-11; PMID:27207802; http://dx.doi.org/10.1182/blood-2015-11-629071.
- Linnemann C, Heemskerk B, Kvistborg P, Kluin RJ, Bolotin DA, Chen X, Bresser K, Nieuwland M, Schotte R, Michels S, et al. High-throughput identification of antigen-specific TCRs by TCR gene capture. Nat Med 2013; 19:1534-41; PMID:24121928; http://dx.doi.org/10.1038/nm.3359
- Lass-Florl C, Roilides E, Loffler J, Wilflingseder D, Romani L. Minireview: host defence in invasive aspergillosis. Mycoses 2013; 56:403-13; PMID:23406508; http://dx.doi.org/10.1111/myc.12052
- Camargo JF, Husain S. Immune correlates of protection in human invasive aspergillosis. Clin Infect Dis 2014; 59:569-77; PMID:24803380; http://dx.doi.org/10.1093/cid/ciu337
- Ferwerda B, Ferwerda G, Plantinga TS, Willment JA, van Spriel AB, Venselaar H, Elbers CC, Johnson MD, Cambi A, Huysamen C, et al. Human dectin-1 deficiency and mucocutaneous fungal infections. N Engl J Med 2009; 361:1760-7; PMID:19864674; http://dx.doi.org/10.1056/NEJMoa0901053
- Cunha C, Di Ianni M, Bozza S, Giovannini G, Zagarella S, Zelante T, D'Angelo C, Pierini A, Pitzurra L, Falzetti F, et al. Dectin-1 Y238X polymorphism associates with susceptibility to invasive aspergillosis in hematopoietic transplantation through impairment of both recipient- and donor-dependent mechanisms of antifungal immunity. Blood 2010; 116:5394-402; PMID:20807886; http://dx.doi.org/10.1182/blood-2010-04-279307
- Cenci E, Mencacci A, Bacci A, Bistoni F, Kurup VP, Romani L. T cell vaccination in mice with invasive pulmonary aspergillosis. J Immunol 2000; 165:381-8; PMID:10861075; http://dx.doi.org/10.4049/jimmunol.165.1.381
- Ito JI, Lyons JM. Vaccination of corticosteroid immunosuppressed mice against invasive pulmonary aspergillosis. J Infect Dis 2002; 186:869-71; PMID:12198627; http://dx.doi.org/10.1086/342509
- Ito JI, Lyons JM, Hong TB, Tamae D, Liu YK, Wilczynski SP, Kalkum M. Vaccinations with recombinant variants of Aspergillus fumigatus allergen Asp f 3 protect mice against invasive aspergillosis. Infect Immun 2006; 74:5075-84; PMID:16926399; http://dx.doi.org/10.1128/IAI.00815-06
- Diaz-Arevalo D, Bagramyan K, Hong TB, Ito JI, Kalkum M. CD4+ T cells mediate the protective effect of the recombinant Asp f3-based anti-aspergillosis vaccine. Infect Immun 2011; 79:2257-66; PMID:21422177; http://dx.doi.org/10.1128/IAI.01311-10
- Bozza S, Gaziano R, Lipford GB, Montagnoli C, Bacci A, Di Francesco P, Kurup VP, Wagner H, Romani L. Vaccination of mice against invasive aspergillosis with recombinant Aspergillus proteins and CpG oligodeoxynucleotides as adjuvants. Microbes Infect 2002; 4:1281-90; PMID:12443892; http://dx.doi.org/10.1016/S1286-4579(02)00007-2
- Bozza S, Clavaud C, Giovannini G, Fontaine T, Beauvais A, Sarfati J, D'Angelo C, Perruccio K, Bonifazi P, Zagarella S, et al. Immune sensing of Aspergillus fumigatus proteins, glycolipids, and polysaccharides and the impact on Th immunity and vaccination. J Immunol 2009; 183:2407-14; PMID:19625642; http://dx.doi.org/10.4049/jimmunol.0900961
- Khanna N, Stuehler C, Conrad B, Lurati S, Krappmann S, Einsele H, Berges C, Topp MS. Generation of a multipathogen-specific T-cell product for adoptive immunotherapy based on activation-dependent expression of CD154. Blood 2011; 118:1121-31; PMID:21642594; http://dx.doi.org/10.1182/blood-2010-12-322610
