ABSTRACT
Drugs such as linezolid that inhibit bacterial protein synthesis may be beneficial in treating infections caused by toxigenic Staphylococcus aureus. As protein synthesis inhibitors have no effect on preformed toxins, neutralization of pathogenic exotoxins with anti-toxin antibodies may be beneficial in conjunction with antibacterial therapy. Herein, we evaluated the efficacy of human-mouse chimeric high-affinity neutralizing anti-staphylococcal enterotoxin B (SEB) antibodies in the treatment of experimental pneumonia caused by SEB-producing S. aureus. Since HLA class II transgenic mice mount a stronger systemic immune response following challenge with SEB and are more susceptible to SEB-induced lethal toxic shock than conventional mice strains, HLA-DR3 transgenic mice were used. Lethal pneumonia caused by SEB-producing S. aureus in HLA-DR3 transgenic mice was characterized by robust T cell activation and elevated systemic levels of several pro-inflammatory cytokines and chemokines. Prophylactic administration of a single dose of linezolid 30 min prior to the onset of infection attenuated the systemic inflammatory response and protected from mortality whereas linezolid administered 60 min after the onset of infection failed to confer significant protection. Human-mouse chimeric high-affinity neutralizing anti-SEB antibodies alone, but not polyclonal human IgG, mitigated this response and protected from death when administered immediately after initiation of infection. Further, anti-SEB antibodies as well as intact polyclonal human IgG, but not its Fab or Fc fragments, protected from lethal pneumonia when followed with linezolid therapy 60 min later. In conclusion, neutralization of superantigens with high-affinity antibodies may have beneficial effects in pneumonia.
Introduction
Staphylococcus aureus is an opportunistic pathogen, capable of causing a spectrum of diseases; from benign skin infections to lethal diseases such as pneumonia, sepsis and toxic shock syndrome.Citation1 There has been an increase in the incidence of serious infections caused by methicillin-resistant S. aureus (MRSA) in recent years both in the community as well as hospitals in the USA,Citation2 and globally,Citation3-6 posing enormous health and economic burdens.Citation7,8 Greater virulence and increased pathogenicity of certain S. aureus strains can be due to higher expression of specific exotoxins.Citation9-15 In addition to exotoxins, several other factors/molecules elaborated by S. aureus also contribute to its immune evasion/persistence.Citation16 Given these observations, antibacterial agents which inhibit bacterial protein synthesis (thereby reducing/preventing exotoxin production) may be more effective than those drugs that inhibit cell wall synthesis in treating infections caused by toxigenic S. aureus.Citation17 Another strategy is to neutralize exotoxins, for example with antitoxin antibodies. This approach may be more effective for the following reasons.
In clinical settings, antibacterial agents are almost always administered following the onset of infection, leaving a considerable time delay between onset of infection and initiation of therapy.Citation18 During this time window, bacteria are actively replicating and producing exotoxins. Antibacterial agents have minimal effect on preformed toxins. In addition, even the most effective protein synthesis inhibitor agent may not completely shut down toxin production in vivo. Therefore, combining traditional antibacterial agents to inhibit toxin production, and anti-toxin agents such as antibodies or small molecule inhibitors to neutralize/antagonize toxin(s) that are already produced, will result in enhanced therapeutic efficacy.
Exotoxins that play major roles in the pathogenesis of S. aureus pneumonia and hence ideal targets for inhibition are α-toxin, Panton-Valentine leukocidin (PVL), and superantigens (SAgs).Citation19 Monoclonal antibodies against α-toxin have been shown to be beneficial in S. aureus pneumonia.Citation20,21 The role of PVL in the pathogenesis of pneumonia is controversial. While some studies support its pathogenic role,Citation22 others have refuted this claim.Citation23,24 Moreover, antibodies against PVL failed to confer protection and even enhanced staphylococcal virulence in a mouse skin abscess model. Hence, the pathogenic role of PVL needs to be established prior to targeting it for inhibition.Citation25 Members of the SAg family have been shown to play a role in the pathogenesis of numerous serious staphylococcal infections, including pneumonia.Citation26-28 Notably, several clinical MRSA isolates produce one or more SAg.Citation29,30 The unique ability of SAgs to robustly stimulate the immune system, followed by induction of immune unresponsiveness or anergy, may divert the immune response against S. aureus, thereby helping in immune evasion.Citation31-34 Therefore, neutralization of preformed SAgs along with inhibition of their production with antibacterial agents in vivo may be an effective approach.
Staphylococcal SAgs may be antagonized using peptide antagonists, soluble TCR Vβ fragments (TCR mimics), or neutralizing antibodies.Citation35-37 Peptide antagonists and TCR mimics have short half-lives in vivo and may be immunogenic to humans, thus causing unwanted side-effects.Citation38 On the other hand, neutralizing antibodies, particularly humanized antibodies, have a longer half-life and could be used in humans without the problem of immunogenicity. In this context, we have generated a panel of high-affinity, human-mouse chimeric monoclonal antibodies against staphylococcal enterotoxin B (SEB),Citation39 a potent SAg expressed by several pathogenic S. aureus strains as well as a potential biological weapon.Citation40,41 These antibodies abolish superantigenicity of SEB and effectively protect from lethal experimental SEB-induced toxic shock syndrome.Citation39,42 Other groups have also described the development of mouse monoclonal as well as human-mouse chimeric neutralizing anti-SEB antibodies, which protected Balb/c mice from shock induced by purified SEB.Citation43-45 One group has also developed lama-derived single domain antibody specific for SEB, which could be used in diagnostic field.Citation46 However, to our knowledge, only one study has tested the efficacy of these humanized antibodies in in vivo infection models.Citation47 In this report, Varshney et al., have shown that humanized anti-SEB antibodies conferred protection from S. aureus sepsis as well as thigh infection caused by toxigenic S. aureus in Balb/c mice.Citation47 However, in these studies, the anti-SEB antibodies were administered prophylactically up to 24 hours prior to infection. Moreover, in these studies, the antibacterial agent vancomycin, which was used in conjunction with antibody therapy, was also initiated 24 hours prior to establishing infection. In addition, Varshney et al., did not evaluate the efficacy of the humanized anti-SEB antibodies in pneumonia, a common and potentially serious infection caused by S. aureus. Most importantly, Varshney et al., tested the efficacy of prophylactic administration of anti-SEB antibodies along with vancomycin in conventional mouse models that have been shown to be resistant to pathogenic effects of SAgs than are humans.Citation11 Therefore, we investigated the efficacy of humanized anti-SEB neutralizing antibodies administered immediately after infection and linezolid given 60 min after infection using a humanized mouse model instead of conventional laboratory mice.
