Introduction
A. fumigatus is the most common mold pathogen in the developed world and commonly causes disease in individuals with an immunodeficiency. During fungal growth, cell wall polysaccharides β-glucan and chitin are exposed, which enables immunological detection by pattern recognition molecules such as the β-glucan binding receptor dectin-1.Citation1 Several immune-regulating chitin receptors have been found including epithelial fibrinogen C domain containing 1 and IgG-Fc-γ receptors.Citation2,3 β-glucan comprises a mixed group of β-D-glucose polysaccharides while chitin is a linear homopolymer consisting of N-acetylglucoseamine (GlcNAc) residues linked by β-1,4 glycosidic bonds.
The three human ficolins (M, L, and H) play essential roles in pathogen recognition and complement activation through the lectin pathway.Citation4 It was recently demonstrated that A. fumigatus infection resulted in decreased fungal clearance and cytokine production in ficolin-A/B double deficient mice although these effects were complement independent.Citation5 Ficolins A and B are mouse homologues of L- and M-ficolin, respectively, while there is no mouse H-ficolin homolog. Different ficolinsCitation6-9 bind A. fumigatus conidia and elicit complement activation, phagocyte activation and modulation of epithelial signaling and L- and H-ficolin are increased in bronchoalveolar fluid in invasive aspergillosis.Citation7,9 However, no direct interaction has been reported between M-ficolin and A. fumigatusCitation10 and the potential role of M-ficolin in immunity against A. fumigatus remains unknown. M-ficolin is primarily produced by peripheral blood leukocytes, bone marrow cells and type II alveolar cells.Citation4 M-ficolin binding is selective for acetylated compounds, including GlcNAc, where recognition and binding occurs through a conserved calcium-dependent binding site, termed S1.Citation11,12
The aim of this study was to investigate the hypothesis that M-ficolin interacts with A. fumigatus through interaction with chitin and β-1,3 glucan and thereby mediates complement activation and potentiates IL-8 secretion of A549 cells, a cell line with characteristics of type II epithelial cells.
Materials and methods
Buffers - TBS: 140 mM NaCl, 10 mM Tris-HCl, and 0.02% (w/v) NaN3, pH 7.4; TBS/Tw: TBS and 0.05% Tween 20 (polyoxyethylene sorbitan monolaurate) (Merck KGaA); TBS/Tw/Ca2+: TBS/Tw, 5 mM CaCl2; TBS/Tw/EDTA: TBS/Tw, 10 mM EDTA; PBS: 137 mM NaCl, 3 mM KCl, 8 mM Na2HPO4, and 1.5 mM KH2PO4, pH 7.4; ELISA coating buffer: 15 mM Na2CO3 and 34.9 mM NaHCO3, pH 9.6; Horseradish peroxidase (HRP) substrate buffer: 35 mM citric acid and 67 mM Na2HPO4, pH 5; B1 buffer: 4 mM barbital, 145 mM NaCl, 2 mM CaCl2, and 1 mM MgCl2; ROSA buffer: 20 mM Tris/Base, 1 M NaCl, 0.05% (v/v) Triton X-100 (Bie & Berntsen), 10 mM CaCl2, and 1 mg/ml human serum albumin (HSA) (10 96 97, CSL Behring); M-ficolin buffer: TBS/Tw, 5 mM EDTA, 100 µg/ml heat-inactivated normal human Ig (beriglobin, CSL Behring), 50 µg/ml bovine Ig (Lampire Biological laboratories), 850 mM NaCl, and 1 mg/ml HSA; Fixative solution (8% formaldehyde in 50 mM PIPES, 25 mM EGTA pH 7.0; 5 mM MgSO4; 5% (v/v) DMSO); Complete medium (CM): RPMI medium 1640, 10% foetal bovine serum, 2 mM L-glutamine, 50 U penicillin/ml, and 50 µg streptomycin/ml (Gibco | Thermo Fisher Scientific, for all cell culture reagents).
Immunohistochemistry – Immunohistochemistry was performed essentially as described previouslyCitation13 using anti-M-ficolin mAb 036–05 (Bioporto Diagnostics A/S). Stained tissue sections were analyzed by a trained pathologist.
Human tissue samples - Human control tissues and tissues from 2 anonymous patients with chronic pulmonary aspergillosis with A. fumigatus pulmonary infection were obtained from the Diagnostic Biobank at the Department of Pathology, Odense University Hospital (Odense, Denmark). The Regional Scientific Ethical Committee for Southern Denmark approved the use of the healthy human tissue sections for research purposes (Ref. No VF20050070), and samples were obtained from patients with written informed consent.
Expression of human rM-ficolin - Human wild-type rM-ficolin was expressed as described previously.Citation14 For the expression of rM-ficolin applied in the complement activation assays, heat-inactivated FBS was used in the cultures.
rM-ficolin ELISA – The rM-ficolin ELISA was a standard sandwich ELISA using 0.5 µg/ml monoclonal anti-M-ficolin antibody (7G1 mAb) as catching antibody and 0.5 µg/ml biotinylated 7G1 antibody as detection antibody.
