ABSTRACT
The global spread of carbapenem-resistant Enterobacteriaceae (CRE) has been fostered by the lack of preemptive screening of patients in healthcare facilities that could prevent patient-to-patient transmission. Outbreaks of CRE infections have led some institutions to implement rigorous screening programs, although controlled comparative data are frequently lacking. Resource limitations and uncertainty regarding the optimal approach has kept many facilities from enacting more active routine surveillance policies that could reduce the prevalence of CRE. The ideal population to target for screening, the frequency of testing, and the preferred test method are components of surveillance programs that remain open to debate. This review discusses the rationale for different screening policies in use and the performance characteristics of laboratory methods available to detect CRE carriage.
Introduction
In light of the limited therapeutic options and substantial mortality associated with infections caused by carbapenem-resistant Enterobacteriaceae (CRE), prevention is of the utmost importance.Citation1,2 There are 2 primary modes of CRE acquisition thought to occur in the healthcare environment: 1) patient-to-patient transmission (i.e., from another patient, through healthcare staff, the proximal environment, or shared equipment); and 2) emergence of resistance, in which carbapenem susceptible isolates within a patient are genetically altered.Citation3 The relative importance each mode plays is not known, but both must be considered to ensure a comprehensive infection prevention strategy. Measures to address patient-to-patient transmission include: 1) hand hygiene, 2) contact isolation precautions, 3) cohorting with dedicated staff, 4) environmental cleaning, 5) decolonization protocols, and 6) surveillance programs to identify asymptomatic carriage.Citation4,5 In contrast, tackling the emergence of resistance requires enforcing antimicrobial stewardship policies to avoid unnecessary use of broad-spectrum agents (especially carbapenems).Citation3 This review will focus on current practices and scientific evidence supporting CRE screening to identify asymptomatic carriers and implement patient-to-patient containment measures. Decisions regarding CRE screening are usually based on the local epidemiology and resources available in the institution. Because multiple measures have been simulteneously employed to control or prevent outbreaks and reduce the rate of CRE infections, the specific impact of active surveillance is unclear.Citation6 Expert opinion rather than high quality scientific evidence guides most CRE screening policies.
Epidemiology of CRE
Carbapenemase-producing (CP) CRE harboring plasmid-encoded resistance genes that easily spread to different species emerged in the mid-1990s.Citation7 The predominant carbapenemase produced by CP-CRE worldwide is the Klebsiella pneumoniae carbapenemase (KPC) family encoded by the blaKPC gene.Citation8 Since its initial recognition, the KPC carbapenemase has been detected in organisms other than K. pneumoniae, including Klebsiella oxytoca, Enterobacter spp., Escherichia coli, Salmonella spp., Serratia spp., Citrobacter freundii, Proteus mirabilis, Acinetobacter baumannii, Pseudomonas aeruginosa, and Pseudomonas putida.Citation7,9 Additional carbapenemases that have been associated with epidemics in Enterobacteriaceae include the Verona integron-encoded metallo-ß-lactamase (VIM), New Delhi metallo-β-lactamase (NDM), Imipenemase (IMP) metallo-β-lactamase, and Oxacillinase-48-type (OXA-48) enzymes.Citation10 As of April 2016, in the United States, only 2 states have not reported KPC-producing CRE to the Center for Disease Control and Prevention (CDC), 157 patients were reported with NDM, 61 patients with OXA-48, 17 patients with VIM, and 10 patients with IMP.Citation11 These numbers are felt to represent the proverbial tip of the iceberg. Plasmid-encoded carbapenemases of primary concern and areas of endemicity are summarized in .
Table 1. Plasmid-encoded carbapenemases of primary concern.Citation7
The food supply is another potential reservoir of CP-CRE with detection reported in retail chicken meat in Egypt and in fresh vegetables and spices imported from Asia.Citation12,13 A blaNDM-1-carrying strain of Acinetobacter was detected in chickens from animal farms in China, and blaOXA producing isolates were detected among cattle in France.Citation14,15 Although it is difficult to quantify the impact of these findings on human CRE infections, emergent efforts to address antimicrobial use in agricultural industry are clearly needed.
In less than a decade, a transposon (Tn4401)-mediated outbreak of KPC-producing CRE has disseminated worldwide.Citation8,13,16 While outbreaks were initially seen mainly in acute-care hospitals, the spread to non-acute care healthcare facilities rapidly followed.Citation17-19 In less developed countries, CRE could also be isolated in community settings among patients with no recent healthcare exposure.Citation12,13 Therefore, prevention of CRE should be targeted through the whole spectrum of the modern continuum of medical care.Citation18 In the Israeli nationwide CRE epidemic for example, a stable decrease in new acquisitions was achieved only after broadening the prevention plan to long-term care facilities (LTCF).Citation20,21 Therefore, this review will focus on screening for CRE among facilities from both acute-care and long-term care facilities.
The use of surveillance cultures has become an essential tool in infection control programs, not only during outbreaks but also as a routine measure in settings endemic for CRE.Citation21 Screening patients to identify asymptomatic colonization, and instituting preemptive contact isolation measures reduces patient-to-patient transmissions and the colonization pressure, and improves patient outcomes by decreasing the delay in initiation of appropriate antimicrobial therapy.Citation22 Active surveillance can estimate the colonization pressure better than cultures obtained from clinical samples alone.Citation23 Moreover, delay in initiation of appropriate antimicrobial therapy is the strongest modifiable independent predictor for mortality in severe sepsis in general, and specifically in CRE infections as well.Citation24,25
Who should be screened and when?
