ABSTRACT
People living with HIV (PLWH) who are treated with effective highly active antiretroviral therapy (HAART) have a similar life expectancy to the general population. Moreover, an increasing proportion of new HIV diagnoses are made in people older than 50 y. The number of older HIV-infected patients is thus constantly growing and it is expected that by 2030 around 70% of PLWH will be more than 50 y old. On the other hand, HIV infection itself is responsible for accelerated immunosenescence, a progressive decline of immune system function in both the adaptive and the innate arm, which impairs the ability of an individual to respond to infections and to give rise to long-term immunity; furthermore, older patients tend to have a worse immunological response to HAART.
In this review we focus on the pathogenesis of HIV-induced immunosenescence and on the clinical management of older HIV-infected patients.
Introduction
The advent of highly active antiretroviral therapy (HAART) deeply improved HIV-infected people's quality of life making possible for young patients with CD4 cell count > 350 cell/mcL to have a life expectancy similar to that of HIV negative individuals.Citation1 Thanks to the prolongation of life expectancy due to HAART,Citation1 the number of people with HIV infection older than 50 y increased during the last years in all Western Countries. According to last CDC report, in 2013 people aged 50 and over accounted for 18% of estimated HIV diagnoses, for 23% of estimated AIDS diagnoses and for 37% of deaths related to AIDS in the United States.Citation2 In 2014, in Italy the 20% of new HIV diagnoses and the 10% of AIDS diagnoses were in people over 50 y old.Citation3
The incidence of infectious and non-infectious comorbidities and overall mortality have been found to be higher in HIV infected patients compared with HIV-negative ones.Citation4 Moreover the duration of HIV infection seems to be a stronger predictor of morbidity and non-infectious comorbidities compared with age at the diagnosis.Citation4
The main problems with older people are the low perception of risk and the stigma of speaking about sex, even with people sexually active. These 2 factors make more difficult to achieve HIV diagnosis and consequently they cause a delay that can lead to a severe immune deficiency. The progression to AIDS is faster in older peopleCitation5-6 mainly because of the immunosenescence due both to age and to HIV infection itself. Moreover, progression to AIDS or occurrence of non-AIDS related comorbidities can be only partially prevented with HAART.Citation7
In this review we focus both on the pathogenesis of immunosenescence in HIV infected patients and on the main challenges involved in the clinical management of older HIV-patients.
Immunology of aging in HIV-infected patients
Immunosenescence and inflammation
Cellular senescence was first described by Hayflick as an irreversible cellular growth arrest following repeated cellular divisions, even in the presence of mitogenic stimuli (“replicative senescence”).Citation8-11 In this status, cells continue to be viable but they cannot proliferate and this process is not reversible. Senescent cells are different both from terminally differentiated cells, which stopped cellular division as a part of their development program, and from quiescent cells, that have the possibility to re-enter the cellular cycle following external stimulation.Citation11 Cellular senescence is mainly mediated by p53/p21 and p16INK4a/retinoblastoma (Rb) tumor suppressor pathways.Citation9,10,12 Different cellular stressors can trigger cellular senescence. In particular telomere shortening, due to consecutive cell divisions, is sensed by the cell as a DNA damage and drives a DNA-damage response (DDR) (“replicative senescence”). DDR together with hypoxia or accumulation of reactive oxygen species (ROS), can provoke “stress-induced senescence” mediated first by p53/p21 and followed, if stress is repeated, by p16INK4a pathways.Citation7,8,11 Finally also the activation of oncogenes induces senescence (“oncogene-induced senescence”).Citation11-12 () As a matter of fact cellular-senescence was an anti-proliferative program to prevent damaged cells to replicate. An increased number of studies draw the attention to the reduction in telomerase length, which is a measure of the proliferation of each single cell, and to mitochondrial damage, which is able to increase oxidative stress and inflammatory pattern, as major drivers for senescence program in all individuals.Citation7,8,13
Figure 1. Cellular pathways leading to cellular senescence. ROS: reactive oxygen species. SASP: senescence-associated secretory phenotype. © The American Society for Clinical Investigation. Adapted by permission of James L. Kirkland. Permission to reuse must be obtained from the rightsholder.Citation9
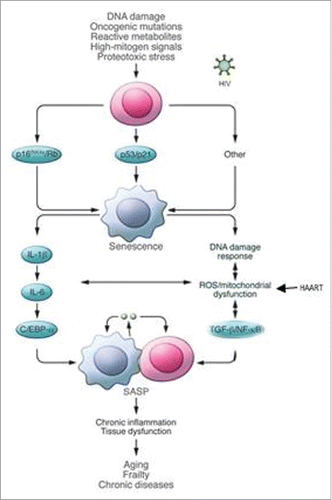
Senescence provokes a cascade of morphological and phenotypic changes in cells: a) increase in size and a flat morphology; b) upregulation of tumor suppressor genes and downregulation of cell-cycle promoting genes; c) production of senescence-associated secretory phenotype (SASP), which are inflammatory cytokines, growth factors and matrix metalloproteinases.Citation8,10,11 In particular Effros et alCitation8 found an increase in pro-inflammatory cytokines (interleukin-6 [IL-6] and tumor necrosis factor-α [TNF-α]) and a reduction in antiviral interferon-γ (IFNγ) (released by CD8+ T-cells). The release of chemokines, activated cytokines and immune modulators (SASP) from senescent cells induces local inflammation attracting cells of immune system. Senescent cells can be removed by innate immune cells (especially macrophages) maintaining tissue homeostasis and restricting tumorigenesis, but they can also accumulate in tissue leading to cancer, aging and fibrosis.Citation11 What drives this process leading to the clearance or not of senescent cells it is not well-known.Citation11 It is supposed to depend both on the kind of trigger (which cell? In which context?) or on the ability of the immune system to get rid of senescent cells.Citation11
The term immunosenescence refers to age-related changes in both the innate and adaptive immune systems associated to morbidity and mortality and reduction in vaccines responses.Citation7,14,15
In older adults, these changes seem to be linked to pro-inflammatory pathway (Inflamm-Aging), even if the mechanism is not completely understood (cellular damage? endogenous activators? hormonal changes?). Main data of immunosenescence associated to increased mortality in elderly derive from OCTO/NONA cohortsCitation15 (2 longitudinal studies in Swedish octogenarians or nonagenarians). In these studies the inversion of the CD4/CD8 ratio (less than 1) and the expansion of terminally differentiated cytotoxic T cells, associated to raised levels of pro-inflammatory cytokines and to seropositivity to cytomegalovirus (CMV),Citation15 were linked to higher morbidity and mortality in healthy elderly individuals.
In HIV infection, both HIV-replication itself and viral coinfections induce immune stimulation and clonal expansion of T-cells. The immune activation results into differentiation and accumulation of nonfunctional senescent cells (CD28− T-cells) and into an inflammatory milieu which perpetuates premature aging.Citation16 The higher level of inflammation in HIV individuals is measured by inflammatory biomarkers: in particular interleukin-6 (IL-6), C-reactive protein (CRP), D-dimer (DD), cystatin C-dimers and cystatin D-dimers.Citation7,13,17-19 In HIV-infected individuals these biomarkers are higher than in uninfected adults. They have been studied in HIV patients during HAART and they remain elevated despite suppressive therapy;Citation7,18 moreover in SMART study IL-6 and DD values predicted both mortality and non-AIDS related events in HIV patients.Citation13,18 Numerous factors are involved in the persistence of inflammation during HIV infection: a) ongoing HIV production; b) viral coinfections and microbial translocation; c) loss of T-regulatory cells; d) irreversible fibrosis of the thymus and lymphoid tissues and e) oxidative stress from smoking, obesity and HAART toxicities.Citation7,13
In spite of the benefit in survival due to HAART, event therapy itself may contribute to cellular activation and senescence. Some nucleoside reverse transcriptase inhibitor (NRTIs), as stavudine or zidovudine, inhibit mitochondria synthesis causing the release of mitochondrial DNA and increasing oxidative damage.Citation7,20 Rodriguez-Mora et al. found a direct HIV damage on mitochondrial DNA mediated by Tat, which was shown to polarize mitochondria and enhance the expression of metabolism-related proteins. According to this mitochondrial dysfunction, Rodriguez-Mora et al. supposed Tat to predispose cells to mitochondrial toxicities due to HAART.Citation20 NRTIs were supposed to inhibit telomerase and consequently accelerate cellular aging.Citation7,21 Protease inhibitors (PIs), especially indinavir, lopinavir and ritonavir, provoke fat redistribution which induces metabolic changes in adipocytes causing inflammation and mitochondrial alterations similar to NRTIs.Citation22,23 Gallego-Escuredo et al. compared adipocyte differentiation after efavirenz and lopinavir/ritonavir treatment, finding that both efavirenz and lopinavir/ritonavir impaired human adipogenesis and increased the expression and secretion of inflammation-related cytokines, but the overall effects were greater with efavirenz.Citation24 Contrarily maraviroc, a chemokine receptor CCR5 antagonist, does not alter adipocyte differentiation and seems to have anti-inflammatory properties exerted by inhibition of the expression and release of pro-inflammatory cytokines.Citation25
Coinfections and pro-inflammatory pattern
HIV itself, especially before the start of HAART, herpesviridae, in particular cytomegalovirus (CMV), and hepatitis viruses cause a prolonged stimulus to the immune system, reducing in particular the capability of proliferation of the T cells and depleting T-cells receptor (TCR) repertoire.Citation7,8,26
CMV causes a lifelong infection able to produce continuous antigenic stimulation thanks to most of viral proteins (751 unique CMV proteins) in infected cells.Citation27 This antigen burden is responsible for a more restricted T-cells repertoire and cause the accumulation of terminally differentiated T-cells, a process called “memory inflation.” CMV infection provokes expansion of CD28- T-cell population both in CD8+ T-cells and in CD4+ T-cells, similarly to the physiologic alterations which happen to T-cell compartment in older patients.Citation8,27,28 CMV stimulates high CD28-CD8+ T-cells response, higher than the CD28-CD4+, causing a reduced CD4:CD8 ratio, hallmark of immunosenescence.Citation27,28 Shortening of telomere length in T-cell phenotype is also associated with CMV infection.Citation27 In addition to the antigenic burden, CMV has developed numerous mechanisms to evade host surveillance as downregulation of HLA I and II of infected cells to escape recognition by CD4+, CD8+ and natural killers (NK). Finally the UL111.5A region of CMV genome encodes for an immunosuppressive interleukin homologous to IL-10. Thereby CMV is able to produce a state of immunosuppression producing a vicious circle in which CMV induces immunosuppression, which in turn facilitates CMV reactivation. This process is seen both in elderly people and in immunosuppressed patients, whether due to iatrogenic suppression as after bone-marrow or solid-organ transplantation or due to acquired causes as in HIV infection.Citation27 Prevalence of CMV coinfection is estimate to be around 90–100% in HIV infected-people. HIV and CMV chronic infections and the consequent inflammation and immune activation, the perturbation of T-cell compartment associated to the supposed role of CMV as “smoking gun” in immunosenescence, make the double infection play a synergic role in accelerated aging.Citation7,14,27,28
Finally, HIV infection causes the depletion of CD4+ T-cells of gut-associated lymphoid tissue (GALT) both by HIV direct effect and by activation-induced cell death. The mucosa-associated damage, due to GALT depletion, promotes a persistent intestinal bacterial translocation which increases chronic inflammation.Citation7-9,13,16 Soluble CD14 (sCD14) is an acknowledged biomarker of microbial products translocation. In particular sCD14 is expressed on the surface of monocytes and macrophages and it is released into circulation after its binding to LPS, which provokes monocytes/macrophages activation. sCD14 levels have been correlated with mortality in treated HIV patients.Citation13,16
Thymic function and T-cells response
T-cell development occurs in thymus, where bone marrow progenitor cells migrate to undergo positive selection (where T cells expressing a TCR that can recognize host MHC proteins on APCs are selected) and negative selection (where T cells recognizing autoreactive or “self” antigens are deleted) processes.
Thymus starts to involute during childhood and continues to involute in adults (at a rate of approximately 1–3% per year)Citation29; for this reason thymus-dependent T-cell creation declines with aging reducing the number of naive T-cells. Under physiologic conditions, thymus releases immature lymphocytes termed “recent thymic emigrants” (RTEs) which maintain a stable pool of naïve T-cells.Citation30 Whether thymus activity is maintained in adulthood is debated but it is accepted that most (approximately 90% of CD4+ T cells) of T cells in older adults are generated from division of cells in the existing T-lymphocyte pool (as RTEs).Citation29,31,32 Consequently, in older adults, most T-cells appear to be antigen-experienced memory T-cells.Citation29,31,32 In thymectomized young adults this pattern was strongly associated with seropositivity to CMV.Citation33
Kohler et al. showed that the surface molecule CD31 (PECAM-1) can be used to discriminate between CD31+thymicnaive and CD31−centralnaive CD4+ T-cells in the peripheral blood of healthy humans.Citation31 Both CD31+thymicnaive and CD31−centralnaive CD4+ T-cells satisfy phenotypic and functional criteria for naive CD4+ T-cells: expression of surface markers, such as CD45RA, CD27, CD28, CCR7 and CD62L and secretion of IL-2 without significant effector cytokine production (interferon-γ and IL4) after stimulation. The excision of the T-cell receptor D locus during TCR development generates signal joint T-cell receptor excision circle (sjTREC). Since TRECs are not replicated during mitosis, the number of TREC per cell decreases during each cell division. The quantification of TRECs therefore reflects the proliferative history of T cells. The content of sjTREC in CD31+thymicnaive cells is greater than in CD31−centralnaive CD4+ T-cells and it is only slightly reduced compared with thymocytes. This strongly suggests that CD31+thymicnaive cells contains T-cell recently sorted form thymus (RTEs), while the reduced sjTREC content of CD31−centralnaive CD4+ T-cells sustains the hypothesis of peripheral proliferation. He et al.Citation30 compared thymic export among healthy controls and HIV-infected patients both with acute (less than 6 months after HIV infection), early chronic (between 6 and 30 months after HIV infection) and chronic HIV-1 infection to assess whether HIV affected thymic export. In this study authors found that HIV-1 infection damaged thymic function, and this impairment was shown to be more severe in the chronic phase of infection than in the earlier periods, during which these alterations may be partially recovered with HAART. In acute HIV-1 infection, the proportion of circulating RTEs was markedly higher in patients with rapid progression than in patients with a slower progression.Citation30 In contrast to that study Rickbaugh et alCitation34 found lower CD31+ expression in CD4+ naive T-cells in patients who progressed to AIDS in one year compared with people who progressed to AIDS within 5 y.