Purification of rM-ficolin from cell culture supernatant (CS) – A total of 40 ml of 50% (v/v) chitin bead slurry (New England Biolabs) was packed in a column and washed with TBS/Tw/Ca2+, 0.5 M NaCl. The rM-ficolin-enriched and serum free CHO cell CS was added to the column connected to an ÄKTA-FPLC (GE Healthcare) and washed. rM-ficolin was eluted with acetate (TBS and 250 mM Na-Acetate, pH 7.4) and EDTA (TBS and 520 mM EDTA).
Fluorescence imaging - A. fumigatus conidia CBS 101355 (Centraalbureau von Schimmelcultures, Utrecht, Netherlands) (5·105/ml) were grown for 14–16 hours in Sabouraud dextrose broth (Difco™, BD Biosciences) to generate hyphae. Grown hyphae were washed twice in 50 mM PIPES, pH 6.7, and fixed in 2 ml fixative solution for 30 min. Purified rM-ficolin was diluted in TBS/Tw/Ca2+ and incubated with the hyphae for 2 hours at room temperature (RT). The hyphae were then washed in TBS/Tw/Ca2+ and incubated with monoclonal 7G1 anti-M-ficolin for 1 hour at 4°C, washed and incubated with FITC-labeled IgG goat-anti-mouse (Dako) for 30 min at 4°C. Then, hyphae were washed, incubated with Alexa Fluor 633-labeled wheat germ agglutinin (WGA) (5 μg/ml) (Life Technologies, Invitrogen, Thermo Scientific) for 30 min at 4°C and then washed again. Images were acquired using an Olympus IX71 fluorescence microscope equipped with 4-laser optics and an F-view fluorescence CCD camera. All images were acquired and processed using Olympus CellF soft imaging software.
Binding of M-ficolin to different A. fumigatus strains - Four A. fumigatus isolates derived from human patients having keratitis were included in this study. They were isolated at the Aravind Eye Hospital and Postgraduate Institute of Ophthalmology (Coimbatore, Tamilnadu, India) and deposited in the Szeged Microbiological Collection (SZMC, Szeged, Hungary, www.szmc.hu) under the following strain numbers: SZMC 2419, SZMC 2421, SZMC 2422, and SZMC 2430. One A. fumigatus isolate (Agricultural Research Service Culture Collection, National Center for Agricultural Utilization Research, Peoria, Illinois USA; NRRL 174) from an unknown source was also included in this study. The fungal isolates were maintained on malt extract slants (0.5% (w/v) malt extract, 0.5% (w/v) yeast extract, 0.5% (w/v) glucose, 1.0% (w/v) KH2PO4, and 1.5% (w/v) agar) at 4 °C. Conidia were incubated for 0 (conidia) or 8 hours (germlings) at 30°C in CM with shaking (150 rev/min), centrifuged (10,000×g, 10 min at 25°C), and washed in TBS/Ca2+ or TBS/EDTA. The suspensions were mixed 1:1 with serum containing Ca2+ or EDTA and prediluted 1:10. The final concentration was 107 conidia or germlings/ml. Positive controls with 10% (v/v) GlcNAc-coated Sepharose beads (CL−4B GE Healthcare) (50% v/v slurry) were added to serum prediluted 1:20 in TBS/Ca2+ or TBS/EDTA. Suspensions were incubated at RT for 1 hour (150 rev/min), centrifuged (10,000 × g, 10 min at 25°C), and supernatants were stored at -80°C until analysis for M-ficolin content by time-resolved fluorometry (TRIFMA) as described previously.Citation15
Preparation of acetylated human serum albumin (acHSA) beads - Cyanogen bromide-activated Sepharose beads 4B (GE Healthcare) were coupled to HSA and subsequently acetylated as previously described.Citation14
Growth of A. fumigatus and preparation of AIF - A. fumigatus conidia (CBS 101355) were grown at 37°C on Sabouraud dextrose agar (Difco™) plates and harvested in PBS/Tw. A. fumigatus AIF was produced essentially as described previouslyCitation16 and tested for endotoxin contamination using limulus amebocyte lysate assay (Lonza). The endotoxin level was <0.5 EU/ml. The amount of A. fumigatus AIF obtained was determined by weighing after vacuum centrifugation at 55°C.
Pull-down assays with rM-ficolin and polysaccharides - Pull-down experiments, which were analyzed by western blotting, were performed with 100 µl 50% (v/v) chitin bead slurry (New England Biolabs), 100 µl 50% (v/v) acHSA bead slurry, 2 mg β-1,3 glucan (curdlan) from Alcaligenes faecalis (Sigma-Aldrich), 2 mg A. fumigatus AIF or 100 µl 50% (v/v) acHSA bead slurry in incubation with 1 ml 7 µg/ml rM-ficolin CS ON (300 rev/min) at 4°C. Pull-down experiments were further performed in the presence of 50 mM glucose, glucosamine, GlcNAc, acetate and propionate or 10 mM EDTA (Sigma-Aldrich).