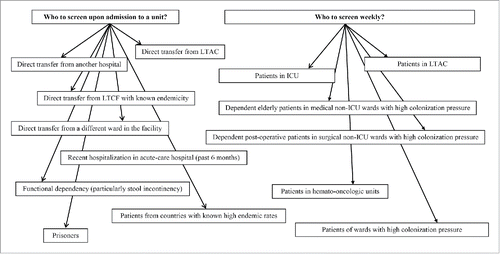
Common risk factors reported for CRE acquisition in acute care hospitals include exposure to antimicrobials (not solely to carbapenems),Citation3,26 high co-morbidity indexes, deteriorated functional status and/or cognition at baseline, recent LTCF stay, and recent invasive procedures or permanent foreign devices.Citation13,26-30 Older age and immunosuppression are additional risk factors seldom reported, but are perceived mainly as confounders, and not as established independent predictors for CRE acquisition.Citation26 Identifiable risk factors for acquisition of CRE in LTCF include prolonged length of stay in acute-care facilities, sharing rooms with known carriers, and a high degree of colonization pressure.Citation21,29,30 Screening for CRE is a practice recommended by many national and international bodies,Citation21,31 although controlled efficacy analyses are lacking. In Israel, the distribution of national guidelines in 2008 requiring all facilities to implement a CRE screening policy, helped curb the nationwide epidemic that began in 2006.Citation21 The CDC has proposed core preventive measures to prevent CP-CRE transmission in United States facilities that includes efforts to detect more colonized patients.Citation32 As with any screening program, decisions of which patients to screen should be based on local epidemiologic data.Citation31 The populations that should be targeted for CRE screening in acute-care hospitals, based on current risk factor stratification,Citation13,26,28 are patients admitted directly from long-term acute-care facilities (LTAC), other LTCFs with known endemicity, or patients who are transferred directly from another acute-care hospital. Recent hospital stay (in the previous 6 months),Citation21 functional dependency,Citation33 inter-ward transfers in the facility, prisoners, and patients from foreign countries with high endemic rates, are additional candidates for CRE screening.Citation21,31 In Israeli LTCFs, screening is recommended for all new admissions that are transferred directly from an acute-care hospital or patients admitted from home with extensive healthcare exposure.Citation18,29,34 Both the CDC and European Society of Clinical Microbiology and Infectious Diseases (ESCMID) support additional periodic screening policies in the facility (e.g., weekly), for hospitalized patients in certain high-risk units (e.g., ICUs, LTACs).Citation31 Populations that are candidates for screening are summarized in .
How to screen?
New laboratory methods have been introduced to improve detection of carbapenemase-producing organisms (CPOs). Determining whether a CRE is carbapenemase producing based on susceptibility testing results can be difficult since the presence of other mechanisms of carbapenem resistance (e.g., porin loss, cephalosporinases) can elevate the MIC. The changing epidemiology of carbapenemases further complicates the task since methods must be optimized to ensure all relevant circulating enzymes associated with CP-CRE are detected. In addition, the role of non-CP CRE, in terms of its epidemiological significance, and the impact of certain infection control measures on its rate of acquisition, is still a matter of ongoing debate.Citation35 While the Israeli ministry of health guidelines requires carbapenemase production testing on all CRE isolates and different infection control measures applied for those who test positive vs. those who test negative,Citation21 the CDC encourages, but does not mandate carbapenemase testing if current carbapenem resistance breakpoints are applied.Citation32
CRE detection from cultures
Enterobacteriaceae isolates with KPC enzymes were not initially recognized because the carbapenem MICs were in the susceptible range when applying breakpoints to susceptibility test results. To address this problem with CP-CRE detection, the Clinical and Laboratory Standards Institute (CLSI) published recommendations in 2009 for Enterobacteriaceae with carbapenem MICs of 2 or 4 µg/ml and resistant to all third-generation cephalosporins to be tested further with the modified Hodge test which has good sensitivity for KPC.Citation36 Problems with this approach included the modified Hodge test not reliably detecting non-KPC enzymes such as NDM and false positives with E. coli, C. freundii, and Enterobacter spp.Citation37-39 Another hindrance with the initial CLSI CP-CRE detection guidance was the more recent discovery that CRE producing OXA-48-type enzymes may be susceptible to third-generation cephalosporins and only resistant to ertapenem. In 2010, CLSI published lower carbapenem breakpoints () based on review of clinical outcome, MIC distribution, and PK/PD data making performance of the modified Hodge test optional for epidemiologic purposes.Citation40 Establishing a carbapenem breakpoint that would reliably distinguish all CP-CRE from those without carbapenemase production is hindered by overlapping susceptibility profiles. The PK/PD data suggest the small population of CP-CRE isolates with carbapenem MICs below the new breakpoint can be effectively treated with a carbapenem.Citation41 However, this is not supported by prospective controlled trials.Citation42
Table 2. CLSI and EUCAST carbapenem susceptibility testing breakpoints (µg/ml)Footnotea.
The European Committee on Antimicrobial Susceptibility Testing (EUCAST) approach is similar, but breakpoints are higher than CLSI and they acknowledge that some CPO may test susceptible.Citation43 This approach of encouraging laboratories to simply rely on the MIC for CPO detection has been met with criticism.Citation44 In 2013, EUCAST responded by adding separate CPO screening cut-off values for infection control and public health purposes () that are considerably lower than the clinical breakpoints.Citation45
In 2015, the CDC CRE surveillance definition was revised to one of 2 criteria: 1) resistance to any carbapenem according to current CLSI breakpoints (MIC ≥2 for ertapenem or ≥4 for doripenem, meropenem, or imipenem) or 2) demonstration of carbapenemase production.Citation32 For bacteria such as Proteus spp, Providencia spp., and Morganella morganii that naturally have elevated MICs to imipenem, a carbapenem other than imipenem must test resistant to meet the CRE surveillance definition. Besides the modified Hodge test, a number of other methods (Carba NP, molecular methods, metallo-β-lactamase testing) are considered acceptable to demonstrate carbapenemase production. Despite sensitivity and specificity issues with the modified Hodge test, it continues to be included as an acceptable method for carbapenemase production because it is widely used by laboratories. CLSI has endorsed the CarbaNP test, a colorimetric pH-based method for detecting carbapenem hydrolysis in isolates, but the sensitivity for OXA-48-type enzymes is poor (38.5%) and the reagents are only stable for 72 hours.Citation46-48 A new carbapenem inactivation method (CIM) appears promising with initial reports of 96-100% sensitivity for KPC, metalloenzymes and OXA-48-like enzymes and results as quickly as 8 hours.Citation49 To perform the CIM, an organism being evaluated for carbapenemase production is incubated in broth containing a carbapenem disk for 2 hours followed by placement of the disk on a lawn of carbapenem susceptible E. coli; no zone of inhibition indicates a positive test for CPO.