In order to mediate their immune stimulating functions, SAgs have to first bind to MHC class II molecules. SAgs bind to mouse MHC class II molecules with 100-fold lower affinity than to human MHC class II molecules.Citation37,48 Therefore, conventional laboratory mice are not only resistant to SAgs but also fail to fully manifest the immunopathogenesis of infections caused by SAg-producing S. aureus.Citation11,49 However, we and others have shown that transgenic mice expressing HLA-DR molecules respond more robustly to SAgs and that their response to pathogenic S. aureus mimic that of humans.Citation37,50-53 Hence, we used HLA-DR3 transgenic mice in the current investigation. Secondly, since SEB binds to mouse MHC class II with lower affinity, even low affinity neutralizing antibodies to SEB could block this interaction and hence, be effective in conventional mice. However, these antibodies may not be protective in humans as SEB binds to human HLA-DR molecules with highest affinity. Hence, higher affinity antibodies may be required to disrupt the interaction of SEB with HLA-DR. Therefore, we envisaged that the effectiveness of administration of neutralizing anti-SEB antibodies and linezolid, a bacteriostatic agent, would be better investigated using humanized mice expressing HLA-DR molecules.
Results and discussion
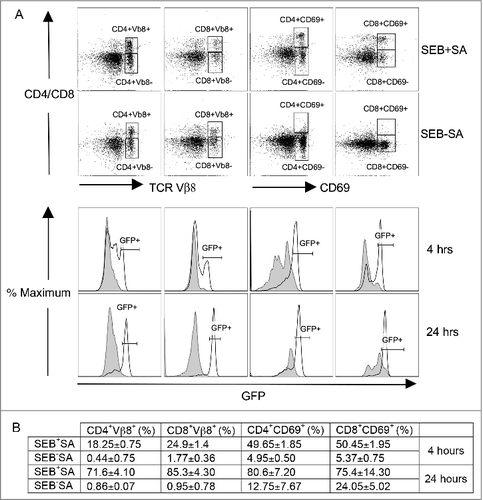
Pneumonia caused by a SAg-producing S. aureus is characterized by early and robust T cell activation
We had previously reported that the S. aureus strain IDRL-7419 which produces SEB, but not its isogenic counterpart IDRL-7420 that does not produce SEB, causes lethal pneumonia in HLA-DR3 transgenic mice.Citation54 We had also demonstrated that this is due to higher susceptibility of HLA-DR3 transgenic mice to the immune-stimulating effects of SEB compared with C57Bl/6 mice.Citation54 To specifically show that SEB produced by the S. aureus strain IDRL-7419 causes early and robust activation of T cells during pneumonia, we generated HLA-DR3.Nur77-eGFP transgenic mice. Experimental pneumonia was induced in HLA-DR3.Nur77-eGFP transgenic mice with SEB-producing S. aureus strain IDRL-7419 (SEB+SA) and the non-SEB-producing isogenic strain, IDRL-7420 (SEB-SA). Mice were sacrificed 4 and 24 hours later, spleens harvested and the expression of eGFP in CD4+ and CD8+ T cell subsets was analyzed by flow cytometry.
The staphylococcal SAg SEB primarily activates CD4+ and CD8+ T cells bearing TCR Vβ8. Therefore, the expression of eGFP within the TCR Vβ8+ sub-populations was examined at 4 and 24 hours. Strong upregulation of eGFP expression in the TCR Vβ8+ sub-population was appreciable in mice infected with S. aureus strains IDRL-7419 (SEB+SA) but not in mice infected with the isogenic strain, IDRL-7420 (SEB-SA) even as early as 4 hours following infection (). By 24 hours, 80–90% of the CD4+ and CD8+ T cells bearing TCR Vβ8+ expressed eGFP in mice infected with SEB + SA compared with only 1–2% in mice infected with SEB-SA (). It should be noted that it would take several days to elicit a conventional peptide-specific CD4+ T cell immune response (as S. aureus is primarily an extracellular pathogen) and even such a response would be limited to far fewer cells; typically the frequency of such peptide-specific T cells would be in the range of 1 in 105 to 1 in 106 cells. As eGFP expression driven by the Nur77 promoter occurs only following signaling through TCR,Citation55 and based on the kinetics of expression of eGFP, it can be inferred that the SAg (i.e., SEB in our model) produced in vivo by the infecting S. aureus strain was responsible for upregulation of eGFP. The same pattern of eGFP expression could be appreciated in CD4+ and CD8+ T cells when gated based on CD69 expression, which is an early T cell activation marker (). Significant CD69 upregulation and eGFP expression within the CD69+ T cell subsets occurred primarily in mice infected with SEB+SA. Taken together, these data clearly established that SEB is readily produced in vivo even at the early stages of pneumonia, causing robust T cell activation.
Figure 1. Superantigens rapidly produced in vivo during staphylococcal pneumonia cause robust T cell activation. HLA-DR3.Nur77-eGFP transgenic mice were intra-tracheally challenged with SEB-producing S. aureus strain IDRL-7419 (SEB+SA) or the non-SEB-producing isogenic strain, IDRL-7420 (SEB-SA). Mice were sacrificed 4 and 24 hours later, spleens harvested and stained with anti-CD4, anti-CD8, anti-Vβ8 and anti-CD69 antibodies. The expression of eGFP within the TCR Vβ8+ (left half) and CD69+ (right half) CD4+ and CD8+ T cell subsets was analyzed by flow cytometry. (A) Representative dot plots at 24 hours showing the gating strategy. Histogram plots show the expression of GFP within CD4+ TCR Vβ8+ and CD8+ TCR Vβ8+ gated cells or GFP expression within CD4+ CD69+ and CD8+ CD69+ gated cells. Gray filled dotted lines represent GFP expression in cells from mice challenged with IDRL-7420 (SEB-SA) and solid black lines represent GFP expression in cells from mice challenged with IDRL-7419 (SEB+SA). Plots from one representative set of experiment is shown. (B) Table shows the percentage (mean ± SE) of GFP positive cells within the gated cells at indicated time points in the histogram plots from panel A, from two mice in each group.
High-affinity, neutralizing human-mouse chimeric anti-SEB antibodies attenuate the systemic inflammatory response that occur during pneumonia caused by SEB-producing S. aureus
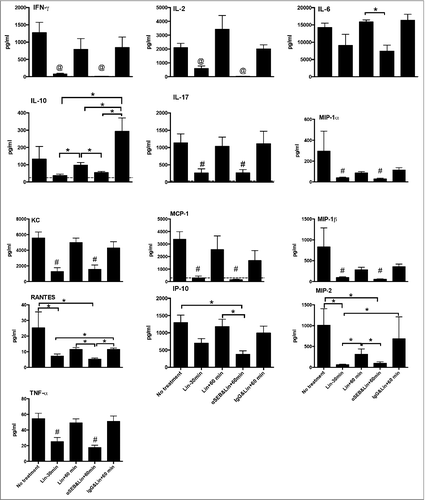
Given the rapidity with which the toxigenic S. aureus strain produced SAg in vivo, we next tested our hypothesis that even a short time gap before the initiation of antibacterial therapy (the bacterial protein synthesis inhibitor, linezolid in this study) following an infection might be sufficient for the bacteria to produce pathological levels of SAg and that passive administration of a pair of anti-SAg antibodies (that bind to distinct epitopes on SEB and synergistically neutralize SEBCitation39,42), along with linezolid may be more effective in neutralizing the pathogenic effects of SAg and conferring protection. HLA-DR3 transgenic mice were assigned to different groups and subjected to various treatments as indicated. Mice were bled 6 hours later to assess the levels of serum cytokine/chemokine levels.