Pull-down experiments, which were analyzed by ELISA, were performed with 10 mg chitin from shrimp shell (Sigma-Aldrich), 10 mg β-1,3 glucan (curdlan), 10 mg A. fumigatus AIF, or 50 µl 50% (v/v) acHSA bead slurry. Beads and particles were washed with TBS/Tw/Ca2+ and mixed with 500 µl rM-ficolin CS diluted to 100 ng/ml in TBS/Tw/Ca2+ and in the presence of 50 mM glucose, glucosamine, GlcNAc, and acetate or 10 mM EDTA. After ON incubation (300 rev/min) at 4°C, the samples were centrifuged (10,000 × g), and 100 µl of the supernatant was analyzed by ELISA.
SDS-PAGE and western blotting - The pelleted particles and beads were washed 3 times in TBS/Tw/Ca2+. Bound protein was eluted by boiling pelleted particles in SDS-PAGE buffer and resolved under non-reduced conditions by SDS-PAGE followed by western blotting using the 7G1 mAb for rM-ficolin detection.
Mannan-MBL-MASP coating of microtiter plates - A 96-well microtiter plate was coated with 10 µg/ml mannan (purified in house from Saccharomyces cerevisiae) in ELISA coating buffer and incubated ON. The wells were blocked with 1 mg/ml HSA in TBS and incubated for 1 hour, followed by washing 3 times in TBS/Tw/Ca2+. Then, 100 μl of normal human serum containing MBL/MASP complexes, diluted 1:25 in ROSA-buffer, was added to each well and incubated for 2 hours at RT. Finally, the wells were washed 3 times before the sample material was loaded as described below.
rM-ficolin-mediated C4 consumption - C4 consumption assays were conducted using chitin beads (New England Biolabs). A total of 10 µl 50% (v/v) chitin bead slurry was washed with TBS/Tw/Ca2+ and incubated ON with 500 µl rM-ficolin CS in serial dilution at 4°C. The samples were washed and incubated end-over-end for 2 hours at RT with 300 µl recombinant MASP-2 prepared as described previouslyCitation14 and diluted to 100 ng/ml in TBS/Tw/Ca2+. The samples were then washed, combined with 300 µl purified human C414 diluted to 80 ng/ml in B1 buffer, and incubated end-over-end for 1.5 hours at 37°C. After centrifugation (10,000 × g), the reaction was stopped by adding 100 µl supernatant to 500 µl TBS/Tw/EDTA, and then loaded onto mannan-MBL-MASP-2-coated microtiter plates (Nunc™ FluoroNunc™, Thermo Scientific) and incubated for 1.5 hours at 37°C, enabling binding of the remaining C4 present in the supernatant. The plate was washed 3 times in TBS/Tw/Ca2+, added a freshly prepared mixture of 2 biotinylated anti-C4 mAbs (Hyb 161–1 and 161–2, BioPorto) in a concentration of 0.5 μg/ml and incubated ON at 4°C. The plate was washed 3 times and Europium3+-labeled streptavidin (Perkin Elmer) diluted 1:1000 in TBS/Tw/EDTA was added. The plate was incubated for 1 hour, washed and 200 μl enhancement buffer (Perkin Elmer) was added. The amount of europium was measured using TRIFMA as described previously.Citation15
rM-ficolin-mediated C4b generation - C4b generation assays were conducted using β-1,3 glucan (curdlan) (Sigma-Aldrich), A. fumigatus AIF and acHSA beads. A total of 0.5 mg curdlan or A. fumigatus AIF or 10 µl 50% (v/v) acHSA bead slurry was washed with TBS/Tw/Ca2+ and incubated ON at 4°C with 500 µl rM-ficolin serially diluted in TBS/Tw/Ca2+. Samples were washed and incubated with 300 µl MASP-2 diluted to 200 ng/ml in TBS/Tw/Ca2+ and incubated end-over-end for 2 hours at RT. Samples were washed in TBS/Tw/Ca2+ and 300 µl C4 diluted to 80 ng/ml in B1 buffer was added. Then, the samples were incubated end-over-end for 1.5 hours at 37°C. After pelleting, 100 µl supernatant was added to 500 µl TBS/Tw/EDTA to stop the reaction and then loaded in duplicate into 96-well microtiter plates (Nunc™ FluoroNunc™, Thermo Scientific) and incubated ON at 4°C. The wells were previously coated with 1 µg/ml anti-C4–1 in 100 µl ELISA coating buffer ON at 4°C and blocked with 200 µl TBS containing 0.1% HSA (v/v) for 2 hours at RT. Polyclonal biotinylated rabbit anti-C4 was used as a detector antibody. The plates were developed using TRIFMA as described previously.Citation15
Growth of A. fumigatus isolates in the presence of rM-ficolin - MBL-deficient human serum was incubated with fungal hyphae for 30 min on ice (150 rev/min), centrifuged (10,000 × g, 10 min at 25°C), and serum supernatant was used for the cultures described beneath. A total of 25 µl 107 conidia was grown ON in RPMI-1640 medium (Sigma-Aldrich) at 4°C (150 rev/min). The resulting fungal hyphae were centrifuged (10,000 × g for 10 min at 25°C) and incubated at 4°C for one hour in 25 µl RPMI with a serial dilution of purified rM-ficolin. Then, 25 µl MBL-deficient serum supernatant was added to the test tubes resulting in final concentrations of rM-ficolin of 1500, 150, 15, 1.5, and 0.15 ng/ml. The pH was adjusted to 7.4 if necessary. The suspensions were incubated at 37°C for 0 and 8 hours (150 rev/min). Finally, pelleted (10,000 × g, 10 min at 25°C) fungal material was lyophilized and weighed.