A significant advancement in CPO detection has occurred with the availability of FDA -cleared molecular methods to detect specific resistance genes (KPC, NDM, VIM, IMP, OXA) from positive blood cultures. Decreased length of stay and lower mortality has been demonstrated when rapid detection of resistance genes is provided in conjunction with antimicrobial stewardship interventions.Citation50 As part of CRE prevention efforts, CDC encourages laboratories to screen CRE for specific carbapenemase enzymes (KPC, NDM, OXA-48-type) if they have the capacity.Citation32 Some countries however, mandate that every CRE be subjected to specific carbapenemase determination by a molecular method, not relying solely on phenotypic determination of the presence of a carbapenemase.Citation21 Although it is prudent to place all patients with CRE in contact isolation, only CP-CRE require more extensive interventions (e.g., screening patient contacts, cohorting patients with dedicated staff).
CRE detection from rectal or perirectal swabs
The Israeli ministry of health allows specimen collection for detection of CRE carriage using a rectal swab, but not a perirectal swab.Citation51 The CDC includes the option of a perirectal swab because this site is preferred by patients and considered safer than an internal rectal swab collection for neutropenic patients. Equal sensitivity of rectal and perirectal swabs for detection of vancomycin-resistant enterococci colonization has been demonstrated,Citation52 but similar data for CRE detection is lacking.
Screening patients for CPO using the CDC method from a rectal or perirectal swab is labor intensive and can take up to 4 days, but utilizes reagents, equipment and skills already available in the clinical laboratory. Commercial chromogenic agars have been developed, but none are FDA cleared, putting the burden on laboratories to perform an extensive validation prior to use. One limitation of studies evaluating performance of CP-CRE screening methods is that a convenience sample of pure cultures rather than rectal or perirectal swabs is often used. In addition, many studies only include KPC-producing strains. Evaluations of culture-based methods performed on rectal or perirectal swabs are summarized in .
Table 3. Evaluations of culture-based CRE screening methods on rectal or perirectal swabs.
The CDC broth enrichment method requires overnight incubation of a rectal swab in 5 ml of trypticase soy broth (TSB) containing a 10 µg carbapenem (meropenem or ertapenem) disk. Subculture of the broth onto MacConkey agar includes the option of adding a carbapenem disk to the agar. Colonies growing around the disk require identification and susceptibility testing or a phenotypic method for carbapenemase production which may lead to a turnaround time of 4 d. The CDC states the method was only validated for E. coli and Klebsiella spp. as reflected in the instruction to only look for lactose fermenting colonies on the MacConkey agar.Citation53 However, detection of bacteria other than non-lactose fermenters can identify important reservoirs of carbapenemase-producing resistance genes.Citation54 The reported sensitivities of the CDC method for KPC and VIM range from 65.6% to 98.8% with specificities of 49.6 to 100%.Citation55-58 The sensitivity of the CDC method for detection of OXA-48 was only 57.6%.Citation59
A screening method with 24-48 hours turnaround time involves direct inoculation on MacConkey agar with placement of carbapenem disks where the first quadrant meets the second quadrant and where the third quadrant meets the fourth.Citation60 The reported sensitivities of MacConkey agar with carbapenem disks ranged from 75.8 to 96.9% with specificities of 73.8 to 100%.Citation51,61-64 Evaluations of KPC carriage using MacConkey agar with imipenem demonstrated sensitivities of 78.3% to 89.5% and specificities of 31.9 to 99.4%.Citation51,55,65,66
Chromogenic agars can shorten turnaround time by eliminating the overnight incubation required for the enrichment step. Examples include Brilliance CRE (Thermo Diagnostics, USA), CHROMagar KPC (CHROMagar, France), chromID Carba (bioMerieux, France), chromID OXA-48 (bioMerieux, France), HardyChrom (Hardy Diagnostics, CA, USA), Supercarba (bioMerieux, France), and SpectraCRE (Thermo Diagnostics, USA). The authors of an extensive review of surveillance methods to detect intestinal carriage of CPOs, favor chromID Carba for culture based screening unless OXA-48 incidence is high which then merits the addition of an OXA-48-specific medium (e.g., chromID OXA-48) or Supercarba.Citation67 A recent evaluation demonstrated good performance of the direct MacConkey method in detecting carbapenem-resistant organisms, but the molecular Check-Direct CPE screen assay for BD MAXDirect and the chromID Carba were most sensitive for CPO detection and a broth enrichment step was not needed.Citation68
Advantages of using molecular assays to screen for carbapenemase genes include labor savings, faster turnaround time, and higher sensitivity than culture-based methods.Citation56,69,70 Commercial molecular assays utilize multiplex PCR, microarray, or isothermal amplification technology to detect carbapenemase genes. The manufacturers include Check-Points Health (Check-Direct CPE), Amplex Biosystems (Eazyplex SuperBug complete A, Eazyplex Superbug complete B) and Cepheid (Xpert Carba-R).Citation71-76 A comparison of Xpert Carba-R, Eazyplex Superbug complete A and Check-Direct CPE kit for detection of carbapenemase genes in isolates demonstrated 100% sensitivity for KPC, NDM, and VIM, but only the CHECK-Direct CPE detected all OXA-48 genes.Citation77 Suboptimal detection of OXA-48-like genes by Xpert Carba-R has also been noted by other investigators.Citation78,79 A multicenter study of the Xpert Carba-R performance on rectal swabs (383 clinical and 250 contrived samples) with comparison to culture and sequencing reported 96.6% sensitivity and 98.6% specificity for detection of IMP-1, VIM, NDM, KPC, and OXA-48.Citation80 A new improved version of Xpert Carba-R with OXA-48 variants OXA-181 and OXA-232 added was recently FDA-approved for performance on rectal swab specimens.
