ABSTRACT
Cytomegalovirus (CMV) remains a major contributor to morbidity and mortality following allogeneic haemopoietic stem cell transplant (HSCT) despite widespread use of viraemia monitoring and pre-emptive antiviral therapy. Uncontrolled viral replication occurs primarily in the first 100 d post transplant but this high risk period can extend to many months if immune recovery is delayed. The re-establishment of a functional population of cellular effectors is essential for control of virus replication and depends on recipient and donor serostatus, the stem cell source, degree of HLA matching and post-transplant factors such as CMV antigen exposure, presence of GVHD and ongoing use of immune suppression. A number of immune monitoring assays exist but have not yet become widely accessible for routine clinical use. Vaccination, adoptive transfer of CMV specific T cells and a number of graft engineering processes are being evaluated to enhance of CMV specific immune recovery post HSCT.
Introduction
Cytomegalovirus (CMV) is a betaherpesvirus that maintains lifelong latency after primary infection. 50 to 90% of the population are CMV seropositive, although this figure varies with age and geographic location. CMV reactivates when host immunity wanes in the context of haemopoietic stem cell transplantation (HSCT) and uncontrolled viral replication can lead to disease in a number of tissues. Despite improvements in monitoring and pre-emptive therapy with antiviral agents, CMV remains a major contributor to post-transplant infectious and overall morbidity and mortality.Citation1 Recovery of immune function is essential to control of reactivation. Here we review what is known about CMV immunity post HSCT, how it can be measured and interventions that may facilitate more rapid immune recovery.
The clinical impact of CMV post-allogeneic HSCT
In recipients of HSCT, CMV is one of the major pathogens responsible for infectious morbidity and mortality. Viraemia usually precedes clinically significant tissue injury. Uncontrolled viral proliferation can cause pneumonitis, gastritis, enteritis, colitis, hepatitis, bone marrow suppression, retinitis and encephalitis.Citation1 CMV disease is defined as the presence of symptoms and signs consistent with CMV end organ infection together with detection of the virus by a validated method including immunohistochemistry or viral culture. PCR positivity in tissue without other evidence of disease is not considered diagnostic of CMV disease.Citation1 CMV infection in HSCT occurs primarily due to reactivation of latent infection in recipients who are seropositive pre transplant. Primary infection of seronegative HSCT recipients occurs largely due to infusion of a stem cell product from a seropositive donor. Transfusion related primary infection is now rare due to the introduction of screening for CMV serostatus of blood donors and the use of leucodepleted blood products.Citation2 The majority of CMV reactivation is observed in the first 100 d post transplant, but late CMV has also become more frequent as a result of conditioning regimens or graft vs. host disease that produce prolonged immune suppression, and prophylactic or pre-emptive therapy that delay immune reconstitution.Citation3-6
Surveillance for viral reactivation and pre-emptive therapy with ganciclovir or foscarnet for asymptomatic viraemia is the most widely accepted approach to management of CMV post HSCT. Therapy is instituted with the aim of limiting viral replication prior to the development of organ damage. Routine surveillance for the presence of CMV viraemia is performed regularly during the period of high risk up to 100 days, and extended in cases of GVHD and ongoing immunosuppression. Ganciclovir used at the onset of detectable viraemia has been shown to significantly reduce the incidence of CMV disease at 100 d when compared with placebo.Citation7 In clinical practice the threshold viral load to trigger antiviral therapy may vary depending on the presence of risk factors such as the type of transplant, the use of corticosteroids or presence of GVHD and physician preference. Threshold triggers are not currently standardised and it is not known whether withholding antiviral therapy may be safe in some subgroups of patients with CMV reactivation such as those with measurable CMV specific cellular immunity. A recent single center retrospective analysis of a CMV monitoring and pre-emptive therapy regimen in 926 transplants with seropositive donors or recipients demonstrates the ongoing burden of CMV. Post-transplant CMV was monitored using quantitative PCR and a treatment threshold viral load of 150IU/ml (using the WHO standard). The CMV reactivation rate was 69% in the first 100 d. Pre-emptive antiviral pharmacotherapy was initiated in almost all these patients but progression to high viral titer (>500 IU/ml) and CMV disease was not prevented in all patients. The cumulative incidence of CMV disease was 11% at one year. The direct CMV disease mortality was low (1%) but overall mortality in patients who reactivated was higher than in those that did not.Citation8 The use of antiviral agents as a pre-emptive strategy or as treatment in such a large proportion of patients comes with significant draw-backs. It is ineffective in some cases due to viral resistance.Citation9,10 Ganciclovir is myelosuppressive while foscarnet is nephrotoxic and causes renal tubular acidosis and electrolyte disturbance.Citation11 The burden of cost of monitoring and treatment with currently available antivirals is high. The overall increment of cost of CMV reactivation compared to no reactivation is estimated in the tens of thousands of dollars.Citation12
Widespread adoption of surveillance and pre-emptive treatment has led to a fall in the number of patients progressing to CMV disease, most commonly pneumonitis which still has a fatality rate of 50%, from 70–90% prior to the availability of antiviral pharmacotherapy.Citation4,13-15 Nonetheless, patients who are seropositive for CMV or who receive transplants from CMV seropositive donors continue to suffer an excess of non-relapse mortality after transplant.Citation16-21
Given the impact of CMV post transplant, measures aimed at prevention and early detection of CMV in HSCT are warranted. Primary prevention includes the early assessment of recipient CMV status, with the use of leukodepleted blood products for all seronegative recipients. Due to the impact on transmission and risk of CMV infection, as well as CMV-specific immune reconstitution (as discussed later), CMV status is an important consideration in HSCT donor selection, particularly in unrelated donor transplantation. Guidelines recommend prioritising CMV seronegative donors for CMV seronegative recipients, and CMV seropositive donors for CMV seropositive recipients.Citation19,21,22
There is a clear need for alternative therapies for CMV. A number of pharmaceutical alternatives are under investigation. Brincidofovir, a lipid conjugated prodrug of cidofovir, showed strong antiviral activity in preclinical and early phase clinical trials. While a significant reduction in the incidence of CMV was shown in a phase III trial, the therapeutic window was narrow with unacceptable gastrointestinal side effects seen at higher dosages.Citation23 Maribavir, a benzimidazole antiviral agent that inhibits viral replication, did not meet the endpoint of prevention of CMV disease.Citation24 Letermovir demonstrates dose dependent antiviral activity and a favorable safety profile and a phase III study is underway.Citation25
CMV immunity in the normal individual
CMV infection results in the development of an adaptive immune response involving humoral and cellular factors. The relative importance of humoral immunity in humans is not clear, with conflicting indirect evidence. Clinical observations imply that humoral immunity at best plays only a part in the control of CMV. The presence of high titer antibodies has been associated with protection from neonatal transmission of the virusCitation26,27 and with improved outcomes in HSCT recipients with CMV reactivation in one study,Citation28 but not in another.Citation29 Hyperimmune globulin used to treat pregnant women with primary CMV infection improves foetal outcomes and abrogates placental pathology,Citation30,31 and is used in solid organ transplant to reduce primary infection. Use of hyperimmune CMV globulin in HSCT recipients is not supported by strong evidence. Nonetheless, it is commonly administered as an adjunct to ganciclovir in pneumonitis where mortality remains high.Citation32
There is overwhelming clinical evidence of the central role of the cellular immune system in defense against CMV. Cellular immune deficiency and predisposition to CMV infection is seen in HSCT recipients,Citation1 advanced HIV infectionCitation33 and in patients treated with lympholytic chemotherapy such as the purine analogs (fludarabine and cladrabine) or the anti-CD52 antibody alemtuzumab.Citation34-36 Reconstitution of CMV cell mediated immunity via adoptive transfer of CMV specific immune effectors has been shown to control CMV infection in both animal modelsCitation37,38 and human trials.Citation39-47
In otherwise healthy individuals, both CD8+ and CD4+ cells targeting multiple CMV peptides are important in the control of infection. The proportion of the immune response devoted to CMV increases with age in seropositive individuals. CMV specific cells comprise up to 10% of circulating CD8+ cells in seropositive individuals,Citation48 a disproportionate allocation given the large number of pathogens to which the immune system must respond. The immunodominant proteins pp65, pp50, glycoproteins IE-1 and IE-2 account for the majority of the T cell repertoire and subdominant responses are present to other CMV proteins including glycoprotein-H and pp28.Citation49-51 CD8+ cells recognize epitopes of CMV proteins in a predictable manner that is HLA determined. The major tegument protein pp65 and immediate early protein-1 (IE-1) are the most extensively studied immune targets in HSCT recipients.