HIV infected more preferably CD31−centralnaive CD4+ T-cells than CD31+thymicnaive CD4+ T-cells. The active proliferation of CD31−centralnaive CD4+ T-cells thereby provides a reservoir for HIV in humans. In HIV patients the CD31+thymicnaive CD4+ cells depletion is more evident than in age-matched healthy controls: this process may explain at least part the reduced TCR repertoire and the consequent detrimental response to neoantigens (infections, cancer, autoimmunity, vaccinations).Citation34,35 Moreover, in older HIV-infected patients (> 50 years) the compensatory increase of CD31-centralnaive CD4+ cells is also reduced compared both to age-matched controls and to younger HIV patients.Citation6,36 The synergistic action of age and HIV infection explain the faster progression to AIDS in older people infected with HIVCitation6,35,36.
In elderly people, the analyses of TCR repertoire revealed decreased diversity if matched with younger people.Citation29,34,37 This finding in addition to developmental and signaling alterations alongside with age-related decrease in T-cell generation are all found in cells from older adults, which contribute to functional deficits. The altered signaling is fostered by the persistence of low quantity of pro-inflammatory cytokines and increased production of ROS, both characteristics of Inflamm-Aging.Citation38 Moreover, the human T-cell compartment lose CD28 expression on CD8 T-cell during the shift from central to effector memory cells.Citation26,34 CD28+ has a central role in the proliferation of CD8+ T-cells as one of the main co-stimulator of T+ cell response during the antigen presentation between APCs and T-cells (in conjunction with TCR recognition by peptide bound to host MHC proteins on APCs).Citation26,29,38 In addition to the loss of CD28, CD8 T-cells express other markers of exhaustion, replicative senescence (mediated by upregulation of p21 and p16, as explained above), or terminal differentiation in the context of aging (such as PD-1, CD57, or KLRG1); moreover, they have increased production of IL6 and TNF-αCitation28 and reduced length of telomerases.Citation8,28,29,38 Recent studies have reported that the senescence phenotype in both CD8 effector memory and CD4 T-cells is strongly associated with irregular signaling via the p38 MAP kinase.Citation29 Altogether, the increased ROS, the skewed TCR repertoire and the loss of CD28 which occur in the aging process provoked an aberrant signaling, leading to immunosenescence, inflamm-aging and their adverse effects.Citation39 Recent studies found that mTOR serine threonine kinase activity plays a role in T-cell activation and differentiation, in particular of naive CD4+ T-cells, leading toward Th1 or Th17 phenotypes.Citation39 TCR/CD28 stimulation controls mTOR signaling pathway. Only few reports on T-cell mTOR in aging are available, based mainly on murine models. Recently, Arnold et al. have reported that TCR stimulation provoked autophagy in human CD8+ T-cells, whereas autophagy was reduced in CD28-CD8+ cells, thus limiting their survival under antigen stimulation. These data strenghten the interconnection between TCR pathways and mTOR activity and the resulting possible relationship to immunosenescence.Citation39
Kovaiou at al.Citation40 studied CD4 subsets in healthy young and elderly volunteers finding increased CD27+CD28+ T-cells (naïve and early-differentiated cells) in younger people, while elderly presented more frequently CD27-CD28- T-cells (fully differentiated cells) demonstrating that the aging process was linked to a shift of CD4 T-cells from naïve and early-differentiated to late differentiated subset. To better differentiate these phenotypes, the authors measured the presence of cell-surface markers, in particular CD45RA, receptors involved in lymphocyte homing [CCR7] and intercellular adhesion and co-stimulation [CD11a] associated with the different cellular subset ability to produce perforin. As expected elderly volunteers showed decreased frequency of CD45RA+ cells (markers of naïve cells) and increased number of CD11a++ cells compared with younger individuals. These data suggest that the CD27+CD28+CD4+ T-cells population is composed of 2 different phenotypes in the 2 groups of volunteers enrolled: a naive antigen-inexperienced subset in younger patients and an antigen-experienced cells of an early differentiation stage in elderly subjects. No differences were found in cytokine production (IL-2, IL-4 and interferon-γ) after in vitro stimulation in each CD4+ T-cell subset between the 2 population considered; whereas CD28-CD8+ T-cells are known to be unable to replicate and to produce IL-2. Finally the analysis of telomere length revealed a significantly shorter length in CD27-CD28-CD4+ cells when compared with CD27+CD28+CD4+ T-cells.Citation40
While CD4+ naïve T-cells decrease with age, memory CD4+ T-cells expand thanks to the continuous antigen presentation and to homeostatic proliferation.Citation40-42 T lymphocytes with specificity for a particular pathogen can persist in the host for many years after the pathogen has been eliminated.Citation43 CD8+ T lymphocytes can persist without re-exposure to antigen, either from exogenous sources or from antigen depots that might be maintained after the resolution of primary infection.Citation43 Memory T-cells survive and undergo homeostatic proliferation in the absence of any MHC molecules. Memory CD8+ T lymphocytes specific for a viral pathogen diminish in frequency in the absence of IL-15 or if they do not express the IL-15 receptor. Memory CD4+ T-cell populations show a similar dependency on IL-7 and IL-15 signals. Despite these similarities, antiviral CD8+ memory T-cell populations typically persist at relatively constant levels over time, whereas virus-specific CD4+ memory T-cells decrease in frequency; this combination provokes a reduction in CD4:CD8 ratio that is enhanced both in aging and in HIV infection.Citation26,29,38 Antigen persistence is required for long-term maintenance of CD4+ memory T-cells.Citation43 Memory T cells can be divided into 2 main subsets, central memory (TCM; cells with high levels of CD62L and CCR7) and effector memory cells (TEM; cells with low levels of CD62L and CCR7, which actively expresses effector functions and traffics to peripheral rather than secondary lymphoid tissues), based on expression of homing and chemokine receptors involved in preferential trafficking to secondary lymphoid organs or peripheral sites, respectively.Citation43,44 Several models of memory T-cell generation have been proposed, including the hypothesis that memory T-cells rapidly distinguish themselves from conventional effector T-cells during the primary immune response. Most data support the model of naïve T-cells differentiating into effector T-cells, then further differentiating into memory T-cells. Comparison of the TCR repertoire of pathogen-specific effector and memory T-cells suggests that these 2 populations share a common ancestry. Memory T-cells appear to have expressed genes encoding effector proteins, further supporting the notion that memory T-cells derive from effector T-cells. During infection, at very early stages of the immune response, a subset of T-cells maintain the capacity to undergo more extensive proliferation.Citation43
HIV-infected patients have several similarities to older people as increased memory cells proliferation compared with naïve cells.Citation16,40,45,46 In particular HIV-1 infection is related to: a) reduction of naive CD4+ and CD8+ T-cells and an increased frequency of memory CD8+ T-cells; b) alterations in expression of CD27 and CD28 in chronic HIV-1 infection (as with aging) regardless of age; c) significantly shorter telomere length of CD28-CD8+ T-cells and d) shift to a replicative senescent phenotype of CD28-CD8+ T-cells. The sum of these changes greatly limits the capability of the CD8+ T-cell compartment to perform an appropriate response against HIV-1 and other antigens. In elderly HIV-infected individuals both aging and HIV-1 infection itself play a role in the loss of naïve CD4+ T-cells, enhancing the changes in CD8+ T-cell phenotypes and the consequent poorer outcome of these subjects.Citation34 Allers et al. found an increased effector memory phenotype in older HIV patients associated to an increased inflammatory cytokines.Citation6
The reduced CD4/CD8 ratio,Citation47 the decreased expression of CD28, the shift from naïve to central memory cells and the reduction of TCR repertoire is much more evident in HIV-infected patients before the introduction of HAART.Citation8 However HAART is not able to normalize these phenomena.Citation8,18 The explanation of these finding may be related to the microbial translocation due to HIV gut mucosal injury, tissue fibrosis, coinfections as cytomegalovirus (CMV) and hepatitis C virus, and homeostatic drive.Citation48 The contribution of each of these phenomena may vary according to the timing of HAART introduction (started during acute or chronic infection), the degree of immune suppression (number of CD4 T-cells), age, comorbidities, and genetic background. IL-6 and DD are the main biomarkers independently associated with morbidity and mortality in HIV-individual on HAART., and are mainly used to measure the chronic activation of the innate immune system.Citation48
B-cells response
The B-cell lineage generates a highly diverse repertoire of rearranged antigen receptor genes and there is evidence that diversity in large populations of B cells is substantially reduced with age and with changes in functional phenotype such as the geriatric syndrome of frailty. Chronic infection with Epstein-Barr virus or CMV also influence B-cell repertoire. Current studies on sequencing the repertoire of purified B-cells revealed evidence for age-related repertoire changes. Moreover as many B-cell functions are dependent on T-cell help, the effects of aging on the B-cell lineage reflect both B-cell intrinsic changes and those resulting from altered T-B-cell interactions (reduced number of recognized antigens and reduced production of antibodiesCitation49). Activation-induced cytidine deaminase (AID) expression on B-cells is a protein fundamental for the heavy chain class switching of B-cells and for somatic hypermutation; AID expression is reduced with age, so that in elderly patients there is a decreased proportion of B cells that underwent class switchingCitation29 and decrease in B cell responses to primary antigenic stimulation.Citation49 Older people have increased proportion of memory B-cells than naive cells,Citation26 with increased gene expression of TNF-α which is negatively correlated with proliferative responses.Citation29
In HIV-infected patients compared with age-matched controls, there is an increase in memory B-cells which are more activated, resulting in polyclonal production of immunoglobulins (Ig), in particular IgG and IgA isotypes. HIV-1 infection causes B-cell hyperactivation and exhaustion through the interaction of HIV-1 virions with CD21 (complement receptor 2) on mature B-cells and through indirect effects of HIV-1 infection (immune activation and follicular-helper T-cell dysfunction).Citation50 B-cell hyperactivation in patients with HIV also leads to accumulation of activated mature (AM) B cells, exhausted “tissue-like” memory (TL) B cells, and immature transitional B cells, whereas resting memory (RM) B cells are depleted.Citation50 Decreased CD21 expression on B cells (CD21low/-) is a marker of B-cell exhaustion which drives the spontaneous production of immunoglobulins and characterizes cells with decreased proliferative capacity (plasma blast).Citation50 HIV-naïve patients have higher values of CD21low/− compared with HIV patients in chronic therapy, nevertheless HAART cannot normalize B cells subpopulations.Citation50-52
Innate response
Neutrophils are short-lived cells that are among the first to reach the site of inflammation.Citation29,39 Neutrophils use a great number of different mechanisms to phagocyte and kill pathogens. Phagocytosis, chemotaxis, generation of neutrophil extracellular traps, free radical production and apoptosis are modified by aging.Citation29,39 These modifications seem to be related to changes in signalosome formation causing reduced cell responses.Citation29 Moreover, even though the number of TLRs is not altered by aging, there still is an impairment in the trafficking of pathway-associated signaling molecules in the plasma membraneCitation39.
Several studies illustrate the increase of activated/inflammatory monocytes, in particular CD14+ CD16+, both in older people and in HIV positive patients.Citation29,53-56 This “activated pattern” is confirmed by the expansion of CD11b expression and by the reduction of CD38, CD62L, and CD115 expression. Plasma levels of soluble CD163 (sCD163) and CXCL10, markers of innate immune activation, increase with age.Citation54 Interestingly, also basal levels of IL-10, an anti-inflammatory cytokine, are higher in older than in younger individuals.Citation29 Van Duin et colleagues registered a reduced production of IL6 and TNF-α following TLR-1/2 stimulation.Citation29,57 While in chronic HIV infection there is an increase in TLR-2 expression and consequent augmented release of pro-inflammatory cytokines.Citation58-59
Myeloid dendritic cells (mDC) are professional APCs with strong antigen-presenting properties able to influence immune activation through secretion of regulatory cytokines.Citation60 TLR function of mDC changes in HIV-infected people depending on the stage of infection.Citation61 While plasmacytoid dendritic cells (pDC) are APCs which can exert antiviral activities mainly by producing abundant amounts of interferon-α (IFN-α).Citation60 pDC function usually decreases both in HIV patients and in older people. TLR function of pDC in chronic and primary HIV infection is related to IFN-α production and induction of NK cytotoxicity.Citation27 The production of inflammatory cytokines, as IL6, IL2, TNF-α and type I interferon, is diminished in older adults compared with younger ones.Citation29 NK cells are defined by the expression of CD56 (or N-CAM) and CD16, receptor for the Fc region of IgG (Fc-R-III).Citation62 The major compartment of peripheral blood NK cells is CD56dimCD16+ (90%) while CD56brightCD16−NK cells represent less than 10% of peripheral blood NK cells. Both these subsets have a high immunoregulatory capability due to cytokine and chemokine production in response to stimulation.Citation62 There is a third compartment of dysfunctional NK cells, CD56−CD16+, also found in a small percentage in peripheral blood of healthy individuals, which is expanded in HIV and in hepatitis C virus infections.Citation62 Changes in NK cells subset of elderly are associated with: a) increased numbers of NK in healthy elderly individuals, b) reduced frequencies of CD56bright NK cells and increase of CD56dimCD16+ NK cells.Citation29,62 These changes drive to a reduction of cytotoxicity-activating receptors and a major susceptibility to infections, also mediated by the reduced ability to respond after stimulation.Citation29,62 In HIV infection NK cells are supposed to have a central role in the complex net of interactions between immune and adaptive responses, as well reported in Scully and Alter's recent paper.Citation63
Treatment of the aging HIV-infected patient
Treatment of HIV infection in both patients diagnosed at an older age and in younger subjects with disease-related biologic senescence represent a challenge for the clinician. While the mechanisms behind aging in these 2 populations may be somewhat different, from the clinical point of view it is necessary to evaluate the patient not only on the base of the chronological age but also considering the biologic age. Nevertheless, elderly HIV-infected patients constitute a specific population in which both disease-related and physiologic mechanisms take place and the choice of treatment must be carefully aimed and tailored to address these issues.