The projected annual reagent and labor cost of PCR to screen 6,860 specimens at an academic medical center with CP-CRE prevalence of 2.7% was approximately 10 times higher than the CDC-recommended phenotypic method ($224,596 vs. $22,818 US dollars).Citation81 However, the longer turnaround time of the culture-based method means a patient may be unnecessarily placed in preemptive isolation for 3 d longer which has been estimated to cost $925 in Canada.Citation82 The cost of CPO transmission that may occur with a less sensitive culture-based screening test must also be considered since CPO isolates with low level resistance (MIC <2 µg/ml) or low inocula at risk for overgrowth by competing organisms may not be detected.Citation67
Conclusions
Preventing the transmission of CP-CRE is a high priority for all institutions. Screening contacts of CP-CRE infected patients is essential to curb transmissions and control outbreaks. However, approaches to routine screening for CRE carriage vary depending on institutional epidemiology, resources and policies. Baseline surveillance is recommended to determine the prevalence and types of carbapenemase enzymes circulating in an institution. Although molecular assays are the most expensive method for CP-CRE screening, the fast turnaround time, high sensitivity, specific genetic information, and availability of FDA-approved or CE-marked assays are appealing. Culture-based methods are not approved by regulatory agencies, less sensitive than molecular methods, labor intensive and slow (up to 4 d to generate results), but have a lower reagent cost. Public health initiatives that focus on the community setting to better understand the factors leading to colonization with CP-CRE are needed. Strategies to reduce the presence of CP-CREs in the environment, animals, and our food supply must be more aggressively pursued.
Disclosure of potential conflicts of interest
S.S.R. has received research funding from bioMerieux, BioFire, Nanosphere, Roche, OpGen, and BD Diagnostics. D.M. has received research funding from Merck, GAMA Healthcare, and SAGE. There was no funding supporting this specific review.
References
- Patel G, Huprikar S, Factor SH, Jenkins SG, Calfee DP. Outcomes of carbapenem-resistant Klebsiella pneumoniae infection and the impact of antimicrobial and adjunctive therapies. Infect Control Hosp Epidemiol 2008; 29:1099-106; PMID:18973455; https://doi.org/10.1086/592412
- Srinivasan A, Patel JB. Klebsiella pneumoniae carbapenemase-producing organisms: an ounce of prevention really is worth a pound of cure. Infect Control Hosp Epidemiol 2008; 29:1107-9; PMID:18973453; https://doi.org/10.1086/594129
- Bogan C, Marchaim D. The role of antimicrobial stewardship in curbing carbapenem resistance. Future Microbiol 2013; 8:979-91; PMID:23902145; https://doi.org/10.2217/fmb.13.73
- Barnes SL, Morgan DJ, Harris AD, Carling PC, Thom KA. Preventing the transmission of multidrug-resistant organisms: modeling the relative importance of hand hygiene and environmental cleaning interventions. Infect Control Hosp Epidemiol 2014; 35:1156-62; PMID:25111924; https://doi.org/10.1086/677632
- Harris AD, Kotetishvili M, Shurland S, Johnson JA, Morris JG, Nemoy LL, Johnson JK. How important is patient-to-patient transmission in extended-spectrum β-lactamase Escherichia coli acquisition. Am J Infect Control 2007; 35:97-101; PMID:17327188; https://doi.org/10.1016/j.ajic.2006.09.011
- Hayden MK, Lin MY, Lolans K, Weiner S, Blom D, Moore NM, Fogg L, Henry D, Lyles R, Thurlow C, et al. Prevention of colonization and infection by Klebsiella pneumoniae carbapenemase-producing enterobacteriaceae in long-term acute-care hospitals. Clin Infect Dis 2015; 60:1153-61; PMID:25537877; https://doi.org/10.1093/cid/ciu1173
- Queenan AM, Bush K. Carbapenemases: the versatile β-lactamases. Clin Microbiol Rev 2007; 20:440-58, table of contents; PMID:17630334; https://doi.org/10.1128/CMR.00001-07
- Munoz-Price LS, Poirel L, Bonomo RA, Schwaber MJ, Daikos GL, Cormican M, Cornaglia G, Garau J, Gniadkowski M, Hayden MK, et al. Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. Lancet Infect Dis 2013; 13:785-96; PMID:23969216; https://doi.org/10.1016/S1473-3099(13)70190-7
- Bennett JW, Herrera ML, Lewis JS, 2nd, Wickes BW, Jorgensen JH. KPC-2-producing Enterobacter cloacae and pseudomonas putida coinfection in a liver transplant recipient. Antimicrob Agents Chemother 2009; 53:292-4; PMID:18852270; https://doi.org/10.1128/AAC.00931-08
- Canton R, Akova M, Carmeli Y, Giske CG, Glupczynski Y, Gniadkowski M, Livermore DM, Miriagou V, Naas T, Rossolini GM, et al. Rapid evolution and spread of carbapenemases among Enterobacteriaceae in Europe. Clin Microbiol Infect 2012; 18:413-31; PMID:22507109; https://doi.org/10.1111/j.1469-0691.2012.03821.x
- Centers for Disease Control and Prevention. Tracking CRE infections. Atlanta, GA: United States Department of Health and Human Services; 2016 April 25 [accessed 2016 Aug 21]. http://www.cdc.gov/hai/organisms/cre/TrackingCRE.html
