 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The objective of this study was to assess the achievement of pharmacokinetic/pharmacodynamic (PK/PD) targets of meropenem (MEM) in critically-ill patients with bloodstream infections (BSI) due to Klebsiella pneumoniae-carbapenemase-producing Klebsiella pneumoniae (KPC-Kp) with MEM minimum inhibitory concentrations (MICs) ≥16 mg/L. Nineteen critically-ill patients with KPC-Kp BSI were given combination therapy including MEM, tigecycline, plus colistin or gentamicin (according to susceptibility testing). MEM was administered as an extended 3-hour infusion of 2 g every 8 hours, or adjusted according to renal function. MEM plasma concentrations were determined by high-performance liquid chromatography. PK/PD targets for MEM were defined as T > 40% 1×MIC and T > 40% 4×MIC. Possible synergisms between MEM and coadministered agents were assessed by time-kill assays based on plasma levels for MEM and on fixed plasma concentrations for the other agents. In none of 19 patients MEM reached any PK/PD target. The actual MEM MICs were 256, 512, and 1024 mg/L in 1, 3, and 15 isolates, respectively. However, theoretically, the PK/PD target of T > 40% 1×MIC could have been achieved in 95%, 68%, 32% and 0% of the isolates for MIC equal to 8, 16, 32, and 64 mg/L, respectively. No synergisms were observed between MEM and coadministered agents. In conclusion, high-dose MEM failed to reach PK/PD targets in 19 patients with BSI due to KPC-Kp with very high MEM MICs. On a theoretical basis, our results suggest a possible usefulness of MEM against resistant blood isolates with MICs up to 32 mg/L.
Introduction
Managing life-threatening infections due to Klebsiella pneumoniae carbapenemase-producing Klebsiella pneumoniae (KPC-Kp) has become a tough challenge in recent years.Citation1-12 Indeed, these infections are often caused by isolates with multidrug-resistant phenotypes, and are associated with high mortality (30–70%).Citation13-17 Dependable therapeutic options often rely on a few antimicrobial drugs administered in combinations, due to a survival benefit over monotherapy reported in multicenter observational studies.Citation13-15,17
Somewhat paradoxically, meropenem (MEM) is almost invariably included in such combinations, since the above cited studies showed that high-dose MEM was able to improve survival from KPC-Kp severe infections, despite in vitro nonsusceptibility (minimum inhibitory concentration [MIC] > 2 mg/L).Citation13-18 However, this effect was clearly detectable only when the resistant KPC-Kp isolate had a MEM MIC ≤ 8 mg/L, while the existence of any benefit above this MIC threshold has still to be confirmed.Citation15-17 Therefore, there is uncertainty about the usefulness of including MEM in combination regimens when the MEM MIC of KPC-Kp strains is ≥ 16 mg/L. Under this light, pharmacokinetic/pharmacodynamic (PK/PD) studies could anticipate valuable theoretical evidence in favor or against this approach, nowadays widely used in the clinical practice, especially in Southern Europe.Citation15,17
Aim of the present study was to assess the achievement of PK/PD targets of high-dose MEM in critically-ill patients with bloodstream infections (BSI) caused by KPC-Kp with high MEM MICs. The possible synergism between MEM and coadministered antibacterials was also investigated.
Patients and methods
From July 2013 to May 2014, a prospective study was conducted at the University of Genoa IRCCS AOU San Martino-IST, a 1,300-bed teaching hospital in Genoa, Italy. The study was approved by the local ethics committee and all enrolled patients or their relatives signed an informed consent to participate in the study according to local regulations. During the study period, all adult patients with a monomicrobial KPC-Kp BSI fulfilling the following criteria were included in the study: (i) at least one blood culture positive for a KPC-Kp isolate with MEM MIC ≥ 16 mg/L by routine susceptibility testing; (ii) signs and symptoms of sepsis, severe sepsis, or septic shock, according to standard definitions;Citation19 (iii) estimated creatinine clearance (CrCl) > 10 mL/min using Cockcroft-Gault formula;Citation20 (iv) no receipt of any renal replacement therapy.