CD4+ cells also play a major role in the control of CMV. Target proteins include pp65, glycoproteins B and H, IE72, IE86 and UL69.Citation52,53 Evidence of the importance of CD4+ cells can be derived from clinical situations in which CD4+ lymphopenia is present, such as HIV infectionCitation33 and in renal transplant recipients where CD4+ lymphopenia is particularly associated with CMV reactivation.Citation54 There is evidence that CD8+ CMV specific cells are insufficient to control CMV in the absence of CD4+ cells.Citation55,56
CMV immunity in HSCT recipients
In the HSCT population, measurable T cell recovery occurs within the first few months of transplant. Risk from CMV is highest in the first 100 d when immunity is in the process of recovery after exposure to lympholytic agents in pre-transplant chemotherapy and transplant conditioning regimens, and in some cases T cell depletion, either ex vivo or in vivo. The number and quality of T cells transferred in the stem cell graft varies with the source (peripheral blood stem cells > bone marrow > cord blood). In patients undergoing spontaneous immune recovery post HSCT both CD8+ and CD4+ T cell subsets recover together, and are quantitatively correlated with control of CMV reactivation.Citation57 Adoptive immunotherapy trials support the assertion that both CD4+ and CD8+ cells are important. Infusion of CD8+ clones was effective in clearing CMV, but cells did not persist in patients who did not develop a CMV specific CD4+ response.Citation58 Einsele demonstrated that by infusing CD4+ cells alone, CD8+ cells were generated in vivo.Citation59
Lack of recovery of immune function in the context of reactivation is associated with prolonged CMV viraemia and adverse outcomes.Citation57,60-62 This is impacted by donor serostatus (faster with D+), donor source (faster with matched related bone marrow, slowest with cord blood transplant), degree of match (slower with mismatch) and conditioning regimen (slower with T cell depletion). Numeric recovery of CMV specific immunity is not sufficient to control viral replication. The capacity to produce multiple cytokines and establish longevity in the recipient (compartmentalised according to functional memory subsets) is also required.Citation63,64
The reactivating CMV virus strains are generally of recipient origin, and control is mediated by donor derived immune effectors.Citation65-67 This explains the differential risk according to donor and recipient serotype. The highest risk group is seropositive recipients (R+) with seronegative donors (D−) in which reactivation occurs in up to 80% of cases. R+/D+ are at moderate risk, R−/D+ at lower risk, with reactivation rate less than 10%. Primary infection in R−/D− transplants is rare.Citation19,60,68-70 In D−R+ scenarios immune recovery is slower but does occur, with evidence that immune recovery is mediated by naïve donor T cells derived from progenitors in the graft.Citation66 It has traditionally been held that T cells from the transplant donor are the sole source of CMV control as recipient immune effectors are ablated by the transplant process. This may be the case for myeloablative transplants but there is evidence that in stem cell transplants conditioned with reduced intensity protocols recipient CMV specific T cells contribute to CMV immunity, particular early after transplant before achievement of full lymphoid chimerism.Citation71,72
Factors influencing CMV immunity post transplant
The immune recovery process is dynamic and influenced by post-transplant events. There is a bidirectional relationship of viraemia to immune recovery. While immune recovery is required for control, the presence of antigen stimulation is required to stimulate clonal proliferation of antigen specific T cells. Seronegative recipients are less likely to develop detectable immunity presumably because the lack of viral reactivation in the recipient does not provide a source of antigen stimulation.Citation57,66,73 In adoptive cell therapy studies, in vivo T cell expansion is associated with episodes of detectable antigenaemia.Citation46 In the absence of GVHD, CMV specific immune recovery is stable and long-lasting. GVHD and associated treatment adversely affect immune recovery and can lead to prolonged problems with CMVCitation74,57 (see ).
Figure 1. CMV immune recovery post-allogeneic HSCT. (A and B) Absence of CMV reactivation does not stimulate clonal expansion of CMV specific T cell clones and detectable CMV immunity is low or undetectable. When CMV-VSTs are administered prophylactically no expansion of the transferred clones is observed. (C and D) Low level CMV reactivation is controlled by CMV-VSTs that recover in the first few months post-HSCT. Prophylactic or pre-emptively administered CMV-VSTs are seen to expand in vivo and produce long-lasting stable immunity that is detectable up to 10 y after transplant. (E) CMV immunity recovers and controls CMV without treatment in the first few months. Subsequent development of GVHD and administration of corticosteroids and other immune suppressive medications results in loss of CMV immunity and recurrent CMV reactivation requires treatment with antiviral pharmacotherapy. (F) After failure to establish an effective cellular immune response spontaneously, either due to treatment (as in E) or donor seronegativity, donor-derived or third party banked CMV-VSTs administered therapeutically can rescue patients refractory to standard therapies.
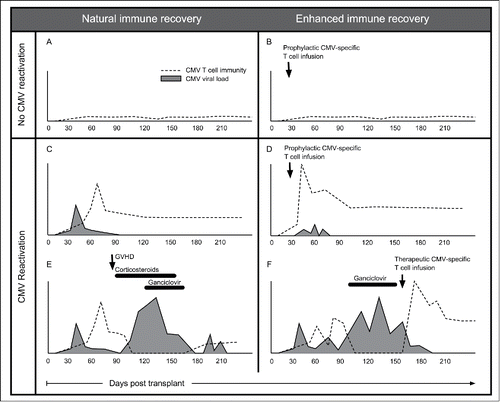
During viral reactivation, massive in vivo expansion of CMV specific clones occurs to the detriment of other immune subsets, such as naïve T cell subsets including recent thymic emigrants.Citation75 These expanded clones are largely CMV specifiC-terminally differentiated effector cells as measured by gene expression analysis and TCR sequencing of single cells sorted by flow cytometry.Citation74 Early clonal expansion produces a period of oligoclonality with a small number of CMV specific clones comprising a large proportion of all CD8+ cells early post transplant. It is possible that this represents expansion of clones transferred with the graft on exposure to antigen in the host.Citation74 Later in the recovery process the TCR diversity expands with new clones derived from stem cell graft progenitors.Citation66 CMV serostatus has a strong impact on the pattern of global immune recovery, such as the ratio of B cells to T and NK cells, in addition to the well known effects on T lymphocytes.Citation76 NK cell subsets recover early post transplant and are involved in the response to CMV, as are Vδ2-negative γδT cells.Citation77,78 It is likely that NK cells contribute to control of CMV infection with expansions of IFN-γ producing NK cell populations reported in response to and after resolution of CMV reactivation post HSCT.Citation79,80
While CMV is the most common virus to cause clinical problems post transplant, concurrent infection with more than one double stranded DNA virus is common in a number of transplant settings, particularly following cord blood and T cell depleted transplant, and is associated with adverse outcomes such as increased overall mortality.Citation81 Reactivation of one or more viruses may reflect global cellular immune deficiencyCitation81-84 or may stem from the fact that CMV infection itself results in contraction of the immune repertoire post transplant thus predisposing to other viral infections.Citation75,81 It is not possible to distinguish these possibilities using presently available information. Recovery of CMV specific immune function has been postulated as a biomarker for overall immune recovery but no causal link between this single pathogen-specific and global immune recovery has been shown.Citation85
There is a growing body of evidence that polymorphisms in the genes encoding molecules involved in the CMV-host interaction affect the incidence and natural history of CMV post transplant. These include SNP (single nucleotide polymorphisms) in the chemokine receptor 5 (CCR5), monocyte chemoattractant protein 1 (MCP-1), interleukin (IL) 10, toll-like receptors (TLR) 8 and 9, dendritic cell-specific molecule-3-grabbing non-integrin (DC-SIGN) and IL-28B genes. SNPs in these genes in donor and recipient have been associated with the occurrence of CMV reactivation, its duration, the peak levels of CMV DNAaemia and the development of CMV disease.Citation86-89 To date study findings have been inconsistent, limited by small numbers and mechanistic data is lacking. Larger confirmatory studies are required before recipient and donor SNPs can be to used for risk stratification or to guide therapeutic interventions.
Detailed assessment of global immune recovery post transplant is currently underway using powerful tools such as multidimensional flow cytometry and mass cytometry. Availability of new methods for single cell analysis, gene expression and bioinformatics tools to map TCR will facilitate ever more detailed interrogation of post-transplant immune function.
Measurement of CMV specific immune function in the clinic
A variety of methods are available to characterize CMV specific immunity. Fluorescently-labeled MHC multimers loaded with individual immunodominant CMV epitopes which bind with high affinity to the TCR allow for specific and rapid identification of CMV specific T cells using multiparameter flow cytometry. However, this approach is limited by the HLA type, knowledge of individual epitopes, the frequency of multimer positive cells and will only give an indication of a proportion of the immune response. Multimer-based assessment provides no indication of functional responsiveness, and thus numeric thresholds may provide false reassurance of immunity in the early post-HSCT population.Citation90-92 Functional assays include the enzyme linked immunoassay (ELISPOT),Citation60 cytokine production assaysCitation57,93,94 and cytotoxicity assays (including Chromium release, degranulation assays or direct vizualization assays).Citation95 ELISPOT has the advantage of reproducibility and is semi-quantitative so it can show change in immune function over time. However it is limited by the lack of individual cellular phenotype information and the need to standardise assays performed at different times. Cytotoxicity assays require large cell numbers and are relevant only for research purposes. Quantitative flow cytometry assays use intracellular flow cytometry to measure production or expression of IFN-γ, TNF, CD107 and IL-2 from CMV-stimulated PBMCs; reacting cells are then quantified using absolute CD3+, 4+ and 8+ cell counts. Lilleri et al used quantitative flow cytometry to assess immune reconstitution over time in 131 patients.Citation57 This study had a relatively high threshold CMV copy number for initiation of pre-emptive treatment (>30,000 copies/µl) so is helpful in understanding the natural history of CMV immune recovery in the absence of therapeutic intervention. It should be noted that the majority of patients were young recipients of bone marrow grafts and few had cord blood or T cell depleted transplants that are associated with higher risk of uncontrolled CMV infection. This study showed that patients with CMV specific immunity above a predetermined cut off (1 and 3 CMV specific CD4+ and CD8+ cells/µl, respectively) were able to control reactivation without the need for antiviral chemotherapy. The only failures were associated with treatment for GVHD. Time to development of both CD4+ and CD8+ immunity was correlated with time to control of CMV. Similar observations have been made in other studies utilizing quantitative or semi-quantitative methods for measurement of CMV immunity.Citation57,93,94
All of these methods are technically demanding and standardisation has not been achieved thus far. Studies using cut-off thresholds for CMV immunity are therefore not easily applied in routine clinical use. The only assay approved for clinical use for CMV immune monitoring is the QuantiFERON-CMV (Qiagen, Valencia, CA, USA), where whole blood is stimulated with CMV peptides and IFN-γ release is quantified by ELISA. This assay is analogous to the QuantiFERON-TB assay that has been widely adopted for tuberculosis. Although QantiFERON-CMV is less sensitive than flow cytometry assays,Citation96 it is simple and reproducible and has been validated in HSCT recipients with similar results to other immune monitoring assays.Citation97 There are no studies that yet conclusively demonstrate the safety of ceasing CMV monitoring based solely on the demonstration of CMV immunity. A future challenge is to integrate assessments of immune recovery into clinical algorithms of CMV management identifying patients in whom monitoring can be reduced or eliminated and in whom pre-emptive antiviral therapy can be safely withheld.