- Abdallah HM, Reuland EA, Wintermans BB, Al Naiemi N, Koek A, Abdelwahab AM, Ammar AM, Mohamed AA, Vandenbroucke-Grauls CM. Extended-Spectrum β-Lactamases and/or carbapenemases-producing enterobacteriaceae isolated from retail chicken meat in Zagazig, Egypt. PLoS One 2015; 10:e0136052; PMID:26284654; https://doi.org/10.1371/journal.pone.0136052
- Schwaber MJ, Carmeli Y. Carbapenem-resistant Enterobacteriaceae: a potential threat. JAMA 2008; 300:2911-3; PMID:19109119; https://doi.org/10.1001/jama.2008.896
- Wang Y, Wu C, Zhang Q, Qi J, Liu H, He T, Ma L, Lai J, Shen Z, Liu Y, et al. Identification of New Delhi metallo-β-lactamase 1 in Acinetobacter lwoffii of food animal origin. PLoS One 2012; 7:e37152; PMID:22629360; https://doi.org/10.1371/journal.pone.0037152
- Tomar JS, Peddinti RK. A. baumannii histone acetyl transferase Hpa2: optimization of homology modeling, analysis of protein-protein interaction and virtual screening. J Biomol Struct Dyn 2016:15:1-12; PMID:27125865; https://doi.org/10.1080/07391102.2016.1172025
- Brennan BM, Coyle JR, Marchaim D, Pogue JM, Boehme M, Finks J, Malani AN, VerLee KE, Buckley BO, Mollon N, et al. Statewide surveillance of carbapenem-resistant enterobacteriaceae in Michigan. Infect Control Hosp Epidemiol 2014; 35:342-9; PMID:24602937; https://doi.org/10.1086/675611
- Won SY, Munoz-Price LS, Lolans K, Hota B, Weinstein RA, Hayden MK. Emergence and rapid regional spread of Klebsiella pneumoniae carbapenemase-producing Enterobacteriaceae. Clin Infect Dis 2011; 53:532-40; PMID:21865189; https://doi.org/10.1093/cid/cir482
- Marchaim D, Chopra T, Bogan C, Bheemreddy S, Sengstock D, Jagarlamudi R, Malani A, Lemanek L, Moshos J, Lephart PR, et al. The burden of multidrug-resistant organisms on tertiary hospitals posed by patients with recent stays in long-term acute care facilities. Am J Infect Control 2012; 40:760-5; PMID:22285709; https://doi.org/10.1016/j.ajic.2011.09.011
- Ben-David D, Masarwa S, Navon-Venezia S, Mishali H, Fridental I, Rubinovitch B, Smollan G, Carmeli Y, Schwaber MJ. Carbapenem-resistant Klebsiella pneumoniae in post-acute-care facilities in Israel. Infect Control Hosp Epidemiol 2011; 32:845-53; PMID:21828964; https://doi.org/10.1086/661279
- Schwaber MJ, Lev B, Israeli A, Solter E, Smollan G, Rubinovitch B, Shalit I, Carmeli Y. Containment of a country-wide outbreak of carbapenem-resistant Klebsiella pneumoniae in Israeli hospitals via a nationally implemented intervention. Clin Infect Dis 2011; 52:848-55; PMID:21317398; https://doi.org/10.1093/cid/cir025
- Schwaber MJ, Carmeli Y. An ongoing national intervention to contain the spread of carbapenem-resistant enterobacteriaceae. Clin Infect Dis 2014; 58:697-703; PMID:24304707; https://doi.org/10.1093/cid/cit795
- Bogan C, Kaye KS, Chopra T, Hayakawa K, Pogue JM, Lephart PR, Bheemreddy S, Lazarovitch T, Zaidenstein R, Perez F, et al. Outcomes of carbapenem-resistant Enterobacteriaceae isolation: matched analysis. Am J Infect Control 2014; 42:612-20; PMID:24837111; https://doi.org/10.1016/j.ajic.2014.02.013
- Bonten MJ. Colonization pressure: a critical parameter in the epidemiology of antibiotic-resistant bacteria. Crit Care 2012; 16:142; PMID:22849650
- Paul M, Shani V, Muchtar E, Kariv G, Robenshtok E, Leibovici L. Systematic review and meta-analysis of the efficacy of appropriate empiric antibiotic therapy for sepsis. Antimicrob Agents Chemother 2010; 54:4851-63; PMID:20733044; https://doi.org/10.1128/AAC.00627-10
- Marchaim D, Chopra T, Perez F, Hayakawa K, Lephart PR, Bheemreddy S, Blunden C, Hujer AM, Rudin S, Shango M, et al. Outcomes and genetic relatedness of carbapenem-resistant enterobacteriaceae at Detroit medical center. Infect Control Hosp Epidemiol 2011; 32:861-71; PMID:21828966; https://doi.org/10.1086/661597
- Marchaim D, Chopra T, Bhargava A, Bogan C, Dhar S, Hayakawa K, Pogue JM, Bheemreddy S, Blunden C, Shango M, et al. Recent exposure to antimicrobials and carbapenem-resistant Enterobacteriaceae: the role of antimicrobial stewardship. Infect Control Hosp Epidemiol 2012; 33:817-30; PMID:22759550; https://doi.org/10.1086/666642
- Marchaim D, Navon-Venezia S, Schwaber MJ, Carmeli Y. Isolation of imipenem-resistant Enterobacter species: emergence of KPC-2 carbapenemase, molecular characterization, epidemiology, and outcomes. Antimicrob Agents Chemother 2008; 52:1413-8; PMID:18227191; https://doi.org/10.1128/AAC.01103-07
- Schwaber MJ, Klarfeld-Lidji S, Navon-Venezia S, Schwartz D, Leavitt A, Carmeli Y. Predictors of carbapenem-resistant Klebsiella pneumoniae acquisition among hospitalized adults and effect of acquisition on mortality. Antimicrob Agents Chemother 2008; 52:1028-33; PMID:18086836; https://doi.org/10.1128/AAC.01020-07
- Bhargava A, Hayakawa K, Silverman E, Haider S, Alluri KC, Datla S, Diviti S, Kuchipudi V, Muppavarapu KS, Lephart PR, et al. Risk factors for colonization due to carbapenem-resistant Enterobacteriaceae among patients exposed to long-term acute care and acute care facilities. Infect Control Hosp Epidemiol 2014; 35:398-405; PMID:24602945; https://doi.org/10.1086/675614
- Schechner V, Kotlovsky T, Tarabeia J, Kazma M, Schwartz D, Navon-Venezia S, Carmeli Y. Predictors of rectal carriage of carbapenem-resistant Enterobacteriaceae (CRE) among patients with known CRE carriage at their next hospital encounter. Infect Control Hosp Epidemiol 2011; 32:497-503; PMID:21515981; https://doi.org/10.1086/659762
