Infectious oral diseases are among the most prevalent in the population worldwide.Citation1,2 The oral cavity has an extensive and complex composition, with approximately 280 species of bacteria having been isolated and named.Citation3 The microbial populations adhering to the dental structure and the host's defense system are in a dynamic balance; however, this system can receive new colonizations and break the cycle of homeostasis to generate an immune response, resulting in inflammation of the surrounding periodontal tissues.Citation4-6 Maintenance of the inflammatory response is determined by the presence of a greater proportion of gram-negative bacteria.Citation7
The periodontopathogen Aggregatibacter actinomycetemcomitans is a gram-negative bacterium that colonizes the gingival sulcus and invades the epithelial tissues by proapoptotic virulence mechanisms and stimulates the expression of proinflammatory cytokines.Citation8 A. actinomycetemcomitans is present in individuals' health conditions in the microbiota, but it can also become the largest colony in cases of aggressive periodontitis, which destroys dental support tissues.Citation9 The qualitative and quantitative relation of subgingival biofilm as well as lifestyle and genetic predisposition are factors that help the disease become established. The genetic diversity of A. actinomycetemcomitans isolates has shown the ability to express and release different virulence factors.Citation10,11 The factors involved in the ability to promote colonization include adhesins, bacteriocins, invasins and antibiotic resistance, which can interact with and adhere to numerous components in the oral cavity.Citation12 Adhesion is mediated by a wide range of adhesins available in the cell wall, in the form of outer membrane proteins, vesicles, fimbriae or amorphous material.Citation13
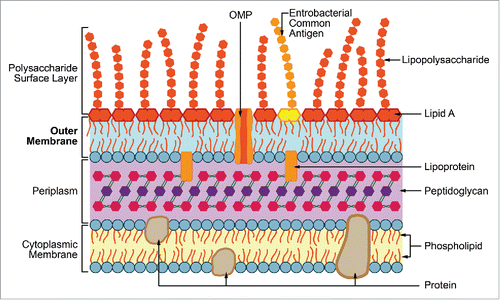
In this issue, Ahlstrand et al.Citation14 highlight an outer membrane lipoprotein of A. actinomycetemcomitans, the bacterial interleukin receptor I (BilRl), using mass spectrometry technique and which is specific to the family Pasteurellaceae. In gram-negative bacteria, such as Escherichia coli, the outer membrane serves as a protective barrier that controls the entry and exit of solutes.Citation10 In addition, it contains other lipopolysaccharides, such as enterobacterial common antigen, and in many conditions a polysaccharide capsule that can be used as an additional layer.Citation11 The outer membrane can anchor organelles and play a fundamental role in the establishment of disease. The illustration () shows the general structure of a cell wall of gram-negative bacterium characterized by an asymmetrical construction and surrounded by an outer membrane protein located on a fine peptidoglycan layer.Citation15
Seen in these terms, the remarkable feature found by the authors is the potential of BilRl to bind the proinflammatory cytokine interleukin IL-1ß. In addition, the publication characterizes BilRI as an intrinsically disordered protein (IDP) for its capacity to interact with other ligands and bind different citokynes, such as IL-8, TNF-, INF-γ and IL-10. The mechanism to regulate IDP ensures its production is strict and feasible only in suitable amounts, no more than necessary. The expression has also been associated with numerous diseases.Citation16 In terms of periodontal disease, little is known about the cytokine sensitization mechanism used by periodontopathogens, and this is important from the point of view that many bacterial species increase their biofilm production and the expression of virulence genes through the mechanism of cytokine binding and sensitization response.Citation17 The individual humoral immune response mechanism against oral microbiota antigens can occur not only at the local level, but also at the systemic level, bringing with it the risk associated with the development of diabetes and cardiovascular diseases, for example.Citation4,11 The adaptive immune system in turn attempts to eliminate the pathogens in the late phase of the infection by establishing an immunological memory.Citation4 Knowledge of how these inflammatory responses occur is important for understanding complex diseases like periodontitis, particularly the receptors directly involved in binding to bacteria.Citation11,18 Several pathogens are specific