ABSTRACT
Maternal antibodies induced by vaccination during pregnancy cross the placental barrier and can close the susceptibility gap to pertussis in young infants up to the start of primary immunization. As not only the quantity but also the quality of circulating antibodies is important for protection, we assessed whether maternal immunization affects the avidity of infant vaccine-induced IgG antibodies, in the frame of a prospective clinical trial on pregnancy vaccination in Belgium. Infants born from Tdap (Boostrix®) vaccinated (N = 55) and unvaccinated (N = 26) mothers were immunized with a hexavalent pertussis containing vaccine (Infanrix Hexa®) at 8, 12 and 16 weeks, followed by a fourth dose at 15 months of age. Right before and one month after this fourth vaccine dose, the avidity of IgG antibodies against diphtheria toxin (DT), tetanus toxin (TT), pertussis toxin (PT), filamentous hemagglutinin (FHA) and pertactin (Prn) was determined using 1.5 M ammonium thiocyanate as dissociating agent.
In both groups, antibody avidity was moderate for TT, PT, FHA and Prn and low for DT after priming. After a fourth dose, antibody avidity increased significantly to high avidity for TT and PT, whereas it remained moderate for FHA and Prn and low for DT. The avidity correlated positively with antibody level in both study groups, yet not significantly for PT. When comparing both study groups, only PT-specific antibodies showed significantly lower avidity in infants born from vaccinated than from unvaccinated mothers after the fourth vaccine dose. The clinical significance of lower avidity of vaccine induced infant antibodies after maternal vaccination, if any, needs further investigation.
Introduction
Pertussis, caused by Bordetella pertussis, is a highly contagious respiratory disease, which can be mortal for newborns and young infants.Citation1,2 In Belgium, the hexavalent vaccine containing diphtheria, tetanus and acellular pertussis is used for primary immunization at 8, 12 and 16 weeks of age with a fourth dose at 15 months of life. To protect these highly susceptible infants from pertussis disease during the first months of life, the National Immunization Technical Advisory Group in Belgium has recommended a trivalent tetanus toxoid, reduced diphtheria toxoid and acellular pertussis (Tdap) vaccine for every pregnant woman between 24 and 32 weeks of gestation since July 2013.Citation3
We previously reported that Tdap vaccination during pregnancy induces high titers of maternal antibodies, passing the placental barrier and closing the susceptibility gap to pertussis, diphtheria and tetanus in young infants up to the start of the primary immunization.Citation4 However, blunting was noticed post-primary vaccination for DT and PT specific responses in infants from vaccinated women.Citation4 At month 15, before the fourth vaccine dose, we observed a steep decay in antibody titers. Antibody levels rose again significantly for all 5 antigens one month after the fourth dose, albeit with lower PT-specific IgG in infants from the maternal vaccine group than in those from the control group.Citation5 The clinical relevance of this blunting effect is not clear, since there are unfortunately no quantifiable correlates of protection for pertussis,Citation6 although antibodies to PT, Prn and fimbriae are thought to be of importance.Citation1
However, other factors such as antibody isotype, affinity, avidity, immunoglobulin gene sequence, and biologic activity (i.e. bactericidal or neutralizing activity) are also important for antibody mediated protection, as extensively documented for Haemophilus influenzae Type b antibodies induced by DTaP/Hib combination vaccines.Citation7,8 Avidity or functional affinity is an important parameter that determines the strength of the antigen-antibody binding.Citation9 Following a T-cell dependent response, somatic hypermutation combined to the selection of high affinity B-cells clones in the germinal center result in the maturation of avidity.Citation10-12 Therefore, measuring antibody avidity can be used to assess a good priming of immunological memory.Citation13 The immune system of neonates is different from that of adults, including weak germinal center B cell responsesCitation14 and rare plasma cells.Citation15 This is illustrated by lower antibody titers induced by vaccination in infants than those observed later in life.Citation10,16,17 While somatic hypermutation is already functional at birth,Citation18 the selection of B cell producing antibody with high avidity is acquired gradually with age.Citation10,12,15,19
Many studies have reported on antibody avidity for pertussis antigensCitation20-24 and tetanus toxoid,Citation10,25 before and after infant immunization, and showed that avidity rises with the number of vaccine doses,Citation20,21,23 and with increasing age.Citation24 In the context of pregnancy vaccination, Abu Raya et al. previously reported a higher antibody avidity to PT in cord samples of newborns of Tdap immunized women during pregnancy, than in newborns of unimmunized women (with very low anti-PT IgG titers).Citation22 In addition, they found significantly higher anti-PT avidity in newborns when maternal vaccination was given at 27–30 weeks rather than beyond 30 weeks of gestation.Citation22 The latter results were not in accordance with our own findingsCitation26 as we found no correlation between avidity and gestational age at vaccination, neither in maternal nor in cord samples.
As far as we know, this is the first report analyzing the impact of maternal immunization on avidity of infant antibodies in response to a booster hexavalent acellular pertussis containing vaccine. We have used a convenience number of samples from our previously published studies.Citation4,5 to assess the avidity of vaccine-induced IgG antibodies to diphtheria toxin (DT), tetanus toxin (TT), pertussis toxin (PT), filamentous hemagglutinin (FHA) and pertactin (Prn) before and one month after the fourth vaccine dose at 15 months of age, in infants born to women immunized with a Tdap vaccine during pregnancy or to unimmunized women.
