ABSTRACT
Children and young adults infected with HIV are at elevated risk for cardiovascular disease (CVD). However, scarce data exist on the utility of non-invasive methods to diagnose subclinical CVD, such as pulse wave velocity (PWV), a non-invasive measure of arterial stiffness. The objectives of this study were to assess the relationship of carotid-femoral PWV with subclinical atherosclerosis measured by carotid intima-media thickness (IMT), compare measurements to healthy controls, and evaluate variables associated with PWV in HIV-infected youth. One hundred and one 8–25 year-old subjects on stable antiretroviral therapy with low-level viremia or an undetectable HIV-1 RNA were enrolled, along with 86 healthy controls similar in age, sex and race. There was no significant difference in PWV between groups (median (Q1, Q3): 5.7 (5.2, 6.3) vs 5.7 (4.9, 6.5) m/s; P = 0.81). Among the HIV-infected subjects, PWV was positively correlated with both internal carotid artery (R = 0.31, P = 0.02) and carotid bulb IMT (R = 0.29, P = 0.01). In multivariable regression, only current alcohol consumption and systolic blood pressure were independently associated with PWV in the HIV-infected group (where current alcohol consumption and higher systolic blood pressure were associated with higher PWV); whereas, age, body mass index, and current marijuana use were associated with PWV in healthy controls. In this study of PWV in HIV-infected youth, measures of arterial stiffness were not different between subjects and controls. However, in HIV-infected youth, there was a significant association between PWV and carotid IMT, as well as between PWV and current alcohol consumption. Thus, PWV may have potential as a useful, non-invasive method to assess CVD risk in HIV-infected youth, but further investigation is needed.
Introduction
Both adultCitation1-3 and pediatricCitation4,5 HIV-infected patients are at a greater risk of cardiovascular disease (CVD) than the general population. To improve risk prediction in the adult population, researchers have used numerous imaging techniques, such as carotid intima-media thickness (IMT) and other tools, such as pulse wave velocity (PWV). The former is considered an excellent surrogate of atherosclerosis, and the latter provides a non-invasive assessment of large artery stiffness. Both have been associated with cardiovascular events and all-cause mortality in the general population.Citation6,7 Additionally, in adult HIV-infected patients, PWV is a reliable marker of subclinical vascular disease.Citation8
While carotid IMT has been a useful tool in assessing CVD risk in HIV-infected children and young adults, it has also proven challenging to assess longitudinal changes in this younger, still growing, population.Citation9 On the other hand, very little is known about the utility of PWV in this population, and given its non-invasive nature, straightforward interpretation, and potential for more consistent measurements over time, evaluating this technique may become vital in assessing CVD risk in young patients. Since the current goal of HIV treatment is long-term virologic suppression with combination antiretroviral therapy (ART), in the current study, we evaluated HIV-infected children and young adults receiving ART with an undetectable HIV-1 RNA or low-level viremia. Our objectives were to: 1) compare PWV measurements in young HIV-infected patients and healthy controls, 2) evaluate the relationship of PWV with carotid IMT as a marker of atherosclerosis, and 3) evaluate which variables were associated with PWV in young HIV-infected patients.
Methods
Study design/population
This was a cross-sectional study investigating PWV in HIV-infected children and young adults on stable ART and healthy controls. HIV-infected children and young adults were recruited from the HIV clinics of University Hospitals Case Medical Center, Cleveland, OH and Grady Health System, Atlanta, GA via electronic medical record system queries and case manager/provider referrals. Subjects were eligible if they were between 8–25 y of age with documented HIV-1 infection on stable combination ART for at least the 12 weeks before enrollment, with ≥ 6 months cumulative duration of ART and with an HIV-1 RNA level <1,000 copies/mL. Exclusion criteria included any acute illness or inflammatory condition, current AIDS-defining clinical condition, chronic inflammatory state besides HIV, other chronic illnesses such as malignancy, or medication use that could affect the results (e.g. chemotherapy agents, systemic steroids). Patients with acute illnesses were eligible after complete resolution of symptoms for ≥ 30 d before enrollment.
The controls were subjects 8–25 y of age who self-reported to have no chronic disease or current/recent illness. HIV-negative status was confirmed with OraQuick Advance Rapid HIV test (OraSure Technologies, Inc., Bethlehem, PA) for subject age 12 and older at the Emory University site, given the high prevalence of HIV in this age group in Atlanta, Georgia.
Healthy controls were recruited aiming to achieve a group with similar characteristics to the HIV-infected subjects. Healthy controls were recruited in multiple ways, including: a) friends or family members of the HIV-infected subjects, b) physician referrals from local pediatric and adult clinics, c) extensive outreach to various local organizations, churches, and schools, and d) recruitment flyers in targeted locations throughout the 2 cities. Controls were recruited from similar geographic and socioeconomic areas as the HIV-infected group, to decrease the risk of potential confounders.
Both HIV-infected subjects and healthy controls were excluded from the study if they were pregnant or lactating.