The primary study endpoint was the achievement of PK/PD targets of high-dose MEM in critically-ill patients with bloodstream infections (BSI) caused by KPC-Kp resistant to MEM (MIC ≥16 mg/L) with respect to their actual MEM MICs. The secondary endpoint was synergism between MEM and coadministered antibacterials.
Antibiotic therapy
MEM was administered as an extended 3-hour infusion, and it was included in 3-drug combinations with tigecycline/gentamicin or tigecycline/colistin according to colistin resistance or susceptibility of KPC-Kp isolates. For patients with CrCl ≥ 40 mL/min, MEM was administered without loading dose at the dosage of 2 g every 8 hours, while for patients with CrCl between 39 and 10 mL/min the given dose was 2 g every 12 hours. Gentamicin was administered at 5–7 mg/kg once-daily, and colistin at 9 million international units (IU) of colistimethate sodium as a loading dose, then 4.5 million IU every 12 hours as a maintenance dose. Standard dose adjustments for decreased renal function were applied for gentamicin and colistin.Citation21,22 Tigecycline was administered at 100 mg as a loading dose, then 50 mg every 12 hours as a maintenance dose.
MEM concentration determination
MEM plasma concentrations were evaluated by validated high-performance liquid chromatography assay.Citation23 MEM plasma concentrations were determined for each patient, after at least 3 infusions of the drug (on the second day of therapy). Blood samples were collected according to the following schemes: (1) immediately after the end of infusion, 1, 3, and 5 hours after the end of infusion when MEM was given every 8 hours; (2) immediately after the end of infusion, 1, 3, 5, and 9 hours after the end of infusion when MEM was given every 12 hours. For each sample, an aliquot of 4 mL of blood was drawn into heparinized tubes, which were centrifuged at 1000 g for 10 min; the resulting plasma was stored at −80°C. Clinical samples, drug-free plasma and calibration standards were extracted as reported elsewhere.Citation23 Fresh human plasma samples were obtained from healthy volunteers for standard samples. Standard solutions for the calibration curves construction were obtained by diluting a stock solution of MEM at a concentration of 1 mg/mL in water. Calibration samples were prepared in pooled samples of blank human plasma and were prepared at 7 different concentrations ranging from 0.5 to 50 µg/ml. The results obtained from the analysis of the calibration points were examined by linear regression. In order to assess whether a calibration point could be accepted, it was back-calculated on the basis of the equation of the corresponding calibration curve; a calibration curve was rejected if more than 2 concentrations or 2 adjacent concentrations deviated more than 20% from the nominal value for the Lower Limit Of Quantification (LLOQ) and by more than 15% for the other concentrations (outliers). The precision and accuracy of the method were determined by performing replicate analyses of quality control plasma samples (1, 5, 25 µg/mL) and LLOQ (0.5 µg/mL). Two replicates of each QC/LLOQ were analyzed on 3 different days and subjected to within- and between-run analysis. Samples with concentrations higher than the upper limit of the calibration were reanalyzed by dilution of the sample. The precision (relative standard deviation of replicate analysis) was calculated using the ANOVA test. The accuracy of the method was calculated by the formula, BIAS = (mean–nominal concentration)/ (nominal concentration X100).Citation24,25
MEM noncompartmental pharmacokinetic analysis
The individual plasma concentration profile of MEM was obtained with a PK software program (Modkine version 1.2; 2001 – Biosoft®, Cambridge UK). The main pharmacokinetic parameters calculated for each patient were maximum plasma concentration (Cmax), area under the curve of plasma concentration vs. time extrapolated to infinity (AUC0-∞), elimination rate constant (Kel), mean residence time (MRT), Volume of distribution (Vd), Clearance (CL) and half-life. Cmax was determined by visual inspection of each plasma concentration time plot. AUC0-∞ was calculated on log-transformed values using the trapezoidal rule and extrapolated to infinity by dividing the last measurable plasma concentration value by Kel. The latter was estimated from the points of the terminal phase of the plasma concentration curve by log-linear regression. The total clearance (CL) was calculated as dose/AUC0-∞, and the volume of distribution (Vd) was calculated as CL*MRT.