IL-8 secretion from A549 lung epithelial cells challenged with A. fumigatus AIF and rM-ficolin - A total of 105 human A549 type II alveolar adenocarcinoma cells were seeded on 24-well plates (Nunc™) in 500 µl CM. The following solutions and suspensions were freshly prepared in serum-free medium and incubated for 1 hour at 37°C: rM-ficolin control, which was the supernatant produced by centrifugation of 2 mg/ml A. fumigatus AIF and 20 µg/ml purified rM-ficolin at 1,000 × g for 5 min; 2 mg/ml A. fumigatus AIF control; and 2 mg/ml A. fumigatus AIF + 5 µg/ml, 10 µg/ml M-ficolin or 20 µg/ml rM-ficolin. The ON cultures of A549 cells were washed twice in cold sterile PBS and then incubated with 200 µl of the appropriate challenge for 6 hours at 37°C. Next, the cell CSs were centrifuged at 1,000 × g for 5 min and stored at -20°C until analysis.
IL-8 ELISA – IL-8 measurements on cell CSs were performed with the Human CXCL8/IL-8 DuoSet ELISA, DY208 (R&D Systems) according to the manufacturer's recommendations.
Statistical analysis - Prism (GraphPad Software, Inc.) version 6.0b was used for all graphs and statistical analyzes. Bindings between rM-ficolin and different A. fumigatus strains or polysaccharides and growth of A. fumigatus isolates with different culture conditions were analyzed by ANOVA with Holm-Sidak's multiple comparisons test. Secretion of IL-8 was analyzed by one-way ANOVA with Tukey's multiple comparison test. P-value < 0.05 was considered statistically significant.
Results
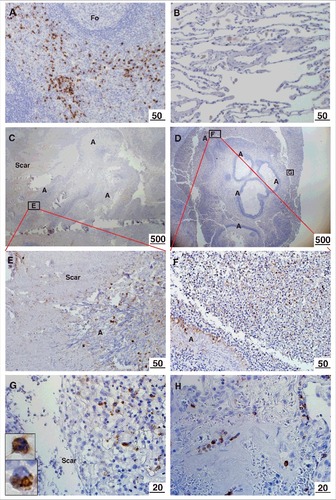
Localization of M-ficolin to the periphery of the aspergilloma - Positive control immunostaining of M-ficolin in monocytes/granulocytes was observed in the spleen (). Weak alveolar macrophage staining was observed in non-infected tissue (). Strong M-ficolin immunoreactivity was detected in monocytes/granulocytes in the interface between fungal balls and the surrounding pulmonary scar tissue () and in all blood vessels (shown from infected lung) (). M-ficolin immunoreactivity was undetectable in scar tissue and in central necrotic zones of fungal balls.
Figure 1. Immunohistochemical localization of M-ficolin to the aspergilloma. Brown staining indicates presence of M-ficolin. (A) Control immunostaining of monocytes/granulocytes in the spleen. (B) Control alveolar tissue. Overview of elongated A. fumigatus fungal balls surrounded by pulmonary scar tissue in patient 1 (C) and patient 2 (D). The boxes indicate the location of images E-G. (E) Pulmonary scar tissue and A. fumigatus mycelial zone. (F-G) Peripheral zone of aspergilloma (Upper insert: granulocyte. Lower insert: monocyte). (H) Pulmonary blood vessels in scar tissue from a lung with A. fumigatus infection. Fo = follicle. A = A. fumigatus. Scar = scar tissue. The lengths of the bars are in micrometers
Characterization of M-ficolin binding to A. fumigatus - Conidia and germlings from 5 different A. fumigatus strains were incubated with purified rM-ficolin, and the residual rM-ficolin in the supernatant after centrifugation was measured (). Conidia and germlings pulled out 40–70% of rM-ficolin in the presence of calcium and binding was significantly calcium-dependent for strains SZMC 2419 (p < 0.01), SZMC 2430 (p < 0.01) and NRRL 174 (p < 0.05).