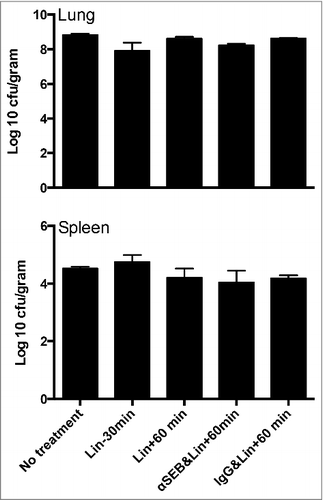
SEB robustly activates T cells by cross-linking their TCR.Citation31,40 Activated T cells produce several cytokines as well as chemokines, which in turn activate other cells of the immune system, leading to a significant elevation in systemic levels of multiple cytokines and chemokines, which is clinically termed as systemic inflammatory response syndrome (SIRS) in humans.Citation31,40 As expected, sera from mice challenged with SEB-producing S. aureus had elevated levels of T cell-derived cytokines including IL-2, IL-17 and IFN-γ as well as other cytokines/chemokines, compared with naïve mice (). Pre-treatment with linezolid caused a significant reduction in these mediators suggesting suppression of SAg production. However, mice that received linezolid an hour after intratracheal challenge with S. aureus had significantly elevated levels of all cytokines and chemokines in their sera which were not different from those in the untreated control group. This suggests that the S. aureus isolate produces sufficient amount of SEB within the first hour following infection to cause a robust systemic inflammatory response. However, in line with our hypothesis, serum levels of most cytokines and chemokines were lower in mice that received anti-SEB antibodies followed by linezolid, but not control human IgG and linezolid, suggesting that anti-SEB antibodies were able to neutralize SEB. We also determined the bacterial load in the lungs and spleen at 6 hours. As shown in , there were no differences in bacterial loads between the different groups at 6 hours. As linezolid is a bacterial protein synthesis inhibitor (i.e., a bacteriostatic agent), this observation is not unexpected. Overall, these observations suggest that elevation in systemic cytokine and chemokines during pneumonia was due to early production of SEB in vivo as neutralization of SEB significantly attenuated this process and even an hour delay in the administration of linezolid did not attenuate the robust systemic inflammatory response.
Figure 2. Prophylactic administration of linezolid as well as humanized anti-SEB antibodies administered immediately after infection followed by linezolid, attenuate systemic inflammatory response in pneumonia. Experimental pneumonia was induced with S. aureus IDRL-7419 in age-matched HLA-DR3 transgenic mice. Mice were left untreated, treated prophylactically with linezolid alone 30 min prior to infection (Lin-30 min), treated with linezolid alone 60 min after infection (Lin+60 min), treated with a mixture of chimeric anti-SEB antibody clones Ch 63 and Ch 82 M (250 μg each) administered immediately following infection and linezolid 60 min after infection (αSEB&Lin + 60 min), or treated with polyclonal human IgG (500 μg) immediately following infection and linezolid 60 min after infection (IgG&Lin + 60 min). Six hours after infection, mice were sacrificed, sera collected and levels of indicated cytokines/chemokine were determined by multiplex assay (EMD Millipore). If shown, the dotted horizontal lines represent the serum concentration of respective analyte in naïve mice. Each bar represents data from 4 to 6 mice/group. @ p < 0.05 by FDR when compared with all other groups. #p < 0.05 by FDR when compared with all other groups except the group with #, *p < 0.05 by FDR when compared with indicated group.
Humanized anti-SEB antibodies protect from lethal pneumonia
We next performed survival experiments to test whether mitigation of systemic inflammatory response by the combination of anti-SEB antibodies and linezolid would translate to heightened protection from lethal pneumonia. The SEB+SA (S. aureus IDRL-7419) was highly lethal to HLA-DR3 transgenic mice, consistent with our prior report (). Moreover, prophylactic administration of just one dose of linezolid 30 min prior to bacterial challenge conferred significant protection over a seven-day period (). As expected, delaying the administration of linezolid by 60 min failed to protect HLA-DR3 mice from lethal pneumonia, as nearly 70% of the animals treated with linezolid after establishing infection died. However, passive administration of anti-SEB antibodies given immediately after the onset of infection significantly improved survival in this group of mice, which correlated with milder systemic inflammatory response (). Unexpectedly, even mice that received polyclonal human IgG and linezolid were protected from lethal pneumonia even though serum cytokine/chemokine levels in this group were significantly elevated at levels similar to those in untreated mice ().
Figure 4. Efficacy of administration of linezolid and humanized anti-SEB antibodies on the outcome of lethal pneumonia caused by SEB-producing S. aureus. Experimental pneumonia was induced in age-matched HLA-DR3 mice by intratracheal inoculation of S. aureus IDRL-7419 (1 × 108 CFU/mouse) in a final volume of 50 μl. Mice were left untreated (n = 31) or treated as follows. Linezolid administered at 200 mg/kg of body weight either 30 min before (Lin-30 min, n = 17) or 60 min after (Lin + 60 min, n = 17) establishment of infection; treated with a mixture of 250 μg each of chimeric anti-SEB antibody clones Ch 63 and Ch 82 M (αSEB mix alone) or 500 μg of polyclonal human IgG alone (IgG alone) immediately following infection; αSEB mix, followed by linezolid 60 min after infection (αSEB&Lin + 60 min, n = 8); polyclonal human IgG followed by linezolid 60 min after infection (IgG&Lin + 60 min, n = 8); intact human IgG derived from a multiple myeloma patient (mmIgG) (n = 6) or Fab (n = 5) or Fc (n = 5) fragments derived from polyclonal human IgG, all at 500 μg/mouse given immediately following infection. Mice were closely monitored; moribund animals were removed and euthanized as per IACUC recommendations. P values of each treatment arm compared with untreated control group by Log-rank (Mantel-Cox) test. Lin-30 min, P = 0.0001; Lin + 60 min, P = 0.60; αSEB&Lin + 60 min, P = 0.012; IgG&Lin + 60 min, P = 0.0005; αSEB alone, P = 0.0040; IgG alone, P = 0.646; mmIgG&Lin + 60 min, P = 0.97, Fab &Lin + 60 min, P = 0.54, Fc &Lin + 60 min P = 0.70.