Materials and Methods
Study population and serum samples
The present study is a spin-off of our previously reported clinical trial in Belgium on Tdap vaccination during pregnancy (Clinicaltrials.gov identifier: NCT01698346) [4,5]. Healthy pregnant women were either vaccinated with the Tdap vaccine (Boostrix®, GSK Biologicals, Rixensart, Belgium) between 22 and 33 (mean gestational age 28.6) weeks of gestation (vaccine group) or received no vaccine (control group) and were followed until vaginal delivery (80% of women). Their infants, born between April 2012 and April 2014, were vaccinated with the hexavalent vaccine (Infanrix hexa®, GSK Biologicals, Rixensart, Belgium) according to the national 8, 12, 16 weeks priming schedule and the fourth dose at 15 months. The original study included 55 infants in the vaccine group and 26 infants in the control group. The remaining available serum samples (46 and 24 at pre-dose 4; 46 and 23 at post-dose 4 in the vaccinated and control group respectively) collected in infants before and one month after the fourth vaccine dose were used to answer the present study question. Extended clinical information can be found in our previous publicationsCitation4,5 No significant differences in demographic characteristics between both study groups were encountered.
Avidity assay
Antibody avidity was measured with the same commercial IgG ELISAs as in our previous studies.Citation4,5 i.e., (Virion/Serion® for anti-PT, Euroimmun® for anti-FHA and anti-Prn, Virotech/Sekisui® for anti-DT and anti-TT) using 1.5 M ammonium thiocyanate (NH4SCN, Sigma-Aldrich 221988, St Louis, MO) as dissociating agent according to a well-established methodCitation20 Briefly, the ELISA was performed in duplicate wells for each antigen, using the same diluted serum sample either left untreated or treated with NH4SCN. For each antigen, 100µl of serum sample dilution (1/101), standards and control sera were incubated in a pre-coated well according to manufacturers' instructions. After incubation and subsequent washing, 100µL of a 1.5 M NH4SCN solution or PBS was added to the appropriate wells. The plate was incubated for 20 min at 37°C, before washing and adding the conjugate solution, followed by the substrate and the stop solution.
IgG concentrations with or without NH4SCN treatment were expressed in international unit IU/mL for all antibodies. The relative avidity index (RAI) was expressed as the percentage between the IgG concentration of the sample treated with NH4SCN and the untreated sample. Using the same criteria as Almanzar et al, a RAI < 40% was considered as low, a RAI between 40% and 60% considered as moderate and a RAI > 60% as high.Citation20
The analytical performance of the avidity test was optimized using different concentrations of NH4SCN ranging from 0 M to 3 M. Five serum samples (diluted 1:101) collected at delivery from women immunized with Tdap were treated for 20 min with NH4SCN at 37°C, the concentration reducing the anti-PT IgG level by 50% was determined to be 1.5 M and this concentration was subsequently used for all avidity analyses. Hendrikx et al previously used NH4SCN concentrations in the same range to test the avidity of anti-PT (1M) and anti-Prn (1.5M) antibodies in Dutch children.Citation27
Laboratory personnel was not blinded to the sample allocation but an external validation for the ELISA was performed by the Canadian Center for Vaccinology in Halifax, Canada and a positive correlation (p < 0.001) between both laboratories was found.Citation4
Statistical analyses
Geometric means RAI were calculated with their 95% confidence intervals. Data distribution was assessed by One-Sample Kolmogorov-Smirnov test for normality. The differences between the time points (maternal, cord, pre- and post-fourth vaccine dose) were tested by paired t-test or alternatively by Wilcoxon matched-pair signed-rank test. The 2 infants' groups were compared by parametric unpaired t-test or its nonparametric alternative Mann-Whitney U test. Pearson correlations were used to analyze the relationship between antibody titers and RAI. All tests were 2-sided. A statistically significant difference was considered with a p-value < 0.05 and GraphPad Prism version 6 was used to analyze data.
As described before,Citation5 multiple linear regression models were used to identify mother and child variables that could potentially influence the RAI at the different time points. Only significant results were reported.
Results
Antibody avidity in infants in pre- and post-fourth vaccine dose
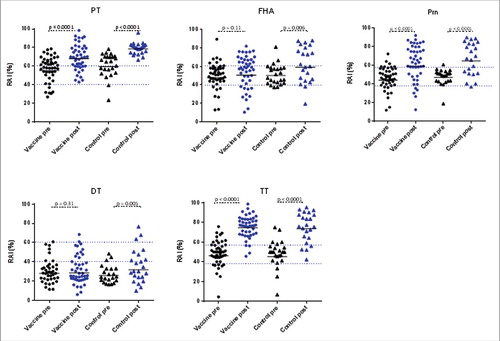
To find out whether the fourth vaccine dose affected antibody avidity, we tested this parameter in an adapted ELISA with 1.5 M NH4SCN as dissociating agent. Prior to the fourth vaccine dose, mean antibody avidity in both groups was moderate for PT, FHA, Prn and TT (RAI between 40% and 60%) and low for DT (less than 40%), with no significant difference between the 2 groups ( and ). At post-dose 4, antibody avidity increased significantly for all antibodies in the control group (), albeit the increase in RAI of DT-specific antibodies was very modest (25.8% pre-dose 4 cfr. 31.8% post-dose 4). In the vaccine group, avidity of antibodies increased significantly for TT, PT and Prn (p < 0.001) but not for DT (p = 0.31) and FHA (p = 0.11) (). After the fourth dose, antibody avidity to TT, PT, FHA and Prn was comparable in both groups, but the avidity to PT was significantly lower in the vaccine than in the control group (p < 0.003) (). Overall antibody avidity was high against TT, PT and Prn, moderate against FHA and low against DT ( and ).