Currently, international guidelines do not recommend specific regimens in older HIV patients, and treatment should be started regardless of CD4 T-cell count.Citation64-68 () Nevertheless, delaying HAART initiation in this population seems to be more harmful than in younger patients even at similar CD4 T-cell tresholds.Citation69 Few large randomized studies enrolled patients >50 y old, so data regarding efficacy and tolerability of HAART in this special population is somewhat scarce, especially considering that by 2030 more than 70% of HIV-infected patients will be more than 50 y old.Citation70
Table 1. Guidelines for older HIV-infected patients.
Clinical outcome
Works evaluating risk of AIDS and death in HIV-infected patients >50 y old showed a higher risk of progression even after adjusting for baseline CD4+ T-cell count, HIV-RNA and stage of disease.Citation71-77 Interestingly, this finding is not explained by differences in virological response, as older HIV-infected patients tend to have a superior virological outcome when compared with younger patients.Citation78-83 Some suggests that these finding may be linked with higher adherence in older patients,Citation78,84-85 but other authors reported a higher risk of inadequate adherence in HIV patients who have other comorbidities and take other medications besides HAART.Citation86-87
While virological response seems not to be influenced by age, at least biologically, older patients have lower CD4+ T-cell gain compared with younger persons,Citation88-95 even when adjusted for antiretroviral therapy regimens. This is particularly relevant considering that lower CD4 T-cell counts are not only linked to HIV-related opportunistic infections and malignancies, but also lead to higher risk of non-AIDS-related comorbidities, adding to the already elevated risk conferred by age.Citation96-108 Moreover, even after an adequate CD4 T-cell increase, the functionality of the immune system remains compromised compare with younger treated HIV+ patients.Citation109-110 Finally, patients who are diagnosed with HIV at an older age have lower CD4 T-cells and less favorable prognosis comparable to younger patients.Citation111
The recent results from the START studyCitation112 highlight how an early treatment started with more than 500 CD4 T-cell was able to reduce the rate of serious AIDS and non-AIDS related events. In a cohort study following people living with HIV in the UK, life expectancy at the age of 35 was comparable to that of general population when optimal virological and immunological (i.e. >350 CD4 T-cell) response was achieved. The study showed that even when virological suppression was obtained, life expectancy was shorter for people with a lower CD4 T-cell count.Citation113 Life expectancy at the age of 20 in HIV-infected patients compared with a non-infected cohort in the US was also recently analyzed.Citation114 While the gap with uninfected population during 1996–2006 was 26 years, this value was reduced to 13 y in the period between 2007 and 2011. Interestingly, starting HAART with more than 500 CD4 T-cells further narrows the gap to 9 years, and patients who are not co-infected with hepatitis B or C, who do not smoke and have a current or past history of drug and/or alcohol abuse have a life expectancy at 20 y old just 6 y lower than uninfected subjects. These findings were also confirmed by other recent reports.Citation115-119 In conclusion, while starting HAART in older HIV-infected patients is undoubtedly recommended, the poorer immunological response seen in this population represent a matter of concern and should be intensively investigated to increase life expectancy of elderly patients.
Drug-drug interactions
Currently, 6 different classes of antiretroviral drugs are approved for clinical use. Antiretrovirals belonging to the same class share similar metabolic pathways, but each drug has its own peculiar pharmacokinetic properties. Protease inhibitors (PIs) inhibit P-glycoprotein (P-gp), cytochrome P450–3A (CYP3A) and other isoenzymes belonging to the same family. Among this class, ritonavir is the most potent and it is currently used to increase plasma levels of other PIs, acting as a so-called booster. Cobicistat, an analog of ritonavir lacking antiviral activity, is a potent inhibitor of CYP3A and P-gp, but its effect is more specific and has no inducing properties. On the other hand, non-nucleoside reverse transcriptase inhibitors (NNRTIs) are inducers of CYP3A. Consequently, PIs usually carry the risk of increasing the concentration of drugs metabolized by CYP3A, while NNRTIs may lower plasma levels of such molecules. Finally, nucleoside reverse transcriptase inhibitors (NRTIs), integrase inhibitors (INIs) and maraviroc are not metabolized by the cytochrome system and therefore carry lower risk of drug-drug interactions.
Older patients are more likely to receive more drugs compared with younger HIV-infected people, and the risk of drug-drug interactions (DDI) is therefore higher in this particular setting.Citation70,120-123 The clinician must face the challenge of selecting an appropriate HAART regimen in a naïve HIV-infected elderly patient and to adapt an ongoing HAART according to concurrent medications in an aging patients already receiving antiretrovirals. illustrate a brief summary of the most relevant interactions between first-line antiretrovirals and commonly prescribed drugs in the aging population.
Table 2. Interactions between first-line antiretrovirals and commonly prescribed drugs in aging population, extracted from68 and http://www.hiv-druginteractions.org/.
Cardiovascular medications
Cardiovascular comorbidities are common among older patients; moreover, both HIV infection and certain antiretrovirals constitute a risk factor for cardiovascular eventsCitation124.
Rivaroxaban, an oral anticoagulant which acts as a direct inhibitor of factor Xa, must not be co-administered with protease inhibitors and cobicistat, as the inhibition of CYP3A4 and P-gp may increase rivaroxaban plasma concentrations, leading to an increased bleeding risk. Moreover, a close international normalized ratio monitoring must be performed when PIs, cobicistat or NNRTIs (with the exception of rilpivirine) are prescribed in people receiving warfarin.
Amiodarone, propafenone, flecainide and lidocaine coadministration with PIs is contraindicated, as serum concentrations of these antiarrhytmics is increased leading to potential serious adverse events. Caution must also be used if using cobicistat.
Among anti-hypertensive medications and drugs used for heart failure, ivabradine, lercanidipine and ranolazine concentrations are increased by concomitant use of PIs or cobicistat, and co-administration should be avoided. Close monitoring is also required with dihydropiridine calcium-channel blockers. Conversely, efavirenz and nevirapine may reduce plasma concentrations of this class of anti-hypertensive.
Finally, digoxin levels may be increased by PIs and cobicistat, and frequent serum digoxin levels monitoring is therefore recommended, while patients already taking digoxin should have their dose halved. In patients receiving rilpivirine, plasma concentrations of this drug may also increase due to inhibition of intestinal P-gp.
Lipid-lowering agents are among the most prescribed medications in both general populations and people living with HIV. While simvastatin and lovastatin coadministration with PIs and cobicistat is contraindicated, other agents as rosuvastatin and atorvastatin may be used if dose-adjusted. Pravastatin and fluvastatin may be safely coadministered with PIs, while the interaction with cobicistat has not been fully evaluated yet.
Gastrointestinal medications
Metformin is the first-line medication for people diagnosed with type 2 diabetes. Cobicistat may increase metformin levels thanks to the inhibition of MATE1 transporter, while rilpivirine may exert the same effect by inhibition of active renal secretion of metformin. Dolutegravir was also demonstrated to increase metformin concentrations, and a dose adjustment is required in case of coadministration.
Gastric pH-lowering agents must be used with caution with most antiretrovirals. Antacids and medications containing polyvalent cations must not be administered concomitantly with atazanavir, rilpivirine and integrase inhibitors. Proton-pump inhibitors must not be coadministered with rilpivirine, and should also be avoided in patients receiving atazanavir, especially unboosted. Finally, anti-H2 receptor antagonist should also not be administered concomitantly with PIs and rilpivirine. Domperidone, an anti-emetic metabolized by CYP3A4, must not be prescribed to patients receiving PIs or cobicistat, due to an increase concentration leading to higher risk of QT prolongation; caution should also be used when coadministered with NNRTIs.
Neurologic and psychiatric medications
Ergot derivatives, commonly used for migraine treatment, should not be used with PIs, cobicistat and NNRTIs because of increased concentrations of dihydroergotamine and ergotamine.
Most antipsychotic drug concentrations are increased if used with PIs and cobicistat, while dose should be adjusted with NNRTIs (with the exception of rilpivirine) because of risk of insufficient drug concentration. In particular, quetiapine carries the higher risk of interactions with PIs and should therefore not be coadministered. Benzodiazepines behave similarly, with risk of overexposure if coadministered with PIs and cobicistat and subtherapeutic levels with NNRTIs. Notably, midazolam and triazolam should not be prescribed to patients receiving these PIs, cobicistat and also efavirenz, because of high risk of overdosing.
Most anti-depressant interacts the same way with PIs, cobicistat and NNRTIs, with the former leading to higher levels and the latter to decreased concentrations of the coadministered drug.
Miscellaneous medications
Boosted PIs and cobicistat increase the concentration of steroid, both systemic and topical, leading to potential serious adverse events such as adrenal insufficiency or Cushing's syndrome. On the contrary, NNRTIs, with the exception of rilpivirine, reduce the concentration of steroids.
Opioid analgesics, such as fentanyl, tramadol or oxycodone, show higher plasma levels when coadministered with PIs or cobicistat, while NNRTIs, again with the exception of rilpivirine, are able to decrease serum concentration of such molecules. Methadone has a remarkable behavior, as it is increased by PIs while cobicistat does not significantly alter plasma concentrations; nevirapine, rilpivirine and especially efavirenz reduce methadone levels in blood, while etravirine does not produce a substantial effect.
Impact of aging on drug toxicity in HIV-infected patients
Multiple factors affect drug absorption and metabolism in older patients. Increase in gastric pH, reduced gastric emptying and gastrointestinal motility associated with reduced splanchnic blood flow influence the absorption of antiretrovirals. Moreover, hepatic and kidney function are known to decline in aging patients, due respectively mainly to decreased activity of the cytochrome P450 system and reduced glomerular filtration and tubular secretion. Finally, the pharmacokinetics of prescribed drugs is also influenced by change in body composition which typically occurs in the elderly, such as increased in body fat and decrease in total body water.Citation125 Special attention should then be made to adjust the dosage of antiretrovirals given these physiologic modifications occurring with age, as several works demonstrated an increase in drug-related adverse events in older patientsvCitation78,126-132
What to start
The decision of an appropriate HAART should be made considering the higher frequency of comorbidities in older patients, which also leads to an higher risk of drug-drug interactions. A recent analysis of a French cohort showed that HIV-infected patients older than 75 y old had a similar rate of viral suppression but significantly more age-linked noncommunicable comorbidities, highlighting the need of a “targeted intervention” in aging HIV patients.Citation71
When choosing a first-line regime in older patients, antiretrovirals with an impact on pre-existing conditions or on potential age-associated comorbidities must be avoided. Moreover, a strict monitoring for potential toxicities and screening for age-related diseases must be constantly be performed. Several works described the issue of comorbidities in older HIV-patients, and a further analysis goes beyond the scope of this review. Regarding the choice of first-line antiretrovirals, in patients with renal impairment (i.e., an estimated creatinine clearance < 70ml/min) regimens containing both TDF and cobicistat should be avoided; moreover, TDF itself must be use with caution, adjusting the dosage if necessary. TDF should also be avoided in case of osteoporosis. In patients with cardio-vascular disease ABC and combinations containing LPV/RTV should be avoided, and in subjects with dyslipidemia PI-based combinations, ABC, EFV and EVG/COBI must be used with caution. Finally, EFV should be avoided in patients with psychiatric comorbidities.
New treatment strategies and immunotherapy in aging HIV patients
Treating older patients with HIV constitutes a multi-faceted challenge which the clinician will face more and more commonly in next few years. Several novel approaches could be considered in the aging population, with the aim of selecting a regimen which could ensure effectiveness and long-term tolerability. Given the already mentioned issue of comorbidities and comedication, one should consider if drug toxicity burden in this particular population may be lowered by using alternative strategies. A possible key player in the immediate future will be the introduction of tenofovir alafenamide (TAF), a pro-drug of tenofovir, with a more favorable renal toxicity profile due to lower plasma levels achieved by higher cellular concentration in lymphoid cells, where TAF is converted in tenofovir diphosphate. The pharmacokinetics properties of this novel antiretroviral may be appealing in aging population.Citation133
In older patients class-sparing regimens are more difficult to introduce, as most of these strategies include PIs or ABC, which should be used with caution in patients with a high cardiovascular risk or dyslipidemia. A recently published study examined the efficacy of cabotegravir (a novel integrase inhibitor, which also has the possibility of being administered as a long-acting formulation) plus rilpivirine as a maintenance therapy,Citation134 which could be an interesting option in aging population. Another possible approach may be based on dolutegravir dual-therapy. While the efficacy of dolutegravir mono-therapy has yet to be fully evaluated, ongoing pilot studies are showing promising results.Citation135-136
The immunological defects associated with immunosenescence need specific considerations, as they may potentially be targeted with immunotherapeutic intervention with either exogenous cytokine administration or by the use of other immune-based therapies.