Given the paradoxical protection conferred by our control polyclonal human IgG, we next investigated whether the control polyclonal human IgG by itself could be protective without linezolid. For this, HLA-DR3 mice were challenged with IDRL-7419 and treated with humanized anti-SEB antibody or polyclonal human IgG immediately following infection. Whereas only 20% of the mice that received anti-SEB antibodies alone died, nearly 85% of mice that received human IgG control antibodies succumbed to pneumonia (). While these results confirmed that the SAg SEB plays a lethal role in pneumonia, the mechanisms by which polyclonal human IgG conferred protection when administered with linezolid still remained unresolved. As the serum levels of cytokines and chemokines in mice treated with polyclonal human IgG were not reduced, we infer that protection is not due to the presence of neutralizing anti-SEB antibodies present in the control IgG preparations. Moreover, polyclonal human IgG preparations do not inhibit SEB-induced T cell proliferation in vitro further ruling out direct neutralization of SEB. [Citation39,42 and additional data not shown]. We therefore hypothesized that the protective effects of polyclonal human IgG was due to the presence of natural antibodies to staphylococcal cell wall components, which may help in opsonization and Fc-mediated clearance of the bacteria from the system. To address this issue, mice were challenged with S. aureus IDRL-7419, immediately following infection injected with the Fab or Fc portions prepared from polyclonal IgG and treated with linezolid 60 minutes later. As shown in , neither administration of Fc nor Fab fragments conferred protection (mortality 4/5 in each group). This suggests that polyclonal human IgG requires both Fab and Fc regions to mediate its protective effect. To further prove that the polyclonality of the human antibodies is mandatory, purified human IgG was obtained from a patient with multiple myeloma, a condition generally characterized by the presence of large quantities of antibodies of single specificity.Citation56 Hence, IgG antibodies from a single myeloma patient are generally monospecific. Mice were challenged with S. aureus IDRL-7419, treated with purified IgG from a myeloma patient (mmIgG) and 60 minutes later treated with linezolid. Interestingly, 5 out of 6 mice succumbed to lethal pneumonia implying that only polyclonal, but not monospecific, IgG is protective ().
In another set of experiments, HLA-DR3 transgenic mice were intratracheally challenged with S. aureus not producing SEB (SEB-SA) at the same dosage as that of S. aureus producing SEB (1×108 cfu/mouse). Subsequently, the mice were left untreated or treated with anti-SEB antibodies, polyclonal human IgG or mmIgG. None of the mice (4–5 mice in each group), either treated with antibodies or left untreated succumbed to pneumonia. This study reaffirmed our earlier report that SAg (SEB in our model) plays a lethal role in pneumoniaCitation54 Moreover, this result also demonstrated that these antibodies are safe and do not potentiate pneumonia caused by S. aureus that do not produce any SAg. In the final set of experiments, we wished to establish the specificity of anti-SEB monoclonal antibodies and to verify that these chimeric antibodies do not have any non-specific anti-staphylococcal activity. For this, HLA-DR3 mice were first challenged with a higher inoculum of the isogenic strain that does not produce any SEB (isolate SEB-SA, 3×108 cfu/mouse) [Note. A higher inoculum of SEB-SA was used because at a lower inoculum, SEB-SA is not lethal to HLA-DR3 mice]. Subsequently, the mice were either left untreated or treated with the anti-SEB antibody mix and closely monitored. Three out of four (75% mortality) mice challenged with SEB-SA and not receiving any antibodies succumbed to pneumonia. At the same time, four out of five (4/5) mice (80% mortality) that were challenged with SEB-SA and treated with anti-SEB antibody mix also succumbed to pneumonia by 48 hours. This experiment clearly concluded that the anti-SEB antibodies do not exert any non-specific anti-staphylococcal activity.
Overall, linezolid attenuates systemic inflammatory response during staphylococcal pneumonia by inhibiting production of SAg consistent with our previous findings.Citation54 Even a short delay in initiation of antibacterial therapy however may be sufficient to allow the production of pathogenic SAg. Neutralizing antibodies to SEB by themselves are protective in pneumonia caused by toxigenic S. aureus producing SEB even when administered immediately after infection. However, considering that diverse toxins may be playing roles in disease pathogenesis depending on the clinical isolate involved, combining neutralizing antibodies against multiple superantigens as well as other pathogenic exotoxins (e.g., anti-α toxin antibodies,Citation20,21) with an antibacterial agent may be a useful approach. Given the efficacy of polyclonal human IgG in our model and the documented presence of natural antibodies to various staphylococcal surface antigens as well as several toxins including superantigens, in healthy humansCitation57-60 intravenous immunoglobulins (IVIg) could possibly be protective in staphylococcal pneumonia as they are in streptococcal toxic shock syndrome.Citation61 Even though not all S. aureus isolates produce SEB, the same concept can be applied to other superantigens. Since passive administration of high affinity anti-SAg antibodies was effective by itself or along with an antibacterial agent in our S. aureus pneumonia model, inducing high affinity antibodies against SAg by vaccination may be beneficial in protecting against serious S. aureus infections. Hence, SAg may be included in vaccine design against S. aureus. Given the success of antitoxin antibodies in treating bacterial diseases such as tetanus,Citation62 their safety and the widespread use of other humanized antibodies in treating a variety of clinical conditions (e.g., rituximab, cetuximab, infliximab, basiliximab),Citation63 there is potential for success with passive antibody therapy in S. aureus disease. Further studies are underway to determine the optimal dosage and window of therapeutic intervention during which administration of anti-SAg antibodies may be effective.
Materials and methods
Mice
AE°.HLA-DR3 transgenic mice expressing HLA-DRA1*0101 and HLA-DRB1*0301 transgenes on the endogenous MHC class II-null background have been previously described.Citation53 HLA-DR3 transgenic mice expressing eGFP transgene under the Nur77 promoter were generated by crossing C57BL/6-Tg(Nr4a1-EGFP/cre)820Khog/J mice (The Jackson Laboratory, Bar Harbor, ME) with AE°.HLA-DR3 transgenic mice. The offspring were genotyped and selectively bred to generate HLA-DR3+.Nur77-eGFP+ mice. In these mice, rapid expression of eGFP in T cells occurs only upon activation through the T cell receptor (TCR) but not following other inflammatory stimuli. Hence, eGFP expression can be used to differentiate TCR-mediated signaling from other inflammatory signals.Citation55 All mice were bred within the barrier facility of Mayo Clinic Immunogenetics Mouse Colony (Rochester, MN) and moved to a conventional facility after weaning. Bacterial infection studies were conducted in a BSL2 bio-containment animal facility when the mice were 8–12 weeks-old. All experiments were approved by the Institutional Animal Care and Use Committee.
Chimeric antibodies and human immunoglobulins
High affinity, human-mouse chimeric anti-SEB neutralizing antibodies Ch 63 and Ch 82 M have been previously described.Citation39,42 These antibodies bind to distinct epitopes on SEB and effectively neutralize the superantigenicity of SEB in vitro and in vivo.Citation39,42 Antibody-secreting clones were grown in roller bottles at Antibody Hybridoma Core Facility at Mayo Clinic, Rochester, MN. Chimeric antibodies were purified from culture supernatants using protein G columns and their neutralizing activity was verified by inhibition of SEB-induced proliferation of splenocytes from HLA-DR3 transgenic mice, as described earlier.Citation39 Polyclonal human immunoglobulin G (IgG) purified from human plasma and their Fab as well as Fc fragments were obtained from Athens Research & Technology (Athens, GA). IgG1 obtained from individual human myeloma plasma was also obtained from the same resource. All of these preparations have been shown to be non-reactive for HBsAg, anti-HCV, anti-HBc and negative for anti-HIV 1 and 2 by FDA approved tests. At least two different lots of human immunoglobulins were tested (IG2010–01, IG2010–02). The lot numbers of Fab, Fc and myeloma IgGs were FAB2013–01, FC2015–01 and IG1K2013–01, respectively.