Table 1. Geometric Mean (GM) of Relative Avidity Index (RA%I) with 95% confidence interval (CI) expressed in percentage (%) for IgG antibodies against PT, FHA, Prn, DT and TT and p-values comparing the 2 study groups of infants either before or one month after the fourth vaccine dose. Differences between both groups were not statistically different except for RAI of anti-PT antibodies after the fourth vaccine dose (p = 0.003).
Figure 1. Distribution of Relative Avidity Index (RAI %) in infant samples before (pre, black symbols) and 1 month after (post, blue symbols) the fourth vaccine dose. Vaccine group = infants born from mothers immunized with Tdap vaccine during pregnancy. Control group = infants born from non-immunized mothers during pregnancy. Black lines indicate the geometric mean of RAI and blue dotted lines represent the avidity classification areas (RAI < 40% was considered as low, a RAI between 40% and 60% considered as moderate and a RAI > 60% as high). P-values show the statistical significance comparing the pre- and the post-dose 4 responses for all 5 antigens in both groups.
Parameters influencing antibody avidity
There was no influence of gestational age on the antibody avidity of infant antibodies measured right before or one month after the fourth vaccine dose. However, a detailed regression analysis of antibody avidity and several other parameters showed for the infants of the vaccine group, a significant decrease in RAI of anti-TT antibodies at pre-booster (month 15) with increasing interval between dose 3 (at 16 weeks of age) and pre-dose 4 blood sampling (p = 0.025). In the vaccine group, avidity of infant anti-Prn antibodies at post-dose 4 (month 16) was influenced significantly by the age of the mother at the time of delivery (p = 0.011), avidity increasing with increasing age of the mother.
In the infants of the control group (but not in the infants of the vaccine group), avidity of anti-DT antibodies at month 15 (pre-booster) increased significantly (p = 0.009) with increasing interval between vaccine dose 3 and the month 15 blood sampling. In the infants of the control group, age of the mother at the moment of the delivery also influenced significantly the antibody avidity at month 16 against Prn (p = 0.032) and FHA (p = 0.029), avidity increasing with increasing age of the mother. Finally, a significant influence of the interval between vaccine dose 4 and the post-dose 4 blood sampling (p = 0.019) was found, avidity against TT increasing with increased time interval.
Correlation between IgG titer and avidity
We have reported before on vaccine-specific IgG antibody titers in this cohort, in the form of geometric mean concentrations.Citation5 Comparing the individual antibody (Fig. S1) and RAI results, Pearson correlation coefficients were calculated. Before the fourth vaccine dose, negative correlation coefficients were found between RAI and antibody levels in the vaccine group for FHA (−0.165, NS), Prn (−0.424, p = 0.003) and TT (−0.332, p = 0.024) and positive coefficients for PT (0.497, NS) and DT (0.228, NS) respectively (). In the control group, a negative correlation was found for TT (−0.125, not significant NS) and a positive for DT (0.930 NS), PT (0.343, NS), FHA (0.412, p = 0.045) and Prn (0.433, p = 0.034). At post-dose4, RAI correlated positively with antibody level in both study groups for all 5 antigens (). In the vaccine group, the following Pearson correlation coefficients were found: PT (0.157, NS), Prn (0.496, p = 0.001), FHA (0.738, p < 0.001), DT (0.581, p < 0.001) and TT (0.395, p = 0.007). In the control group, the positive correlation coefficients were for PT (0.287, NS), Prn (0.864, p < 0.001), FHA (0.850, p < 0.001), DT (0.447, p = 0.032) and TT (0.809, p < 0.001).
Table 2. Correlations between IgG titer (IU/ml) and Relative Avidity Index (% RAI) before (pre-dose 4) and 1 month after (post-dose 4) the fourth vaccine dose. Vaccine group = infants born from mothers immunized with Tdap vaccine during pregnancy. Control group = infants born from non-immunized mothers during pregnancy. R2: Pearson correlation coefficient.
Evolution of avidity and antibody titer to PT from delivery up to 1 month after the fourth vaccine dose in the vaccine group
In a pilot study, we previously analyzed in the vaccine group, the PT specific antibody titer and avidity in serum from women at delivery and in the umbilical cord.Citation26 shows these results (obtained using the same ELISA and avidity testing protocol) together with the avidity and IgG levels in infants before and after the fourth vaccine dose. At delivery, avidity was moderate in women (serum) and newborns (cord blood) (geometric mean of RAI of 51.9% and 46.9% respectively) and was significantly higher in women than in their infants (paired t-test, p < 0.0001), while geometric mean concentration was higher in infants' cord blood (101 IU/mL) than in mothers' serum at delivery (31.4 IU/mL). The reason why avidity of anti-PT antibodies was higher in mothers than in infants at time of birth is not clear, but may be related to the fact that antibody avidity of cord blood results from continuous transplacental transfer during the second and third trimester of pregnancy, whereas avidity in serum of mothers at delivery measures only the end point of a progressive maturation after the maternal booster vaccination.
Figure 2. Geometric mean concentration of IgG (IU/mL) and geometric mean of relative avidity index (%) of PT-specific antibodies, at delivery in women immunized with Tdap vaccine during pregnancy (Maternal) and in their infants at birth (Cord), before the fourth vaccine dose (Vaccine pre) and one month after the fourth vaccine dose (Vaccine post) at 15 months of age.