- Guidance for control of infections with carbapenem-resistant or carbapenemase-producing Enterobacteriaceae in acute care facilities. MMWR Morb Mortal Wkly Rep 2009; 58:256-60; PMID:19300408
- Centers for Disease Control and Prevention. Facility guidance for control of carbapenem-resistant Enterobacteriaceae (CRE), November 2015 update - CRE toolkit. Atlanta (GA): United States Department of Health and Human Services; 2015 Nov [accessed 2016 Aug 20]. http://www.cdc.gov/hai/pdfs/cre/CRE-guidance-508.pdf
- Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of Illness in the Aged. the index of Adl: a standardized measure of biological and psychosocial function. Jama 1963; 185:914-9; PMID:14044222; https://doi.org/10.1001/jama.1963.03060120024016
- Friedman ND, Kaye KS, Stout JE, McGarry SA, Trivette SL, Briggs JP, Lamm W, Clark C, MacFarquhar J, Walton AL, et al. Health care–associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med 2002; 137:791-7; PMID:12435215; https://doi.org/10.7326/0003-4819-137-10-200211190-00007
- Lerner A, Adler A, Abu-Hanna J, Cohen Percia S, Kazma Matalon M, Carmeli Y. Spread of KPC-producing carbapenem-resistant Enterobacteriaceae: the importance of super-spreaders and rectal KPC concentration. Clin Microbiol Infect 2015; 21:470e1-7; PMID:25636943; https://doi.org/10.1016/j.cmi.2014.12.015
- Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; Nineteenth informational supplement. CLSI document M100-S19. Wayne (PA): Clinical and Laboratory Standards Institute; 2009
- Carbapenem-resistant Enterobacteriaceae containing New Delhi metallo-β-lactamase in two patients - Rhode Island, March 2012. MMWR Morb Mortal Wkly Rep 2012; 61:446-8; PMID:22717513
- Thomson KS. Extended-spectrum-β-lactamase, AmpC, and Carbapenemase issues. J Clin Microbiol 2010; 48:1019-25; PMID:20181902; https://doi.org/10.1128/JCM.00219-10
- Mathers AJ, Carroll J, Sifri CD, Hazen KC. Modified Hodge test versus indirect carbapenemase test: prospective evaluation of a phenotypic assay for detection of Klebsiella pneumoniae carbapenemase (KPC) in Enterobacteriaceae. J Clin Microbiol 2013; 51:1291-3; PMID:23390272; https://doi.org/10.1128/JCM.03240-12
- Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; Twenty-second informational supplement. CLSI document M100-S22. Wayne (PA): Clinical and Laboratory Standards Institute; 2012.
- Dudley MN. Rationale for the 2010 revised susceptibility breakpoints for cephalosporins, aztreonam, and carbapenems for enterobacteriaceae. J Pediatric Infect Dis Soc 2012; 1:166-8; PMID:26619172; https://doi.org/10.1093/jpids/pis046
- Ku K, Pogue JM, Moshos J, Bheemreddy S, Wang Y, Bhargava A, Campbell M, Khandker N, Lephart PR, Chopra T, et al. Retrospective evaluation of colistin versus tigecycline for the treatment of Acinetobacter baumannii and/or carbapenem-resistant Enterobacteriaceae infections. Am J Infect Control 2012; 40:983-7; PMID:22440526; https://doi.org/10.1016/j.ajic.2011.12.014
- European Committee on Antimicrobial Susceptibility Testing. EUCAST clinical breakpoints. Basil, Switzerland: European Society of Clinical Microbiology and Infectious Diseases; 2016 April 17 [accessed 2016 Aug 20]. http://www.eucast.org
- Livermore DM, Andrews JM, Hawkey PM, Ho PL, Keness Y, Doi Y, Paterson D, Woodford N. Are susceptibility tests enough, or should laboratories still seek ESBLs and carbapenemases directly? J Antimicrob Chemother 2012; 67:1569-77; PMID:22461311; https://doi.org/10.1093/jac/dks088
- Giske CG, Martinez-Martinez L, Canton R, Stefani S, Skov R, Glupczynski Y, Nordmann P, Wooton M, Miriagou V, Simonsen GS, et al. EUCAST guidelines for detection of resistance mechanisms and specific resistances of clinical and/or epidemiological importance. Basel, Switzerland: European Society of Clinical Microbiology and Infectious Diseases; 2013 Dec [accessed 2016 Aug 20]. http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Resistance_mechanisms/EUCAST_detection_of_resistance_mechanisms_v1.0_20131211.pdf
- Tijet N, Boyd D, Patel SN, Mulvey MR, Melano RG. Evaluation of the Carba NP test for rapid detection of carbapenemase-producing Enterobacteriaceae and Pseudomonas aeruginosa. Antimicrob Agents Chemother 2013; 57:4578-80; PMID:23817380; https://doi.org/10.1128/AAC.00878-13
- Vasoo S, Cunningham SA, Kohner PC, Simner PJ, Mandrekar JN, Lolans K, Hayden MK, Patel R. Comparison of a novel, rapid chromogenic biochemical assay, the Carba NP test, with the modified Hodge test for detection of carbapenemase-producing Gram-negative bacilli. J Clin Microbiol 2013; 51:3097-101; PMID:23824767; https://doi.org/10.1128/JCM.00965-13
- Osterblad M, Hakanen AJ, Jalava J. Evaluation of the Carba NP test for carbapenemase detection. Antimicrob Agents Chemother 2014; 58:7553-6; PMID:25246404; https://doi.org/10.1128/AAC.02761-13
- van der Zwaluw K, de Haan A, Pluister GN, Bootsma HJ, de Neeling AJ, Schouls LM. The carbapenem inactivation method (CIM), a simple and low-cost alternative for the Carba NP test to assess phenotypic carbapenemase activity in gram-negative rods. PLoS One 2015; 10:e0123690; PMID:25798828; https://doi.org/10.1371/journal.pone.0123690