Interventions to improve CMV immune reconstitution
Given the impact on mortality and cost, attention has focused on ways of improving CMV specific immunity as a means of improving overall outcome in transplant recipients.
Vaccination
The observation that antigenaemia is associated with improved CMV specific immune reconstitution implies that vaccination with antigen may improve immunity early post transplant at the time when patients are at risk of CMV.Citation98 No CMV vaccine has regulatory approval for prevention of CMV infection in normal individuals but a number of vaccine candidates are under investigation. A recombinant glycoprotein B vaccine was trialled in seronegative and seropositive solid organ transplant recipients prior to transplant with significant improvement shown in antibody titres in both serogroups, along with a reduction in duration of viraemia and antiviral treatment required in D+/R− patients who reactivated post transplant.Citation99 ASP0113 is a bivalent vaccine containing 2 plasmids that encode CMV glycoprotein B and tegument protein PP65. In a phase 2 randomized placebo trial of ASP0113 in which 40 allo HSCT CMV seropositive patients were administered the vaccine prior to conditioning and at 1, 3 and 6 mths post transplant there was a reduction in overall CMV viraemia, delay in viremic onset and reduced risk of recurrence. However there was no reduction in use of antiviral therapy and no significant improvement in measured CMV specific immunity.Citation100 A planned HSCT donor vaccination arm for this study had to be abandoned due to logistical problems with identifying and vaccinating donors prior to transplant. This compound is now under investigation in a phase 3 study with planned recruitment of 500 participants (Clinicaltrials.gov ID NCT01877655). An alternative vaccine candidate is a peptide vaccine conjugated to a toll like receptor agonist that has been administered to 18 HLA-A201 CMV seropositive allogeneic stem cell transplant recipients at day 28 and day 56 as part of a randomized phase 1 clinical trial. A CMV-specific CD8+ T cell response was noted without an antibody response and both risk of CMV reactivation and duration of CMV antiviral therapy were reduced compared to the control arm. An increase in relapse-free survival was also demonstrated.Citation101
Adoptive transfer of CMV specific T cells
Infusion of unmanipulated donor lymphocytes after transplant can improve antiviral immunity but it does so at the cost of increased GVHD.Citation102 CMV specific immune reconstitution via adoptive transfer of ex vivo isolated or expanded donor derived virus specific T cells (CMV-VSTs) has now been performed successfully in a number of clinical trials.Citation39-47,58,59 CMV-VSTs can be manufactured in a variety of ways. Time consuming ex vivo culture methods using limiting dilution cloning or EBV transformed lymphocytes as antigen presenting cells (APCs) have largely been replaced with more rapid techniques using alternative APCs (activated monocytes, rapidly matured monocyte derived DCs or artificial APCs), various antigen sources and shorter culture times. Ex vivo culture duration can range from 10 d to a number of weeks. Direct isolation methods using activation markers, cytokine capture or multimer selection are able to generate a clinical product within 48 hours. There are no studies comparing these methods directly, but it appears that cellular persistence depends on the presence of CD4+ cells either within the adoptive cell product or generated from the stem cell graft.Citation103 Adoptive cell therapy appears safe, with no evidence that it increases the risk of GVHD in single arm studies and a cohort study.Citation104 Two randomized studies of prophylactic/preemptive adoptive immunotherapy have been performed using direct capture methods for generation of donor-derived CMV VSTs (CMV∼IMPACT and CMV∼ASPECT) that have been reported in abstract form.Citation105,106 Adoptive therapy appeared to be safe in both studies, and a significant expansion of CMV-specific cells was reported over controls for the ASPECT study. However, reductions in the rates of reactivation, recurrence and duration of therapy in the IMPACT study failed to reach significance, likely contributed to by a lower than expected CMV event rate in the control group.
Routine prophylaxis with transplant donor-derived CMV-VSTs is currently expensive, and may not be needed in patients who would never reactivate CMV. Thus, a pre-emptive strategy using rapidly generated CMV-VSTs may be a preferable approach although the methodology for cell generation is not yet widely available. An alternative approach is to use partially-HLA matched banked third party CMV-VSTs. To date, the treatment of over 75 patients with active CMV infection and disease utilizing banked non-donor derived unrelated (third party) CMV-VSTs have been reported.Citation107-110 The 3 largest studies showed a high complete response rate, and did not flag any safety concerns; in particular the rate of GVHD was not higher than expected. In contrast to donor-derived adoptive transfer, these third party partially-matched donor cells do not appear to persist beyond a few weeks, yet effect long-term CMV control. With a median follow up of 6 months, few patients required retreatment with antiviral therapy after the final dose of third party cells.Citation109,110 Further studies of third party cells are needed to elucidate the mechanisms of this clinical effect.
Engineering of the graft to improve immune recovery
As an alternative to the use of VSTs, graft engineering strategies may maintain antiviral immunity while reducing the risk of GVHD associated with transplantation of unmanipulated stem cell products. These include add-back of ex vivo allodepleted T cells and naïve T cell depletion of stem cell products via CD45RA or TCRα/β depletion.Citation111-113 Incorporation of safety switches into T cells prior to infusion allows selective deletion of gene-modified cells if GVHD occurs.Citation114,115 Other immunomodulatory approaches to GVHD include induction of anergy or administration of regulatory T cells but the effect of these strategies on CMV immunity has not been studied.Citation116,117
CMV immunity and AML relapse
Donor seropositivity for CMV has been associated with lower relapse rate in AML in a number of observational studies dating back to the mid 1980s.Citation118-123 CMV reactivation in patients undergoing transplant for AML was associated with reduced relapse risk with a proposed hypothesis of immune mediated anti-leukemic activity. In response to these studies the CIBMTR has performed an analysis of the impact of CMV reactivation on relapse in 9,469 patients with haematological malignancies, including 5,310 with AML.Citation19 No association was found between CMV reactivation before day 100 and reduced risk of relapse. An association of CMV reacivation with reduced AML relapse risk in a transplant subgroup cannot be absolutely excluded. In contrast, CMV reactivation was associated with increased transplant related mortality and reduced overall survival.
Conclusion and future directions
With few exceptions, studies show that recovery of both CD4+ and CD8+ cellular immunity are required for protection from CMV viral replication. CMV seropositive recipients with donors who are seropositive for CMV have fewer, shorter and later viraemic episodes compared with those with seronegative donors. Regular monitoring with pre-emptive antiviral therapy has reduced the rate of CMV disease and direct mortality but at a cost of morbidity and financial burden. Despite clear efficacy in minimizing CMV tissue infection, pre-emptive anti-CMV therapy has not eliminated the adverse effect of CMV reactivation on non-relapse and overall mortality and CMV remains a major contributor to poor post-transplant outcomes. It is not yet clear which patients with CMV reactivation can safely be left to clear virus without pharmacological intervention. The use of more specific measurements of immunity may facilitate better case selection for pharmacotherapy. Graft engineering strategies promise to preserve graft mediated anti-CMV activity but more rapid reconstitution of CMV immunity after transplant seems most likely to result from donor and/or recipient vaccination and adoptive immunotherapy.