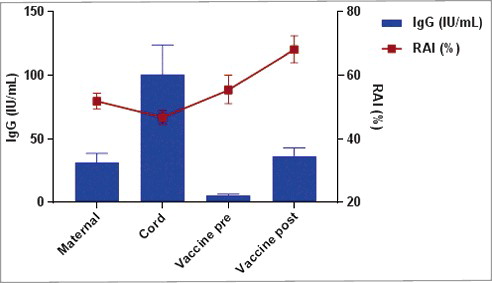
In infants, anti-PT antibody avidity was moderate (55.4%) before the fourth vaccine dose (and significantly higher (p < 0.0001) than in cord) but not significantly different from maternal antibody avidity at delivery (p = 0.1379), and increased to RAI of 68% after the fourth vaccine dose. Avidity of infant anti-PT antibodies post fourth vaccine dose was significantly higher than avidity of maternal (at delivery) and cord anti-PT antibodies (p < 0.0001). In infants, anti-PT IgG level at month 15 was low (5.4 IU/mL) and one month later mean anti-PT IgG level had increased to 36.3 IU/mL. This infant anti-PT level at month 16 was not statistically different from maternal (at delivery) anti-PT IgG level (p = 0.9315).
Discussion
To evaluate the efficacy of a vaccine, it is important to consider antibody levels as well as antibody avidity.Citation28 In this study, we used an elution ELISA modified by a chaotropic agent to assess the avidity of specific antibodies to PT, FHA, Prn, DT and TT in infants born to vaccinated and unvaccinated mothers. The choice of the chaotropic agent is a critical issue when evaluating antibody avidity.Citation23 A number of reports have measured avidity with different chaotropic agents,Citation20-23,29 and NH4SCN proved to be easy to use in a modified ELISA assay and convenient to measure the strength of antigen-antibody complex.Citation20 In our study we used a 1.5 M concentration of NH4SCN to determine the relative avidity index RAI for all 5 vaccine antigens and we used criteria defined by Almanzar et al. for interpretation.Citation20 Although these cut-off values defining low (RAI < 40%) and high (RAI > 60%) avidity are arbitrary to some extent, as the results depend on the assay conditions,Citation20 within a defined setting these criteria enable a monitoring of the avidity of the same antibody specificity over time. Also, use of 1.5 M NH4SCN allowed us to discriminate between the different vaccine antigens, and in particular diphtheria-specific antibodies were found to remain of low avidity in both infant groups, despite the fact that antibody concentrations increased dramatically after the fourth vaccine dose. Interestingly, avidity of anti-DT antibodies increased significantly (p = 0.009) with increasing interval between vaccine dose 3 and the pre-dose 4 blood sampling in the control group, indicating that in this infant population, age is an important factor for antibody maturation (independent of vaccine dose).
In this spin-off study of a previously reported clinical trial in Belgium on Tdap vaccination during pregnancy, we assessed antibody avidity in infants born from Tdap immunized women during pregnancy (vaccine group) before and 1 month after a fourth hexavalent vaccine dose as compared with infants from unimmunized women during pregnancy (control group). In a previous pilot study limited to pertussis toxin only, we found that antibodies in cord blood of infants from the vaccine group had a moderate avidity i.e., 46.9%,Citation26 whereas Abu Raya et al. reported a higher avidity (RAI = 73.8%) in cord blood samples after maternal vaccination.Citation22,30 The higher RAI values of the Israeli study may have been the result of differences between the maternal cohorts in terms of priming and natural exposure to Bordetella pertussis, or in the time of vaccination during pregnancy.Citation31,32 More likely, differences in laboratory technique used to determine the relative avidity may explain the discrepancy. Indeed, in contrast to Abu Raya et al. who used 0.25 M of NH4SCN,Citation22 we used a 6-fold higher concentration of 1.5 M NH4SCN, concentration also used in previous studies by Almanzar et al. and Prelog et al..Citation20,21 As the avidity index is indeed a relative value, depending on the NH4SCN concentration used, RAI values will vary in function of the precise assay conditions and can only be compared with other values generated under the same conditions.
All currently used infant subunit vaccines delivered before 6 months of age need a series of vaccine doses to elicit protection.Citation33 In the present study, a significant increase in antibody avidity was found in the control group for all 5 vaccine-induced antibodies after the fourth vaccine dose, albeit that the increase in avidity of DT specific antibodies was very modest and resulting avidity remained low (RAI 31.8%). Moreover, the expected increase of RAI after re-exposure of the same antigen to a primed immune systemCitation20 was not observed for FHA and DT in the vaccine group, with avidity remaining moderate for FHA (50.5%) and low (28.6%) for DT specific antibodies. Furthermore, although avidity of anti-PT antibodies increased to high after the fourth vaccine dose in both study groups, it was significantly lower (p = 0.003) in infants from the maternal vaccine group compared with those from the control group. The clinical relevance of this blunting effect is not clear since there are unfortunately no protective antibody levels known for pertussis, i.e., no threshold antibody (nor avidity) levels.Citation6
Blunting of the antibody response in infants to primary infant vaccination has been described by others, and by our group, after maternal Tdap vaccination during pregnancy.Citation4,5,34-36 This blunting of pertussis immune responses was reported to resolve with a fourth infant vaccine dose,Citation34,35 although we have still observed interference for PT-specific antibodies after the fourth vaccine doseCitation5 as well as for their avidity. Diphtheria-specific IgG antibody response was also found to be lower in the vaccine group than in the control group at week 20 after the first 3 immunizations (p = 0.002)Citation4 and at month 16 after the fourth vaccination dose (p = 0.023).Citation5 Ladhani et al have described a similar interference of maternal vaccination on DT-specific antibodies and on some of the serotype specific responses to the pneumococcal CRM (a naturally occurring diphtheria toxin variant) - conjugated vaccinesCitation36 and suggested that the administration of a diphtheria containing vaccine in pregnancy may influence the CRM-197 conjugated vaccine responses in infants. In that view, the present results showing adequate IgG antibody responses to infant diphtheria toxoid, yet low avidity of the induced antibodies could be explained by an interference by the maternal Tdap vaccination as well.