- Walker T, Dumadag S, Lee CJ, Lee SH, Bender JM, Cupo Abbott J, She RC. Clinical impact of laboratory implementation of verigene BC-GN microarray-based assay for detection of gram-negative bacteria in positive blood cultures. J Clin Microbiol 2016; 54:1789-96; PMID:27098961; https://doi.org/10.1128/JCM.00376-16
- Adler A, Navon-Venezia S, Moran-Gilad J, Marcos E, Schwartz D, Carmeli Y. Laboratory and clinical evaluation of screening agar plates for detection of carbapenem-resistant Enterobacteriaceae from surveillance rectal swabs. J Clin Microbiol 2011; 49:2239-42; PMID:21471338; https://doi.org/10.1128/JCM.02566-10
- Weinstein JW, Tallapragada S, Farrel P, Dembry LM. Comparison of rectal and perirectal swabs for detection of colonization with vancomycin-resistant enterococci. J Clin Microbiol 1996; 34:210-2; PMID:8748308
- Centers for Disease Control and Prevention. Laboratory protocol for detection of carbapenem-resistant or carbapenemase-producing, Klebsiella spp. or E. coli from rectal swabs. Atlanta, GA: United States Department of Health and Human Services; 2009 [accessed 2016 Aug 20]. https://www.cdc.gov/HAI/pdfs/labSettings/Klebsiella_or_Ecoli.pdf
- Gniadek TJ, Carroll KC, Simner PJ. Carbapenem-resistant non-glucose-fermenting gram-negative Bacilli: the missing piece to the puzzle. J Clin Microbiol 2016; 54:1700-10; PMID:26912753; https://doi.org/10.1128/JCM.03264-15
- Papadimitriou-Olivgeris M, Bartzavali C, Christofidou M, Bereksi N, Hey J, Zambardi G, Spiliopoulou I. Performance of chromID(R) CARBA medium for carbapenemases-producing Enterobacteriaceae detection during rectal screening. Eur J Clin Microbiol Infect Dis 2014; 33:35-40; PMID:23912722; https://doi.org/10.1007/s10096-013-1925-6
- Vasoo S, Cunningham SA, Kohner PC, Mandrekar JN, Lolans K, Hayden MK, Patel R. Rapid and direct real-time detection of blaKPC and blaNDM from surveillance samples. J Clin Microbiol 2013; 51:3609-15; PMID:23966498; https://doi.org/10.1128/JCM.01731-13
- Lolans K, Calvert K, Won S, Clark J, Hayden MK. Direct ertapenem disk screening method for identification of KPC-producing Klebsiella pneumoniae and Escherichia coli in surveillance swab specimens. J Clin Microbiol 2010; 48:836-41; PMID:20071553; https://doi.org/10.1128/JCM.01988-09
- Vrioni G, Daniil I, Voulgari E, Ranellou K, Koumaki V, Ghirardi S, Kimouli M, Zambardi G, Tsakris A. Comparative evaluation of a prototype chromogenic medium (ChromID CARBA) for detecting carbapenemase-producing Enterobacteriaceae in surveillance rectal swabs. J Clin Microbiol 2012; 50:1841-6; PMID:22461675; https://doi.org/10.1128/JCM.06848-11
- Zarakolu P, Day KM, Sidjabat HE, Kamolvit W, Lanyon CV, Cummings SP, Paterson DL, Akova M, Perry JD. Evaluation of a new chromogenic medium, chromID OXA-48, for recovery of carbapenemase-producing Enterobacteriaceae from patients at a university hospital in Turkey. Eur J Clin Microbiol Infect Dis 2015; 34:519-25; PMID:25308827; https://doi.org/10.1007/s10096-014-2255-z
- Blackburn J, Tsimiklis C, Lavergne V, Pilotte J, Grenier S, Gilbert A, Lefebvre B, Domingo M-C, Tremblay C, Bourgault A-M, et al. Carbapenem disks on MacConkey agar in screening methods for detection of carbapenem-resistant Gram-negative rods in stools. J Clin Microbiol 2013; 51:331-3; PMID:23135936; https://doi.org/10.1128/JCM.02878-12
- Samra Z, Bahar J, Madar-Shapiro L, Aziz N, Israel S, Bishara J. Evaluation of CHROMagar KPC for rapid detection of carbapenem-resistant Enterobacteriaceae. J Clin Microbiol 2008; 46:3110-1; PMID:18632915; https://doi.org/10.1128/JCM.00249-08
- Hindiyeh M, Smollen G, Grossman Z, Ram D, Davidson Y, Mileguir F, Vax M, Ben David D, Tal I, Rahav G, et al. Rapid detection of blaKPC carbapenemase genes by real-time PCR. J Clin Microbiol 2008; 46:2879-83; PMID:18614657; https://doi.org/10.1128/JCM.00661-08
- Vasoo S, Lolans K, Li H, Prabaker K, Hayden MK. Comparison of the CHROMagar KPC, Remel Spectra CRE, and a direct ertapenem disk method for the detection of KPC-producing Enterobacteriaceae from perirectal swabs. Diagn Microbiol Infect Dis 2014; 78:356-9; PMID:24439449; https://doi.org/10.1016/j.diagmicrobio.2013.08.016
- Pournaras S, Zarkotou O, Poulou A, Kristo I, Vrioni G, Themeli-Digalaki K, Tsakris A. A combined disk test for direct differentiation of carbapenemase-producing enterobacteriaceae in surveillance rectal swabs. J Clin Microbiol 2013; 51:2986-90; PMID:23843486; https://doi.org/10.1128/JCM.00901-13
- Schechner V, Straus-Robinson K, Schwartz D, Pfeffer I, Tarabeia J, Moskovich R, Chmelnitsky I, Schwaber MJ, Carmeli Y, Navon-Venezia S. Evaluation of PCR-based testing for surveillance of KPC-producing carbapenem-resistant members of the Enterobacteriaceae family. J Clin Microbiol 2009; 47:3261-5; PMID:19675211; https://doi.org/10.1128/JCM.02368-08
- Panagea T, Galani I, Souli M, Adamou P, Antoniadou A, Giamarellou H. Evaluation of CHROMagar KPC for the detection of carbapenemase-producing Enterobacteriaceae in rectal surveillance cultures. Int J Antimicrob Agents 2011; 37:124-8; PMID:21177079; https://doi.org/10.1016/j.ijantimicag.2010.10.010