T > 40% 1×MIC and T > 40% 4×MIC were defined as PK/PD targets. For each patients and for different MIC thresholds, the time during which MEM concentrations were higher than MIC (T> 1×MIC and 4×MIC) was calculated as follows:Citation26
Strain identification and antibiotic susceptibility testing
Routine identification and antibiotic susceptibility testing were carried out using the Vitek-2 AES automated system (BioMérieux, Marcy-l′Etoile, France). In addition, in vitro activity of meropenem, colistin, tigecycline, and gentamicin were confirmed by the broth microdilution method and interpreted according to the European Committee on Antimicrobial Susceptibility Testing (EUCAST) guidelines (Breakpoint for interpretation of MIC and zone diameters. Version 5.0, 2015; www.eucast.org). Identification of KPC carbapenemase-encoding gene (blaKPC-) was achieved by PCR and sequencing using primers and conditions reported elsewhere.Citation27 Multilocus sequence typing (MLST) was performed for all isolates as previously described.Citation27
Time-kill experiments
The activities of the antimicrobial combinations were determined in triplicate for each isolates with the time-kill methods. Antimicrobial combinations to be tested were selected for each strain on the basis of the therapy received by patients. Thirteen strains were tested against meropenem plus tigecycline, meropenem plus gentamicin, tigecycline plus gentamicin, and meropenem plus tigecycline plus gentamicin. Six strains were tested against meropenem plus tigecycline, meropenem plus colistin, tigecycline plus colistin, and colistin plus tigecycline plus meropenem
Time-kill experiments were performed by adding the antibiotics to log-phase bacterial cultures diluted to 106-107 CFU/mL growing in 250 ml flasks at 37°C. Just before the antibiotics were added and at 2, 6, and 24 h thereafter, bacterial counts were carried out. Survivors were evaluated by determining CFU on agar plates. The antibiotics were tested alone and in combination. MEM was used at specific concentrations obtained for each patients on the basis of the measured area under the concentration-time curve (AUC0–8/8 h for patients receiving MEM every 8 hours and AUC0–12/12 h for patients receiving MEM every 12 hours), while tigecycline, gentamicin and colistin were used at fixed concentrations, according to standard plasma levels (0.125, 2, and 12 mg\L for tigecycline, colistin, and gentamicin, re-spectively).Citation28,29
Antibiotic interactions were interpreted as synergism if the combinations, compared with the most effective single antibiotic, caused at least a ≥2 log10 decrease in colony count. Antagonism was defined as a ≥2 log10 increase in colony count, while indifference was defined as a <2 log10 change (increase or decrease). When a combination of 3 antibiotics was analyzed, interactions were interpreted as above comparing the combination with both the most effective single antibiotics and the most effective combination of 2 antibiotics.
Results
Nineteen patients were enrolled in the study. Their mean age was 62 y (SD ± 13 ), 12/19 were males (63%). The clinical characteristics and outcome of patients are reported in . shows MEM PK parameters in the study population. Most patients (15/19) received 2 g of MEM every 8 hours, while 3/19 were treated with 2 g of MEM every 12 hours because of impaired renal function. A morbidly obese patient (body mass index [BMI] > 40 kg/m2) with normal renal function was given 3 g of MEM every 8 hours.
Table 1. Characteristics and outcome of 19 patients with bloodstream infection due to Klebsiella pneumoniae carbapenemase-producing Klebsiella pneumonia (KPC-Kp).
Table 2. Pharmacokinetic parameters of high-dose meropenem in 19 patients with bloodstream infection due to Klebsiella pneumoniae carbapenemase-producing Klebsiella pneumoniae.