Full protection against diphtheria is achieved when IgG anti-DT titers are above 0.1 IU/ml.Citation37 All infants in our study had anti-DT IgG titers > 0.1 IU/ml and were sufficiently protected at month 15 before the fourth vaccine dose. However, RAI of anti-DT antibodies was low (in both groups) and remained so after the fourth vaccine dose. Booy et al.Citation38 and Tiru et al.Citation39 have reported on widening of the 3 DTP primary dose intervals and showed that antibody responses to 3 doses were lower following a shorter 8–12–16 week schedule than following a 8–16–24 week or a 3–5–12 months schedule respectively. With the rapid vaccination schedule, most infants failed to have good response to DT, which is a relatively weak vaccine antigen.Citation38,39 To our knowledge, avidity of the anti-DT antibodies was not tested in these previous studies, but a comparison of anti-DT avidity in large cohorts of infants vaccinated with these different vaccination schedules would certainly be interesting.
Pediatric vaccination provides adequate protection against diphtheria in infants and adolescents, but a significant susceptible population with low anti-DT levels was reported in Europe among adults and the elderly.Citation40 With a good vaccination coverage, diphtheria infections could be asymptomatic or less severe, but, as for pertussis, asymptomatic carriers of toxin-producing strains may contaminate unvaccinated or incompletely vaccinated infants.Citation41 A sad example was the recent death of an unvaccinated child last year in Belgium.Citation42
After the fourth vaccine dose, antibody levels increased significantly in both groups and this increase correlated positively with increases in RAI in both study groups (p < 0.05), even if the correlation was not significant for PT. Before the fourth vaccine dose however, RAI in the vaccine group correlated negatively with the antibody levels for Prn and TT, showing that high antibody levels do not necessarily mean high avidity and vice versa. In the vaccine group, regression analysis showed a significant influence of the interval between the third vaccine dose and the pre-fourth dose blood sampling on the avidity of tetanus-specific antibodies (p = 0.025) at month 15, the TT RAI decreasing with increasing interval. In contrast, in the control group, avidity of DT-specific antibodies increased significantly (p = 0.009) with increasing interval between the third vaccine dose and the pre-fourth dose blood sampling. It is not clear for the moment why these correlations were different between the 2 study groups. Another interesting observation was the fact that the age of the mother at delivery influenced antibody avidity of Prn-specific antibodies measured at month 16 in infants of both groups. Obviously, analysis of larger cohorts of infants would be needed to confirm these observations.
Throughout the first 2 y of life, there is a gradual acquisition of immune competence and a progressive affinity maturation of IgG antibodies after immunization.Citation43 For pertussis toxin, Prelog et al. have reported on moderate and high avidity of anti-PT antibodies respectively before and after the adolescent booster vaccine dose.Citation21 The infants in our 2 study groups also had moderate avidity antibodies against PT before the fourth dose (55.4% and 59.6% respectively in the vaccine and control group) which increased to high avidity (68.1% and 78.6%) after the fourth vaccine dose, indicating a good priming of B-cell memoryCitation21 to PT in both groups and providing evidence of additional avidity maturation with the number of vaccine doses. For technical reasons, serum samples could not be tested for antibody avidity after the completed primary vaccination at month 5, and therefore comparison with antibody avidity at month 15, before the fourth vaccine dose, and analysis of the impact of increasing age on avidity maturation, as reported by Ibrahim et alCitation24 could not be performed.
In conclusion, among the 5 vaccine antigens, PT and TT specific antibodies increased to the highest avidity, although there was a minor, but significant, negative effect of maternal vaccination on the PT-specific avidity as observed with antibody level.Citation4,5 Unfortunately, no correlates of protection exist for pertussis. More specifically, the relative importance of anti-PT IgG antibodies for protection against pertussis is not clear and could be questioned on the basis of the low and rapidly decaying antibody responses, induced after vaccinationCitation44 and the high anti-PT titers detected in pertussis patients. On the other hand, higher pertussis antibodies in general have been associated with enhanced protection against disease.Citation44-46 Further analysis of the bactericidal properties and opsonizing potential of pertussis-specific antibodies induced in the context of maternal immunization is needed to clarify whether the minor blunting of PT-specific infant immune responses and of avidity of induced antibodies, related to maternal vaccination has any clinical relevance.
Limitations of the study
This study was limited by the reduced number of infants in the control group, making strict randomization impossible. Another limitation is the fact that serum samples could for practical reasons not be tested for avidity at month 5 after the first 3 vaccine doses. Laboratory personnel was not blinded to the sample allocation.