Figure 2. Characterization of M-ficolin binding to A. fumigatus. The binding between M-ficolin and A. fumigatus strains NRRL 174 (174), SZMC 2419 (2419), SZMC 2421 (2421), SZMC 2422 (2422) and SZMC 2430 (2430) was on conidia (0 hours) and germlings (8 hours) using pull-down assays in the presence of (A) 5 mM Ca2+ or (B) 10 mM EDTA. The data are triplicates from 2 independently performed experiments. Data shown are mean ± SEM. Significance was determined using 2-way ANOVA with Holm-Sidak's multiple comparison test, #p < 0.05, ##p < 0.01, ####p < 0.001. (C) Light microscopy of growing hyphae, original magnification 100×. (D) Light microscopy of growing hyphae, original magnification 400×. (E-F) Localization of regions recognized by M-ficolin (green) and (G-H) localization of chitin (WGA, red). (I-J) Overlay images. The lengths of the bars are in micrometers
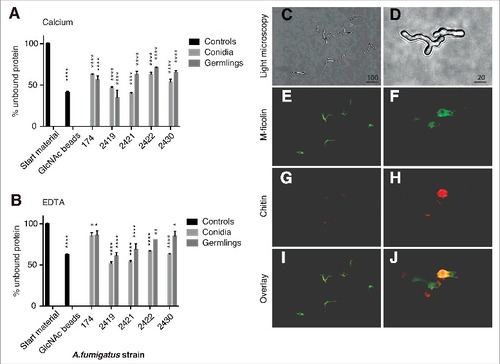
Next, fluorescence microscopy showed that rM-ficolin bound to the surface of the A. fumigatus hyphae and mother-bud, while the tip of polarized growing buds were undetected (). Chitin (WGA) was localized to sites of septum formation, the mother-bud, and to evolving hyphae with polarized growth (). The binding of rM-ficolin to the hyphae was partially co-localized with chitin (WGA) in the mother-bud () and was inhibited by co-incubation with GlcNAc (data not shown). rM-ficolin also bound regions with low chitin content.
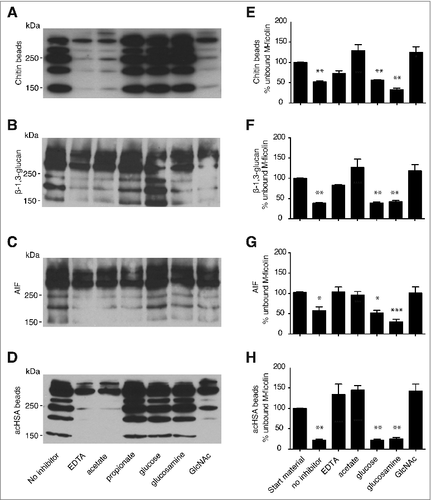
Binding of rM-ficolin to chitin, β-1,3 glucan and A. fumigatus AIF – Pull-down assays showed binding of rM-ficolin to chitin beads, β-1,3 glucan, A. fumigatus AIF, and acHSA beads (). These bindings were partially inhibited by EDTA, acetate, and GlcNAc, as shown by the disappearance of the lowest MW rM-ficolin bands. The presence of the non-acetylated compounds glucose, glucosamine, and propionate showed no or weak inhibition. In a separate set of similar pull-down experiments, quantitative ELISA showed that the concentration of rM-ficolin in the supernatant was significantly reduced by chitin, β-1,3 glucan, A. fumigatus AIF, and acHSA beads (). The presence of inhibitors appeared to affect the interaction with assay detection antibodies. This resulted in apparent inhibition > 100% in some ELISA assays.
Figure 3. Pull-down assays with rM-ficolin binding the polysaccharides chitin, β-glucan and A. fumigatus AIF. Western blotting assays using the monoclonal anti-M-ficolin 7G1 antibody to detect rM-ficolin in the pellet resulting from incubation with (A) chitin beads, (B) β-1,3 glucan, (C) A. fumigatus AIF and (D) acHSA beads (control). The binding was performed in the presence of 10 mM EDTA, 50 mM acetate, propionate, glucose, glucosamine, or GlcNAc. The results are representative of 3 independent experiments. rM-ficolin was further measured by ELISA in the supernatant resulting from incubation with (E) chitin, (F) β-1,3 glucan and (G) A. fumigatus AIF and (H) acHSA beads. The data are from 3 independent experiments. The data shown are mean ± SEM. The data were analyzed by one-way ANOVA with Holm-Sidak's multiple comparisons test, #p < 0.05, ##p < 0.01, ###p < 0.001
M-ficolin-mediated complement activation – Two different assays were used to demonstrate dose-dependent complement activation; a “C4 consumption” assay () and a “C4b deposition” assay (), respectively. Dose-dependent rM-ficolin-mediated complement activation was observed in response to chitin beads, β-1,3 glucan and A. fumigatus AIF (), while no detectable C4b deposition by the known M-ficolin ligand acHSA was observed using the same conditions ().