According to routine Vitek-2 results, all isolates exhibited a MEM MICs > 16 mg/L. By broth microdilution method, actual MEM MICs of isolates turned out to be 256, 512, and 1024 mg/L in 1, 3, and 15 of the 19 isolates, respectively. Two out of 19 isolates were resistant to gentamicin (11%, MICs range: 2–32 mg/L), 13/19 were resistant to colistin (68%, MICs range: 0.25 – 64 mg/L), and 3/19 were resistant to tigecycline (18%, MICs range: 1–32 mg/L). Gentamicin-resistant strains were susceptible to colistin and colistin-resistant strains were susceptible to gentamicin. All strains were KPC-Kp. In particular, 16 of them produced KPC-3 (84%) and 3 produced KPC-2 (16%). Most isolates (18/19) belonged to sequence type 258 (ST258), and one belonged to ST307.
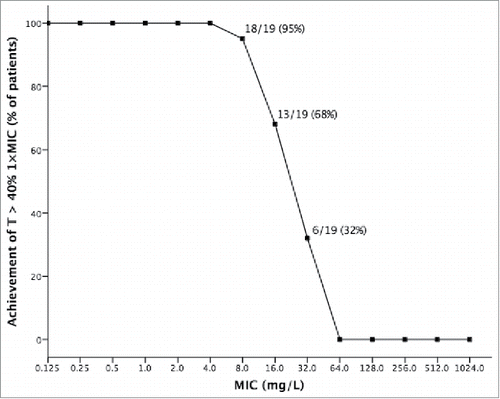
MEM did not achieve the PK/PD targets of T > 40% 1×MIC and T > 40% 4×MIC in any of the enrolled patients. However, interestingly, on the basis of measured levels, T > 40% 1×MIC (but not T > 40% 4×MIC) could have been attained in 68%, and 32% of patients for hypothetical MEM MICs of 16, and 32 mg/L, respectively. The relationship between T > 40% 1×MIC and either actual or hypothetical MEM MICs is further detailed in .
Figure 1. Achievement of T > 40% 1×MIC by measured meropenem levels in 19 critically-ill patients with BSI due to KPC-Kp, according to different hypothetical meropenem MICs. Actual meropenem MICs of all 19 isolates turned out to be far higher than those compatible with the achievement of such a PK/PD target, ranging from 256 to 1024 mg/L.
Additionally, possible synergisms between MEM and coadministered antibacterials were evaluated. Results concerning the activities of administered combinations are detailed in . Although in 18/19 cases (95%) 3-drug combinations determined a further reduction in colony counts when compared with the most effective double-antibiotic combinations, no synergism was detected. Similarly, in subgroup analyses the 2-drug combinations of tigecycline plus colistin and meropenem plus gentamicin seemed to perform better than their counterparts (meropenem plus colistin and tigecycline plus gentamicin, respectively), but the overall effect was indifferent.
Table 3. In vitro interactions of administered antibacterials by time-kill methods against blood Klebsiella pneumoniae carbapenemase-producing Klebsiella pneumoniae isolates of 19 critically-ill patients.
Discussion
High-dose MEM administered as an extended 3-hour infusion did not achieve any of the predefined PK/PD targets in 19 critically-ill patients with BSI due KPC-Kp with high MEM MICs, ranging from 256 to 1024 mg/L. In addition, no synergism was found between MEM and coadministered agents by time-kill assays.