- Viau R, Frank KM, Jacobs MR, Wilson B, Kaye K, Donskey CJ, Perez F, Endimiani A, Bonomo RA. Intestinal carriage of carbapenemase-producing organisms: Current status of surveillance methods. Clin Microbiol Rev 2016; 29:1-27; PMID:26511484; https://doi.org/10.1128/CMR.00108-14
- Simner PJ, Martin I, Opene B, Tamma PD, Carroll KC, Milstone AM. Evaluation of multiple methods for detection of gastrointestinal colonization of carbapenem-resistant organisms from rectal swabs. J Clin Microbiol 2016; 54:1664-7; PMID:27053674; https://doi.org/10.1128/JCM.00548-16
- Doyle D, Peirano G, Lascols C, Lloyd T, Church DL, Pitout JD. Laboratory detection of Enterobacteriaceae that produce carbapenemases. J Clin Microbiol 2012; 50:3877-80; PMID:22993175; https://doi.org/10.1128/JCM.02117-12
- Singh K, Mangold KA, Wyant K, Schora DM, Voss B, Kaul KL, Hayden MK, Chundi V, Peterson LR. Rectal screening for Klebsiella pneumoniae carbapenemases: comparison of real-time PCR and culture using two selective screening agar plates. J Clin Microbiol 2012; 50:2596-600; PMID:22622443; https://doi.org/10.1128/JCM.00654-12
- Nijhuis R, Samuelsen O, Savelkoul P, van Zwet A. Evaluation of a new real-time PCR assay (Check-Direct CPE) for rapid detection of KPC, OXA-48, VIM, and NDM carbapenemases using spiked rectal swabs. Diagn Microbiol Infect Dis 2013; 77:316-20; PMID:24135412; https://doi.org/10.1016/j.diagmicrobio.2013.09.007
- van der Zee A, Roorda L, Bosman G, Fluit AC, Hermans M, Smits PH, van der Zanden AG, Te Witt R, Bruijnesteijn van Coppenraet LE, Cohen Stuart J, et al. Multi-centre evaluation of real-time multiplex PCR for detection of carbapenemase genes OXA-48, VIM, IMP, NDM and KPC. BMC Infect Dis 2014; 14:27; PMID:24422880; https://doi.org/10.1186/1471-2334-14-27
- Garcia-Fernandez S, Morosini MI, Marco F, Gijon D, Vergara A, Vila J, Ruiz-Garbajosa P, Cantón R. Evaluation of the eazyplex(R) SuperBug CRE system for rapid detection of carbapenemases and ESBLs in clinical Enterobacteriaceae isolates recovered at two Spanish hospitals. J Antimicrob Chemother 2015; 70:1047-50; PMID:25428926
- Cuzon G, Naas T, Bogaerts P, Glupczynski Y, Nordmann P. Evaluation of a DNA microarray for the rapid detection of extended-spectrum β-lactamases (TEM, SHV and CTX-M), plasmid-mediated cephalosporinases (CMY-2-like, DHA, FOX, ACC-1, ACT/MIR and CMY-1-like/MOX) and carbapenemases (KPC, OXA-48, VIM, IMP and NDM). J Antimicrob Chemother 2012; 67:1865-9; PMID:22604450; https://doi.org/10.1093/jac/dks156
- Braun SD, Monecke S, Thurmer A, Ruppelt A, Makarewicz O, Pletz M, Reiβig A, Slickers P, Ehricht R. Rapid identification of carbapenemase genes in gram-negative bacteria with an oligonucleotide microarray-based assay. PLoS One 2014; 9:e102232
- Tenover FC, Canton R, Kop J, Chan R, Ryan J, Weir F, Ruiz-Garbajosa P, LaBombardi V, Persing DH. Detection of colonization by carbapenemase-producing Gram-negative Bacilli in patients by use of the Xpert MDRO assay. J Clin Microbiol 2013; 51:3780-7; PMID:24006011; https://doi.org/10.1128/JCM.01092-13
- Findlay J, Hopkins KL, Meunier D, Woodford N. Evaluation of three commercial assays for rapid detection of genes encoding clinically relevant carbapenemases in cultured bacteria. J Antimicrob Chemother 2015; 70:1338-42; PMID:25630646; https://doi.org/; https://doi.org/10.1093/jac/dku571
- Decousser JW, Poirel L, Desroches M, Jayol A, Denamur E, Nordmann P. Failure to detect carbapenem-resistant Escherichia coli producing OXA-48-like using the Xpert Carba-R assay(R). Clin Microbiol Infect 2015; 21:e9-10; PMID:25682281; https://doi.org/10.1016/j.cmi.2014.09.006
- Lafeuille E, Laouira S, Sougakoff W, Soulier-Escrihuela O, Leconte J, Garrec H, Tourret J, Jarlier V, Robert J. Detection of OXA-48-like carbapenemase genes by the Xpert(R) Carba-R test: room for improvement. Int J Antimicrob Agents 2015; 45:441-2; PMID:25601530; https://doi.org/10.1016/j.ijantimicag.2014.12.009
- Tato M, Ruiz-Garbajosa P, Traczewski M, Dodgson A, McEwan A, Humphries R, Hindler J, Veltman J, Wang H, Cantón R. Multisite evaluation of cepheid xpert carba-R assay for detection of carbapenemase-producing organisms in rectal swabs. J Clin Microbiol 2016; 54:1814-9; PMID:27122379; https://doi.org/10.1128/JCM.00341-16
- Mathers AJ, Poulter M, Dirks D, Carroll J, Sifri CD, Hazen KC. Clinical microbiology costs for methods of active surveillance for Klebsiella pneumoniae carbapenemase-producing Enterobacteriaceae. Infect Control Hosp Epidemiol 2014; 35:350-5; PMID:24602938; https://doi.org/10.1086/675603
- Rajapakse N, Vayalumkal J, Lam-Li D, Pearce C, Rees G, Kamhuka L, Peirano G, Pidhorney C, Ledgerwood D, Alfieri N, et al. Pilot testing of an out-of-country medical care questionnaire with screening and cost analysis of preemptive isolation for carbapenem-resistant Enterobacteriaceae in a large Canadian health region. Infect Control Hosp Epidemiol 2014; 35:450-1; PMID:24602958; https://doi.org/10.1086/675616
- Girlich D, Bouihat N, Poirel L, Benouda A, Nordmann P. High rate of faecal carriage of extended-spectrum β-lactamase and OXA-48 carbapenemase-producing Enterobacteriaceae at a university hospital in Morocco. Clin Microbiol Infect 2014; 20:350-4; PMID:23927757; https://doi.org/10.1111/1469-0691.12325