Figure 4. Functional interactions between rM-ficolin and fungal polysaccharides. (A) Concentration-dependent rM-ficolin-mediated chitin complement C4 consumption assay and complement C4b generation assays for (B) β-1,3 glucan, (C) A. fumigatus AIF and (D) acHSA (control). Dry weight (mg) of A. fumigatus (E) NRRL 174 and (F) SZMC 2430 cultures before and after an 8-hours incubation in 50% MBL-deficient serum and in the presence of various concentrations (0–1500 ng/ml) of rM-ficolin. (G) IL-8 secretion in A549 cell CS collected 6 hours after challenge with rM-ficolin alone or after incubation with A. fumigatus AIF or increasing concentrations of rM-ficolin opsonized A. fumigatus AIF. Blank control = serum free medium. The data shown are mean ± SEM of quadruplicate measurements representative of 2 (A) and duplicates from 3 (B-G) independent experiments, #p < 0.5, ##, $$p < 0.01, ###, $$$p < 0.001. #relative to background, #relative to A. fumigatus AIF control, $relative to rM-ficolin control
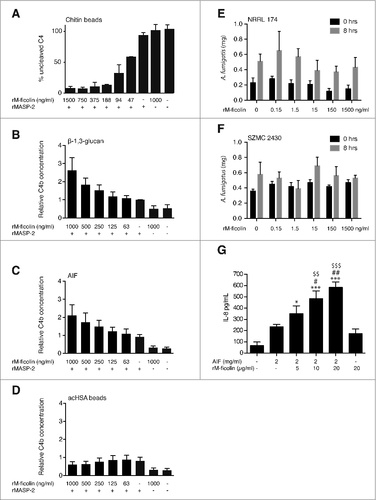
No rM-ficolin modulation of A. fumigatus growth – The ability of rM-ficolin to mediate growth inhibition of A. fumigatus clinical isolates was tested in cultures containing 50% serum. No rM-ficolin-dependent growth inhibition was detected after 8 hours-old culture of A. fumigatus ().
rM-ficolin-enhanced AIF stimulation of lung epithelial cell IL-8 secretion – rM-ficolin opsonization of A. fumigatus AIF induced a significant and dose-dependent increase in the A549 lung epithelial cell secretion of IL-8 compared with challenge with un-opsonized AIF and rM-ficolin alone after 6 hours of treatment ().
Discussion
In the present study, we investigated the possible role of M-ficolin in the recognition of fungal cell wall polysaccharides, which are exposed during fungal growth. We found that M-ficolin is present in human lung with aspergilloma and binds A. fumigatus calcium-dependently. M-ficolin further binds cell wall components chitin, β-1,3 glucan and A. fumigatus AIF and mediates complement activation, but provides no initial growth disadvantage of A. fumigatus. Finally, we found that rM-ficolin opsonization of A. fumigatus AIF increases IL-8 secretion in A549 lung epithelial cells.
M-ficolin immunoreactivity was located to monocytes/granulocytes in the vicinity of the pulmonary aspergilloma in accordance with a role in limiting the growth in a surface reaction. This was an intriguing observation, but it did not reveal whether soluble M-ficolin could react with A. fumigatus.
Previous studies have reported that M-ficolin does not bind A. fumigatus conidia,Citation10 however, we detected binding to conidia of 5 different A. fumigatus strains. This discrepancy between observations may be due to the high variability in the sialic acid ligand density on conidia of A. fumigatus strains.Citation17 However, the focus of this study was related to recognition of polysaccharides in the cell wall of growing fungus and we initially envisioned chitin as the main M-ficolin ligand because chitin is a polymer of the known ligand GlcNAc.
Purified rM-ficolin bound directly to the growing A. fumigatus hyphal cell wall but was only partially co-localized to chitin-rich zones, which suggests that M-ficolin recognizes alternative A. fumigatus ligands as well. Following, we studied the most abundant polysaccharide of the fungal cell wall, β-1,3 glucan, after determining that M-ficolin interactions with the growing fungal cell wall were observed for various different A. fumigatus strains. Our demonstration of binding of rM-ficolin to β-1,3 glucan is a novel observation, as no other non-acetylated compound has been demonstrated as a ligand for M-ficolin.
We further demonstrated functional interaction with A. fumigatus AIF, which mainly consists of a branched β-1,3/1,6 glucan backbone, but also comprises linear β-1,3/1,4 glucan and chitin.Citation16 The binding profiles of rM-ficolin to chitin, β-1,3 glucan, and A. fumigatus AIF were highly similar to each other and to the binding profile for the positive control acHSA with inhibition by acetate, GlcNAc, and EDTA. The acetylated small molecule propionate was moreover included as inhibitor in some experiments. Thus, the M-ficolin binding profiles showed specificity and indicated involvement of the conserved ficolin S1 binding site, which mediates interaction with GlcNAc, N-acetylgalactose and N-acetylneuramic acid.Citation12 However, due to the unexpected interaction with β-1,3 glucan we cannot rule out that additional binding sites may exist and support S1-mediated binding of this polysaccharide. Such additional interactions may further be suggested based on the non-significant inhibition by EDTA seen in some of A. fumigatus strains in the performed pull-down assays. Following, successful complement activation by the polysaccharides was performed using physiologically relevant concentrations of rM-ficolin that, however, were insufficient to mediate complement activation by the control ligand acHSA.
We observed no apparent growth inhibition of A. fumigatus with increasing concentrations of M-ficolin. The assay was performed in the presence of 50% MBL-deficient serum, which was preincubated with fungal hyphae to remove potential anti-fungal antibodies. The negative outcome suggests that M-ficolin-mediated complement activation either may not result in a functional complement membrane attack complex or predominantly may occur with free polysaccharide particles liberated from dying cells. However, this observation does not exclude potential complement-mediated effects on inflammation and opsonization.