Induction of pneumonia
The S. aureus strain IDRL-7419 [RN6734, containing the intact cloned SEB pRN5543::seb (pRN7114), referred to as the SEB + SA strain] expresses only SEB but not any other SAg. The S. aureus strain IDRL-7420 [RN6734, containing a derivative with a large 3′ deletion in SEB, pRN5543::seb(b.2) (pRN7116), referred to as SEB-SA], is an isogenic control for IDRL-7419 and does not produce any SAg including SEB.Citation54,64,65 RN6734 produces α-toxin and δ-toxin.Citation66 We have shown previously that IDRL-7419 produces equivalent levels of SEB compared with clinical isolatesCitation65 Only the SEB+SA, but not the SEB-SA, induces lethal pneumonia in HLA-DR3 transgenic at the inoculum tested, whereas C57Bl/B6 mice are not susceptible to lethal pneumonia.Citation54
Experimental pneumonia was induced following our previously published protocol.Citation54 Briefly, single colonies of S. aureus strains IDRL-7419 (SEB+SA) or IDRL-7420 (SEB-SA) picked from agar plates containing chloramphenicol (20 µg/ml) were inoculated into tryptic soy broth (TSB) containing chloramphenicol (20 µg/ml) and grown over night at 37°C with shaking. The next day, the bacterial density was determined using McFarland standards for broth cultures, the required volume of the bacterial culture was taken out, spun and the supernatant discarded. The bacterial pellet was resuspended in required volume of 0.1% agar in saline to achieve 20×108 CFU per ml. Quantitative culture for each bacterial strain was made with a small aliquot to confirm the inoculation dose used. Subsequently, while under ketamine and xylazine anesthesia, HLA-DR3 transgenic mice were challenged intratracheally with 1–2 × 108 cfu. As IDRL-7420, which does not produce SEB, is not as lethal as IDRL-7419, which produces SEB, in some experiments to check the specificity of anti-SEB antibodies, 3 × 108 cfu of S. aureus IDRL-7420 was used to induce lethal pneumonia. Treatments were given at the time points indicated. Animals were monitored closely and moribund animals were removed from the study. The dose of antibodies was determined from other similar murine studies.Citation47 All injections were done by the intraperitoneal route.
Measurement of serum cytokines and chemokines
Immediately after euthanasia, blood was collected by cardiac puncture in serum separation tubes, spun and sera stored at −80°C until assayed. Concentrations of various cytokines and chemokines in the sera were determined using a mouse cytokine/chemokine magnetic bead multiplex assay following the manufacturer's guidelines (EMD Millipore, Billerica, MA) using MAGPIX multiplexing platform (Luminex, Austin, TX).
Preparation of splenocytes for flow cytometry
Briefly, spleens were placed on sterile disposable 100 μM filters, which were fitted on to sterile 50 ml conical tubes. The spleens were crushed with the rubber end of a 2 ml syringe plunger. The filters were rinsed with 5 ml phosphate buffered saline (PBS). Cells were pelleted by centrifugation. Red blood cells were removed by ammonium chloride lysis. Splenocytes were washed again in PBS and the pellets resuspended in PBS. Cells were subsequently processed for flow cytometry.
CD4+ and CD8+ T cells were identified with PerCP-conjugated anti-CD4 (clone - GK1.5) and APC-conjugated anti-CD8 (clone - 53–6.7) antibodies. TCR Vβ8+ and CD69+ T cells subsets within CD4+ and CD8+ gates were identified with PE-conjugated anti-Vβ8 (clone - F23.1) or PE-conjugated anti-CD69 (clone – H12F3) antibodies. All antibodies are from BD Biosciences ™, San Diego, CA. Expression of eGFP within the gated subpopulations were identified using a BD Accuri™ C6 flow cytometer. Data analysis and generation of dot and histogram plots were done using FlowJo Software V10.0.8 (Tree Star, Ashland, OR).
Determination of bacterial load in organs
Organs were collected aseptically and placed into a sterile stomacher bag with 1 ml of TSB, homogenized for 120 sec and vortexed an additional 30 seconds. One hundred microliters of this homogenate was mixed with 900 μl of saline to generate 1 in 10 dilution. Six additional serial 10-fold dilutions were made in saline. One hundred microliters of the samples from each dilution was plated on a blood agar plate and incubated overnight in a CO2 incubator. The number of colonies in any plate with 10–100 colonies was counted. Based on the dilution factor and the weight of the tissue, CFU per gram of lung tissue was calculated.Citation54
Statistical analyses
Kaplan Meier survival curves were generated using GraphPad prism Version 6 (GraphPad Prism, Inc., San Diego, CA). Statistical comparisons using data from cytokine and chemokine analyses, among the groups were made using Kruskall wallis test. If the overall test among the groups was statistically significant, further pairwise comparisons were made using Wilcoxon rank-sum tests. P-values less than 0.05 were considered statistically significant. Where indicated, False Discovery Rate (FDR) corrected p-values were reported to adjust for multiple comparisons in this study. This analysis was performed using SAS software version 9.3 (SAS Inc. Cary, NC).
Abbreviations
| eGFP | = | enhanced green fluorescent protein |
| HLA | = | Human leukocyte antigen |
| IDRL | = | Infectious Disease Research Laboratory |
| SEB-SA | = | Staphylococcus aureus not producing SEB |
| SEB | = | Staphylococcal enterotoxin B |
| SEB+SA | = | Staphylococcus aureus producing SEB |
| TCR | = | T cell receptor |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We are thankful to Julie Hanson and her crew for excellent mice husbandry and Michelle Smart for characterizing the transgenic mice.
Funding
This study was funded by NIH grants AI101172 (GR) and AI68741 (GR and CSD).