Interleukin(IL)-2 and IL-7 were the most extensively studied cytokines in the context of HIV infection. IL-2 is a molecule primarily produced by CD4 T-cell; alongside its major role as T-cell proliferation promoter this cytokine act as a crucial molecule in immune system differentiation and development, influencing also cytokine secretion, T regulatory cells development, B and NK-cells replication.Citation137 IL-2 production impairment was one of the first immunological defects described in HIV infection.Citation138 Two randomized international trials evaluated the use of intermittent IL-2 administration in patients receiving HAART. While CD4 T-cell increase was substantial, there was no effect on clinical outcome (deaths, opportunistic infections). Moreover, patients receiving IL-2 had more frequent grade 4 side effects than patients receiving HAART alone.Citation139 The somewhat paradoxical effect of IL-2 may be explained by the increase of distinct CD4+CD25+ expressing high level of FOXP3, defined as cytokine-induced naïve (CEN) T-cells, which could act as T regulatory cells with suppressor activity.Citation140-143 IL-7 is another key cytokine which role is impaired in HIV infection, mostly thank to impairment of receptor signaling via CD127 (a component of IL-7 receptor [IL-7R]).Citation144–145 IL-7 is crucial for T cell development and homeostasis thanks to its anti-apoptotic and co-stimulatory proliferative signalsCitation146 and in untreated patients with HIV infection high levels of IL-7 reflects a compensatory loop mechanism to contrast HIV-induced lymphopenia ().Citation147 A first phase I/IIa randomized dose-escalation trial showed that IL-7 treatment was able to provide a stable increase in CD4 T-cells in HAART-treated patients. The T-cell receptor diversity was shown to be broadened and thymic output was increased by IL-7 administration, possibly paving the way for an improvement of the immune function. Finally, IL-7 was well-tolerated with a favorable toxicity profile.Citation148 Two subsequent phase II trials confirmed these findings, but a large proportion of patients developed anti-recombinant-human-IL-7 binding antibodies (including neutralizing antibodies in more than a third of the subjects), although without any impact on CD4 T-cell response.Citation149 Finally, in an unrelated study aimed at evaluating a possible eradication strategy, IL-7 administration associated with HAART intensification was shown to induce an increase in HIV-DNA and in CD4 T-cell subsets harboring the majority of HIV reservoir (T central and transitional memory).Citation150 This finding led to concerns regarding the expansion of HIV reservoir in patients treated with IL-7. Other cytokines are being explored as potential therapeutic tool in HIV. Another cytokine with a relevant role in HIV infection is IL-15, which physiologically influences the host defense against intracellular pathogens but also regulates T, B and NK-cells survival. In HIV infection, the injection of IL-15 enhanced the survival and effector function of HIV-specific CD8+ T-cells and, when exogenously increased by administration of a IL-15 coding plasmid, was shown to be a potent adjuvant by stimulating CD8 T-cell response and longevity as well as increasing IFNγ production.Citation151-152 Finally, IL-21 is a cytokine involved in CD8 T-cell proliferation and activation and controls the differentiation of naïve CD4 T-cell in Th17 cells; this molecule is defective in SIV-infected rhesus macaques and this deficit is associated with Th17 cell depletion.Citation153 In untreated rhesus macaques with SIV infection the administration of IL-21 led to an increase in the cytotoxic potential of T cells without enhancing cellular activation,Citation154 and in HAART-receiving animals drove a reduction of immune activation and a restoration of intestinal Th17 and Th22.155
Figure 2. Role of interleukin7 (IL-7). IL-7 is a stromal cell-derived cytokine that provides continuous signals in all levels of lymphocytes maturation. Ag: antigen.
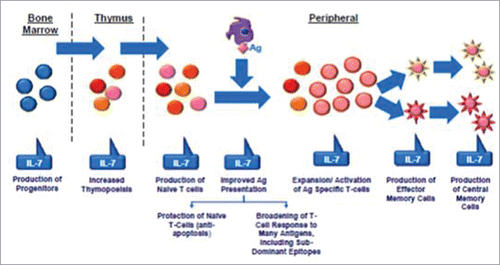
Another issue related to chronic HIV infection is represented by the interplay of immune senescence and immune activation. Theoretically, approaches aimed at reducing immune activation may prove to be beneficial in breaking the vicious circle of chronic inflammation and immunological dysfunction. Chloroquine and its derivate hydroxychloroquine are 2 antimalarial drugs which are also used in autoimmune diseases such as systemic erythematous lupus thanks to their immune modulatory activities. These molecules mainly acts on different TLRs (especially TLR9 and TLR4) and are able to decrease proinflammatory cytokine release such as IL-6, IL-18 and TNFa.Citation156-157 Several works explored the use of chloroquine or hydroxychloroquine in HIV-infected patients in HAART-treated and HAART-naïve patients, showing contrasting results. In some studies this molecule was shown to reduce immune activation markers (i.e., IL-6, TNF-α and LPS) and CD8 T-cells expressing CD38+HLA-DR+ and Ki-67,Citation158-161 while other researchers found no effect on immune-activation.Citation162-164
A possible target is also represented by TNF itself, which may be antagonized by several molecules currently in use for rheumatologic diseases. A study in rhesus macaques showed that adalimumab, a human anti-TNF monoclonal antibody, was able to reduce infiltration of polymorphonuclear cells into the T-cell zone of lymphoid tissues, diminishing also lymphoid tissue fibrosis; nevertheless, no effect on plasma immune activation was proven.Citation165 Interestingly, a very recent case report described the efficacy of infliximab, another anti-TNF monoclonal antibody, in treating immune reconstitution inflammatory syndrome in HIV-patients coinfected with tuberculosis,Citation166 possibly highlighting the efficacy of this approach to control excessive immune activation.
Another molecule whose immune-regulatory properties were tested in HIV infection is cyclosporine, which inhibits nuclear factor of activated T-cells (NFAT) reducing T-cell activation and proliferation. However, while an initial report in early HIV infection suggested a possible immunological benefit,Citation167 other studies showed no substantial benefit.Citation168-169 Moreover, mycophenolic acid (an inhibitor of T-cell proliferation which block the synthesis of guanosine nucleotides acting as an inhibitor of the enzyme monophosphate dehydrogenase) and rapamycin (which blocks mTOR, leading to a reduction in T-cell activation and proliferation) were also suggested as possible therapeutic approaches to reduce immune activation in HIV patients, but currently no significant results on immune activation were demonstrated, regardless of some reports of clinical benefit in HIV-infected patients.Citation170-171
Other possible immune-based therapeutic approaches include the blockade of molecules involved in immune checkpoints such as programmed death (PD)-1. PD-1 has a central role in virus-specific CD8 T-cell exhaustion, it is upregulated in most CD8 T-cell populations during HIV infection and correlates with the level of CD8 T-cell exhaustion and with disease progression.Citation172-173 Several studies on animal models showed how PD-1 blockade was able to enhance proliferation of CD4 and CD8 T-cells, especially HIV-specific clones; moreover, an increased proliferation of resting memory B-cells and a reduction in microbial translocation were also demonstrated in animals treated with anti-PD-1 antibodies.Citation174 Other potential immune checkpoints target for immune intervention may be CTLA-4, Lag-3, Tim-3 and TIGIT and several investigators are exploring therapeutic options directed at these molecules.
Conclusions
Life expectancy of people living with HIV increased thanks to the efficacy of antiretroviral therapy and to improved care of HIV-related conditions. Thus, the management of older patients with HIV infection represents an increasingly prevalent issue. Furthermore, HIV itself is able to accelerate this process contributing to premature aging, further impairing an already damaged immunological system and contributing to the emergence of comorbidities.
The clinician must be able to adapt to this new scenario by selecting an appropriate treatment taking into consideration all the players involved in an aging HIV-infected patients, being able not only to adequately manage infection-related issues but also to identify and care for age-associated conditions.
Abbreviations
| 3TC | = | lamivudine |
| ABC | = | abacavir |
| D | = | comedication decrease concentration of ART |
| DTG | = | dolutegravir |
| E | = | comedication increases concentration of ART |
| EVG/COBI | = | elvitegravir/cobicistat |
| FTC | = | emtricitabine |
| RAL | = | raltegravir |
| RPV | = | rilpivirine |
| RV/r | = | darunavir/ritonavir |
| TAF | = | tenofovir alafenamide |
| TDF | = | Tenofovir Disoproxil Fumarate |
| ↑ | = | ART increases concentration of the comedication |
| ↓ | = | ART decreases concentration of the comedication |
| = | = | no significant interactions |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- May MT, Gompels M, Delpech V, Porter K, Orkin C, Kegg S, Hay P, Johnson M, Palfreeman A, Gilson R, et al. Impact on life expectancy of HIV-1 positive individuals of CD4+ cell count and viral load response to antiretroviral therapy. AIDS 2014; 28:1193-202; PMID:24556869; https://doi.org/10.1097/QAD.0000000000000243
- Centers for Disease Control and Prevention (CDC). Diagnoses of HIV infection in the United States and dependent areas: HIV surveillance report. 2014. Available at http://www.cdc.gov/hiv/group/age/olderamericans/index.html
- Centro Operativo Aids. Notiziario ISS-COA volume 28, numero 9. 2015. Available at http://www.iss.it/binary/ccoa/cont/HIV_AIDS_DIC_2015.pdf
- Guaraldi G, Zona S, Brothers TD, Carli F, Stentarelli C, Dolci G, Santoro A, Beghetto B, Menozzi M, Mussini C, et al. Aging with HIV vs. HIV seroconversion at older age: a diverse population with distinct comorbidity profiles. PLoS One 2015; 10(4):e011853; https://doi.org/10.1371/journal.pone.0118531
- Cao W, Jamieson BD, Hultin LE, Hultin PM, Effros RB, Detels R. Premature aging of T cells is associated with faster HIV-1 disease progression. J Acquir Immune Defic Syndr 2009; 50:137-147; PMID: 19131896; https://doi.org/10.1097/QAI.0b013e3181926c28
- Allers K, Bösel D, Epple HJ, Karcher H, Schmidt W, Kunkel D, Geelhaar-Karsch A, Schinnerling K, Moos V, Schneider T. Effect of age on the CD4+ T-cell impairment in HIV-infected persons. J Acquir Immune Defic Syndr 2014; 66:7-15; PMID: 24378723; https://doi.org/10.1097/QAI.0000000000000097
- Deeks SG. HIV infection, inflammation, immunosenescence and aging. Annu Rev Med 2011; 62:141-55; PMID: 21090961; https://doi.org/10.1146/annurev-med-042909-093756
- Effros RB. Telomere/telomerase dynamics within the human immune system: effect of chronic infection and stress. Exp Gerontol 2011; 46(2–3):135-40; PMID: 20833238; https://doi.org/10.1016/j.exger.2010.08.027
- Tchkonia T, Zhu Y, van Deursen J, Campisi J, Kirkland J. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J Clin Invest 2013; 123(3):966-72; PMID: 23454759; https://doi.org/10.1172/JCI64098
- Zhu Y, L. Amstrong J, Tchkonia T, Kirkland J. Cellular senescence and the senescent secretory phenotype in age-related chronic diseases. Curr Opin Clin Metab Care 2014, 17:324-328; https://doi.org/10.1097/MCO.0000000000000065
- Lujambio A. To clear, or not to clear (senescent cells)? That is the question Bioessays 2016; 38:S56-S64; PMID: 27417123; https://doi.org/10.1002/bies.201670910