H-ficolin is reported to increase A. fumigatus induced IL-8 secretion from A549 cells.Citation9 Whether this could also be achieved following M-ficolin opsonization was previously unknown. We observed potentiation of IL-8 secretion following A549 cell challenge with rM-ficolin-opsonized A. fumigatus AIF. Thus, our data support that M-ficolin mediates the initiation of inflammation and enhancement of neutrophil recruitment.
Effects of M-ficolin modulation of phagocyte activity may further be anticipated, but were not explored. A model of the M-ficolin-mediated effects observed in this study is provided in .
Figure 5. Schematic depiction of the interaction between M-ficolin-opsonized A. fumigatus and the innate immune system of the host. M-ficolin interacts with different life stages of A. fumigatus. The M-ficolin ligand on the surface of resting conidia (1) is unknown, however the effects of opsonization may be overlapping with other A. fumigatus life stages. Swollen conidia (2), germlings (3) and hyphae forming stages (4) expose cell wall polysaccharides β-glucan and chitin, which mediates M-ficolin interaction. M-ficolin enhances fungal polysaccharide-mediated pulmonary epithelial IL-8 secretion involved in phagocyte recruitment and enhances complement activation. The complement activation does not result in the formation of membrane attack complexes (MAC). Possible effects of M-ficolin-enhanced activation of phagocyte functions are unknown
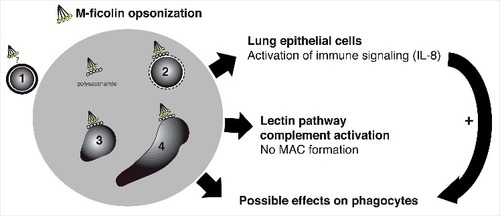
In summary, the data are in support of recent in vivo data showing reduced fungal clearance in ficolin-deficiency.Citation5 These first observations of binding of rM-ficolin to fungal polysaccharides, including the novel M-ficolin ligands chitin and β-1,3 glucan and resulting modulation of human epithelial cells, may be essential for efficient immune activation during fungal infection of the human lung.
Abbreviations
| acBSA | = | acetylated BSA |
| A. fumigatus | = | Aspergillus fumigatus |
| AIF | = | alkali-insoluble fraction |
| BSA | = | bovine serum albumin |
| CHO cells | = | Chinese hamster ovary cells |
| CS | = | culture supernatant |
| CM | = | complete medium |
| FReDs | = | fibrinogen-related domains |
| GlcNAc | = | N-acetylglucosamine |
| IL-8 | = | interleukin 8 |
| MASP-2 | = | MBL-associated serine protease 2 |
| MBL | = | mannan-binding lectin |
| ON | = | overnight |
| rM-ficolin | = | recombinant M-ficolin |
| RT | = | room temperature. |
| S. typhimurium | = | Salmonella typhimurium |
| TRIFMA | = | time-resolved fluorometry |
| WGA | = | wheat germ agglutinin |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
Jensen-K, Lund-KP, Christensen-KB, Holm-AT, Jepsen-CS, Dubey-LK and Galgóczy-L performed experiments; Jensen-K, Lund-KP and Sorensen-GL wrote the manuscript; Moeller-JB, Schlosser-A, Thiel-S, Holmskov-U and Sorensen-GL designed experiments; Thiel-S and Sorensen-GL conceived study; all co-authors approved final version of the manuscript.
Funding
We would like to thank technician Lisbeth Jensen, Aarhus University, and Karen E. Olsen and Ole Nielsen, Department of Pathology, Odense University Hospital, for all of the help and support with providing and interpreting the immunohistochemical sections. L.G. was supported by a Lise Meitner fellowship (M1776-B20) from the Austrian Science Fund (FWF) and the Postdoctoral Excellence Program (PD 120808) of the Hungarian National Research, Development and Innovation Office (NKFI Office). The Danish Research Council for Independent Research further supported this study.