MEM has been reported to improve survival from infections caused by KPC-Kp with slightly increased MEM MICs, if administered in combination with one or more active agents and despite in vitro nonsusceptibility (MICs > 2 mg/L).Citation13-15,17,18 On the basis of a literature review, Daikos et al. first suggested that this approach could improve survival of patients in case of isolates with MEM MICs of 4 mg/L.Citation18 Then, Tumbarello et al. confirmed these findings in an Italian multicenter cohort of 125 patients with KPC-Kp BSI.Citation14 Recently, results from the same cohort have been updated.Citation17 Among 661 patients with KPC-Kp infections, mostly BSI, MEM-based combinations significantly improved survival when MEM MICs were > 2 and ≤ 8 mg/L.Citation17 Similar results were found in a Greek study of 167 patients with KPC-Kp BSI.Citation15 However, it should be noted that in all these studies no effort was made to evaluate the actual MEM MIC of isolates with MIC above 8 mg/L, since higher values were lumped together. In reality, MEM MICs > 8 mg/L might include several MIC levels ranging from 16 mg/L to likely much higher values. This possibly prevented the authors from observing a beneficial effect on survival for MEM MICs slightly exceeding 8 mg/L, and the issue remains controversial. Although only large dedicated studies, preferably randomized clinical trials, can definitely address this issue, valuable theoretical evidence in support or against the use of MEM in such a gray zone could be provided by targeted PK/PD analyses.
In our selected population of critically-ill patients with KPC-Kp BSI, high-dose MEM did not achieve either T > 40% 1×MIC or T > 40% 4×MIC in any of the enrolled subjects. Since MEM plasma levels were far below those necessary for the achievement of these targets, the possibility of any activity appears very unlikely for KPC-Kp with MEM MICs as high as 256-1024 mg/L, even by taking into account the high inter-patient variability of PK parameters in critically-ill subjects.Citation30,31 Nonetheless, it is worth noting that T > 40% 1×MIC could have been theoretically attained in a fairly large patient proportion of our patients for hypothetical MEM MICs of 8 and 16 mg/L (95% and 68%, respectively) and still in 32% of cases with MIC up to 32 mg/L. Although even these hypothetical MICs would have not allowed to reach T > 40% 4×MIC, which is necessary for MEM to exert an optimal bactericidal activity according to some literature data,Citation32,33 our results suggest that MEM might retain some bactericidal activity per se against KPC-Kp with MEM MICs up to 32 mg/L, albeit not at its maximum and only in some cases.
It should be noted that possible synergisms between MEM and coadministered agents could have supported the use of MEM in our patients even if PK/PD targets were not reached. This possibility is in line with previous reports of synergistic effects between MEM and either colistin or tigecycline.Citation34-37 However, we did not detect any synergism between MEM and coadministered agents against the isolates collected from our patients. Of note, although with the inherent limits of approximation and static model, we tried to individualize time-kill assays on the basis of PK/isolate pairs, in order to better reproduce the pharmacodynamics of antibiotic interactions and increase their clinical relevancy.
We should acknowledge that the 30-day mortality in the patients included in this study is lower than that reported in other studies, including ours,Citation7,14-18 and also seems incoherent with our PK/PD findings. However, this study was not designed for studying the direct effect of MEM MICs on mortality, but to provide PK/PD data as a first step in the process. The low mortality we found in this small group of patients might well be due to chance. Indeed, no related statistically or clinically significant conclusion could be obtained from a study including only 19 patients, and information about mortality was just reported for descriptive reasons. Hopefully, our PK/PD results could be an aid for designing larger clinical studies which can adequately address this critical issue.
This study has some important limitations. First, our results should be considered tentative, since the number of cases was too limited for broad generalization. Another important limitation is that our analysis was limited to plasma concentrations of MEM, and both the attainment of PK/PD targets and MEM interactions with other agents might be different in patients with foci of infection other than blood.