- Chaudhary N, Staab JF, Marr KA. Healthy human T-Cell Responses to Aspergillus fumigatus antigens. PloS One 2010; 5:e9036; PMID:20174463; http://dx.doi.org/10.1371/journal.pone.0009036
- Hebart H, Bollinger C, Fisch P, Sarfati J, Meisner C, Baur M, Loeffler J, Monod M, Latgé JP, Einsele H. Analysis of T-cell responses to Aspergillus fumigatus antigens in healthy individuals and patients with hematologic malignancies. Blood 2002; 100:4521-8; PMID:12393638; http://dx.doi.org/10.1182/blood-2002-01-0265
- Ramadan G, Davies B, Kurup VP, Keever-Taylor CA. Generation of Th1 T cell responses directed to a HLA Class II restricted epitope from the Aspergillus f16 allergen. Clin Exp Immunol 2005; 139:257-67; PMID:15654824; http://dx.doi.org/10.1111/j.1365-2249.2005.02699.x
- Jolink H, Meijssen IC, Hagedoorn RS, Arentshorst M, Drijfhout JW, Mulder A, Claas FH, van Dissel JT, Falkenburg JH, Heemskerk MH. Characterization of the T-cell-mediated immune response against the Aspergillus fumigatus proteins Crf1 and catalase 1 in healthy individuals. J Infect Dis 2013; 208:847-56; PMID:23698813; http://dx.doi.org/10.1093/infdis/jit237
- Stuehler C, Nowakowska J, Bernardini C, Topp MS, Battegay M, Passweg J, Khanna N. Multispecific Aspergillus T cells selected by CD137 or CD154 induce protective immune responses against the most relevant mold infections. J Infect Dis 2015; 211:1251-61; PMID:25367298
- Jolink H, Hagedoorn RS, Lagendijk EL, Drijfhout JW, van Dissel JT, Falkenburg JH, Heemskerk MH. Induction of A. fumigatus-specific CD4-positive T cells in patients recovering from invasive aspergillosis. Haematologica 2014; 99:1255-63; PMID:24747947; http://dx.doi.org/10.3324/haematol.2013.098830
- Potenza L, Vallerini D, Barozzi P, Riva G, Forghieri F, Beauvais A, Beau R, Candoni A, Maertens J, Rossi G, et al. Characterization of specific immune responses to different Aspergillus antigens during the course of invasive Aspergillosis in hematologic patients. PloS One 2013; 8:e74326; PMID:24023936; http://dx.doi.org/10.1371/journal.pone.0074326
- Tramsen L, Koehl U, Tonn T, Latge JP, Schuster FR, Borkhardt A, Uharek L, Quaritsch R, Beck O, Seifried E, et al. Clinical-scale generation of human anti-Aspergillus T cells for adoptive immunotherapy. Bone Marrow Transplant 2009; 43:13-9; PMID:18762764; http://dx.doi.org/10.1038/bmt.2008.271
- Tramsen L, Schmidt S, Boenig H, Latge JP, Lass-Florl C, Roeger F, Seifried E, Klingebiel T, Lehrnbecher T. Clinical-scale generation of multi-specific anti-fungal T cells targeting Candida, Aspergillus and mucormycetes. Cytotherapy 2013; 15:344-51; PMID:23579059; http://dx.doi.org/10.1016/j.jcyt.2012.11.014
- Stuehler C, Khanna N, Bozza S, Zelante T, Moretti S, Kruhm M, Lurati S, Conrad B, Worschech E, Stevanović S, et al. Cross-protective TH1 immunity against Aspergillus fumigatus and Candida albicans. Blood 2011; 117:5881-91; PMID:21441461; http://dx.doi.org/10.1182/blood-2010-12-325084
- Perruccio K, Tosti A, Burchielli E, Topini F, Ruggeri L, Carotti A, Capanni M, Urbani E, Mancusi A, Aversa F, et al. Transferring functional immune responses to pathogens after haploidentical hematopoietic transplantation. Blood 2005; 106:4397-406; PMID:16123217; http://dx.doi.org/10.1182/blood-2005-05-1775