cytokine receptors, and this can result in changes in the properties of the bacteria, altering the biofilm formation, the expression of virulence genes, and they can also change the inflammatory response reactions of the host.Citation19 Pro-inflammatory cytokines play a key role in the host's response to microbial infections, such as IL-1b and TNF-α, which are essential for the regulation process.Citation20,21 These same mediators contribute to the induction of bone resorption, signaling an increase in collagenase secretion, the regulation of prostaglandin E2 expression, and they can also be produced by numerous types of cells such as neutrophils, macrophages, natural killer cells, etc. directly in the periodontium.Citation22,23 Clinical studies have shown a high expression of these markers in patients with periodontal disease.Citation21 These cytokines may induce bone resorption, affecting the production of essential osteoclast differentiation and the resulting bone destruction. The stimulatory factors regulating this process are cytokines such as interleukin-1 (IL-1), macrophage colony-stimulating factor (MCSF), monocytes and T cells.Citation22,24 The treatment of periodontal disease is based on virulence factors, the microorganisms that are established in the processes of health and disease; therefore, the treatments must aim to control these microorganisms.Citation4,8,9 It is necessary to understand the role of bacterial biofilm in the etiology of periodontal diseases, as the severity and progression of these diseases are determined in large part by factors related to the host's response.Citation25,26
The results regarding BiIRI are noteworthy because they show what an IDP is and that gene deletion causes a reduction in the internalization of the cytokine IL-1B by the microorganism A. actinomycetemcomitans; an IDP acts as a nonspecific agent that causes the surface concentration. The authors also conclude that in small cytokine concentrations, the role of BiIRI is to promote the entry of molecules onto the surface of the microorganism. The way in which cytokine transfer and protein-binding occur showed a weak affinity, suggesting a possible involvement of BiIRI in the mechanisms that regulate the disease. These findings could also lead in future to a better understanding of the individual functions of bacterial species in the formation of periodontal disease and the mechanisms used by A. actinomycetemcomitans for cytokine sensitization. This study also creates an environment conducive to future investigations involving structural data with molecular dynamics, presenting an ideal situation for a better understanding of how other bacterial species can express cytokine receptors similar to BilRI.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
I am grateful to Dr. Eleftherios Mylonakis, Dr. Thomas J. Silhavy, and the Virulence editorial team for all your support.
References
- Petersen PE, Ogawa H. The global burden of periodontal disease: towards integration with chronic disease prevention and control. Periodontol 2012; 60:15-39; PMID:22909104; http://dx.doi.org/10.1111/j.1600-0757.2011.00425.x
- Petersen PE, Bourgeois D, Ogawa H, Estupinan-Day S, Ndiaye C. The global burden of oral diseases and risks to oral health. Bull World Health Organ 2005; 83:661-9; PMID:16211157
- Dewhirst FE, Chen T, Izard J, Paster BJ, Tanner ACR, Yu W-H, Lakshmanan A, Wade WG. The human oral microbiome. J Bacteriol 2010; 192:5002-17. PMID:20656903; http://dx.doi.org/10.1128/JB.00542-10
- Darveau RP. Periodontitis: a polymicrobial disruption of host homeostasis. Nat Rev Microbiol 2010; 8:481-90. PMID:20514045; http://dx.doi.org/10.1038/nrmicro2337
- Seymour RA. Effects of medications on the periodontal tissues in health and disease. Periodontol 2006; 40:120-9; PMID:16398689; http://dx.doi.org/10.1111/j.1600-0757.2005.00137.x
- Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet (London, England) 2005; 366:1809-20. PMID:16298220; http://dx.doi.org/10.1016/S0140-6736(05)67728-8
- Dzink JL, Tanner ACR, Haffajee AD, Socransky SS. Gram negative species associated with active destructive periodontal lesions. J Clin Periodontol 1985; 12:648-59. PMID:3863838; http://dx.doi.org/10.1111/j.1600-051X.1985.tb00936.x