- Larsson LG. Oncogene-and tumor suppressor gene-mediated suppression of cellular senescence. Semin Cancer Biol 2011; 21:367-376; PMID: 22037160; https://doi.org/10.1016/j.semcancer.2011.10.005
- OAR working group in HIV and Aging. HIV and aging: state of knowledge and areas of critical need for research. J Acquir Immune Defic Syndr 2012; 60:S1-18; PMID: 22688010; https://doi.org/10.1097/QAI.0b013e31825a3668
- Pathai S, Bajillan H, Landay AL, High KP. Is HIV a model of accelerated or accentuated aging? J Gerontol A Biol Sci Med Sci 2014; 69(7):833-42; PMID: 24158766; https://doi.org/10.1093/gerona/glt168
- Wikby A, Nilsson BO, Forsey R, Thompson J, Strindhall J, Löfgren S, Ernerudh J, Pawelec G, Ferguson F, Johansson B. The immune risk phenotype is associated with IL-6 in the terminal decline stage: findings from the Swedish NONA immune longitudinal study of very late life functioning. Mech Ageing Dev. 2006; 127(8):695-704; PMID: 16750842; https://doi.org/10.1016/j.mad.2006.04.003
- Desai S, Landay A. Early immune senescence in HIV disease. Curr HIV/AIDS Rep 2010; 7:4-10; PMID: 20425052; https://doi.org/10.1007/s11904-009-0038-4
- Tenorio AR, Zheng Y, Bosch RJ, Krishnan S, Rodriguez B, Hunt PW, Plants J, Seth A, Wilson CC, Deeks SG, et al. Soluble markers of inflammation and coagulation but not T-cell activation predict non-AIDS-defining morbid events during suppressive antiretroviral treatment. J Infect Dis 2014 15; 210(8):1248-59; PMID: 24795473; https://doi.org/10.1093/infdis/jiu254
- Grund B, Baker JV, Deeks SG, Wolfson J, Wentworth D, Cozzi-Lepri A, Cohen CJ, Phillips A, Lundgren JD, Neaton JD, INSIGHT SMART/ESPRIT/SILCAAT Study Group. Relevance of interleukin-6 and D-dimer for serious non-AIDS morbidity and death among HIV-positive adults on suppressive antiretroviral therapy. PLoS One 2016; 11(5):e0155100; PMID: 27171281; https://doi.org/10.1371/journal.pone.0155100
- Chiappetta S, Ripa M, Galli L, Razzari C, Longo V, Galli A, Faioni EM, Nozza S, Lazzarin A, Tambussi G. Soluble endothelial protein C receptor (sEPCR) as an inflammatory biomarker in naive HIV-infected patients during ART. J Antimicrob Chemother 2016; 71(6):1627-31; PMID: 26888911; https://doi.org/10.1093/jac/dkw010
- Rodríguez-Mora S, Mateos E, Moran M, Má Martín, López JA, Calvo E, Terrón MC, Luque D, Muriaux D, Alcamí J, et al. Intracellular expression of Tat alters mitochondrial functions in T cells: a potential mechanism to understand mitochondrial damage during HIV-1 replication. Retrovirology 2015; 12:78; PMID: 26376973; https://doi.org/10.1186/s12977-015-0203-3
- Strahl C, Blackburn EH. Effects of reverse transcriptase inhibitors on telomere length and telomerase activity in two immortalized human cell lines. Mol. Cell Biol 1996; 16:53-65; PMID: 8524329; https://doi.org/10.1128/MCB.16.1.53
- Caron M, Auclair M, Vigouroux C, Glorian M, Forest C, Capeau J. The HIV protease inhibitor indinavir impairs sterol regulatory element-binding protein-1 intranuclear localization, inhibits preadipocyte differentiation, and induces insulin resistance. Diabetes. 2001; 50(6):1378-88; PMID: 11375339; https://doi.org/10.2337/diabetes.50.6.1378
- Zha BS, Wan X, Zhang X, Zha W, Zhou J, Wabitsch M, Wang G, Lyall V, Hylemon PB, Zhou H. HIV protease inhibitors disrupt lipid metabolism by activating endoplasmic reticulum stress and inhibiting autophagy activity in adipocytes. PLoS One 2013; 8(3):e59514; PMID: 23533630; https://doi.org/10.1371/journal.pone.0059514
- Gallego-Escuredo JM, Del Mar Gutierrez M, Diaz-Delfin J, Domingo JC, Mateo MG, Domingo P, Giralt M, Villarroya F. Differential effects of efavirenz and lopinavir/ritonavir on human adipocyte differentiation, gene expression and release of adipokines and pro-inflammatory cytokines. Curr HIV Res 2010; 8(7):545-53; PMID: 21073442; https://doi.org/10.2174/157016210793499222
- Díaz-Delfín J, Domingo P, Giralt M, Villarroya F. Maraviroc reduces cytokine expression and secretion in human adipose cells without altering adipogenic differentiation. Cytokine 2013; 61(3):808-15; PMID: 23357304; https://doi.org/10.1016/j.cyto.2012.12.013
- Effros RB, Fletcher CV, Gebo K, Halter JB, Hazzard WR, Horne FM, Huebner RE, Janoff EN, Justice AC, Kuritzkes D, et al. Aging and infectious diseases: workshop on HIV infection and aging: what is known and future research directions. Clin. Infect. Dis 2008; 47:542-553; PMID: 18627268; https://doi.org/10.1086/590150
- Söderberg-Nauclér C, Fornara O, Rahbar A. Cytomegalovirus driven immunosenescence-An immune phenotype with or without clinical impact? Mech Ageing Dev 2016
- Effros RB. The silent war of CMV in aging and HIV infection. Mech Ageing Dev 2015 PMID: 26404009
- Bandaranayake T, Shaw A. Host resistance and immune aging. Clin Geriatr Med 2016 32 415-432; PMID: 27394014; https://doi.org/10.1016/j.cger.2016.02.007
- He S, Zhang Z, Fu Y, Qin C, Li S, Han X, Xu J, Liu J, Jiang Y, Shang H. Thymic function is most severely impaired in chronic HIV-1 infection, but individuals with faster disease progression during early HIV-1 infection expressed lower levels of RTEs. J Acquir Immune Defic Syndr 2015; 70(5):472-8; PMID: 26569175; https://doi.org/10.1097/QAI.0000000000000801
- Kohler S, Thiel A. Life after the thymus: CD31+ and CD31-human naïve CD4+ T-cell subsets. Blood 2009; 113:769-774; PMID: 18583570; https://doi.org/10.1182/blood-2008-02-139154
- den Braber I, Mugwagwa T, Vrisekoop N, Westera L, Mögling R, de Boer AB, Willems N, Schrijver EH, Spierenburg G, Gaiser K, et al. Maintenance of peripheral naive T cells is sustained by thymus output in mice but not humans. Immunity 2012; 36:288-297; PMID: 22365666; https://doi.org/10.1016/j.immuni.2012.02.006
- Sauce D, Larsen M, Fastenackels S, Duperrier A, Keller M, Grubeck-Loebenstein B, Ferrand C, Debré P, Sidi D, Appay V. Evidence of premature immune aging in patients thymectomized during early childhood. J Clin Invest 2009; 119:3070-8; PMID: 19770514; https://doi.org/10.1172/JCI39269
- Rickabaugh TM, Jamieson BD. A challenge for the future: aging and HIV infection. Immunol Res 2010; 48:59-71; PMID: 20734158; https://doi.org/10.1007/s12026-010-8167-9
- Kohler S, Wagner U, Pierer M, Kimmig S, Oppmann B, Möwes B, Jülke K, Romagnani C, Thiel A. Post-thymic in vivo proliferation of naive CD4+ T cells constrains the TCR repertoire in healthy human adults. Eur J Immunol 2005; 35:1987-1994; PMID: 15909312; https://doi.org/10.1002/eji.200526181
- Rickabaugh TM, Kilpatrick RD, Hultin LE, Hultin PM, Hausner MA, Sugar CA, Althoff KN, Margolick JB, Rinaldo CR, Detels R, et al. The dual impact of HIV-1 infection and aging on naïve CD4+ T-cells: additive and distinct patterns of impairment. Plos ONE 2011; 6(1):e16459; PMID: 21298072; https://doi.org/10.1371/journal.pone.0016459
- Britanova OV, Putintseva EV, Shugay M, Merzlyak EM, Turchaninova MA, Staroverov DB, Bolotin DA, Lukyanov S, Bogdanova EA, Mamedov IZ, et al. Age-related decrease in TCR repertoire diversity measured with deep and normalized sequence profiling. J Immunol 2014; 192(6):2689-98; PMID: 24510963; https://doi.org/10.4049/jimmunol.1302064
- Chou JP, Effros RB. T cell replicative senescence in human aging. Curr Pharm Des 2013; 19(9):1680-1698. PMID: 23061726
- Fulop T, Le Page A, Fortin C, Witkowski JM, Dupuis G, Larbi A. Cellular signaling in the aging immune system. Current Opinion in Immunology 2014; 29:105-111; PMID: 24934647; https://doi.org/10.1016/j.coi.2014.05.007
- Kovaiou RD, Weiskirchner I, Keller M, Pfister G, Cioca DP, Grubeck-Loebenstein B. Age-related differences in phenotype and function of CD4+ T cells are due to a phenotypic shift from naive to memory effector CD4+ T cells. Int Immunol 2005; 17(10):1359-66; PMID: 16141244; https://doi.org/10.1093/intimm/dxh314
- Jackola DR, Ruger JK, Miller RA. Age-associated changes in human T cell phenotype and function. Aging (Milano) 1994; 6:25-34. PMID: 8043623
- Bisset LR, Lung TL, Kaelin M, Ludwig E, Dubs RW. Reference values for peripheral blood lymphocyte phenotypes applicable to the healthy adult population in Switzerland. Eur J Haematol 2004; 72:203-212; PMID: 14962239; https://doi.org/10.1046/j.0902-4441.2003.00199.x
- Mandell Douglas, and Bennett's Principles and Practice of Infectious Diseases, 8th Edition
- Murray AJ, Kwon KJ, Farber DL, Siliciano RF. The latent reservoir for HIV-1: how immunologic memory and clonal expansion contribute to HIV-1 persistence. J Immunol 2016;197(2):407-17; PMID: 27382129; https://doi.org/10.4049/jimmunol.1600343
- Appay V, Dunbar PR, Callan M, Klenerman P, Gillespie GM, Papagno L, Ogg GS, King A, Lechner F, Spina CA, et al. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat Med 2002; 8:379-385; PMID: 11927944; https://doi.org/10.1038/nm0402-379
- Effros RB. Impact of the hayflick limit on T cell responses to infection: lessons from aging and HIV disease. Mech Ageing Dev 2004; 125:103-106; PMID: 15037010; https://doi.org/10.1016/j.mad.2003.11.003
- Sainz T, Serrano-Villar S, Díaz L, González Tomé MI, Gurbindo MD, de José MI, Mellado MJ, Ramos JT, Zamora J, Moreno S, et al. The CD4/CD8 ratio as a marker T-cell activation, senescence and activation/exhaustion in treated HIV-infected children and young adults. AIDS 2013; 27(9):1513-6; PMID: 23435292; https://doi.org/10.1097/QAD.0b013e32835faa72
- Sandler NG, Sereti I. Can early therapy reduce inflammation? Curr Opin HIV AIDS 2014; 9:72-79; PMID: 24247669; https://doi.org/10.1097/COH.0000000000000020
- Stacy S, Krolick KA, Infante AJ, Kraig E. Immunological memory and late onset autoimmunity. Mech Ageing Dev 2002; 123(8):975-85; PMID: 12044946; https://doi.org/10.1016/S0047-6374(02)00035-0
- Abudulai LN, Fernandez S, Corscadden K, Hunter M, Kirkham LA, Post JJ, French MA. Chronic HIV-1 infection induces B-Cell dysfunction that is incompletely resolved by long-term antiretroviral therapy. J Acquir Immune Defic Syndr 2016; 71(4):381-9; PMID: 26914910; https://doi.org/10.1097/QAI.0000000000000869
- Hu Z, Luo Z, Wan Z, Wu H, Li W, Zhang T, Jiang W. HIV-associated memory B cell perturbations. Vaccine 2015; 33(22):2524-2529; PMID: 25887082; https://doi.org/10.1016/j.vaccine.2015.04.008
- Pogliaghi M, Ripa M, Pensieroso S, Tolazzi M, Chiappetta S, Nozza S, Lazzarin A, Tambussi G, Scarlatti G. Beneficial effects of cART initiated during primary and chronic HIV-1 infection on immunoglobulin-expression of memory B-cell subsets. PLoS One 2015; 10(10):e0140435; PMID: 26474181; https://doi.org/10.1371/journal.pone.0140435
- Hearps AC, Maisa A, Cheng WJ, Angelovich TA, Lichtfuss GF, Palmer CS, Landay AL, Jaworowski A, Crowe SM. HIV infection induces age-related changes to monocytes and innate immune activation in young men that persist despite combination antiretroviral therapy. AIDS 2012; 26:843-853; PMID: 22313961; https://doi.org/10.1097/QAD.0b013e328351f756
- Hearps AC, Martin GE, Angelovich TA, Cheng WJ, Maisa A, Landay AL, Jaworowski A, Crowe SM. Aging is associated with chronic innate immune activation and dysregulation of monocyte phenotype and function. Aging Cell 2012; 11:867-875; PMID: 22708967; https://doi.org/10.1111/j.1474-9726.2012.00851.x
- Seidler S, Zimmermann HW, Bartneck M, Trautwein C, Tacke F. Age-dependent alterations of monocyte subsets and monocyte-related chemokine pathways in healthy adults. BMC Immunol 2010; 11:30; PMID: 20565954; https://doi.org/10.1186/1471-2172-11-30
- Nyugen J, Agrawal S, Gollapudi S, Gupta S. Impaired functions of peripheral blood monocyte subpopulations in aged humans. J Clin Immunol 2010; 30:806-813; PMID: 20703784; https://doi.org/10.1007/s10875-010-9448-8