Abbreviations
| DT | = | diphtheria toxin |
| FHA | = | filamentous hemagglutinin |
| IgG | = | immunoglobulin G |
| M | = | molar |
| PT | = | pertussis toxin |
| Prn | = | pertactin |
| RAI | = | relative avidity index |
| Tdap vaccine | = | trivalent Diphtheria and Tetanus Toxoids and Acellular Pertussis Vaccine |
| TT | = | tetanus toxin |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
KVIR_S_1296998.zip
Download Zip (85.4 KB)Acknowledgments
The authors would like to express their gratitude to all participating women and infants, to Aline Bontenakel for blood collection as well as to Caroline Rodeghiero and to Christophe Van den Poel for laboratory assistance.
Funding
This study was supported by VLIR-UOS (Flemish Interuniversity Council) (ZEIN2012Z131), FWO (Fund for Scientific Research-Flanders) (G.A032.12N) and the Belgian Ministry of Social Affairs through a fund within the Health Insurance System in the frame of the Belgian National Reference Center for Bordetella and National Reference Center for Toxigenic Corynebacteria. RNC was partially funded by Belgian Science Policy (BELSPO). EL is beneficiary of a postdoctoral mandate fellowship from the FWO (FWO 12D6114N).
References
- Cherry JD. Pertussis: challenges today and for the future. PLoS Pathog 2013; 9:e1003418; PPATHOGENS-D-13-00807 [pii]; PMID:23935481; http://dx.doi.org/10.1371/journal.ppat.1003418
- He Q, Mertsola J. Factors contributing to pertussis resurgence. Future Microbiol 2008; 3:329-39; PMID:18505398; http://dx.doi.org/10.2217/17460913.3.3.329
- Superior Health Council (2013) https://www.zorg-en-gezondheid.be/sites/default/files/atoms/files/vaccinatie_fiche_volw_kinkhoest_20130830.pdf
- Maertens K, Cabore RN, Huygen K, Hens N, Van DP, Leuridan E. Pertussis vaccination during pregnancy in Belgium: Results of a prospective controlled cohort study 2. Vaccine 2016; 34:142-50; S0264-410X(15)01556-X [pii]; PMID:26592142; http://dx.doi.org/10.1016/j.vaccine.2015.10.100
- Maertens K, Cabore RN, Huygen K, Vermeiren S, Hens N, Van DP, Leuridan E. Pertussis vaccination during pregnancy in Belgium: Follow-up of infants until 1 month after the fourth infant pertussis vaccination at 15 months of age. Vaccine 2016; 34:3613-9; S0264-410X(16)30231-6 [pii]; PMID:27142328; http://dx.doi.org/10.1016/j.vaccine.2016.04.066
- Plotkin SA. Complex correlates of protection after vaccination. Clin Infect Dis 2013; 56:1458-65; cit048 [pii]; PMID:23386629; http://dx.doi.org/10.1093/cid/cit048
- Poolman J, Kaufhold A, De GD, Goldblatt D. Clinical relevance of lower Hib response in DTPa-based combination vaccines. Vaccine 2001; 19:2280-5; S0264410×0000517X [pii]; PMID:11257348; http://dx.doi.org/10.1016/S0264-410X(00)00517-X
- Denoel PA, Goldblatt D, De V, I, Jacquet JM, Pichichero ME, Poolman JT. Quality of the Haemophilus influenzae type b (Hib) antibody response induced by diphtheria-tetanus-acellular pertussis/Hib combination vaccines. Clin Vaccine Immunol 2007; 14:1362-9. CVI.00154-07 [pii]; PMID:17699836; http://dx.doi.org/10.1128/CVI.00154-07
- Dimitrov JD, Lacroix-Desmazes S, Kaveri SV. Important parameters for evaluation of antibody avidity by immunosorbent assay. Anal Biochem 2011; 418:149-51; S0003-2697(11)00461-1 [pii]; PMID:21803020; http://dx.doi.org/10.1016/j.ab.2011.07.007
- Marchant A, Pihlgren M, Goetghebuer T, Weiss HA, Ota MO, Schlegel-Hauter SE, Whittle H, Lambert PH, Newport MJ, Siegrist CA. Predominant influence of environmental determinants on the persistence and avidity maturation of antibody responses to vaccines in infants. J Infect Dis 2006; 193:1598-605; JID35412 [pii]; PMID:16652290; http://dx.doi.org/10.1086/503775
- Yu YH, Lin KI. Factors that regulate the generation of antibody-secreting plasma cells. Adv Immunol 2016; 131:61-99; S0065-2776(16)30020-7 [pii]; PMID:27235681
- French DL, Laskov R, Scharff MD. The role of somatic hypermutation in the generation of antibody diversity. Science 1989; 244:1152-7; PMID:2658060; http://dx.doi.org/10.1126/science.2658060
- Goldblatt D, Vaz AR, Miller E. Antibody avidity as a surrogate marker of successful priming by Haemophilus influenzae type b conjugate vaccines following infant immunization. J Infect Dis 1998; 177:1112-5; PMID:9534995; http://dx.doi.org/10.1086/517407
- Siegrist CA, Aspinall R. B-cell responses to vaccination at the extremes of age. Nat Rev Immunol 2009; 9:185-94; nri2508 [pii]; PMID:19240757; http://dx.doi.org/10.1038/nri2508