In conclusion, high-dose MEM failed to reach PK/PD targets in patients with BSI due to KPC-Kp with very high MEM MICs, ranging from 256 to 1024 mg/L. Our results also suggest that MEM-based combinations might not offer any benefit in terms of bactericidal activity in comparison to non MEM-based regimens in case of resistant blood isolates with MEM MIC higher than 32 mg/L. For these reasons, we think MEM use in treatment of BSI due to KPC-Kp with MEM MICs > 16 mg/L should rely on more detailed information about actual MEM MICs of locally prevalent KPC-Kp clones.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Munoz-Price LS, Poirel L, Bonomo RA, Schwaber MJ, Daikos GL, Cormican M, Cornaglia G, Garau J, Gniadkowski M, Hayden MK, et al. Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. Lancet Infect Dis 2013; 13:785-96; PMID:23969216; http://dx.doi.org/10.1016/S1473-3099(13)70190-7
- Nordmann P, Dortet L, Poirel L. Carbapenem resistance in Enterobacteriaceae: here is the storm! Trends Mol Med 2012; 18:263-72; PMID:22480775; http://dx.doi.org/10.1016/j.molmed.2012.03.003
- Hirsch EB, Tam VH. Detection and treatment option for Klebsiella pneumoniae carbapenemases (KPCs): an emerging cause of multidrug-resistant infection. J Antimicrob Chemother 2010; 65:1119-25; PMID:20378670; http://dx.doi.org/10.1093/jac/dkq108
- Giacobbe DR, Del Bono V, Marchese A, Viscoli C. Early carbapenem-resistant Klebsiella pneumoniae bacteraemia: should we expand the screening? Clin Microbiol Infect 2014; 20:O1157-O1158; PMID:25366247; http://dx.doi.org/10.1111/1469-0691.12804
- Gupta N, Limbago BM, Patel JB, Kallen AJ. Carbapenem-resistant Enterobacteriaceae: epidemiology and prevention. Clin Infect Dis 2011; 53:60-7; PMID:21653305; http://dx.doi.org/10.1093/cid/cir202
- Marchain D, Chopra T, Pogue JM, Perez F, Hujer AM, Rudin S, Endimiani A, Navon-Venezia S, Hothi J, Slim J, et al. Outbreak of colistin-resistant, carbapenem-resistant Klebsiella pneumoniae in metropolitan Detroit, Michigan. Antimicrob Agents Chemother 2011; 55:593-9; PMID:21115786; http://dx.doi.org/10.1128/AAC.01020-10
- Alicino C, Giacobbe DR, Orsi A, Tassinari F, Trucchi C, Sarteschi G, Copello F, Del Bono V, Viscoli C, Icardi G. Trends in the incidence of carbapenem-resistant Klebsiella pneumoniae bloodstream infections: a 8-year retrospective study in a large teaching hospital in northern Italy. BMC Infect Dis 2015; 15:415; PMID:26464061; http://dx.doi.org/10.1186/s12879-015-1152-0
- Giani T, Arena F, Vaggelli G, Conte V, Chiarelli A, Henrici De Angelis L, Fornaini R, Grazzini M, Niccolini F, Pecile P, et al. Large Nosocomial Outbreak of Colistin-Resistant, Carbapenemase-Producing Klebsiella pneumoniae Traced to Clonal Expansion of an mgrB Deletion Mutant. J Clin Microbiol 2015; 53:3341-4; PMID:26202124; http://dx.doi.org/10.1128/JCM.01017-15
- Endimiani A, Hujer AM, Perez F, Bethel CR, Hujer KM, Kroeger J, Oethinger M, Paterson DL, Adams MD, Jacobs MR, et al. Characterization of blaKPC-containing Klebsiella Pneumoniae isolates detected in different institutions in the eastern USA. J Antimicrob Chemother 2009; 63:427-37; PMID:19155227; http://dx.doi.org/10.1093/jac/dkn547
- Jiang Y, Wei Z, Wang Y, Hua X, Feng Y, Yu Y. Tracking a hospital outbreak of KPC-producing ST11 Klebsiella pneumoniae with whole genome sequencing. Clin Microbiol Infect 2015; 21:1001-7; PMID:26166545; http://dx.doi.org/10.1016/j.cmi.2015.07.001