- van Duin D, Mohanty S, Thomas V, Ginter S, Montgomery RR, Fikrig E, Allore HG, Medzhitov R, Shaw AC. Age-associated defect in human TLR-1/2 function. J Immunol 2007; 178:970-975; PMID: 17202359; https://doi.org/10.4049/jimmunol.178.2.970
- Chang JJ, Lacas A, Lindsay RJ, Doyle EH, Axten KL, Pereyra F, Rosenberg ES, Walker BD, Allen TM, Altfeld M. Differential regulation of toll-like receptor pathways in acute and chronic HIV-1 infection. AIDS 2012; 26:533-541; PMID: 22210629; https://doi.org/10.1097/QAD.0b013e32834f3167
- Heggelund L, Müller F, Lien E, Yndestad A, Ueland T, Kristiansen KI, Espevik T, Aukrust P, Frøland SS. Increased expression of toll-like receptor 2 on monocytes in HIV infection: possible roles in inflammation and viral replication. Clin Infect Dis 2004; 39:264-269; PMID: 15307037; https://doi.org/10.1086/421780
- Huang J, Yang Y, Al-Mozaini M, Burke PS, Beamon J, Carrington MF, Seiss K, Rychert J, Rosenberg ES, Lichterfeld M, et al. Dendritic cell dysfunction during primary HIV-1 infection. J Infect Dis 2011; 204(10):1557-62; PMID: 21969335; https://doi.org/10.1093/infdis/jir616
- Zapata HJ, Shaw AC. Aging of the human innate immune system in HIV infection. Curr Opin Immunol 2014; 0:127-136; https://doi.org/10.1016/j.coi.2014.06.007
- Pera A, Campos C, López N, Hassouneh F, Alonso C, Tarazona R, Solana R. Immunosenescence: implications for response to infection and vaccination in older people. Maturitas. 2015; 82(1):50-5 ; PMID: 26044074; https://doi.org/10.1016/j.maturitas.2015.05.004
- Scully E, Alter G. NK cells in HIV disease. Curr HIV/AIDS Rep. 2016; 13(2):85-94; PMID: 27002078; https://doi.org/10.1007/s11904-016-0310-3
- Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. Available at http://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf
- WHO. Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV 30 September 2015. Available at http://www.who.int/hiv/pub/guidelines/earlyrelease-arv/en/
- WHO. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. June 2016. Available at http://www.who.int/hiv/pub/arv/arv-2016/en/
- European AIDS Clinical Society. EACS Guidelines 8.0. Available at http://www.eacsociety.org/guidelines/eacs-guidelines/eacs-guidelines.html
- BHIVA guidelines for the treatment of HIV-1-positive adults with ART 2015 (2016 interim update). Available at http://www.bhiva.org/HIV-1-treatment-guidelines.aspx
- Edwards JK, Cole SR, Westreich D, Mugavero MJ, Eron JJ, Moore RD, Mathews WC, Hunt P, Williams C, Centers for AIDS Research Network of Integrated Clinical Systems investigators. Age at entry into care, timing of antiretroviral therapy initiation, and 10-year mortality among HIV-seropositive adults in the United States. Clin Infect Dis 2015; 61(7):1189-95; PMID: 26082505; https://doi.org/10.1093/cid/civ463
- Smit M, Brinkman K, Geerlings S, Smit C, Thyagarajan K, Sighem Av, de Wolf F, Hallett TB, ATHENA observational cohort. Future challenges for clinical care of an ageing population infected with HIV: a modelling study. Lancet Infect Dis 2015; 15(7):810-8; PMID: 26070969; https://doi.org/10.1016/S1473-3099(15)00056-0
- Allavena C, Bernaud C, Lariven S, Valantin M, Ferry T, Cuzin L, Naqvi A, Hanf M, Cabié A, for the DatAIDS study group. Ageing with HIV: emerging importance of chronic comorbidities in patients over 75. Conference on Retroviruses and Opportunistic Infections (CROI), February 22–25, 2016, Boston. Abstract 709
- Collaboration of Observational HIV Epidemiological Research Europe (COHERE) Study Group, Sabin CA, Smith CJ, d'Arminio Monforte A, Battegay M, Gabiano C, Galli L, Geelen S, Gibb D, Guiguet M, et al. Response to combination antiretroviral therapy: variation by age. AIDS 2008; 22(12):1463-73; PMID: 18614870; https://doi.org/10.1097/QAD.0b013e3282f88d02
- Ewings FM, Bhaskaran K, McLean K, Hawkins D, Fisher M, Fidler S, Gilson R, Nock D, Brettle R, Johnson M, et al. Survival following HIV infection of a cohort followed up from seroconversion in the UK. AIDS 2008; 22(1):89-95; PMID: 18090396; https://doi.org/10.1097/QAD.0b013e3282f3915e
- May M, Sterne JA, Sabin C, Costagliola D, Justice AC, Thiébaut R, Gill J, Phillips A, Reiss P, Hogg R, Ledergerber B, et al. Prognosis of HIV-1-infected patients up to 5 years after initiation of HAART: collaborative analysis of prospective studies. AIDS 2007; 21(9):1185-97; PMID: 17502729; https://doi.org/10.1097/QAD.0b013e328133f285
- Mussini C, Manzardo C, Johnson M, Ad Monforte, Uberti-Foppa C, Antinori A, Gill MJ, Sighinolfi L, Borghi V, Lazzarin A, et al. Patients presenting with AIDS in the HAART era: a collaborative cohort analysis. AIDS 2008; 22(18):2461-9; PMID: 19005269; https://doi.org/10.1097/QAD.0b013e328314b5f1
- Greenbaum AH, Wilson LE, Keruly JC, Moore RD, Gebo KA. Effect of age and HAART regimen on clinical response in an urban cohort of HIV-infected individuals. AIDS 2008; 22(17):2331-9; PMID: 18981772; https://doi.org/10.1097/QAD.0b013e32831883f9
- O'Brien D, Spelman T, Greig J, McMahon J, Ssonko C, Casas E, Mesic A, Du Cros P, Ford N. Risk factors for mortality during antiretroviral therapy in older populations in resource-limited settings. J Int AIDS Soc 2016; 19(1):20665; PMID: 26782169; https://doi.org/10.7448/IAS.19.1.20665
- Silverberg MJ, Leyden W, Horberg MA, DeLorenze GN, Klein D, Quesenberry CP Jr. Older age and the response to and tolerability of antiretroviral therapy. Arch Intern Med 2007; 167(7):684-91; PMID: 17420427; https://doi.org/10.1001/archinte.167.7.684
- Grabar S, Kousignian I, Sobel A, Le Bras P, Gasnault J, Enel P, Jung C, Mahamat A, Lang JM, Costagliola D. Immunologic and clinical responses to highly active antiretroviral therapy over 50 years of age. Results from the French Hospital Database on HIV. AIDS 2004; 18(15):2029-38
- Porter K, Walker S, Hill T, Anderson J, Leen C, Johnson M, Gazzard B, Walsh J, Fisher M, Orkin C, et al. Changes in outcome of persons initiating highly active antiretroviral therapy at a CD4 count less than 50 Cells/mm3. J Acquir Immune Defic Syndr 2008; 47(2):202-5; PMID: 17971709; https://doi.org/10.1097/QAI.0b013e31815b1291
- Mussini C, Manzardo C, Johnson M, Ad Monforte, Uberti-Foppa C, Antinori A, Gill MJ, Sighinolfi L, Borghi V, et al. Patients presenting with AIDS in the HAART era: a collaborative cohort analysis. AIDS 2008; 22(18):2461-9; PMID: 19005269; https://doi.org/10.1097/QAD.0b013e328314b5f1
- Lampe FC, Gatell JM, Staszewski S, Johnson MA, Pradier C, Gill MJ, de Lazzari E, Dauer B, Youle M, Fontas E, Krentz HB, Phillips AN. Changes over time in risk of initial virological failure of combination antiretroviral therapy: a multicohort analysis, 1996 to 2002. Arch Intern Med 2006; 166(5):521-8.
- Hall HI, Frazier EL, Rhodes P, Holtgrave DR, Furlow-Parmley C, Tang T, Gray KM, Cohen SM, Mermin J, Skarbinski J. Differences in human immunodeficiency virus care and treatment among subpopulations in the United States. JAMA Intern Med 2013; 173(14):1337-44; PMID: 23780395; https://doi.org/10.1001/jamainternmed.2013.6841
- Ghidei L, Simone MJ, Salow MJ, Zimmerman KM, Paquin AM, Skarf LM, Kostas TR, Rudolph JL. Aging, antiretrovirals, and adherence: a meta analysis of adherence among older HIV-infected individuals. Drugs Aging 2013; 30(10):809-19; PMID: 23959913; https://doi.org/10.1007/s40266-013-0107-7
- Hinkin CH, Hardy DJ, Mason KI, Castellon SA, Durvasula RS, Lam MN, Stefaniak M. Medication adherence in HIV-infected adults: effect of patient age, cognitive status, and substance abuse. AIDS 2004; 18 Suppl 1:S19-25; PMID: 15075494; https://doi.org/10.1097/00002030-200418001-00004
- Krentz HB, Gill MJ. The impact of non-antiretroviral polypharmacy on the continuity of antiretroviral therapy (ART) among HIV patients. AIDS Patient Care STDS 2016; 30(1):11-7; PMID: 26544766; https://doi.org/10.1089/apc.2015.0199
- Abara WE, Adekeye OA, Xu J, Heiman HJ, Rust G. Correlates of combination antiretroviral adherence among recently diagnosed older HIV-infected adults between 50 and 64 years. AIDS Behav 2016; 20(11):2674-81; PMID: 26885812
- Engsig FN, Zangerle R, Katsarou O, Dabis F, Reiss P, Gill J, Porter K, Sabin C, Riordan A, Fätkenheuer G, et al. Long-term mortality in HIV-positive individuals virally suppressed for >3 years with incomplete CD4 recovery. Clin Infect Dis 2014; 58(9):1312-21; PMID: 24457342; https://doi.org/10.1093/cid/ciu038
- Goetz MB, Boscardin WJ, Wiley D, Alkasspooles S. Decreased recovery of CD4 lymphocytes in older HIV-infected patients beginning highly active antiretroviral therapy. AIDS 2001; 15(12):1576-9; PMID: 11504992; https://doi.org/10.1097/00002030-200108170-00017
- Kaufmann GR, Furrer H, Ledergerber B, Perrin L, Opravil M, Vernazza P, Cavassini M, Bernasconi E, Rickenbach M, Hirschel B, et al. Characteristics, determinants, and clinical relevance of CD4 T cell recovery to <500 cells/microL in HIV type 1-infected individuals receiving potent antiretroviral therapy. Clin Infect Dis 2005; 41(3):361-72; PMID: 16007534; https://doi.org/10.1086/431484
- Florence E, Lundgren J, Dreezen C, Fisher M, Kirk O, Blaxhult A, Panos G, Katlama C, Vella S, Phillips A, EuroSIDA Study Group. Factors associated with a reduced CD4 lymphocyte count response to HAART despite full viral suppression in the EuroSIDA study. HIV Med 2003; 4(3):255-62; PMID: 12859325; https://doi.org/10.1046/j.1468-1293.2003.00156.x
- Baker JV, Peng G, Rapkin J, Krason D, Reilly C, Cavert WP, Abrams DI, MacArthur RD, Henry K, Neaton JD. Poor initial CD4+ recovery with antiretroviral therapy prolongs immune depletion and increases risk for AIDS and non-AIDS diseases. J Acquir Immune Defic Syndr 2008; 48(5):541-6; PMID: 18645520; https://doi.org/10.1097/QAI.0b013e31817bebb3
- Hunt PW, Deeks SG, Rodriguez B, Valdez H, Shade SB, Abrams DI, Kitahata MM, Krone M, Neilands TB, Brand RJ, et al. Continued CD4 cell count increases in HIV-infected adults experiencing 4 years of viral suppression on antiretroviral therapy. AIDS 2003; 17(13):1907-15; PMID: 12960823; https://doi.org/10.1097/00002030-200309050-00009
- Li X, Margolick JB, Jamieson BD, Rinaldo CR, Phair JP, Jacobson LP. CD4+ T-cell counts and plasma HIV-1 RNA levels beyond 5 years of highly active antiretroviral therapy. J Acquir Immune Defic Syndr 2011; 57(5):421-8; PMID: 21602699; https://doi.org/10.1097/QAI.0b013e31821e9f21
- Althoff KN, Justice AC, Gange SJ, Deeks SG, Saag MS, Silverberg MJ, Gill MJ, Lau B, Napravnik S, Tedaldi E, et al. Virologic and immunologic response to HAART, by age and regimen class. AIDS 2010; 24(16):2469-79; PMID: 20829678; https://doi.org/10.1097/QAD.0b013e32833e6d14
- Weber R, Sabin CA, Friis-Møller N, Reiss P, El-Sadr WM, Kirk O, Dabis F, Law MG, Pradier C, De Wit S, et al. Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Arch Intern Med 2006; 166(15):1632-41; PMID: 16908797; https://doi.org/10.1001/archinte.166.15.1632
- Study Group on Death Rates at High CD4 Count in Antiretroviral Naive Patients, Lodwick RK, Sabin CA, Porter K, Ledergerber B, van Sighem A, Cozzi-Lepri A, Khaykin P, Mocroft A, Jacobson L, et al. Death rates in HIV-positive antiretroviral-naive patients with CD4 count greater than 350 cells per microL in Europe and North America: a pooled cohort observational study. Lancet 2010; 376(9738):340-5; PMID: 20638118; https://doi.org/10.1016/S0140-6736(10)60932-4
- Phillips AN, Neaton J, Lundgren JD. The role of HIV in serious diseases other than AIDS. AIDS 2008; 22(18):2409-18. https://doi.org/10.1097/QAD.0b013e3283174636; PMID: 19005264; https://doi.org/10.1097/QAD.0b013e3283174636