Abbreviations
| CMV | = | cytomegalovirus |
| CMV-VSTs | = | CMV virus specific T cells |
| GVHD | = | graft versus host disease |
| HSCT | = | haemopoietic stem cell transplant |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Ljungman P, Hakki M, Boeckh M. Cytomegalovirus in hematopoietic stem cell transplant recipients. Hematol Oncol Clin North Am 2011; 25:151-69; http://dx.doi.org/10.1016/j.hoc.2010.11.011
- Thiele T, Krüger W, Zimmermann K, Ittermann T, Wessel A, Steinmetz I, Dölken G, Greinacher A. Transmission of cytomegalovirus (CMV) infection by leukoreduced blood products not tested for CMV antibodies: a single-center prospective study in high-risk patients undergoing allogeneic hematopoietic stem cell transplantation (CME). Transfusion 2011; 51:2620-6; PMID:21645009; http://dx.doi.org/10.1111/j.1537-2995.2011.03203.x
- Gandhi MK, Khanna R. Human cytomegalovirus: clinical aspects, immune regulation, and emerging treatments. Lancet Infect Dis 2004; 4:725-38; PMID:15567122; http://dx.doi.org/10.1016/S1473-3099(04)01202-2
- Boeckh MJ, Leisenring W, Riddell SR, Bowden RA, Huang ML, Myerson D, Stevens-Ayers T, Flowers ME, Cunningham T, Corey L. Late cytomegalovirus disease and mortality in recipients of allogeneic hematopoietic stem cell transplants: importance of viral load and T-cell immunity. Blood 2003; 101:407-14; PMID:12393659; http://dx.doi.org/10.1182/blood-2002-03-0993
- Ozdemir E, Saliba RM, Champlin RE, Couriel DR, Giralt SA, de Lima M, Khouri IF, Hosing C, Kornblau SM, Anderlini P, et al. Risk factors associated with late cytomegalovirus reactivation after allogeneic stem cell transplantation for hematological malignancies. Bone Marrow Transplantation 2007; 40:125-36; PMID:17530009; http://dx.doi.org/10.1038/sj.bmt.1705699
- Nguyen Q, Champlin R, Giralt S, Rolston K, Raad I, Jacobson K, Ippoliti C, Hecht D, Tarrand J, Luna M, et al. Late cytomegalovirus pneumonia in adult allogeneic blood and marrow transplant recipients. Clin Infect Dis 1999; 28:618-23; PMID:10194088; http://dx.doi.org/10.1086/515146
- Goodrich JM, Mori M, Gleaves CA, Du Mond C, Cays M, Ebeling DF, Buhles WC, DeArmond B, Meyers JD. Early treatment with ganciclovir to prevent cytomegalovirus disease after allogeneic bone marrow transplantation. N Eng J Med 1991; 325:1601-7; PMID:1658652; http://dx.doi.org/10.1056/NEJM199112053252303
- Green ML, Leisenring W, Stachel D, Pergam SA, Sandmaier BM, Wald A, Corey L, Boeckh MJ. Efficacy of a viral load-based, risk-adapted, preemptive treatment strategy for prevention of Cytomegalovirus disease after hematopoietic cell transplantation. Biol Blood Marrow Transplantation 2012; 18:1687-99; PMID:22683614; http://dx.doi.org/10.1016/j.bbmt.2012.05.015
- Erice A, Gil-Roda C, Pérez JL, Balfour HH, Sannerud KJ, Hanson MN, Boivin G, Chou S. Antiviral susceptibilities and analysis of UL97 and DNA polymerase sequences of clinical cytomegalovirus isolates from immunocompromised patients. J Infect Dis 1997; 175:1087-92; PMID:9129070; http://dx.doi.org/10.1086/516446
- Herling M, Schröder L, Awerkiew S, Chakupurakal G, Holtick U, Kaiser R, Pfister H, Scheid C, Di Cristanziano V. Persistent CMV infection after allogeneic hematopoietic stem cell transplantation in a CMV-seronegative donor-to-positive recipient constellation: Development of multidrug resistance in the absence of anti-viral cellular immunity. J Clin Virol 2016; 74:57-60; PMID:26672492; http://dx.doi.org/10.1016/j.jcv.2015.11.033
- Paul S, Dummer S. Topics in clinical pharmacology: ganciclovir. Am J Med Sci 1992; 304:272-7; PMID:1329509; http://dx.doi.org/10.1097/00000441-199210000-00010
- Jain NA, Lu K, Ito S, Muranski P, Hourigan CS, Haggerty J, Chokshi PD, Ramos C, Cho E, Cook L, et al. The clinical and financial burden of pre-emptive management of cytomegalovirus disease after allogeneic stem cell transplantation—implications for preventative treatment approaches. Cytotherapy 2014; 16:927-33; PMID:24831837; http://dx.doi.org/10.1016/j.jcyt.2014.02.010
- Schmidt GM, Horak DA, Niland JC, Duncan SR, Forman SJ, Zaia JA. A randomized, controlled trial of prophylactic ganciclovir for cytomegalovirus pulmonary infection in recipients of allogeneic bone marrow transplants; The City of Hope-Stanford-Syntex CMV Study Group. N Eng J Med 1991; 324:1005-11; PMID:1848679; http://dx.doi.org/10.1056/NEJM199104113241501
- Konoplev S, Champlin RE, Giralt S, Ueno NT, Khouri I, Raad I, Rolston K, Jacobson K, Tarrand J, Luna M, et al. Cytomegalovirus pneumonia in adult autologous blood and marrow transplant recipients. Bone Marrow Transplantation 2001; 27:877-81; PMID:11477447; http://dx.doi.org/10.1038/sj.bmt.1702877
- Green ML, Leisenring W, Xie H, Mast TC, Cui Y, Sandmaier BM, Sorror ML, Goyal S, Ozkok S, Yi J, et al. Cytomegalovirus viral load and mortality after haemopoietic stem cell transplantation in the era of pre-emptive therapy: a retrospective cohort study. Lancet Haematol 2016; 3(3):e119-27: 1–9
- Broers AE, van Der Holt R, van Esser JW, Gratama JW, Henzen-Logmans S, Kuenen-Boumeester V, Löwenberg B, Cornelissen JJ. Increased transplant-related morbidity and mortality in CMV-seropositive patients despite highly effective prevention of CMV disease after allogeneic T-cell-depleted stem cell transplantation. Blood 2000; 95:2240-5; PMID:10733491
- Craddock C, Szydlo RM, Dazzi F, Olavarria E, Cwynarski K, Yong A, Brookes P, de la Fuente J, Kanfer E, Apperley JF, et al. Cytomegalovirus seropositivity adversely influences outcome after T-depleted unrelated donor transplant in patients with chronic myeloid leukaemia: the case for tailored graft-versus-host disease prophylaxis. Br J Haematol 2001; 112:228-36; PMID:11167809; http://dx.doi.org/10.1046/j.1365-2141.2001.02519.x
- Boeckh MJ, Nichols WG. The impact of cytomegalovirus serostatus of donor and recipient before hematopoietic stem cell transplantation in the era of antiviral prophylaxis and preemptive therapy. Blood 2004; 103:2003-8; PMID:14644993; http://dx.doi.org/10.1182/blood-2003-10-3616
- Teira P, Battiwalla M, Ramanathan M, Barrett AJ, Ahn KW, Chen M, Green JS, Saad A, Antin JH, Savani BN, et al. Early cytomegalovirus reactivation remains associated with increased transplant related mortality in the current era: a CIBMTR analysis. Blood 2016; 127(20):2427-38: blood-2015-11-679639-41
- Ljungman P, Brand R, Einsele H, Frassoni F, Niederwieser D, Cordonnier C. Donor CMV serologic status and outcome of CMV-seropositive recipients after unrelated donor stem cell transplantation: an EBMT megafile analysis. Blood 2003; 102:4255-60; PMID:12933590; http://dx.doi.org/10.1182/blood-2002-10-3263
- Ljungman P, Brand R, Hoek J, de la Cámara R, Cordonnier C, Einsele H, Styczynski J, Ward KN, Cesaro S. Transplantation IDWPotEGfBaM. Donor cytomegalovirus status influences the outcome of allogeneic stem cell transplant: a study by the European group for blood and marrow transplantation. Clin Infect Dis 2014; 59:473-81; PMID:24850801; http://dx.doi.org/10.1093/cid/ciu364
- Schmidt-Hieber M, Labopin M, Beelen D, Volin L, Ehninger G, Finke J, Socie G, Schwerdtfeger R, Kröger N, Ganser A, et al. CMV serostatus has still an important prognostic impact in de novo acute leukemia patients after allogeneic stem cell transplantation: a report from the acute leukemia working party of EBMT. Blood 2013; 122(19):3359-64; PMID:24037724