- Herbert BA, Novince CM, Kirkwood KL. Aggregatibacter actinomycetemcomitans, a potent immunoregulator of the periodontal host defense system and alveolar bone homeostasis. Mol Oral Microbiol 2016; 31:207-27. PMID:26197893; http://dx.doi.org/10.1111/omi.12119
- Åberg CH, Kelk P, Johansson A. Aggregatibacter actinomycetemcomitans: virulence of its leukotoxin and association with aggressive periodontitis. Virulence 2015; 6:188-95. PMID:25494963; http://dx.doi.org/10.4161/21505594.2014.982428
- Johansson A. Aggregatibacter actinomycetemcomitans leukotoxin: a powerful tool with capacity to cause imbalance in the host inflammatory response. Toxins (Basel) 2011; 3:242-59. PMID:22069708; http://dx.doi.org/10.3390/toxins3030242
- Alexander C, Rietschel ET. Bacterial lipopolysaccharides and innate immunity. J Endotoxin Res 2001; 7:167-202. PMID:11581570; http://www.ncbi.nlm.nih.gov/pubmed/11581570
- Fives-Taylor PM, Meyer DH, Mintz KP, Brissette C. Virulence factors of Actinobacillus actinomycetemcomitans. Periodontol 2000; 20:136-67; http://dx.doi.org/10.1111/j.1600-0757.1999.tb00161.x
- Ellis TN, Kuehn MJ. Virulence and immunomodulatory roles of bacterial outer membrane vesicles. Microbiol Mol Biol Rev 2010; 74:81-94. PMID:20197500; http://dx.doi.org/10.1128/MMBR.00031-09
- Ahlstrand T, Tuominen H, Beklen A, Torittu A, Oscarsson J, Sormunen R, Pöllänen MT, Permi P, Ihalin R. A novel intrinsically disordered outer membrane lipoprotein of Aggregatibacter actinomycetemcomitans binds various cytokines and plays a role in biofilm response to interleukin-1β and interleukin-8. Virulence 2017; 8(2): 115-134; PMID:27459270; http://dx.doi.org/10.1080/21505594.2016.1216294
- Silhavy TJ, Kahne D, Walker S. The bacterial cell envelope. Cold Spring Harb Perspect Biol 2010; 2:a000414. PMID:20452953; http://dx.doi.org/10.1101/cshperspect.a000414
- Dinarello CA, Simon A, van der Meer JWM. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discov 2012; 11:633-52. PMID:22850787; http://dx.doi.org/10.1038/nrd3800
- Henderson B, Poole S, Wilson M. Bacterial modulins: a novel class of virulence factors which cause host tissue pathology by inducing cytokine synthesis. Microbiol Rev 1996; 60:316-41. PMID:8801436; http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=239446&tool=pmcentrez&rendertype=abstract
- Nikaido H. Molecular basis of bacterial outer membrane permeability revisited. Microbiol Mol Biol Rev 2003; 67:593-656. PMID:14665678; http://dx.doi.org/10.1128/MMBR.67.4.593-656.2003
- Paino A, Ahlstrand T, Nuutila J, Navickaite I, Lahti M, Tuominen H, Välimaa H, Lamminmäki U, Pöllänen MT, Ihalin R, et al. Identification of a novel bacterial outer membrane interleukin-1β-binding protein from aggregatibacter actinomycetemcomitans. PLoS One 2013; 8:e70509. PMID:23936223; http://dx.doi.org/10.1371/journal.pone.0070509
- Kornman KS, Crane A, Wang HY, di Giovine FS, Newman MG, Pirk FW, Wilson TG, Higginbottom FL, Duff GW. The interleukin-1 genotype as a severity factor in adult periodontal disease. J Clin Periodontol 1997; 24:72-7. PMID:9049801; http://dx.doi.org/10.1111/j.1600-051X.1997.tb01187.x
- Gomes FIF, Aragão MGB, Barbosa FCB, Bezerra MM, de Paulo Teixeira Pinto V, Chaves HV. Inflammatory cytokines interleukin-1β and tumour necrosis factor-α - novel biomarkers for the detection of periodontal diseases: a literature review. J oral Maxillofac Res 2016; 7:e2. PMID:27489606; http://www.ncbi.nlm.nih.gov/pubmed/27489606
- Crotti TN, Dharmapatni AASSK, Alias E, Haynes DR. Osteoimmunology: Major and costimulatory pathway expression associated with chronic inflammatory induced bone loss. J Immunol Res 2015; 2015:281287. PMID:26064999; http://dx.doi.org/10.1155/2015/281287
- Graves DT, Li J, Cochran DL. Inflammation and uncoupling as mechanisms of periodontal bone loss. J Dent Res 2011; 90:143-53. PMID:21135192; http://dx.doi.org/10.1177/0022034510385236
- Ohshiba T, Miyaura C, Inada M, Ito A. Role of RANKL-induced osteoclast formation and MMP-dependent matrix degradation in bone destruction by breast cancer metastasis. Br J Cancer 2003; 88:1318-26. PMID:12698202; http://dx.doi.org/10.1038/sj.bjc.6600858
- Salvi GE, Lang NP. Host response modulation in the management of periodontal diseases. J Clin Periodontol 2005; 32(Suppl 6):108-29. PMID:16128833; http://dx.doi.org/10.1111/j.1600-051X.2005.00785.x
- Moore WE, Moore LV. The bacteria of periodontal diseases. Periodontol 2000 [Internet] 1994; 5:66-77. PMID:9673163; http://dx.doi.org/10.1111/j.1600-0757.1994.tb00019.x