- Goulet JL, Fultz SL, Rimland D, Butt A, Gibert C, Rodriguez-Barradas M, Bryant K, Justice AC. Aging and infectious diseases: do patterns of comorbidity vary by HIV status, age, and HIV severity? Clin Infect Dis 2007; 45(12):1593-601; PMID: 18190322; https://doi.org/10.1086/523577
- Monforte Ad, Abrams D, Pradier C, Weber R, Reiss P, Bonnet F, Kirk O, Law M, De Wit S, Friis-Møller N, Phillips AN, Sabin CA, Lundgren JD, Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) Study Group. HIV-induced immunodeficiency and mortality from AIDS-defining and non-AIDS-defining malignancies. AIDS 2008; 22(16):2143-53; PMID: 18832878; https://doi.org/10.1097/QAD.0b013e3283112b77
- Triant VA, Regan S, Lee H, Sax PE, Meigs JB, Grinspoon SK. Association of immunologic and virologic factors with myocardial infarction rates in a US healthcare system. J Acquir Immune Defic Syndr 2010; 55(5):615-9; PMID: 20827215; https://doi.org/10.1097/QAI.0b013e3181f4b752
- Lichtenstein KA, Armon C, Buchacz K, Chmiel JS, Buckner K, Tedaldi EM, Wood K, Holmberg SD, Brooks JT, HIV Outpatient Study (HOPS) Investigators. Low CD4+ T cell count is a risk factor for cardiovascular disease events in the HIV outpatient study. Clin Infect Dis 2010; 51(4):435-47; PMID: 20597691; https://doi.org/10.1086/655144
- Data Collection on Adverse Events of Anti-HIV drugs (D:A:D) Study Group, Smith C, Sabin CA, Lundgren JD, Thiebaut R, Weber R, Law M, Monforte Ad, Kirk O, Friis-Moller N, et al. Factors associated with specific causes of death amongst HIV-positive individuals in the D:A:D study. AIDS 2010; 24(10):1537-48. PMID: 20453631
- Castilho JL, Shepherd BE, Koethe J, Turner M, Bebawy S, Logan J, Rogers WB, Raffanti S, Sterling TR. CD4+/CD8+ ratio, age, and risk of serious noncommunicable diseases in HIV-infected adults on antiretroviral therapy. AIDS 2016; 30(6):899-908; PMID: 26959354; https://doi.org/10.1097/QAD.0000000000001005
- Shepherd L, Borges Á, Ledergerber B, Domingo P, Castagna A, Rockstroh J, Knysz B, Tomazic J, Karpov I, Kirk O, Lundgren J, Mocroft A, EuroSIDA in EuroCOORD. Infection-related and -unrelated malignancies, HIV and the aging population. HIV Med 2016; 17(8): 590-600; PMID: 26890156
- Opportunistic Infections Project Team of the Collaboration of Observational HIV Epidemiological Research in Europe (COHERE) in EuroCoord, Young J, Psichogiou M, Meyer L, Ayayi S, Grabar S, Raffi F, Reiss P, Gazzard B, Sharland M, et al. CD4 cell count and the risk of AIDS or death in HIV-Infected adults on combination antiretroviral therapy with a suppressed viral load: a longitudinal cohort study from COHERE. PLoS Med 2012; 9(3):e1001194; PMID: 22448150; https://doi.org/10.1371/journal.pmed.1001194
- Zoufaly A, Cozzi-Lepri A, Reekie J, Kirk O, Lundgren J, Reiss P, Jevtovic D, Machala L, Zangerle R, Mocroft A, et al. Immuno-virological discordance and the risk of non-AIDS and AIDS events in a large observational cohort of HIV-patients in Europe. PLoS One 2014; 9(1):e87160; PMID: 24498036; https://doi.org/10.1371/journal.pone.0087160
- Lapadula G, Chatenoud L, Gori A, Castelli F, Di Giambenedetto S, Fabbiani M, Maggiolo F, Focà E, Ladisa N, Sighinolfi L, et al. Risk of severe non AIDS events is increased among patients unable to increase their CD4+ T-cell counts >200+/μl despite effective HAART. PLoS One 2015; 10(5):e0124741; PMID: 26020949; https://doi.org/10.1371/journal.pone.0124741
- Kasahara TM, Hygino J, Andrade RM, Monteiro C, Sacramento PM, Andrade AF, Bento CA. Poor functional immune recovery in aged HIV-1-infected patients following successfully treatment with antiretroviral therapy. Hum Immunol 2015; 76(10):701-10; PMID: 26429325; https://doi.org/10.1016/j.humimm.2015.09.023
- Kalayjian RC, Spritzler J, Matining RM, Fiscus SA, Gross BH, Francis IR, Pollard RB, Lederman MM, Landay A. Older HIV-infected patients on antiretroviral therapy have B-cell expansion and attenuated CD4 cell increases with immune activation reduction. AIDS 2013; 27(10):1563-71; PMID: 24047762; https://doi.org/10.1097/QAD.0b013e32835fabc2
- Asher I, Guri KM, Elbirt D, Bezalel SR, Maldarelli F, Mor O, Grossman Z, Sthoeger ZM. Characteristics and outcome of patients diagnosed with HIV at older age. Medicine (Baltimore) 2016; 95(1):e2327; PMID: 26735534; https://doi.org/10.1097/MD.0000000000002327
- INSIGHT START Study Group, Lundgren JD, Babiker AG, Gordin F, Emery S, Grund B, Sharma S, Avihingsanon A, Cooper DA, Fätkenheuer G, et al. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med 2015; 373(9):795-807; PMID: 26192873; https://doi.org/10.1056/NEJMoa1506816
- May MT, Gompels M, Delpech V, Porter K, Orkin C, Kegg S, Hay P, Johnson M, Palfreeman A, Gilson R, et al. Impact on life expectancy of HIV-1 positive individuals of CD4+ cell count and viral load response to antiretroviral therapy. AIDS 2014; 28(8):1193-202; PMID: 24556869; https://doi.org/10.1097/QAD.0000000000000243
- Marcus JL, Chao CR, Leyden WA, Xu L, Quesenberry CP Jr, Klein DB, Towner WJ, Horberg MA, Silverberg MJ. Narrowing the gap in life expectancy between HIV-infected and HIV-uninfected individuals with access to care. J Acquir Immune Defic Syndr 2016; 73(1):39-46.
- Legarth RA, Ahlström MG, Kronborg G, Larsen CS, Pedersen C, Pedersen G, Mohey R, Gerstoft J, Obel N. Long-term mortality in HIV-infected individuals 50 years or older: a nationwide, population-based cohort study. J Acquir Immune Defic Syndr 2016; 71(2):213-8; PMID: 26334734; https://doi.org/10.1097/QAI.0000000000000825
- Siddiqi AE, Hall HI, Hu X, Song R. Population-Based Estimates of Life Expectancy After HIV Diagnosis. United States 2008 – 2011. J Acquir Immune Defic Syndr. 2016
- Patterson S, Cescon A, Samji H, Chan K, Zhang W, Raboud J, Burchell AN, Cooper C, Klein MB, Rourke SB, Loutfy MR, Machouf N, Montaner JS, Tsoukas C, Hogg RS, CANOC collaboration. Life expectancy of HIV-positive individuals on combination antiretroviral therapy in Canada. BMC Infect Dis 2015; 15:274; PMID: 26183704; https://doi.org/10.1186/s12879-015-0969-x
- Nsanzimana S, Remera E, Kanters S, Chan K, Forrest JI, Ford N, Condo J, Binagwaho A, Mills EJ. Life expectancy among HIV-positive patients in Rwanda: a retrospective observational cohort study. Lancet Glob Health 2015; 3(3):e169-77; PMID: 25701995; https://doi.org/10.1016/S2214-109X(14)70364-X
- Helleberg M, May MT, Ingle SM, Dabis F, Reiss P, Fätkenheuer G, Costagliola D, d'Arminio A, Cavassini M, Smith C, et al. Smoking and life expectancy among HIV-infected individuals on antiretroviral therapy in Europe and North America. AIDS 2015; 29(2):221-9; PMID: 25426809; https://doi.org/10.1097/QAD.0000000000000540
- Gimeno-Gracia M, Crusells-Canales MJ, Javier Armesto-Gómez F, Rabanaque-Hernández MJ. Prevalence of concomitant medications in older HIV+ patients and comparison with general population. HIV Clin Trials 2015; 16(3):117-24; PMID: 25978302; https://doi.org/10.1179/1528433614Z.0000000012
- Moore HN, Mao L, Oramasionwu CU. Factors associated with polypharmacy and the prescription of multiple medications among persons living with HIV (PLWH) compared to non-PLWH. AIDS Care 2015; 27(12):1443-8; PMID: 26608408; https://doi.org/10.1080/09540121.2015.1109583
- Patel R, Moore T, Cooper V, McArdle C, Perry N, Cheek E, Gainsborough N, Fisher M. An observational study of comorbidity and healthcare utilisation among HIV-positive patients aged 50 years and over. Int J STD AIDS; 27(8):628-37; 0956462415589524
- Marzolini C, Back D, Weber R, Furrer H, Cavassini M, Calmy A, Vernazza P, Bernasconi E, Khoo S, Battegay M, et al. Ageing with HIV: medication use and risk for potential drug-drug interactions. J Antimicrob Chemother 2011; 66(9):2107-11; PMID: 21680580; https://doi.org/10.1093/jac/dkr248
- Nou E, Lo J, Hadigan C, Grinspoon SK. Pathophysiology and management of cardiovascular disease in patients with HIV. Lancet Diabetes Endocrinol 2016; 4(7):598-610; pii: S2213-8587(15)00388-5. PMID: 26873066
- Schoen JC, Erlandson KM, Anderson PL. Clinical pharmacokinetics of antiretroviral drugs in older persons. Expert Opin Drug Metab Toxicol. 2013; 9(5):573-88; PMID: 23514375; https://doi.org/10.1517/17425255.2013.781153
- Harb GE, Alldredge BK, Coleman R, Jacobson MA. Pharmacoepidemiology of adverse drug reactions in hospitalized patients with human immunodeficiency virus disease. J Acquir Immune Defic Syndr 1993; 6:919-26 PMID: 8315576
- Mehta U, Durrheim DN, Blockman M, Kredo T, Gounden R, Barnes KI. Adverse drug reactions in adult medical inpatients in a South African hospital serving a community with a high HIV/AIDS prevalence: prospective observational study. Br J Clin Pharmacol 2008; 65(3):396-406; PMID: 18070223; https://doi.org/10.1111/j.1365-2125.2007.03034.x
- Masenyetse LJ, Manda SO, Mwambi HG. An assessment of adverse drug reactions among HIV positive patients receiving antiretroviral treatment in South Africa. AIDS Res Ther 2015; 12:6; PMID: 25745501; https://doi.org/10.1186/s12981-015-0044-0
- Sabin CA, Smith CJ, Delpech V, Anderson J, Bansi L, Gilson R, Schwenk A, Leen C, Gazzard B, Porter K, et al. The associations between age and the development of laboratory abnormalities and treatment discontinuation for reasons other than virological failure in the first year of highly active antiretroviral therapy. HIV Med 2009; 10(1):35-43; PMID: 19018876; https://doi.org/10.1111/j.1468-1293.2008.00654.x
- Lodwick RK, Smith CJ, Youle M, Lampe FC, Tyrer M, Bhagani S, Chaloner C, Sabin CA, Johnson MA, Phillips AN. Stability of antiretroviral regimens in patients with viral suppression. AIDS 2008; 22(9):1039-46; PMID: 18520347; https://doi.org/10.1097/QAD.0b013e3282fec415
- Prosperi MC, Fabbiani M, Fanti I, Zaccarelli M, Colafigli M, Mondi A, et al. Predictors of first-line antiretroviral therapy discontinuation due to drug- related adverse events in HIV-infected patients: a retrospective cohort study. BMC Infect Dis 2012; 12:296; PMID: 23145925; https://doi.org/10.1186/1471-2334-12-296
- Orlando G, Meraviglia P, Valsecchi L, Mainini A, Schiavini M, Merli S, et al. cART durability and causes for treatment switching or discontinuation in HIV-positive patients older than 50 years of age. J Acquir Immune Defic Syndr 2010; 55(2):e12-4; PMID: 20859084; https://doi.org/10.1097/QAI.0b013e3181ef791b
- Antela A, Aguiar C, Compston J, Hendry BM, Boffito M, Mallon P, Pourcher-Martinez V, Di Perri G. The role of tenofovir alafenamide in future HIV management. HIV Med 2016; 17 Suppl 2:4-16; PMID: 26952360; https://doi.org/10.1111/hiv.12401
- Margolis DA, Brinson CC, Smith GH, de Vente J, Hagins DP, Eron JJ, Griffith SK, St Clair MH, Stevens MC, Williams PE, Ford SL, Stancil BS, Bomar MM, Hudson KJ, Smith KY, Spreen WR, LAI116482 Study Team. Cabotegravir plus rilpivirine, once a day, after induction with cabotegravir plus nucleoside reverse transcriptase inhibitors in antiretroviral-naive adults with HIV-1 infection (LATTE): a randomised, phase 2b, dose-ranging trial. Lancet Infect Dis 2015; 15(10):1145-55; PMID: 26201299; https://doi.org/10.1016/S1473-3099(15)00152-8
- Figueroa MI, Sued O, Patterson Gun A, Rolón MJ, Cahn P. Dolutegravir-lamivudine as initial therapy in HIV-infected ARV-naive patients: first results of the PADDLE trial. Program and abstracts of the 15th European AIDS Conference; October 21–24, 2015; Barcelona, Spain. Abstract 1066
- Babafemi T. Dolutegravir antiretroviral strategy to promote improvement and reduce drug exposure (ASPIRE). 2014. Available at: https://clinicaltrials.gov/ct2/show/NCT02263326
- Klatzmann D, Abbas AK. The promise of low-dose interleukin-2 therapy for autoimmune and inflammatory diseases. Nat Rev Immunol 2015; 15(5):283-94; PMID: 25882245; https://doi.org/10.1038/nri3823