- Marty FM, Winston DJ, Rowley SD, Vance E, Papanicolaou GA, Mullane KM, Brundage TM, Robertson AT, Godkin S, Momméja-Marin H, et al. CMX001 to prevent cytomegalovirus disease in hematopoietic-cell transplantation. N Eng J Med 2013; 369:1227-36; PMID:24066743; http://dx.doi.org/10.1056/NEJMoa1303688
- Marty FM, Ljungman P, Papanicolaou GA, Winston DJ, Chemaly RF, Strasfeld L, Young JA, Rodriguez T, Maertens J, Schmitt M, et al. Maribavir prophylaxis for prevention of cytomegalovirus disease in recipients of allogeneic stem-cell transplants: a phase 3, double-blind, placebo-controlled, randomised trial. Lancet Infect Dis 2011; 11:284-92; PMID:21414843; http://dx.doi.org/10.1016/S1473-3099(11)70024-X
- Chemaly RF, Ullmann AJ, Stoelben S, Richard MP, Bornhäuser M, Groth C, Einsele H, Silverman M, Mullane KM, Brown J, et al. Letermovir for cytomegalovirus prophylaxis in hematopoietic-cell transplantation. N Eng J Med 2014; 370:1781-9; PMID:24806159; http://dx.doi.org/10.1056/NEJMoa1309533
- Boppana SB, Britt WJ. Antiviral antibody responses and intrauterine transmission after primary maternal cytomegalovirus infection. J Infect Dis 1995; 171:1115-21; PMID:7751685; http://dx.doi.org/10.1093/infdis/171.5.1115
- Nigro G, Adler SP, La Torre R, Best AM, Group CC. Passive immunization during pregnancy for congenital cytomegalovirus infection. N Eng J Med 2005; 353:1350-62; PMID:16192480; http://dx.doi.org/10.1056/NEJMoa043337
- Schoppel K, Schmidt C, Einsele H, Hebart H, Mach M. Kinetics of the antibody response against human cytomegalovirus-specific proteins in allogeneic bone marrow transplant recipients. J Infect Dis 1998; 178:1233-43; PMID:9780241; http://dx.doi.org/10.1086/314428
- Volpi A, Pica F, Gentile G, Capobianchi A, Fraschetti M, Martino P. Neutralizing antibody response against human cytomegalovirus in allogeneic bone marrow-transplant recipients. J Infect Dis 1999; 180:1747-8; PMID:10515847; http://dx.doi.org/10.1086/315092
- Adler SP, Nigro G. Findings and conclusions from CMV hyperimmune globulin treatment trials. J Clin Virol 2009; 46:S54-7; PMID:19781985; http://dx.doi.org/10.1016/j.jcv.2009.08.017
- Maidji E, Nigro G, Tabata T, McDonagh S, Nozawa N, Shiboski S, Muci S, Anceschi MM, Aziz N, Adler SP, et al. Antibody treatment promotes compensation for human cytomegalovirus-induced pathogenesis and a hypoxia-like condition in placentas with congenital infection. Am J Pathol 2010; 177:1298-310; PMID:20651234; http://dx.doi.org/10.2353/ajpath.2010.091210
- Jacobsen N, Schäfer U, Ostendorf P, Kubaneck B, Wolf H. Intravenous hyperimmune globulin prophylaxis against cytomegalovirus interstitial pneumonitis after allogenic bone marrow transplantation. Tokai J Exp Clin Med 1985; 10:193-5; PMID:3010510
- Yust I, Fox Z, Burke M, Johnson A, Turner D, Mocroft A, Katlama C, Ledergerber B, Reiss P, Kirk O, et al. Retinal and extraocular cytomegalovirus end-organ disease in HIV-infected patients in Europe: a EuroSIDA study, 1994-2001. Eur J Clin Microbiol Infect 2004; 23:550-9
- Montillo M, Tedeschi A, Petrizzi VB, Ricci F, Crugnola M, Spriano M, Spedini P, Ilariucci F, Uziel L, Attolico I, et al. An open-label, pilot study of fludarabine, cyclophosphamide, and alemtuzumab in relapsed/refractory patients with B-cell chronic lymphocytic leukemia. Blood 2011; 118:4079-85; PMID:21772050; http://dx.doi.org/10.1182/blood-2011-05-351833
- Grigg A, Chapman R, Szer J. Fatal CMV pneumonia associated with steroid therapy after autologous transplantation in patients previously treated with fludarabine. Bone Marrow Transplantation 1998; 21:619-21; PMID:9543067; http://dx.doi.org/10.1038/sj.bmt.1701126
- Orlandi EM, Baldanti F, Citro A, Pochintesta L, Gatti M, Lazzarino M. Monitoring for cytomegalovirus and Epstein-Barr virus infection in chronic lymphocytic leukemia patients receiving i.v. fludarabine-cyclophosphamide combination and alemtuzumab as consolidation therapy. Haematologica 2008; 93:1758-60; PMID:18790800; http://dx.doi.org/10.3324/haematol.13265
- Berger C, Berger M, Anderson D, Riddell SR. A non-human primate model for analysis of safety, persistence, and function of adoptively transferred T cells. J Medical Primatol 2011; 40:88-103; http://dx.doi.org/10.1111/j.1600-0684.2010.00451.x
- Wang X, Berger C, Wong CW, Forman SJ, Riddell SR, Jensen MC. Engraftment of human central memory-derived effector CD8+ T cells in immunodeficient mice. Blood 2011; 117:1888-98; PMID:21123821; http://dx.doi.org/10.1182/blood-2010-10-310599
- Riddell SR, Greenberg P. Therapeutic reconstitution of human viral immunity by adoptive transfer of cytotoxic T lymphocyte clones. Curr Topics Microbiol Immunol 1994; 189:9-34; PMID:7924439
- Micklethwaite KP, Hansen A, Foster AE, Snape E, Antonenas V, Sartor M, Shaw P, Bradstock K, Gottlieb DJ. Ex-Vivo expansion and Prophylactic infusion of CMV-pp65 peptide specific cytotoxic T-Lymphocytes following allogeneic haemopoietic stem cell transplantation. Biol Blood Marrow Transplantation 2007; 13:707-14; PMID:17531781; http://dx.doi.org/10.1016/j.bbmt.2007.02.004
- Micklethwaite KP, Clancy L, Sandher U, Hansen A, Blyth E, Antonenas V, Sartor M, Bradstock K, Gottlieb DJ. Prophylactic infusion of cytomegalovirus-specific cytotoxic T lymphocytes stimulated with Ad5f35pp65 gene-modified dendritic cells after allogeneic hemopoietic stem cell transplantation. Blood 2008; 112:3974; PMID:18768783; http://dx.doi.org/10.1182/blood-2008-06-161695
- Rooney CM, Smith C, Ng CY, Loftin SK, Sixbey JW, Gan Y, Srivastava DK, Bowman LC, Krance RA, Brenner MK, et al. Infusion of cytotoxic T cells for the prevention and treatment of Epstein-Barr virus-induced lymphoma in allogeneic transplant recipients. Blood 1998; 92:1549-55; PMID:9716582
- Leen AM, Myers GD, Sili U, Huls MH, Weiss H, Leung KS, Carrum G, Krance RA, Chang CC, Molldrem JJ, et al. Monoculture-derived T lymphocytes specific for multiple viruses expand and produce clinically relevant effects in immunocompromised individuals. Nat Med 2006; 12:1160-6; PMID:16998485; http://dx.doi.org/10.1038/nm1475
- Peggs KS, Thomson K, Samuel E, Dyer G, Armoogum J, Chakraverty R, Pang K, Mackinnon S, Lowdell MW. Directly selected cytomegalovirus-reactive donor T cells confer rapid and safe systemic reconstitution of virus-specific immunity following stem cell transplantation. Clin Infect Dis 2011; 52:49-57; PMID:21148519; http://dx.doi.org/10.1093/cid/ciq042
- Peggs KS. Adoptive T cell immunotherapy for cytomegalovirus. Exp Opin Biol Therapy 2009; 9:725-36; PMID:19456207; http://dx.doi.org/10.1517/14712590902967588
- Blyth E, Clancy L, Simms R, Ma CK, Burgess J, Deo S, Byth K, Dubosq M-C, Shaw PJ, Micklethwaite KP, et al. Donor-derived CMV-specific T cells reduce the requirement for CMV-directed pharmacotherapy after allogeneic stem cell transplantation. Blood 2013; 121:3745-58; PMID:23435462; http://dx.doi.org/10.1182/blood-2012-08-448977
- Clancy L, Blyth E, Simms R, Micklethwaite KP, Ma CK, Burgess J, Antonenas V, Shaw P, Gottlieb DJ. CMV-specific cytotoxic T lymphocytes can be efficiently expanded from G-CSF mobilised haemopoietic progenitor cell products ex vivo and safely transferred to stem cell transplant recipients to facilitate immune reconstitution. Biol Blood Marrow Transplantation 2013; 19(5): 725-734.