- Matos DC, Silva AM, Neves PC, Martins RM, Homma A, Marcovistz R. Pattern of functional antibody activity against Haemophilus influenzae type B (Hib) in infants immunized with diphtheria-tetanus-pertussis/Hib Brazilian combination vaccine. Braz J Med Biol Res 2009; 42:1242-7; S0100-879×2009005000039 [pii]; PMID:19893995; http://dx.doi.org/10.1590/S0100-879X2009005000039
- Lieberman JM, Greenberg DP, Wong VK, Partridge S, Chang SJ, Chiu CY, Ward JI. Effect of neonatal immunization with diphtheria and tetanus toxoids on antibody responses to Haemophilus influenzae type b conjugate vaccines. J Pediatr 1995; 126:198-205; S0022-3476(95)70545-7 [pii]; PMID:7844665; http://dx.doi.org/10.1016/S0022-3476(95)70545-7
- Prince HE, Lieberman JM, Cherry JD. Age-related differences in patterns of increased Bordetella pertussis antibodies. Clin Vaccine Immunol 2012; 19:545-50; CVI.05725-11 [pii]; PMID:22357646; http://dx.doi.org/10.1128/CVI.05725-11
- Marchant A, Kollmann TR. Understanding the ontogeny of the immune system to promote immune-mediated health for life. Front Immunol 2015; 6:77; PMID:25755655; http://dx.doi.org/10.3389/fimmu.2015.00077
- Schallert N, Pihlgren M, Kovarik J, Roduit C, Tougne C, Bozzotti P, Del GG, Siegrist CA, Lambert PH. Generation of adult-like antibody avidity profiles after early-life immunization with protein vaccines. Eur J Immunol 2002; 32:752-60; PMID:11870619; http://dx.doi.org/10.1002/1521-4141
- Almanzar G, Ottensmeier B, Liese J, Prelog M. Assessment of IgG avidity against pertussis toxin and filamentous hemagglutinin via an adapted enzyme-linked immunosorbent assay (ELISA) using ammonium thiocyanate. J Immunol Methods 2013; 387:36-42; S0022-1759(12)00290-6 [pii]; PMID:23022630; http://dx.doi.org/10.1016/j.jim.2012.09.008
- Prelog M, Almanzar G, Rieber N, Ottensmeier B, Zlamy M, Liese J. Differences of IgG antibody avidity after an acellular pertussis (aP) booster in adolescents after a whole cell (wcP) or aP primary vaccination. Vaccine 2013; 31:387-93; S0264-410X(12)01578-2 [pii]; PMID:23142306; http://dx.doi.org/10.1016/j.vaccine.2012.10.105
- Abu RB, Bamberger E, Almog M, Peri R, Srugo I, Kessel A. Immunization of pregnant women against pertussis: the effect of timing on antibody avidity. Vaccine 2015; 33:1948-52; S0264-410X(15)00248-0 [pii]; PMID:25744227; http://dx.doi.org/10.1016/j.vaccine.2015.02.059
- Barkoff AM, Grondahl-Yli-Hannuksela K, Vuononvirta J, Mertsola J, Kallonen T, He Q. Differences in avidity of IgG antibodies to pertussis toxin after acellular pertussis booster vaccination and natural infection. Vaccine 2012; 30:6897-902; S0264-410X(12)01302-3 [pii]; PMID:22981763; http://dx.doi.org/10.1016/j.vaccine.2012.09.003
- Ibrahim NM, El-Kady EM, Eissa SA, Wahby AF. Assessment of antibody level and avidity against Bordetella pertussis in a cohort of Egyptian individuals aged 1–18 years 1. J Adv Res 2016; 7:105-11; S2090-1232(15)00032-6 [pii]; PMID:26843976; http://dx.doi.org/10.1016/j.jare.2015.03.002
- Aboud S, Matre R, Lyamuya EF, Kristoffersen EK. Levels and avidity of antibodies to tetanus toxoid in children aged 1–15 years in Dar es Salaam and Bagamoyo, Tanzania. Ann Trop Paediatr 2000; 20:313-22; PMID:11219170; http://dx.doi.org/10.1080/02724936.2000.11748153
- Maertens K, Hoang TH, Cabore RN, Leuridan E. Avidity of maternal pertussis antibodies after vaccination during pregnancy. Vaccine 2015; 33(42):5489; S0264-410X(15)00748-3 [pii].
- Hendrikx LH, Berbers GA, Veenhoven RH, Sanders EA, Buisman AM. IgG responses after booster vaccination with different pertussis vaccines in Dutch children 4 years of age: effect of vaccine antigen content. Vaccine 2009; 27:6530-6; S0264-410X(09)01237-7 [pii]; PMID:19729085; http://dx.doi.org/10.1016/j.vaccine.2009.08.052
- Andersson U, Bird G, Britton S. A sequential study of human B lymphocyte function from birth to two years of age 313. Acta Paediatr Scand 1981; 70:837-42; PMID:6275657; http://dx.doi.org/10.1111/j.1651-2227.1981.tb06236.x
- Stenger RM, Smits M, Kuipers B, Kessen SF, Boog CJ, van Els CA. Fast, antigen-saving multiplex immunoassay to determine levels and avidity of mouse serum antibodies to pertussis, diphtheria, and tetanus antigens. Clin Vaccine Immunol 2011; 18:595-603; CVI.00061-10 [pii]; PMID:21325488; http://dx.doi.org/10.1128/CVI.00061-10
- Abu RB, Srugo I, Bamberger E, Kessel A. The avidity of pertussis antibodies following gestational acellular pertussis immunization: Reply to Maertens. Vaccine 2015; 33(42):5490-1; S0264-410X(15)00763-X [pii].