- Lane HC, Fauci AS. Immunologic abnormalities in the acquired immunodeficiency syndrome. Annu Rev Immunol 1985; 3:477-500; PMID: 2415141; https://doi.org/10.1146/annurev.iy.03.040185.002401
- INSIGHT-ESPRIT Study Group; SILCAAT Scientific Committee, Abrams D, Lévy Y, Losso MH, Babiker A, Collins G, Cooper DA, Darbyshire J, Emery S, et al. Interleukin-2 therapy in patients with HIV infection. N Engl J Med 2009; 361(16):1548-59; PMID: 19828532; https://doi.org/10.1056/NEJMoa0903175
- Kovacs JA, Vogel S, Metcalf JA, Baseler M, Stevens R, Adelsberger J, Lempicki R, Hengel RL, Sereti I, Lambert L, et al. Interleukin-2 induced immune effects in human immunodeficiency virus-infected patients receiving intermittent interleukin-2 immunotherapy. Eur J Immunol 2001; 31(5):1351-60; PMID: 11465092; https://doi.org/10.1002/1521-4141(200105)31:5%3c1351::AID-IMMU1351%3e3.0.CO;2-9
- Sereti I, Anthony KB, Martinez-Wilson H, Lempicki R, Adelsberger J, Metcalf JA, Hallahan CW, Follmann D, Davey RT, Kovacs JA, et al. IL-2-induced CD4+ T-cell expansion in HIV-infected patients is associated with long-term decreases in T-cell proliferation. Blood 2004; 104(3):775-80; PMID: 15090457; https://doi.org/10.1182/blood-2003-12-4355
- Sereti I, Imamichi H, Natarajan V, Imamichi T, Ramchandani MS, Badralmaa Y, Berg SC, Metcalf JA, Hahn BK, Shen JM, et al. In vivo expansion of CD4CD45RO-CD25 T cells expressing foxP3 in IL-2-treated HIV-infected patients. J Clin Invest 2005; 115(7):1839-47; PMID: 15937547; https://doi.org/10.1172/JCI24307
- Weiss L, Letimier FA, Carriere M, Maiella S, Donkova-Petrini V, Targat B, Benecke A, Rogge L, Levy Y. In vivo expansion of naive and activated CD4+CD25+FOXP3+ regulatory T cell populations in interleukin-2-treated HIV patients. Proc Natl Acad Sci U S A 2010; 107(23):10632-7; PMID: 20498045; https://doi.org/10.1073/pnas.1000027107
- MacPherson PA, Fex C, Sanchez-Dardon J, Hawley-Foss N, Angel JB. Interleukin-7 receptor expression on CD8(+) T cells is reduced in HIV infection and partially restored with effective antiretroviral therapy. J Acquir Immune Defic Syndr 2001; 28(5):454-7; PMID: 11744834; https://doi.org/10.1097/00042560-200112150-00008
- Paiardini M, Cervasi B, Albrecht H, Muthukumar A, Dunham R, Gordon S, Radziewicz H, Piedimonte G, Magnani M, Montroni M, et al. Loss of CD127 expression defines an expansion of effector CD8+ T cells in HIV-infected individuals. J Immunol 2005; 174(5):2900-9; PMID: 15728501; https://doi.org/10.4049/jimmunol.174.5.2900
- Mackall CL, Fry TJ, Gress RE. Harnessing the biology of IL-7 for therapeutic application. Nat Rev Immunol 2011; 11(5):330-42; PMID: 21508983; https://doi.org/10.1038/nri2970
- Fry TJ, Mackall CL. Interleukin-7: master regulator of peripheral T-cell homeostasis? Trends Immunol 2001; 22(10):564-71; PMID: 11574281; https://doi.org/10.1016/S1471-4906(01)02028-2
- Lévy Y, Sereti I, Tambussi G, Routy JP, Lelièvre JD, Delfraissy JF, Molina JM, Fischl M, Goujard C, Rodriguez B, et al. Effects of recombinant human interleukin 7 on T-cell recovery and thymic output in HIV-infected patients receiving antiretroviral therapy: results of a phase I/IIa randomized, placebo-controlled, multicenter study. Clin Infect Dis 2012; 55(2):291-300; PMID: 22550117; https://doi.org/10.1093/cid/cis383
- Thiébaut R, Jarne A, Routy JP, Sereti I, Fischl M, Ive P, Speck RF, D'Offizi G, Casari S, Commenges D, et al. Repeated cycles of recombinant human interleukin 7 in HIV-infected patients with low CD4 T-cell reconstitution on antiretroviral therapy: results of 2 phase II multicenter studies. Clin Infect Dis 2016; 62(9):1178-85; PMID: 26908786; https://doi.org/10.1093/cid/ciw065
- Katlama C, Lambert-Niclot S, Assoumou L, Papagno L, Lecardonnel F, Zoorob R, Tambussi G, Clotet B, Youle M, Achenbach CJ, et al. Treatment intensification followed by interleukin-7 reactivates HIV without reducing total HIV DNA: a randomized trial. AIDS 2016; 30(2):221-30; PMID: 26684819; https://doi.org/10.1097/QAD.0000000000000894
- Mueller YM, Bojczuk PM, Halstead ES, Kim AH, Witek J, Altman JD, Katsikis PD. IL-15 enhances survival and function of HIV-specific CD8+ T cells. Blood 2003; 101(3):1024-9; PMID: 12393488; https://doi.org/10.1182/blood-2002-07-1957
- Ramanathan MP, Kutzler MA, Kuo YC, Yan J, Liu H, Shah V, Bawa A, Selling B, Sardesai NY, Kim JJ, et al. Coimmunization with an optimized IL15 plasmid adjuvant enhances humoral immunity via stimulating B cells induced by genetically engineered DNA vaccines expressing consensus JEV and WNV E DIII. Vaccine 2009; 27(32):4370-80; PMID: 19497647; https://doi.org/10.1016/j.vaccine.2009.01.137
- Micci L, Cervasi B, Ende ZS, Iriele RI, Reyes-Aviles E, Vinton C, Else J, Silvestri G, Ansari AA, Villinger F, et al. Paucity of IL-21-producing CD4(+) T cells is associated with Th17 cell depletion in SIV infection of rhesus macaques. Blood 2012; 120(19):3925-35; PMID: 22990011; https://doi.org/10.1182/blood-2012-04-420240
- Pallikkuth S, Rogers K, Villinger F, Dosterii M, Vaccari M, Franchini G, Pahwa R, Pahwa S. Interleukin-21 administration to rhesus macaques chronically infected with simian immunodeficiency virus increases cytotoxic effector molecules in T cells and NK cells and enhances B cell function without increasing immune activation or viral replication. Vaccine 2011; 29(49):9229-38; PMID: 21996099; https://doi.org/10.1016/j.vaccine.2011.09.118
- Micci L, Ryan ES, Fromentin R, Bosinger SE, Harper JL, He T, Paganini S, Easley KA, Chahroudi A, Benne C, et al. Interleukin-21 combined with ART reduces inflammation and viral reservoir in SIV-infected macaques. J Clin Invest 2015; 125(12):4497-513; PMID: 26551680; https://doi.org/10.1172/JCI81400
- Hong Z, Jiang Z, Liangxi W, Guofu D, Ping L, Yongling L, Wendong P, Minghai W. Chloroquine protects mice from challenge with CpG ODN and LPS by decreasing proinflammatory cytokine release. Int Immunopharmacol 2004; 4(2):223-34; PMID: 14996414; https://doi.org/10.1016/j.intimp.2003.12.006
- Wozniacka A, Lesiak A, Narbutt J, McCauliffe DP, Sysa-Jedrzejowska A. Chloroquine treatment influences proinflammatory cytokine levels in systemic lupus erythematosus patients. Lupus 2006; 15(5):268-75; PMID: 16761500; https://doi.org/10.1191/0961203306lu2299oa
- Sperber K, Louie M, Kraus T, Proner J, Sapira E, Lin S, Stecher V, Mayer L. Hydroxychloroquine treatment of patients with human immunodeficiency virus type 1. Clin Ther 1995; 17(4):622-36; PMID: 8565026; https://doi.org/10.1016/0149-2918(95)80039-5
- Sperber K, Chiang G, Chen H, Ross W, Chusid E, Gonchar M, Chow R, Liriano O. Comparison of hydroxychloroquine with zidovudine in asymptomatic patientsi nfected with human immunodeficiency virus type 1. Clin Ther 1997; 19(5):913-23; PMID: 9385480; https://doi.org/10.1016/S0149-2918(97)80045-8
- Murray SM, Down CM, Boulware DR, Stauffer WM, Cavert WP, Schacker TW, Brenchley JM, Douek DC. Reduction of immune activation with chloroquine therapy during chronic HIV infection. J Virol 2010; 84(22):12082-6; PMID: 20844049; https://doi.org/10.1128/JVI.01466-10
- Piconi S, Parisotto S, Rizzardini G, Passerini S, Terzi R, Argenteri B, Meraviglia P, Capetti A, Biasin M, Trabattoni D, et al. Hydroxychloroquine drastically reduces immune activation in HIV-infected, antiretroviral therapy-treated immunologic nonresponders. Blood 2011; 118(12):3263-72; PMID: 21576701; https://doi.org/10.1182/blood-2011-01-329060
- Paton NI, Goodall RL, Dunn DT, Franzen S, Collaco-Moraes Y, Gazzard BG, Williams IG, Fisher MJ, Winston A, Fox J, et al. Effects of hydroxychloroquine on immune activation and disease progression among HIV-infected patients not receiving antiretroviral therapy: a randomized controlled trial. JAMA 2012; 308(4):353-61; PMID: 22820788; https://doi.org/10.1001/jama.2012.6936
- Routy JP, Angel JB, Patel M, Kanagaratham C, Radzioch D, Kema I, Gilmore N, Ancuta P, Singer J, Jenabian MA. Assessment of chloroquine as a modulator of immune activation to improve CD4 recovery in immune nonresponding HIV-infected patients receiving antiretroviral therapy. HIV Med 2015; 16(1):48-56; PMID: 24889179; https://doi.org/10.1111/hiv.12171
- Jacobson JM, Bosinger SE, Kang M, Belaunzaran-Zamudio P, Matining RM, Wilson CC, Flexner C, Clagett B, Plants J, Read S, et al. The effect of chloroquine on immune activation and interferon signatures associated with HIV-1. AIDS Res Hum Retroviruses 2016; 32(7):636-47; PMID: 26935044; https://doi.org/10.1089/aid.2015.0336
- Tabb B, Morcock DR, Trubey CM, Quiñones OA, Hao XP, Smedley J, Macallister R, Piatak M Jr, Harris LD, Paiardini M, et al. Reduced inflammation and lymphoid tissue immunopathology in rhesus macaques receiving anti-tumor necrosis factor treatment during primary simian immunodeficiency virus infection. J Infect Dis 2013; 207(6):880-92; PMID: 23087435; https://doi.org/10.1093/infdis/jis643
- Hsu DC, Faldetta KF, Pei L, Sheikh V, Utay NS, Roby G, Rupert A, Fauci AS, Sereti I. A paradoxical treatment for a paradoxical condition: infliximab use in three cases of mycobacterial IRIS. Clin Infect Dis 2016; 62(2):258-61; PMID: 26394669; https://doi.org/10.1093/cid/civ841
- Rizzardi GP, Harari A, Capiluppi B, Tambussi G, Ellefsen K, Ciuffreda D, Champagne P, Bart PA, Chave JP, Lazzarin A, et al. Treatment of primary HIV-1 infection with cyclosporin a coupled with highly active antiretroviral therapy. J Clin Invest 2002; 109(5):681-8; PMID: 11877476; https://doi.org/10.1172/JCI0214522
- Lederman MM, Smeaton L, Smith KY, Rodriguez B, Pu M, Wang H, Sevin A, Tebas P, Sieg SF, Medvik K, et al. Cyclosporin A provides no sustained immunologic benefit to persons with chronic HIV-1 infection starting suppressive antiretroviral therapy: results of a randomized, controlled trial of the AIDS clinical trials group A5138. J Infect Dis 2006; 194(12):1677-85; PMID: 17109339; https://doi.org/10.1086/509261
- Markowitz M, Vaida F, Hare CB, Boden D, Mohri H, Hecht FM, Kalayjian RC, Conrad A, Mildvan D, Aberg J, et al. The virologic and immunologic effects of cyclosporine as an adjunct to antiretroviral therapy in patients treated during acute and early HIV-1 infection. J Infect Dis 2010; 201(9):1298-302; PMID: 20235838; https://doi.org/10.1086/651664
- Coull JJ, Turner D, Melby T, Betts MR, Lanier R, Margolis DM. A pilot study of the use of mycophenolate mofetil as a component of therapy for multidrug-resistant HIV-1 infection. J Acquir Immune Defic Syndr 2001; 26(5):423-34; PMID: 11391161; https://doi.org/10.1097/00126334-200104150-00004
- Di Benedetto F, Di Sandro S, De Ruvo N, Montalti R, Ballarin R, Guerrini GP, Spaggiari M, Guaraldi G, Gerunda G. First report on a series of HIV patients undergoing rapamycin monotherapy after liver transplantation. Transplantation 2010; 89(6):733-8; PMID: 20048692; https://doi.org/10.1097/TP.0b013e3181c7dcc0
- Trautmann L, Janbazian L, Chomont N, Said EA, Gimmig S, Bessette B, Boulassel MR, Delwart E, Sepulveda H, Balderas RS, et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med 2006; 12(10):1198-202; PMID: 16917489; https://doi.org/10.1038/nm1482
- Hoffmann M, Pantazis N, Martin GE, Hickling S, Hurst J, Meyerowitz J, Willberg CB, Robinson N, Brown H, Fisher M, et al. Exhaustion of activated CD8 T cells predicts disease progression in primary HIV-1 infection. PLoS Pathog 2016; 12(7):e1005661; PMID: 27415828; https://doi.org/10.1371/journal.ppat.1005661
- Velu V, Shetty RD, Larsson M, Shankar EM. Role of PD-1 co-inhibitory pathway in HIV infection and potential therapeutic options. Retrovirology 2015; 12:14; PMID: 25756928; https://doi.org/10.1186/s12977-015-0144-x