- Kontopidou F, Giamarellou H, Katerelos P, Maragos A, Kioumis I, Trikka-Graphakos E, Valakis C, Maltezou HC. Group for the Study of KPC-producing Klebsiella pneumoniae infections in intensive care units. Infections caused by carbapenem-resistant Klebsiella pneumoniae among patients in intensive care units in Greece: a multi-centre study on clinical outcome and therapeutic options. Clin Microbiol Infect 2014; 20:O117-O123; PMID:23992130; http://dx.doi.org/10.1111/1469-0691.12341
- Corcione S, Rocchetti A, Argentero PA, Raso R, Zotti CM, De Rosa FG, Ghisetti V. A one-year survey of carbapenemase-producing Klebsiella pneumoniae in Italy: beyond the ICU. Clin Microbiol Infect 2015; 21:e11-e13; PMID:25682279; http://dx.doi.org/10.1016/j.cmi.2014.09.012
- Qureshi ZA, Paterson DL, Potoski BA, Kilayko MC, Sandovsky G, Sordillo E, Polsky B, Adams-Haduch JM, Doi Y. Treatment outcome of bacteremia due to KPC-producing Klebsiella pneumoniae: superiority of combination antimicrobial regimens. Antimicrob Agents Chemother 2012; 56:2108-13; PMID:22252816; http://dx.doi.org/10.1128/AAC.06268-11
- Tumbarello M, Viscoli C, Viale P, Trecarichi EM, Tumietto F, Marchese A, Spanu T, Ambretti S, Ginocchio F, Cristini F, et al. Predictors of mortality in bloodstream infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae: importance of combination therapy. Clin Infect Dis 2012; 55:943-50; PMID:22752516
- Daikos GL, Tsaousi S, Tzouvelekis LS, Anyfantis I, Psichogiou M, Argyropoulou A, Stefanou I, Sypsa V, Miriagou V, Nepka M, et al. Carbapenemase-producing Klebsiella pneumoniae bloodstream infections: lowering mortality by antibiotic combination schemes and the role of carbapenems. Antimicrob Agents Chemother 2014; 58:2322-8; PMID:24514083
- Giacobbe DR, Del Bono V, Trecarichi EM, De Rosa FG, Giannella M, Bassetti M, Bartoloni A, Losito AR, Corcione S, Bartoletti M, et al. Risk factors for bloodstream infections due to colistin-resistant KPC-producing Klebsiella pneumoniae: results from a multicenter case-control-control study. Clin Microbiol Infect 2015; 21:1106.e1-8; PMID:26278669
- Tumbarello M, Trecarichi EM, De Rosa FG, Giannella M, Giacobbe DR, Bassetti M, Losito AR, Bartoletti M, Del Bono V, Corcione S, et al. Infections caused by KPC-producing Klebsiella pneumoniae: differences in therapy and mortality in a multicentric study. J Antimicrob Chemother 2015; 70:2133-43; PMID:25900159
- Daikos GL, Markogiannakis A. Carbapenemase-producing Klebsiella pneumoniae: (when) might we still consider treating with carbapenem? Clin Microbiol Infect 2011; 17:1135-41; PMID:21635663
- Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med 2003; 31:1250-6; PMID:12682500
- Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron 1976; 16:31-41; PMID:1244564
- Nicolau DP, Freeman CD, Belliveau PP, Nightingale CH, Ross JW, Quintiliani R. Experience with a Once-Daily Aminoglycoside Program Administered to 2,184 Adult Patients. Antimicrob Agents Chemother 1995; 39:650-5; PMID:7793867
- Plachouras D, Karvanen M, Friberg LE, Papadomichelakis E, Antoniadou A, Tsangaris I, Karaiskos I, Poulakou G, Kontopidou F, Armaganidis A, et al. Population pharmacokinetic analysis of colistin methanesulfonate and colistin after intravenous administration in critically ill patients with infections caused by gram-negative bacteria. Antimicrob Agents Chemother 2009; 53:3430-6; PMID:19433570