References
- Gersuk GM, Underhill DM, Zhu L, Marr KA. Dectin-1 and TLRs permit macrophages to distinguish between different Aspergillus fumigatus cellular states. J Immunol 2006; 176:3717-24; PMID:16517740; https://doi.org/https://doi.org/10.4049/jimmunol.176.6.3717
- Thomsen T, Moeller JB, Schlosser A, Sorensen GL, Moestrup SK, Palaniyar N, Wallis R, Mollenhauer J, Holmskov U. The recognition unit of FIBCD1 organizes into a noncovalently linked tetrameric structure and uses a hydrophobic funnel (S1) for acetyl group recognition. J Biol Chem 2010; 285:1229-38; PMID:19892701; https://doi.org/https://doi.org/10.1074/jbc.M109.061523
- Becker KL, Aimanianda V, Wang X, Gresnigt MS, Ammerdorffer A, Jacobs CW, Gazendam RP, Joosten LA, Netea MG, Latgé JP, et al. Aspergillus Cell Wall Chitin Induces Anti- and Proinflammatory Cytokines in Human PBMCs via the Fc-γ Receptor/Syk/PI3K Pathway. MBio 2016; 7:e01823-15; PMID:27247234; https://doi.org/https://doi.org/10.1128/mBio.01823-15
- Thomsen T, Schlosser A, Holmskov U, Sorensen GL. Ficolins and FIBCD1: soluble and membrane bound pattern recognition molecules with acetyl group selectivity. Mol Immunol 2011; 48:369-81; PMID:21071088; https://doi.org/https://doi.org/10.1016/j.molimm.2010.09.019
- Genster N, Praestekjaer Cramer E, Rosbjerg A, Pilely K, Cowland JB, Garred P. Ficolins promote fungal clearance in vivo and modulate the inflammatory cytokine response in host defense against aspergillus fumigatus. J Innate Immun 2016; 8(6):579-588; PMID:27467404; https://doi.org/https://doi.org/10.1159/000447714
- Bidula S, Kenawy H, Ali YM, Sexton D, Schwaeble WJ, Schelenz S. Role of ficolin-A and lectin complement pathway in the innate defense against pathogenic Aspergillus species. Infect Immun 2013; 81:1730-40; PMID:23478320; https://doi.org/https://doi.org/10.1128/IAI.00032-13
- Bidula S, Sexton DW, Abdolrasouli A, Shah A, Reed A, Armstrong-James D, Schelenz S. The serum opsonin L-ficolin is detected in lungs of human transplant recipients following fungal infections and modulates inflammation and killing of Aspergillus fumigatus. J Infect Dis 2015; 212:234-46; PMID:25612732; https://doi.org/https://doi.org/10.1093/infdis/jiv027
- Bidula S, Sexton DW, Schelenz S. Serum opsonin ficolin-A enhances host-fungal interactions and modulates cytokine expression from human monocyte-derived macrophages and neutrophils following Aspergillus fumigatus challenge. Med Microbiol Immunol 2016; 205:133-42; PMID:26337048; https://doi.org/https://doi.org/10.1007/s00430-015-0435-9
- Bidula S, Sexton DW, Yates M, Abdolrasouli A, Shah A, Wallis R, Reed A, Armstrong-James D, Schelenz S. H-ficolin binds Aspergillus fumigatus leading to activation of the lectin complement pathway and modulation of lung epithelial immune responses. Immunology 2015; 146:281-91; PMID:26133042; https://doi.org/https://doi.org/10.1111/imm.12501
- Ma YJ, Doni A, Romani L, Jurgensen HJ, Behrendt N, Mantovani A, Garred P. Ficolin-1-PTX3 complex formation promotes clearance of altered self-cells and modulates IL-8 production. J Immunol 2013; 191:1324-33; PMID:23817411; https://doi.org/https://doi.org/10.4049/jimmunol.1300382
- Frederiksen PD, Thiel S, Larsen CB, Jensenius JC. M-ficolin, an innate immune defence molecule, binds patterns of acetyl groups and activates complement. Scand J Immunol 2005; 62:462-73; PMID:16305643; https://doi.org/https://doi.org/10.1111/j.1365-3083.2005.01685.x
- Gout E, Garlatti V, Smith DF, Lacroix M, Dumestre-Perard C, Lunardi T, Martin L, Cesbron JY, Arlaud GJ, Gaboriaud C, et al. Carbohydrate recognition properties of human ficolins: glycan array screening reveals the sialic acid binding specificity of M-ficolin. J Biol Chem 2010; 285:6612-22; PMID:20032467; https://doi.org/https://doi.org/10.1074/jbc.M109.065854
- Kjaer TR, Hansen AG, Sorensen UB, Nielsen O, Thiel S, Jensenius JC. Investigations on the pattern recognition molecule M-ficolin: quantitative aspects of bacterial binding and leukocyte association. J Leukoc biol 2011; 90:425-37; PMID:21730084; https://doi.org/https://doi.org/10.1189/jlb.0411201
- Kjaer TR, Hansen AG, Sorensen UB, Holm AT, Sorensen GL, Jensenius JC, Thiel S. M-ficolin binds selectively to the capsular polysaccharides of Streptococcus pneumoniae serotypes 19B and 19C and of a Streptococcus mitis strain. Infect Immun 2013; 81:452-9; PMID:23184524; https://doi.org/https://doi.org/10.1128/IAI.01148-12
- Wittenborn T, Thiel S, Jensen L, Nielsen HJ, Jensenius JC. Characteristics and biological variations of M-ficolin, a pattern recognition molecule, in plasma. J Innate Immun 2010; 2:167-80; PMID:20375634; https://doi.org/https://doi.org/10.1159/000218324
- Fontaine T, Simenel C, Dubreucq G, Adam O, Delepierre M, Lemoine J, Vorgias CE, Diaquin M, Latge JP. Molecular organization of the alkali-insoluble fraction of aspergillus fumigatus cell wall. J Biol Chem 2000; 275:41528; PMID:11134062
- Wasylnka JA, Simmer MI, Moore MM. Differences in sialic acid density in pathogenic and non-pathogenic Aspergillus species. Microbiol 2001; 147:869-77; https://doi.org/https://doi.org/10.1099/00221287-147-4-869