- Sylwester AW, Mitchell BL, Edgar JB, Taormina C, Pelte C, Ruchti F, Sleath PR, Grabstein KH, Hosken NA, Kern F, et al. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J Exp Med 2005; 202:673-85; PMID:16147978; http://dx.doi.org/10.1084/jem.20050882
- Wills MR, Okecha G, Weekes MP, Gandhi MK, Sissons PJ, Carmichael AJ. Identification of naive or antigen-experienced human CD8(+) T cells by expression of costimulation and chemokine receptors: analysis of the human cytomegalovirus-specific CD8(+) T cell response. J Immunol 2002; 168:5455-64; PMID:12023339; http://dx.doi.org/10.4049/jimmunol.168.11.5455
- Maecker H, Maino V. Analyzing t-cell responses to cytomegalovirus by cytokine flow cytometry. Human Immunology 2004; 65:493-9; PMID:15172449; http://dx.doi.org/10.1016/j.humimm.2004.02.004
- Wills MR, Carmichael AJ, Mynard K, Jin X, Weekes MP, Plachter B, Sissons JG. The human cytotoxic T-lymphocyte (CTL) response to cytomegalovirus is dominated by structural protein pp65: frequency, specificity, and T-cell receptor usage of pp65-specific CTL. J Virol 1996; 70:7569-79; PMID:8892876
- Beninga J, Kropff B, Mach M. Comparative analysis of fourteen individual human cytomegalovirus proteins for helper T cell response. J General Virol 1995; 76:153-60; PMID:7844526; http://dx.doi.org/10.1099/0022-1317-76-1-153
- Beninga J, Kalbacher H, Mach M. Analysis of T helper cell response to glycoprotein H (gpUL75) of human cytomegalovirus: evidence for strain-specific T cell determinants. J Infect Dis 1996; 173:1051-61; PMID:8627054; http://dx.doi.org/10.1093/infdis/173.5.1051
- Sund F, Lidehäll A-K, Claesson K, Foss A, Tötterman TH, Korsgren O, Eriksson BM. CMV-specific T-cell immunity, viral load, and clinical outcome in seropositive renal transplant recipients: a pilot study. Clin Transplantation 2010; 24:401-9; PMID:19222507; http://dx.doi.org/10.1111/j.1399-0012.2009.00976.x
- Gamadia LE, Rentenaar RJ, Baars PA, Remmerswaal EB, Surachno S, Weel JF, Toebes M, Schumacher TN, ten Berge IJ, van Lier RA. Differentiation of cytomegalovirus-specific CD8(+) T cells in healthy and immunosuppressed virus carriers. Blood 2001; 98:754-61; PMID:11468176; http://dx.doi.org/10.1182/blood.V98.3.754
- Gamadia LE, Remmerswaal EB, WeelJF, Bemelman F, van Lier RA, ten Berge IJ. Primary immune responses to human CMV: a critical role for IFN-gamma-producing CD4+ T cells in protection against CMV disease. Blood 2003; 101:2686-92; PMID:12411292; http://dx.doi.org/10.1182/blood-2002-08-2502
- Lilleri D, Gerna G, Zelini P, Chiesa A, Rognoni V, Mastronuzzi A, Giorgiani G, Zecca M, Locatelli F. Monitoring of human cytomegalovirus and virus-specific T-cell response in young patients receiving allogeneic hematopoietic stem cell transplantation. PloS One 2012; 7:e41648; PMID:22848556; http://dx.doi.org/10.1371/journal.pone.0041648
- Riddell SR, Walter BA, Gilbert MJ, Greenberg PD. Selective reconstitution of CD8+ cytotoxic T lymphocyte responses in immunodeficient bone marrow transplant recipients by the adoptive transfer of T cell clones. Bone Marrow Transplantation 1994; 14:S78-84; PMID:7728132
- Einsele H, Roosnek E, Rufer N, Sinzger C, Riegler S, Loffler J, Grigoleit GU, Moris A, Rammensee H-G, Kanz L, et al. Infusion of cytomegalovirus (CMV)-specific T cells for the treatment of CMV infection not responding to antiviral chemotherapy. Blood 2002; 99:3916-22; PMID:12010789; http://dx.doi.org/10.1182/blood.V99.11.3916
- Ganepola S, Gentilini C, Hilbers U, Lange T, Rieger K, Hofmann J, Maier M, Liebert UG, Niederwieser D, Engelmann E, et al. Patients at high risk for CMV infection and disease show delayed CD8+ T-cell immune recovery after allogeneic stem cell transplantation. Bone Marrow Transplantation 2007; 39:293-9; PMID:17262060; http://dx.doi.org/10.1038/sj.bmt.1705585
- Tormo N, Solano C, Benet I, Clari MA, Nieto J, de la Camara R, López J, López-Aldeguer N, Hernández-Boluda JC, Remigia MJ, et al. Lack of prompt expansion of cytomegalovirus pp65 and IE-1-specific IFNgamma CD8+ and CD4+ T cells is associated with rising levels of pp65 antigenemia and DNAemia during pre-emptive therapy in allogeneic hematopoietic stem cell transplant recipients. Bone Marrow Transplantation 2010; 45:543-9; PMID:19617905; http://dx.doi.org/10.1038/bmt.2009.172
- Tormo N, Solano C, Benet I, Nieto J, de la Cámara R, Garcia-Noblejas A, Clari MÁ, Chilet M, López J, Hernández-Boluda JC, et al. Kinetics of cytomegalovirus (CMV) pp65 and IE-1-specific IFNgamma CD8+ and CD4+ T cells during episodes of viral DNAemia in allogeneic stem cell transplant recipients: potential implications for the management of active CMV infection. J Medical Virol 2010; 82:1208-15; PMID:20513086; http://dx.doi.org/10.1002/jmv.21799
- Scheinberg P, Melenhorst JJ, Brenchley JM, Hill BJ, Hensel NF, Chattopadhyay PK, Roederer M, Picker LJ, Price DA, Barrett AJ, et al. The transfer of adaptive immunity to CMV during hematopoietic stem cell transplantation is dependent on the specificity and phenotype of CMV-specific T cells in the donor. Blood 2009; 114:5071-80; PMID:19776383; http://dx.doi.org/10.1182/blood-2009-04-214684
- Luo XH, Huang XJ, Liu KY, Xu LP, Liu DH. Protective immunity transferred by infusion of CMV-specific CD8+ T cells within donor graftsits associations with CMV reactivation following unmanipulated allogeneic hematopoietic stem cell transplantation CMV-specific CD8+ T cells within donor grafts. Biol Blood Marrow Transplantation 2010; 16(7):994-1004
- Winston DJ, Huang ES, Miller MJ, Lin CH, Ho WG, Gale RP, Champlin RE. Molecular epidemiology of cytomegalovirus infections associated with bone marrow transplantation. Annals Internal Med 1985; 102:16-20; PMID:2981496; http://dx.doi.org/10.7326/0003-4819-102-1-16
- Gandhi MK, Wills MR, Okecha G, Day EK, Hicks R, Marcus RE, Sissons JG, Carmichael AJ. Late diversification in the clonal composition of human cytomegalovirus-specific CD8+ T cells following allogeneic hemopoietic stem cell transplantation. Blood 2003; 102:3427-38; PMID:12869514; http://dx.doi.org/10.1182/blood-2002-12-3689
- Gandhi MK, Wills MR, Sissons JG, Carmichael AJ. Human cytomegalovirus-specific immunity following haemopoietic stem cell transplantation. Blood Rev 2003; 17:259-64; PMID:14556781; http://dx.doi.org/10.1016/S0268-960X(03)00028-6
- Nichols WG, Corey L, Gooley T, Davis C, Boeckh M. High risk of death due to bacterial and fungal infection among cytomegalovirus (CMV)-seronegative recipients of stem cell transplants from seropositive donors: Evidence for indirect effects of primary CMV infection. J Infect Dis 2002; 185:273-82; PMID:11807708; http://dx.doi.org/10.1086/338624
- Ljungman P, Aschan J, Lewensohn-Fuchs I, Carlens S, Larsson K, Lonnqvist B, Mattsson J, Sparrelid E, Winiarski J, Ringden O. Results of different strategies for reducing: Cytomegalovirus-associated mortality in allogeneic stem cell transplant recipients. Transplantation 1998; 66:1330-4; PMID:9846518; http://dx.doi.org/10.1097/00007890-199811270-00012
- Broers AE, van Der Holt R, van Esser JW, Gratama JW, Henzen-Logmans S, Kuenen-Boumeester V, Lowenberg B, Cornelissen JJ. Increased transplant-related morbidity and mortality in CMV-seropositive patients despite highly effective prevention of CMV disease after allogeneic T-cell-depleted stem cell transplantation. Blood 2000; 95:2240-5; PMID:10733491
- Sellar RS, Vargas FA, Henry JY, Verfuerth S, Charrot S, Beaton B, Chakraverty R, Quezada SA, Mackinnon S, Thomson KJ, et al. CMV promotes recipient T-cell immunity following reduced-intensity T-cell-depleted HSCT, significantly modulating chimerism status. Blood 2015; 125:731-9; PMID:25499763; http://dx.doi.org/10.1182/blood-2014-07-589150
- Stevanović S, van Bergen CA, van Luxemburg-Heijs SA, van der Zouwen B, Jordanova ES, Kruisselbrink AB, van de Meent M, Harskamp JC, Claas FH, Marijt EW, et al. HLA class II upregulation during viral infection leads to HLA-DP-directed graft-versus-host disease after CD4+ donor lymphocyte infusion. Blood 2013; 122:1963-73; PMID:23777765; http://dx.doi.org/10.1182/blood-2012-12-470872