References
- Lowy FD. Staphylococcus aureus Infections. N Engl J Med 1998; 339:520-32; PMID:9709046; http://dx.doi.org/10.1056/NEJM199808203390806
- DeLeo FR, Otto M, Kreiswirth BN, Chambers HF. Community-associated meticillin-resistant Staphylococcus aureus. Lancet 2010; 375:1557-68; PMID:20206987; http://dx.doi.org/10.1016/S0140-6736(09)61999-1
- Otter JA, French GL. Molecular epidemiology of community-associated meticillin-resistant Staphylococcus aureus in Europe. Lancet Infect Dis 2010; 10:227-39; PMID:20334846; http://dx.doi.org/10.1016/S1473-3099(10)70053-0
- Bassetti M, Nicco E, Mikulska M. Why is community-associated MRSA spreading across the world and how will it change clinical practice? Int J Antimicrob Agents 2009; 34:S15-S9; PMID:19560669; http://dx.doi.org/10.1016/S0924-8579(09)70544-8
- Schlievert PM. Staphylococcal toxic shock syndrome: still a problem. Med J Aust 2005; 182:651-2; PMID:16116687
- Tierno PM, Jr, Schlievert PM, Tripp TJ, Peterson ML. Reemergence of Staphylococcal Toxic Shock Syndrome in the United States since 2000. J Clin Microbiol 2005; 43:2032-3; PMID:15815055; http://dx.doi.org/10.1128/JCM.43.4.2032-2033.2005
- Yok-Al Que, Moreill P. Staphylococcus aureus (Including Staphylococcal Toxic Shock). In: Mandell GL, Bennett JE, Dolin R, eds. Mandell, Douglas, and Bennett's principles and practice of infectious diseases. Philadelphia: Churchill Livingstone, Elsevier, 2009.
- Melamed A, Sorvillo F. The burden of sepsis-associated mortality in the United States from 1999 to 2005: an analysis of multiple-cause-of-death data. Critical Care 2009; 13:R28; PMID:19250547; http://dx.doi.org/10.1186/cc7733
- Sugiyama Y, Okii K, Murakami Y, Yokoyama T, Takesue Y, Ohge H, Sueda T, Hiyama E. Changes in the agr Locus Affect Enteritis Caused by Methicillin-Resistant Staphylococcus aureus. J Clin Microbiol 2009; 47:1528-35; PMID:19297601; http://dx.doi.org/10.1128/JCM.01497-08
- Schlievert PM, Strandberg KL, Lin YC, Peterson ML, Leung DY. Secreted virulence factor comparison between methicillin-resistant and methicillin-sensitive Staphylococcus aureus, and its relevance to atopic dermatitis. J Allergy Clin Immunol 2010; 125:39-49; PMID:20109735; http://dx.doi.org/10.1016/j.jaci.2009.10.039
- Schlievert PM. Cytolysins, superantigens, and pneumonia due to community-associated methicillin-resistant Staphylococcus aureus. J Infect Dis 2009; 200:676-8; PMID:19653828; http://dx.doi.org/10.1086/605333
- Nagao M, Okamoto A, Yamada K, Hasegawa T, Hasegawa Y, Ohta M. Variations in amount of TSST-1 produced by clinical methicillin resistant Staphylococcus aureus (MRSA) isolates and allelic variation in accessory gene regulator (agr) locus. BMC Microbiol 2009; 9:52; PMID:19272162; http://dx.doi.org/10.1186/1471-2180-9-52
- Cai Y, Kong F, Wang Q, Tong Z, Sintchenko V, Zeng X, Gilbert GL. Comparison of single- and multilocus sequence typing and toxin gene profiling for characterization of methicillin-resistant Staphylococcus aureus. J Clin Microbiol 2007; 45:3302-8; PMID:17715374; http://dx.doi.org/10.1128/JCM.01082-07
- Shukla SK, Karow ME, Brady JM, Stemper ME, Kislow J, Moore N, Wroblewski K, Chyou P-H, Warshauer DM, Reed KD, et al. Virulence Genes and Genotypic Associations in Nasal Carriage, Community-Associated Methicillin-Susceptible and Methicillin-Resistant USA400 Staphylococcus aureus Isolates. J Clin Microbiol 2010; 48:3582-92; PMID:20668125; http://dx.doi.org/10.1128/JCM.00657-10
- Mushtaq F, Hildrew S, Okugbeni G, Ellis RW. Necrotizing haemorrhagic pneumonia proves fatal in an immunocompetent child due to Panton–Valentine Leucocidin, toxic shock syndrome toxins 1 and 2 and enterotoxin C-producing Staphylococcus aureus. Acta Pædiatrica 2008; 97:985-7; http://dx.doi.org/10.1111/j.1651-2227.2008.00797.x
- Pozzi C, Lofano G, Mancini F, Soldaini E, Speziale P, De Gregorio E, Rappuoli R, Bertholet S, Grandi G, Bagnoli F. Phagocyte subsets and lymphocyte clonal deletion behind ineffective immune response to Staphylococcus aureus. FEMS Microbiol Rev 2015; 39:750; PMID:25994610; http://dx.doi.org/10.1093/femsre/fuv024
- van Langevelde P, van Dissel J, Meurs C, Renz J, Groeneveld P. Combination of flucloxacillin and gentamicin inhibits toxic shock syndrome toxin 1 production by Staphylococcus aureus in both logarithmic and stationary phases of growth. Antimicrob Agents Chemother 1997; 41:1682-5; PMID:9257741
- Lodise TP, McKinnon PS, Swiderski L, Rybak MJ. Outcomes Analysis of Delayed Antibiotic Treatment for Hospital-Acquired Staphylococcus aureus Bacteremia. Clin Infect Dis 2003; 36:1418-23; PMID:12766837; http://dx.doi.org/10.1086/375057
- Moskowitz SM, Wiener-Kronish JP. Mechanisms of bacterial virulence in pulmonary infections. Curr Opin Crit Care 2010; 16:8-12; PMID:19956071; http://dx.doi.org/10.1097/MCC.0b013e3283354710
- Ragle BE, Bubeck Wardenburg J. Anti-Alpha-Hemolysin Monoclonal Antibodies Mediate Protection against Staphylococcus aureus Pneumonia. Infect Immun 2009; 77:2712-8; PMID:19380475; http://dx.doi.org/10.1128/IAI.00115-09
- Hua L, Hilliard JJ, Shi Y, Tkaczyk C, Cheng LI, Yu X, Datta V, Ren S, Feng H, Zinsou R, et al. Assessment of an Anti-Alpha-Toxin Monoclonal Antibody for Prevention and Treatment of Staphylococcus aureus-Induced Pneumonia. Antimicrob Agents and Chemother 2014; 58:1108-17; PMID:24295977; http://dx.doi.org/10.1128/AAC.02190-13
- Labandeira-Rey M, Couzon F, Boisset S, Brown EL, Bes M, Benito Y, Barbu EM, Vazquez V, Höök M, Etienne J, et al. Staphylococcus aureus Panton-Valentine Leukocidin Causes Necrotizing Pneumonia. Science 2007; 315:1130-3; PMID:17234914; http://dx.doi.org/10.1126/science.1137165
- Bubeck Wardenburg J, Palazzolo-Ballance AM, Otto M, Schneewind O, DeLeo FR. Panton-Valentine Leukocidin Is Not a Virulence Determinant in Murine Models of Community-Associated Methicillin-Resistant Staphylococcus aureus Disease. J Infect Dis 2008; 198:1166-70; PMID:18729780; http://dx.doi.org/10.1086/592053
- Voyich Jovanka M, Otto M, Mathema B, Braughton Kevin R, Whitney Adeline R, Welty D, Long RD, Dorward David W, Gardner Donald J, Lina G, et al. Is Panton-Valentine Leukocidin the Major Virulence Determinant in Community Associated Methicillin Resistant Staphylococcus aureus Disease?. J Infect Dis 2006; 194:1761-70; PMID:17109350; http://dx.doi.org/10.1086/509506
- Yoong P, Pier GB. Antibody-mediated enhancement of community-acquired methicillin-resistant Staphylococcus aureus infection. Proc Natl Acad Sci U S A 2010; 107:2241-6; PMID:20133867; http://dx.doi.org/10.1073/pnas.0910344107
- Parker D, Ryan CL, Alonzo F, Torres VJ, Planet PJ, Prince AS. CD4+ T Cells Promote the Pathogenesis of Staphylococcus aureus Pneumonia. J Infect Dis 2015; 211:835-45; PMID:25240171; http://dx.doi.org/10.1093/infdis/jiu525
- Tilahun AY, Karau MJ, Clark CR, Patel R, Rajagopalan G. The impact of tacrolimus on the immunopathogenesis of staphylococcal enterotoxin-induced systemic inflammatory response syndrome and pneumonia. Microbes Infect 2012; 14:528-36; PMID:22273732; http://dx.doi.org/10.1016/j.micinf.2012.01.001
- Spaulding AR, Salgado-Pabón W, Merriman JA, Stach CS, Ji Y, Gillman AN, Peterson ML, Schlievert PM. Vaccination Against Staphylococcus aureus Pneumonia. J Infect Dis 2014; 209:1955-62; PMID:24357631; http://dx.doi.org/10.1093/infdis/jit823
- CDC. Four Pediatric Deaths From Community-Acquired Methicillin-Resistant Staphylococcus aureus, Minnesota and North Dakota, 1997–1999. MMWR Morb Mortal Week Rep 1999; 48:707-10.