- Kumaresan PR, Manuri PR, Albert ND, Maiti S, Singh H, Mi T, Roszik J, Rabinovich B, Olivares S, Krishnamurthy J, et al. Bioengineering T cells to target carbohydrate to treat opportunistic fungal infection. Proc Natl Acad Sci U S A 2014; 111:10660-5; PMID:25002471; http://dx.doi.org/10.1073/pnas.1312789111
- Singh H, Figliola MJ, Dawson MJ, Olivares S, Zhang L, Yang G, Maiti S, Manuri P, Senyukov V, Jena B, et al. Manufacture of clinical-grade CD19-specific T cells stably expressing chimeric antigen receptor using Sleeping Beauty system and artificial antigen presenting cells. PloS One 2013; 8:e64138; PMID:23741305; http://dx.doi.org/10.1371/journal.pone.0064138
- Curran KJ, Pegram HJ, Brentjens RJ. Chimeric antigen receptors for T cell immunotherapy: current understanding and future directions. J Gene Med 2012; 14:405-15; PMID:22262649; http://dx.doi.org/10.1002/jgm.2604
- Maus MV, Grupp SA, Porter DL, June CH. Antibody-modified T cells: CARs take the front seat for hematologic malignancies. Blood 2014; 123:2625-35; PMID:24578504; http://dx.doi.org/10.1182/blood-2013-11-492231
- Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, Chew A, Gonzalez VE, Zheng Z, Lacey SF, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 2014; 371:1507-17; PMID:25317870; http://dx.doi.org/10.1056/NEJMoa1407222
- Turtle CJ, Hanafi LA, Berger C, Gooley TA, Cherian S, Hudecek M, Sommermeyer D, Melville K, Pender B, Budiarto TM, et al. CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J Clin Invest 2016; 126:2123-38; PMID:27111235; http://dx.doi.org/10.1172/JCI85309
- Cunha C, Carvalho A, Esposito A, Bistoni F, Romani L. DAMP signaling in fungal infections and diseases. Front Immunol 2012; 3:286; PMID:22973279; http://dx.doi.org/10.3389/fimmu.2012.00286
- Marin V, Cribioli E, Philip B, Tettamanti S, Pizzitola I, Biondi A, Biagi E, Pule M. Comparison of different suicide-gene strategies for the safety improvement of genetically manipulated T cells. Hum Gene Ther Methods 2012; 23:376-86; PMID:23186165; http://dx.doi.org/10.1089/hgtb.2012.050
- Jena B, Dotti G, Cooper LJ. Redirecting T-cell specificity by introducing a tumor-specific chimeric antigen receptor. Blood 2010; 116:1035-44; PMID:20439624; http://dx.doi.org/10.1182/blood-2010-01-043737
- de Witte MA, Kierkels GJ, Straetemans T, Britten CM, Kuball J. Orchestrating an immune response against cancer with engineered immune cells expressing alphabetaTCRs, CARs, and innate immune receptors: an immunological and regulatory challenge. Cancer Immunol Immunother 2015; 64:893-902; PMID:25990073; http://dx.doi.org/10.1007/s00262-015-1710-8
- Ben-Ami R, Lewis RE, Kontoyiannis DP. Immunocompromised hosts: immunopharmacology of modern antifungals. Clin Infect Dis 2008; 47:226-35; PMID:18540822; http://dx.doi.org/10.1086/589290
- Lamaris GA, Lewis RE, Chamilos G, May GS, Safdar A, Walsh TJ, Raad II, Kontoyiannis DP. Caspofungin-mediated β-glucan unmasking and enhancement of human polymorphonuclear neutrophil activity against Aspergillus and non-Aspergillus hyphae. J Infect Dis 2008; 198:186-92; PMID:18500936; http://dx.doi.org/10.1086/589305
- Cenci E, Mencacci A, Del Sero G, Bistoni F, Romani L. Induction of protective Th1 responses to Candida albicans by antifungal therapy alone or in combination with an interleukin-4 antagonist. J Infect Dis 1997; 176:217-26; PMID:9207370; http://dx.doi.org/10.1086/514027