- Oliveira G, Ruggiero E, Stanghellini MT, Cieri N, D'Agostino M, Fronza R, Lulay C, Dionisio F, Mastaglio S, Greco R, et al. Tracking genetically engineered lymphocytes long-term reveals the dynamics of T cell immunological memory. Sci Translational Med 2015; 7:317ra198; PMID:26659572
- Link CS, Eugster A, Heidenreich F, Rücker-Braun E, Schmiedgen M, Oelschlägel U, Kühn D, Dietz S, Fuchs Y, Dahl A, et al. Abundant CMV reactive clonotypes in the CD8(+) T cell receptor α repertoire following allogeneic transplantation. Clin Exp Immunol 2016; 184(3):389-402: n/a-n/a; PMID:26800118
- Suessmuth Y, Mukherjee R, Watkins B, Koura DT, Finstermeier K, Desmarais C, Stempora L, Horan JT, Langston A, Qayed M, et al. CMV reactivation drives posttransplant T-cell reconstitution and results in defects in the underlying TCRβ repertoire. Blood 2015; 125:3835-50; PMID:25852054; http://dx.doi.org/10.1182/blood-2015-03-631853
- Itzykson R, Robin M, Moins-Teisserenc H, Delord M, Busson M, Xhaard A, de Fontebrune FS, Peffault de Latour R, Toubert A, Socie G. Cytomegalovirus shapes long-term immune reconstitution after allogeneic stem cell transplantation. Haematologica 2015; 100:114-23; PMID:25261095; http://dx.doi.org/10.3324/haematol.2014.113415
- Knight A, Madrigal AJ, Grace S, Sivakumaran J, Kottaridis P, Mackinnon S, Travers PJ, Lowdell MW. The role of Vδ2-negative γδ T cells during cytomegalovirus reactivation in recipients of allogeneic stem cell transplantation. Blood 2010; 116:2164-72; PMID:20576814; http://dx.doi.org/10.1182/blood-2010-01-255166
- Storek J. Immunological reconstitution after hematopoietic cell transplantation - its relation to the contents of the graft. Exp Opin Biol Therapy 2008; 8:583-97; PMID:18407763; http://dx.doi.org/10.1517/14712598.8.5.583
- Foley B, Cooley S, Verneris MR, Pitt M, Curtsinger J, Luo X, Lopez-Vergès S, Lanier LL, Weisdorf D, Miller JS. Cytomegalovirus reactivation after allogeneic transplantation promotes a lasting increase in educated NKG2C+ natural killer cells with potent function. Blood 2012; 119:2665-74; PMID:22180440; http://dx.doi.org/10.1182/blood-2011-10-386995
- Muñoz-Cobo B, Solano C, Benet I, Costa E, Remigia MJ, de la Cámara R, Nieto J, López J, Amat P, Garcia-Noblejas A, et al. Functional profile of cytomegalovirus (CMV)-specific CD8+ T cells and kinetics of NKG2C+ NK cells associated with the resolution of CMV DNAemia in allogeneic stem cell transplant recipients. J Medical Virol 2012; 84:259-67; http://dx.doi.org/10.1002/jmv.22254
- Schabert VF, Mozaffari E, Lee YC, Casciano R. Double-stranded DNA (dsDNA) viral infections among allogeneic hematopoietic cell transplant (HCT) recipients in the first year after transplant. Blood 2015; 126:3296
- Huang Y-TH, Kim SJ, Lee YJ, Burack D, Nichols P, Maloy M, Tamari R, Perales MA, Giralt SA, Castro-Malaspina HR, et al. Infection burden of double stranded DNA (dsDNA) viruses after CD34+ selected, T-Cell depleted (TCD) hematopoietic cell transplantation (HCT) for myeloid malignancies at memorial sloan kettering cancer center (MSK). Blood 2015; 126:4320; http://dx.doi.org/10.1182/blood-2015-05-646687
- Mozaffari E, Lin J, Lingohr-Smith M. Hospitalizations and mortality associated with dsDNA viral infections among hematopoietic cell transplant recipients. Blood 2015; 126:2073
- Hill JA, Mayer BT, Xie H, Leisenring WM, Milano F, Delaney C, Huang ML, Stevens-Ayers TL, Jerome KR, Nichols G, et al. Detection of multiple Double-Stranded DNA viruses after cord blood transplantation is frequent and persistent. Blood 2015; 126:3104
- Noviello M, Forcina A, Veronica V, Crocchiolo R, Stanghellini MT, Carrabba M, Greco R, Vago L, Giglio F, Assanelli A, et al. Early recovery of CMV immunity after HLA-haploidentical hematopoietic stem cell transplantation as a surrogate biomarker for a reduced risk of severe infections overall. Bone Marrow Transplantation 2015; 50:1262-4; PMID:26076126; http://dx.doi.org/10.1038/bmt.2015.132
- Bravo D, Solano C, Giménez E, Remigia MJ, Corrales I, Amat P, Navarro D. Effect of the IL28B Rs12979860 C/T polymorphism on the incidence and features of active cytomegalovirus infection in allogeneic stem cell transplant patients. J Medical Virol 2014; 86:838-44; PMID:24374819; http://dx.doi.org/10.1002/jmv.23865
- Corrales I, Giménez E, Solano C, Amat P, de la Cámara R, Nieto J, Garcia-Noblejas A, Navarro D. Incidence and dynamics of active cytomegalovirus infection in allogeneic stem cell transplant patients according to single nucleotide polymorphisms in donor and recipient CCR5, MCP-1, IL-10, and TLR9 genes. J Medical Virol 2015; 87:248-55; PMID:25132583; http://dx.doi.org/10.1002/jmv.24050
- Mezger M, Steffens M, Semmler C, Arlt E-M, Zimmer M, Kristjanson G-I, Wienker TF, Toliat MR, Kessler T, Einsele H, et al. Investigation of promoter variations in dendritic cell-specific ICAM3-grabbing non-integrin (DC-SIGN) (CD209) and their relevance for human cytomegalovirus reactivation and disease after allogeneic stem-cell transplantation. Clin Microbiol Infect 2008; 14:228-34; PMID:18076668; http://dx.doi.org/10.1111/j.1469-0691.2007.01902.x
- Xiao HW, Luo Y, Lai XY, Shi JM, Tan YM, He JS, Xie WZ, Zheng WY, Ye XJ, Yu XH, et al. Donor TLR9 gene tagSNPs influence susceptibility to aGVHD and CMV reactivation in the allo-HSCT setting without polymorphisms in the TLR4 and NOD2 genes. Bone Marrow Transplantation 2014; 49:241-7; PMID:24121213; http://dx.doi.org/10.1038/bmt.2013.160
- Ozdemir E, St John LS, Gillespie G, Rowland-Jones S, Champlin RE, Molldrem JJ, Komanduri KV. Cytomegalovirus reactivation following allogeneic stem cell transplantation is associated with the presence of dysfunctional antigen-specific CD8+ T cells. Blood 2002; 100:3690-7; PMID:12393402; http://dx.doi.org/10.1182/blood-2002-05-1387
- Guerrero A, Riddell SR, Storek J, Stevens-Ayers T, Storer B, Zaia JA, Forman S, Negrin RS, Chauncey T, Bensinger W, et al. Cytomegalovirus viral load and virus-specific immune reconstitution after peripheral blood stem cell versus bone marrow transplantation. Biol Blood Marrow Transplantation 2012; 18:66-75; PMID:21664286; http://dx.doi.org/10.1016/j.bbmt.2011.05.010
- Morita-Hoshi Y, Heike Y, Kawakami M, Sugita T, Miura O, Kim S-W, Mori S-I, Fukuda T, Tanosaki R, Tobinai K, et al. Functional analysis of cytomegalovirus-specific T lymphocytes compared to tetramer assay in patients undergoing hematopoietic stem cell transplantation. Bone Marrow Transplantation 2008; 41:515-21; PMID:18026143; http://dx.doi.org/10.1038/sj.bmt.1705932
- Gimenez E, Muñoz-Cobo B, Solano C, Amat P, de la Camara R, Nieto J, López J, Remigia MJ, Garcia-Noblejas A, Navarro D. Functional patterns of cytomegalovirus (CMV) pp65 and immediate early-1-specific CD8(+) T cells that are associated with protection from and control of CMV DNAemia after allogeneic stem cell transplantation. Transplant Infect Dis 2015; 17:361-70; PMID:25850900; http://dx.doi.org/10.1111/tid.12391
- Pelák O, Stuchlý J, Król L, Hubacek P, Keslová P, Sedláček P, Formánková R, Starý J, Hrušák O, Kalina T. Appearance of CMV specific T-cells predicts fast resolution of viremia post hematopoietic Stem cell transplantation. Cytometry B Clin Cytom 2015: n/a-n/a
- Lacey SF, Gallez-Hawkins G, Crooks M, Martinez J, Senitzer D, Forman SJ, Spielberger R, Zaia JA, Diamond DJ. Characterization of cytotoxic function of CMV-pp65-specific CD8+ T-lymphocytes identified by HLA tetramers in recipients and donors of stem-cell transplants. Transplantation 2002; 74:722-32; PMID:12352893; http://dx.doi.org/10.1097/00007890-200209150-00023
- Clari MÁ, Muñoz-Cobo B, Solano C, Benet I, Costa E, Remigia MJ, Bravo D, Amat P, Navarro D. Performance of the QuantiFERON-cytomegalovirus (CMV) assay for detection and estimation of the magnitude and functionality of the CMV-specific gamma interferon-producing CD8(+) T-cell response in allogeneic stem cell transplant recipients. Clin Vaccine Immunol 2012; 19:791-6; http://dx.doi.org/10.1128/CVI.05633-11
- Tey SK, Kennedy GA, Cromer D, Davenport MP, Walker S, Jones LI, Crough T, Durrant ST, Morton JA, Butler JP, et al. Clinical assessment of anti-viral CD8+ T cell immune monitoring using QuantiFERON-CMV® assay to identify high risk allogeneic hematopoietic stem cell transplant patients with CMV infection complications. PloS One 2013; 8:e74744; PMID:24146744; http://dx.doi.org/10.1371/journal.pone.0074744