- Fey PD, Said-Salim B, Rupp ME, Hinrichs SH, Boxrud DJ, Davis CC, Kreiswirth BN, Schlievert PM. Comparative molecular analysis of community- or hospital-acquired methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 2003; 47:196-203; PMID:12499191; http://dx.doi.org/10.1128/AAC.47.1.196-203.2003
- Proft T, Fraser JD. Bacterial superantigens. Clin Exp Immunol 2003; 133:299-306; PMID:12930353; http://dx.doi.org/10.1046/j.1365-2249.2003.02203.x
- Foster TJ. Immune evasion by staphylococci. Nat Rev Micro 2005; 3:948-58; http://dx.doi.org/10.1038/nrmicro1289
- Fraser J, Arcus V, Kong P, Baker E, Proft T. Superantigens - powerful modifiers of the immune system. Mol Med Today 2000; 6:125-32; PMID:10689316; http://dx.doi.org/10.1016/S1357-4310(99)01657-3
- Thomas D, Dauwalder O, Brun V, Badiou C, Ferry T, Etienne J, Vandenesch F, Lina G. Staphylococcus aureus Superantigens Elicit Redundant and Extensive Human V{beta} Patterns. Infect Immun 2009; 77:2043-50; PMID:19255190; http://dx.doi.org/10.1128/IAI.01388-08
- Buonpane RA, Churchill HRO, Moza B, Sundberg EJ, Peterson ML, Schlievert PM, Kranz DM. Neutralization of staphylococcal enterotoxin B by soluble, high-affinity receptor antagonists. Nat Med 2007; 13:725-9; PMID:17515896; http://dx.doi.org/10.1038/nm1584
- Arad G, Hillman D, Levy R, Kaempfer R. Superantigen antagonist blocks Th1 cytokine gene induction and lethal shock. J Leukoc Biol 2001; 69:921-7; PMID:11404377
- DaSilva L, Welcher BC, Ulrich RG, Aman MJ, David CS, Bavari S. Humanlike immune response of human leukocyte antigen-DR3 transgenic mice to staphylococcal enterotoxins: a novel model for superantigen vaccines. J Infect Dis 2002; 185:1754-60; PMID:12085321; http://dx.doi.org/10.1086/340828
- Krakauer T. Therapeutic Down-Modulators of Staphylococcal Superantigen-Induced Inflammation and Toxic Shock. Toxins 2010; 2:1963-83; PMID:22069668; http://dx.doi.org/10.3390/toxins2081963
- Tilahun ME, Rajagopalan G, Shah-Mahoney N, Lawlor RG, Tilahun AY, Xie C, Natarajan K, Margulies DH, Ratner DI, Osborne BA, et al. Potent neutralization of staphylococcal enterotoxin B by synergistic action of chimeric antibodies. Infect Immun 2010; 78:2801-11; PMID:20308304; http://dx.doi.org/10.1128/IAI.01121-09
- Dinges MM, Orwin PM, Schlievert PM. Exotoxins of Staphylococcus aureus. Clin Microbiol Rev 2000; 13:16-34; PMID:10627489; http://dx.doi.org/10.1128/CMR.13.1.16-34.2000
- Lee VT, Chang AH, Chow AW. Detection of staphylococcal enterotoxin B among toxic shock syndrome (TSS)- and non-TSS-associated Staphylococcus aureus isolates. J Infect Dis 1992; 166:911-5; PMID:1527429; http://dx.doi.org/10.1093/infdis/166.4.911
- Tilahun ME, Kwan A, Natarajan K, Quinn M, Tilahun AY, Xie C, Margulies DH, Osborne BA, Goldsby RA, Rajagopalan G. Chimeric Anti-Staphylococcal Enterotoxin B Antibodies and Lovastatin Act Synergistically to Provide In Vivo Protection against Lethal Doses of SEB. PLoS One 2011; 6:e27203; PMID:22102880; http://dx.doi.org/10.1371/journal.pone.0027203
- Varshney AK, Wang X, Cook E, Dutta K, Scharff MD, Goger MJ, Fries BC. Generation, characterization, and epitope mapping of neutralizing and protective monoclonal antibodies against staphylococcal enterotoxin B-induced lethal shock. J Biol Chem 2011; 286:9737-47; PMID:21233204; http://dx.doi.org/10.1074/jbc.M110.212407
- Karauzum H, Chen G, Abaandou L, Mahmoudieh M, Boroun AR, Shulenin S, Devi VS, Stavale E, Warfield KL, Zeitlin L, et al. Synthetic Human Monoclonal Antibodies toward Staphylococcal Enterotoxin B (SEB) Protective against Toxic Shock Syndrome. J Biol Chem 2012; 287:25203-15; PMID:22645125; http://dx.doi.org/10.1074/jbc.M112.364075
- Drozdowski B, Zhou Y, Kline B, Spidel J, Chan YY, Albone E, Turchin H, Chao Q, Henry M, Balogach J, et al. Generation and characterization of high affinity human monoclonal antibodies that neutralize staphylococcal enterotoxin B. J Immune Based Ther Vaccines 2010; 8:9-; PMID:21176153; http://dx.doi.org/10.1186/1476-8518-8-9
- Graef RR, Anderson GP, Doyle KA, Zabetakis D, Sutton FN, Liu JL, Serrano-González J, Goldman ER, Cooper LA. Isolation of a Highly Thermal Stable Lama Single Domain Antibody Specific for Staphylococcus aureus Enterotoxin B. BMC Biotechnol 2011; 11:86-; PMID:21933444; http://dx.doi.org/10.1186/1472-6750-11-86
- Varshney AK, Wang X, Scharff MD, MacIntyre J, Zollner RS, Kovalenko OV, Martinez LR, Byrne FR, Fries BC. Staphylococcal Enterotoxin B–Specific Monoclonal Antibody 20B1 Successfully Treats Diverse Staphylococcus aureus Infections. J Infect Dis 2013; 208:2058-66; PMID:23922375; http://dx.doi.org/10.1093/infdis/jit421
- Fraser JD. High-affinity binding of staphylococcal enterotoxins A and B to HLA-DR. Nature 1989; 339:221-3; PMID:2785644; http://dx.doi.org/10.1038/339221a0