- Eberhardt CS, Blanchard-Rohner G, Lemaitre B, Boukrid M, Combescure C, Othenin-Girard V, Chilin A, Petre J, de Tejada BM, Siegrist CA. Maternal Immunization Earlier in Pregnancy Maximizes Antibody Transfer and Expected Infant Seropositivity Against Pertussis 2. Clin Infect Dis 2016; 62:829-36. ciw027 [pii]; PMID:26797213; http://dx.doi.org/10.1093/cid/ciw027
- Abu RB, Srugo I, Bamberger E. Optimal timing of immunization against pertussis during pregnancy 1. Clin Infect Dis 2016; 63(1):143-4; ciw233 [pii].
- Siegrist CA. Neonatal and early life vaccinology. Vaccine 2001; 19:3331-46; S0264410×01000287 [pii]; PMID:11348697; http://dx.doi.org/10.1016/S0264-410X(01)00028-7
- Munoz FM, Bond NH, Maccato M, Pinell P, Hammill HA, Swamy GK, Walter EB, Jackson LA, Englund JA, Edwards MS, et al. Safety and immunogenicity of tetanus diphtheria and acellular pertussis (Tdap) immunization during pregnancy in mothers and infants: a randomized clinical trial. JAMA 2014; 311:1760-9; 1866102 [pii]; PMID:24794369; http://dx.doi.org/10.1001/jama.2014.3633
- Ladhani SN, Andrews NJ, Southern J, Jones CE, Amirthalingam G, Waight PA, England A, Matheson M, Bai X, Findlow H, et al. Antibody responses after primary immunization in infants born to women receiving a pertussis-containing vaccine during pregnancy: single arm observational study with a historical comparator. Clin Infect Dis 2015; 61:1637-44. civ695 [pii]; PMID:26374816; http://dx.doi.org/10.1093/cid/civ695
- Hardy-Fairbanks AJ, Pan SJ, Decker MD, Johnson DR, Greenberg DP, Kirkland KB, Talbot EA, Bernstein HH. Immune responses in infants whose mothers received Tdap vaccine during pregnancy. Pediatr Infect Dis J 2013; 32:1257-60; PMID:23799518; http://dx.doi.org/10.1097/INF.0b013e3182a09b6a
- Scheifele DW, Ochnio JJ. The Immunological Basis for Immunization Series/ Module 2: Diphtheria Update 2009. [pp.1-128]; WHO.
- Booy R, Aitken SJ, Taylor S, Tudor-Williams G, Macfarlane JA, Moxon ER, Ashworth LA, Mayon-White RT, Griffiths H, Chapel HM. Immunogenicity of combined diphtheria, tetanus, and pertussis vaccine given at 2, 3, and 4 months versus 3, 5, and 9 months of age. Lancet 1992; 339:507-10; 0140–6736(92)90336-2 [pii]; PMID:1346876; http://dx.doi.org/10.1016/0140-6736(92)90336-2
- Tiru M, Hallander HO, Gustafsson L, Storsaeter J, Olin P. Diphtheria antitoxin response to DTP vaccines used in Swedish pertussis vaccine trials, persistence and projection for timing of booster. Vaccine 2000; 18:2295-306; S0264-410X(99)00539-3 [pii]; PMID:10717350; http://dx.doi.org/10.1016/S0264-410X(99)00539-3
- Edmunds WJ, Pebody RG, Aggerback H, Baron S, Berbers G, Conyn-van Spaendonck MA, Hallander HO, Olander R, Maple PA, Melker HE, et al. The sero-epidemiology of diphtheria in Western Europe. ESEN Project. European Sero-Epidemiology Network. Epidemiol Infect 2000; 125:113-25; PMID:11057967; http://dx.doi.org/10.1017/S0950268899004161
- Huygen K. Development of human monoclonal antibodies to diphtheria toxin: A solution for the increasing lack of equine DAT for therapeutic use? Virulence 2016; 7:613-5; PMID:27196732; http://dx.doi.org/10.1080/21505594.2016.1190062
- European Centre for Disease Prevention and Control. A fatal case of diphtheria in Belgium 2016. 24 March 2016: ECDC; 2016. http://ecdc.europa.eu/en/publications/Publications/RRA-Diphtheria-Belgium.pdf
- ACOG Committee Opinion No. 566: Update on immunization and pregnancy: tetanus, diphtheria, and pertussis vaccination. Obstet Gynecol 2013; 121:1411-4; 00006250-201306000-00044 [pii]; PMID:23812487; http://dx.doi.org/10.1097/01.AOG.0000431054.33593.e3
- Storsaeter J, Hallander HO, Gustafsson L, Olin P. Levels of anti-pertussis antibodies related to protection after household exposure to Bordetella pertussis. Vaccine 1998; 16:1907-16; S0264410×98002278 [pii]; PMID:9796042; http://dx.doi.org/10.1016/S0264-410X(98)00227-8
- Taranger J, Trollfors B, Lagergard T, Sundh V, Bryla DA, Schneerson R, Robbins JB. Correlation between pertussis toxin IgG antibodies in postvaccination sera and subsequent protection against pertussis. J Infect Dis 2000; 181:1010-3; JID990862 [pii]; PMID:10720524; http://dx.doi.org/10.1086/315318
- Heininger U, Riffelmann M, Bar G, Rudin C, von Konig CH. The protective role of maternally derived antibodies against Bordetella pertussis in young infants. Pediatr Infect Dis J 2013; 32:695-8; PMID:23429559; http://dx.doi.org/10.1097/INF.0b013e318288b610