- Harris AE, Styczynski J, Bodge M, Mohty M, Savani BN, Ljungman P. Pretransplant vaccinations in allogeneic stem cell transplantation donors and recipients: an often-missed opportunity for immunoprotection? Bone Marrow Transplantation 2015; 50:899-903; PMID:25798674; http://dx.doi.org/10.1038/bmt.2015.49
- Goardon N, Marchi E, Atzberger A, Quek L, Schuh A, Soneji S, Woll P, Mead A, Alford KA, Rout R, et al. Coexistence of LMPP-like and GMP-like leukemia stem cells in acute myeloid leukemia. Cancer Cell 2011; 19:138-52; PMID:21251617; http://dx.doi.org/10.1016/j.ccr.2010.12.012
- Kharfan-Dabaja MA, Boeckh MJ, Wilck MB, Langston AA, Chu AH, Wloch MK, Guterwill DF, Smith LR, Rolland AP, Kenney RT. A novel therapeutic cytomegalovirus DNA vaccine in allogeneic haemopoietic stem-cell transplantation: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Infect Dis 2012; 12:290-9; PMID:22237175; http://dx.doi.org/10.1016/S1473-3099(11)70344-9
- Nakamura R, Rosa CL, Longmate J, Drake J, Slape C, Zhou Q, Lampa MG, O'Donnell M, Cai JL, Farol L, et al. Viraemia, immunogenicity, and survival outcomes of cytomegalovirus chimeric epitope vaccine supplemented with PF03512676 (CMVPepVax) in allogeneic haemopoietic stem-cell transplantation: randomised phase 1b trial. Lancet Haematol 2016; 3:e87-98; PMID:26853648; http://dx.doi.org/10.1016/S2352-3026(15)00246-X
- Doubrovina E, Oflaz-Sozmen B, Prockop SE, Kernan NA, Abramson S, Teruya-Feldstein J, Hedvat C, Chou JF, Heller G, Barker JN, et al. Adoptive immunotherapy with unselected or EBV-specific T cells for biopsy proven EBV+ lymphomas after allogeneic hematopoietic cell transplants. Blood 2012; 119:2644-56; PMID:22138512; http://dx.doi.org/10.1182/blood-2011-08-371971
- Cobbold M, Khan N, Pourgheysari B, Tauro S, McDonald D, Osman H, Assenmacher M, Billingham L, Steward C, Crawley C, et al. Adoptive transfer of cytomegalovirus-specific CTL to stem cell transplant patients after selection by HLA-peptide tetramers. J Exp Med 2005; 202:379-86; PMID:16061727; http://dx.doi.org/10.1084/jem.20040613
- Blyth E, Clancy L, Simms R, Ma CK, Burgess J, Deo S, Byth K, Dubosq MC, Shaw P, Micklethwaite KP, et al. Donor-derived CMV specific T-cells reduce the requirement for CMV-directed pharmacotherapy after allogeneic stem cell transplantation. Blood 2013; 121:3745-58; PMID:23435462; http://dx.doi.org/10.1182/blood-2012-08-448977
- Chen F, Peniket A, Tholouli E, Bloor A. CMV-specific T-cell therapy improves immune reconstitution following unrelated donor HSCT: results of a randomized controlled trial. Biol Blood Marrow Transplantation 2014; 22:782-95
- Peggs KS, Tholouli E, Chakraverty R, Nikolousis E. CMV∼ impact: results of a randomized controlled trial of immuno-prophylactic adoptive cellular therapy following sibling donor allogeneic HSCT. Blood 2014; 124(21): 1109.
- Feuchtinger T, Opherk K, Bethge WA, Topp MS, Schuster FR, Weissinger EM, Mohty M, Or R, Maschan M, Schumm M, et al. Adoptive transfer of pp65-specific T cells for the treatment of chemorefractory cytomegalovirus disease or reactivation after haploidentical and matched unrelated stem cell transplantation. Blood 2010; 116:4360-7; PMID:20625005; http://dx.doi.org/10.1182/blood-2010-01-262089
- Uhlin M, Gertow J, Uzunel M, Okas M, Berglund S, Watz E, Brune M, Ljungman P, Maeurer M, Mattsson J. Rapid salvage treatment with virus-specific T-cells for therapy-resistant disease. Clin Infect Dis 2012; 55(8):1064-73; PMID:22806594
- Leen AM, Bollard CM, Mendizabal AM, Shpall EJ, Szabolcs P, Antin JH, Kapoor N, Pai SY, Rowley SD, Kebriaei P, et al. Multicenter study of banked third-party virus-specific T cells to treat severe viral infections after hematopoietic stem cell transplantation. Blood 2013; 121:5113-23; PMID:23610374; http://dx.doi.org/10.1182/blood-2013-02-486324
- Withers B, Blyth E, Clancy L, Burgess J, Simms R, Micklethwaite K, Gottlieb D. Third-party donor virus-specific T Cells are efficacious in the treatment of refractory viral infection following allogeneic HSCT, but may not persist post-infusion. Blood 2015; 126:623
- Bleakley M, Heimfeld S, Loeb KR, Jones LA, Chaney C, Seropian S, Gooley TA, Sommermeyer F, Riddell SR, Shlomchik WD. Outcomes of acute leukemia patients transplanted with naive T cell-depleted stem cell grafts. J Clin Invest 2015; 125:2677-89; PMID:26053664; http://dx.doi.org/10.1172/JCI81229
- Triplett BM, Shook DR, Eldridge P, Li Y, Kang G, Dallas M, Hartford C, Srinivasan A, Chan WK, Suwannasaen D, et al. Rapid memory T-cell reconstitution recapitulating CD45RA-depleted haploidentical transplant graft content in patients with hematologic malignancies. Bone Marrow Transplantation 2015; 50:968-77; PMID:25665048; http://dx.doi.org/10.1038/bmt.2014.324
- Maschan M, Shelikhova L, Ilushina M, Kurnikova E, Boyakova E, Balashov D, Persiantseva M, Skvortsova Y, Laberko A, Muzalevskii Y, et al. TCR-α/β and CD19 depletion and treosulfan-based conditioning regimen in unrelated and haploidentical transplantation in children with acute myeloid leukemia. Bone Marrow Transplantation 2016; 51(5):668-74
- Vago L, Oliveira G, Bondanza A, Noviello M, Soldati C, Ghio D, Brigida I, Greco R, Lupo Stanghellini MT, Peccatori J, et al. T-cell suicide gene therapy prompts thymic renewal in adults after hematopoietic stem cell transplantation. Blood 2012; 120:1820-30; PMID:22709689; http://dx.doi.org/10.1182/blood-2012-01-405670
- Tey SK, Dotti G, Rooney CM, Heslop HE, Brenner MK. Inducible caspase 9 suicide gene to improve the safety of allodepleted T cells after haploidentical stem cell transplantation. Biol Blood Marrow Transplantation 2007; 13:913-24; PMID:17640595; http://dx.doi.org/10.1016/j.bbmt.2007.04.005
- Guinan EC, Boussiotis VA, Neuberg D, Brennan LL, Hirano N, Nadler LM, Gribben JG. Transplantation of anergic histoincompatible bone marrow allografts. N Eng J Med 1999; 340:1704-14; PMID:10352162; http://dx.doi.org/10.1056/NEJM199906033402202
- Brunstein CG, Miller JS, Cao Q, McKenna DH, Hippen KL, Curtsinger J, DeFor T, Levine BL, June CH, Rubinstein P, et al. Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: safety profile and detection kinetics. Blood 2011; 117:1061-70; PMID:20952687; http://dx.doi.org/10.1182/blood-2010-07-293795
- Green ML, Leisenring WM, Xie H, Walter RB, Mielcarek M, Sandmaier BM, Riddell SR, Boeckh MJ. CMV reactivation after allogeneic HCT and relapse risk: evidence for early protection in acute myeloid leukemia. Blood 2013; 122:1316-24; PMID:23744585; http://dx.doi.org/10.1182/blood-2013-02-487074
- Elmaagacli AH, Steckel NK, Koldehoff M, Hegerfeldt Y, Trenschel R, Ditschkowski M, Christoph S, Gromke T, Kordelas L, Ottinger HD, et al. Early human cytomegalovirus replication after transplantation is associated with a decreased relapse risk: evidence for a putative virus-versus-leukemia effect in acute myeloid leukemia patients. Blood 2011; 118:1402-12; PMID:21540462; http://dx.doi.org/10.1182/blood-2010-08-304121
- Behrendt CE, Rosenthal J, Bolotin E, Nakamura R, Zaia J, Forman SJ. Donor and recipient CMV serostatus and outcome of pediatric allogeneic HSCT for acute leukemia in the era of CMV-preemptive therapy. Biol Blood Marrow Transplantation 2009; 15:54-60; PMID:19135943; http://dx.doi.org/10.1016/j.bbmt.2008.10.023
- Ito S, Pophali P, CO W, Koklanaris EK, Superata J, Fahle GA, Childs R, Battiwalla M, Barrett AJ. CMV reactivation is associated with a lower incidence of relapse after allo-SCT for CML. Bone Marrow Transplantation 2013; 48:1313-6; PMID:23562969; http://dx.doi.org/10.1038/bmt.2013.49
- Jang JE, Kim SJ, Cheong JW, Hyun SY, Kim YD, Kim YR, Kim JS, Min YH. Early CMV replication and subsequent chronic GVHD have a significant anti-leukemic effect after allogeneic HSCT in acute myeloid leukemia. Annals Hematol 2015; 94:275-82; PMID:25135450; http://dx.doi.org/10.1007/s00277-014-2190-1
- Ramanathan M, Teira P, Battiwalla M, Barrett J, Ahn KW, Chen M, Green J, Laughlin M, Lazarus HM, Marks D, et al. Impact of early CMV reactivation in cord blood stem cell recipients in the current era. Bone Marrow Transplantation 2016; 51(8):1113-20; PMID:27042847