- Tilahun AY, Marietta EV, Wu TT, Patel R, David CS, Rajagopalan G. Human leukocyte antigen class II transgenic mouse model unmasks the significant extrahepatic pathology in toxic shock syndrome. Am J Pathol 2011; 178:2760-73; PMID:21641398; http://dx.doi.org/10.1016/j.ajpath.2011.02.033
- Rajagopalan G, Sen M, David CS. In vitro and in vivo evaluation of staphylococcal superantigen peptide antagonisits. Infect Immun 2004; 72:6733-7; PMID:15501813; http://dx.doi.org/10.1128/IAI.72.11.6733-6737.2004
- Tilahun AY, Chowdhary VR, David CS, Rajagopalan G. Systemic Inflammatory Response Elicited by Superantigen Destabilizes T Regulatory Cells, Rendering Them Ineffective during Toxic Shock Syndrome. J Immunol 2014; 193:2919-30; PMID:25092888; http://dx.doi.org/10.4049/jimmunol.1400980
- Tilahun AY, Holz M, Wu TT, David CS, Rajagopalan G. Interferon gamma-dependent intestinal pathology contributes to the lethality in bacterial superantigen-Induced toxic shock syndrome. PLoS One 2011; 6:e16764; PMID:21304813; http://dx.doi.org/10.1371/journal.pone.0016764
- Tilahun AY, Marietta EV, Wu TT, Patel R, David CS, Rajagopalan G. Human leukocyte antigen class II transgenic mouse model unmasks the significant extrahepatic pathology in toxic shock syndrome. Am J Pathol 2011; 178:2760-73; PMID:21641398; http://dx.doi.org/10.1016/j.ajpath.2011.02.033
- Karau MJ, Tilahun A, Schmidt S, Clark CR, Patel R, Rajagopalan G. Linezolid is Superior to Vancomycin in Experimental Pneumonia Caused by Superantigen-Producing Staphylococcus aureus in HLA class II Transgenic Mice. Antimicrob Agents Chemother 2012; 56:5401-5; PMID:22850509; http://dx.doi.org/10.1128/AAC.01080-12
- Moran AE, Holzapfel KL, Xing Y, Cunningham NR, Maltzman JS, Punt J, Hogquist KA. T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. J Exp Med 2011; 208:1279-89; PMID:21606508; http://dx.doi.org/10.1084/jem.20110308
- Kuehl WM, Bergsagel PL. Molecular pathogenesis of multiple myeloma and its premalignant precursor. J Clin Invest 2012; 122:3456-63; PMID:23023717; http://dx.doi.org/10.1172/JCI61188
- Skurnik D, Kropec A, Roux D, Theilacker C, Huebner J, Pier GB. Natural Antibodies in Normal Human Serum Inhibit Staphylococcus aureus Capsular Polysaccharide Vaccine Efficacy. Clin Infect Dis 2012; 55:1188-97; PMID:22806596; http://dx.doi.org/10.1093/cid/cis624
- Lehar SM, Pillow T, Xu M, Staben L, Kajihara KK, Vandlen R, DePalatis L, Raab H, Hazenbos WL, Hiroshi Morisaki J, et al. Novel antibody–antibiotic conjugate eliminates intracellular S. aureus. Nature 2015; 527:323-8; PMID:26536114; http://dx.doi.org/10.1038/nature16057
- Dryla A, Prustomersky S, Gelbmann D, Hanner M, Bettinger E, Kocsis B, Kustos T, Henics T, Meinke A, Nagy E. Comparison of antibody repertoires against Staphylococcus aureus in healthy individuals and in acutely infected patients. Clin Diagn Lab Immunol 2005; 12:387-98; PMID:15753252
- Moravcova S, Kyle B, Shanahan H, Giannaris S, Smith A, Hamilton-Davies C. Variability of anti-staphylococcal antibodies in healthy volunteers and pre-cardiac surgery patients. Perioper Med 2016; 5:13; PMID:27239299; http://dx.doi.org/10.1186/s13741-016-0039-y
- Linnér A, Darenberg J, Sjölin J, Henriques-Normark B, Norrby-Teglund A. Clinical Efficacy of Polyspecific Intravenous Immunoglobulin Therapy in Patients With Streptococcal Toxic Shock Syndrome: A Comparative Observational Study. Clin Infect Dis 2014; 59:851-7; http://dx.doi.org/10.1093/cid/ciu449
- de Barros Miranda-Filho D, de Alencar Ximenes RA, Barone AA, Vaz VL, Vieira AG, Albuquerque VMG. Randomised controlled trial of tetanus treatment with antitetanus immunoglobulin by the intrathecal or intramuscular route. BMJ Br Med J 2004; 328:615; http://dx.doi.org/10.1136/bmj.38027.560347.7C
- Jarboe J, Gupta A, Saif W. Therapeutic Human Monoclonal Antibodies Against Cancer. In: Steinitz M, ed. Human Monoclonal Antibodies: Methods and Protocols. Totowa, NJ: Humana Press, 2014:61-77.
- Kim CK, Karau MJ, Greenwood-Quaintance KE, Tilahun AY, Krogman A, David CS, Pritt B, Patel R, Rajagopalan G. Superantigen-Producing Staphylococcus aureus Elicits Systemic Immune Activation in a Murine Wound Colonization Model. Toxins 2015; 7:5308-19; PMID:26670252; http://dx.doi.org/10.3390/toxins7124886
- Chung J-W, Greenwood-Quaintance KE, Karau MJ, Tilahun A, Khaleghi SR, Chowdhary VR, David CS, Patel R, Rajagopalan G. Superantigens Produced by Catheter-Associated Staphylococcus aureus Elicit Systemic Inflammatory Disease in the Absence of Bacteremia. J Leukoc Biol 2015; 98:271-81; PMID:25979434; http://dx.doi.org/10.1189/jlb.4A1214-577RR
- Adhikari RP, Novick RP. Regulatory organization of the staphylococcal sae locus. Microbiology 2008; 154:949-59; PMID:18310041; http://dx.doi.org/10.1099/mic.0.2007/012245-0