- Legrand T, Chhun S, Rey E, Blanchet B, Zahar JR, Lanternier F, Pons G, Jullien V. Simultaneous determination of three carbapenem antibiotics in plasma by HPLC with ultraviolet detection. J Chromatogr B Analyt Technol Biomed Life Sci 2008; 875:551-6; PMID:18848512
- International Conference on Harmonization (ICH). Harmonised tripartite guideline: Validation of analytical procedures: text and methodology Q2(R1) 6 November 1996. ICH Official web site. Available:http://www.ich.org. Accessed 2015 May 8
- Shah VP, Midha KK, Dighe S. Conference report: analytical methods validation: bioavailability, bioequivalence, and pharmacokinetic studies. J Pharm Sci-US 1992; 81:309-12
- Kim MK, Xuan D, Quintiliani R, Nightingale CH, Nicolau DP. Pharmacokinetic and pharmacodynamic profile of high dose extended interval piperacillin-tazobactam. J Antimicrob Chemother 2001; 48:259-67; PMID:114-81298
- Marchese A., Coppo E, Barbieri R, Debbia E. Emergence of KPC-2 carbapenemase-producing Klebsiella pneumoniae strains and spread of an isolate of sequence type 258 in the neuro-rehabilitation unit of an Italian hospital. J Chemother 2010; 22:212-4; PMID:20566429
- Gaibani P, Lombardo D, Lewis RE, Mercuri M, Bonora S, Landini MP, Ambretti S. In vitro activity and post-antibiotic effects of colistin in combination with other antimicrobials against colistin-resistant KPC-producing Klebsiella pneumoniae bloodstream isolate. J Antimicrob Chemother 2014; 69:1856-65; PMID:24648503
- Goncalves-Pereira J, Martins A, P∫voa P. Parmacokinetics of gentamicin in critically ill patients: pilot study evaluating the first dose. Clin Microbiol Infect 2010; 16:1258-63; PMID:19832713
- Nicolau DP. Pharmacokinetic and pharmacodynamics properties of meropenem. Clin Infect Dis 2008; 47(Suppl 1):32-40
- Binder L, Schwörer H, Hoppe S, Streit F, Neumann S, Beckmann A, Wachter R, Oellerich M, Walson PD. Pharmacokinetics of meropenem in critically ill patients with severe infections. Ther Drug Monit 2013; 35:63-70; PMID:23318279
- Taccone FS, Lattere P, Dugernier T, Spapen H, Delattre I, Wittebole X, De Backer D, Layeux B, Wallemacq P, Vincent JL, et al. Insufficient β-lactam concentrations in the early phase of severe sepsis and septic shock. Critical Care 2010; 14:R126; PMID:20594297
- Drusano GL. Antimicrobial pharmacodynamics: critical interactions of ‘bug and drug’. Nat Rev Microbiol 2004; 2:289-300; PMID:15031728
- Pankey GA, Ashcraft DS. The detection of synergy between meropenem and polymyxin B against meropenem-resistant Acinetobacter baumannii using Etest and time-kill assay. Diagn Microbiol Infect Dis 2009; 63:228-32; PMID:19150711
- Pankey GA, Ashcraft DS. Detection of synergy using the combuination of polymyxin B with either meropenem or rifampin against carbapenemase-producing Klebsiella pneumoniae. Diagn Microbiol Infect Dis 2011; 70:561-4; PMID:21767715
- Petersen PJ, Labthavikul P, Jones CH, Bradford PA. In vitro antibacterial activities of tigecyline in combination with other antimicrobial agents determined by chequerboard and time-kill kinetic analysis. J Antimicrob Chemother 2006; 57:573-6; PMID:16431863
- Vouillamoz J, Moreillon P, Giddey M, Entenza JM. In vitro activities of tigecycline combined with other antimicrobials against multiresistant Gram-positive and Gram-negative pathogens. J Antimicrob Chemother 2008; 61:371-4; PMID:18033780