The study was reviewed and approved by the Institutional Review Boards of University Hospital Case Medical Center, Emory University, and Grady Health System. All parents or legal guardians and subjects ≥ 18 y of age gave written informed consent to participate in the study. Subjects aged 17 y or older signed a written consent along with their parent or legal guardian. Subjects between the ages of 8–10 y gave verbal assent and those 11–16 y gave written assent.
Study assessments
Clinical evaluations
For HIV-infected subjects and healthy controls, relevant data were obtained by questionnaire, including demographics, current and past medical history, alcohol intake, tobacco use, and drug habits. Further information was also collected from the HIV-infected subjects' medical records including past and current medical diagnoses, CD4 nadir, detailed past and current antiretroviral (ARV) and non-ARV medication use, HIV diagnosis date, and acquisition method (perinatal or horizontal). Targeted physical examination, weight and height measurements were obtained in all subjects.
Laboratory evaluations
After at least an 8-hour fast, blood was collected from all subjects for real-time measurements of insulin, glucose, and lipoprotein profile. Insulin resistance was calculated using the homeostasis model assessment of insulin resistance (HOMA-IR; [fasting glucose (mg/dl)× fasting insulin (mU/ml)]/405).Citation10 Absolute CD4+ T-cell count and plasma HIV-1 RNA level were concomitantly measured as markers of HIV disease activity.
Cardiovascular measurements
Cardiovascular measurements were performed by experienced technicians who were all blinded to subjects' HIV status. Brachial artery systolic (SBP) and diastolic (DBP) blood pressure were measured with a Dinamap digital automatic blood pressure measurement device (Atlanta site) or using a manual Sphygmomanometer (Cleveland site) using the appropriate cuff size after the subjects rested in a supine position for at least 10 minutes. Pulse wave velocity measurements were subsequently taken with a SphygmoCor CPV Clinical System (AtCor Medical) following the manufacturer's protocol and corrected for heart rate. Pulse wave velocity measurements meeting the manufacturer quality control criteria were collected in triplicate and averaged. Higher velocity measurements correspond to greater arterial stiffness.
Carotid intima-media thickness (IMT) was measured bilaterally at the level of the common carotid artery (CCA), internal carotid artery (ICA), and carotid bulb, using a Philips iU22 ultrasound system with a L9–3 MHz linear array transducer according to the consensus protocol of the American Society of Echocardiography.Citation11 Ten-second cine-loops were obtained of the distal 1 cm of the CCA at 3 separate angles (anterior, lateral, and posterior) and of the ICA and carotid bulb at the optimum angle of insonation bilaterally. The far wall IMT was then measured offline using semi-automated edge detection software (Medical Imaging Applications LLC, Coralville, IA). The CCA measurements from the left side were averaged to produce a single mean measurement for that side. This process was repeated for the right side. Then, the measurements from the left and right side were averaged to produce a single mean. This process was then repeated for both the ICA and bulb IMT. All images were reviewed by a cardiologist who was blinded to the subjects' HIV status.
Statistical analyses
Demographic, clinical and laboratory characteristics are described by study group, and HIV-related characteristics are described for the HIV-infected subjects. Continuous measures are described by median and first and third quartiles, and nominal variables are described as frequencies or percentages. Normally distributed variables were compared using Student's t-tests, and non-normally distributed variables were compared using Wilcoxon rank sum tests. Pulse wave velocity and carotid IMT were also analyzed adjusted for systolic blood pressure using least squares means.
Associations between PWV and variables of interest were assessed using Spearman correlation coefficients for continuous variables and appropriate 2-sample tests for dichotomous variables for the HIV-infected and control groups separately. A multivariable regression model was then performed with the HIV-infected subjects and healthy controls combined to determine if a study arm was independently associated with PWV. Variables were included in the model if they were significantly associated with PWV in either study arm. These variables included age, body mass index, sex, systolic blood pressure, alcohol consumption, smoking, marijuana use, and study arm. No further steps were taken to reduce the model, so all variables could be seen relative to one another.
Multivariable regression analyses were then conducted separately for the 2 study arms by applying a stepwise process to determine variables independently associated with PWV. Variables were again included in the regression models if they were statistically significantly associated with PWV within each study arm or deemed clinically relevant. For the HIV-infected group, these variables included age, systolic blood pressure, alcohol consumption, cumulative tenofovir use, sex, current CD4 count, CD4/CD8 ratio, cumulative antiretroviral duration, and current tenofovir use. For the healthy control group, these variables included age, body mass index, marijuana use, systolic blood pressure, alcohol consumption and smoking. In the first step, any variable with P ≥ 0.5 was removed. The final models were then constructed by eliminating any variables with P > 0.1, such that only variables with P ≤ 0.1 were included.
Finally, associations between PWV and carotid IMT were assessed using Spearman correlation coefficients for the HIV-infected group and healthy controls separately.
Statistical analyses were performed using SAS 9.4 (The SAS Institute, Cary, NC). Statistical significance was set at the 0.05 level, including for the final regression models.
Results
Study enrollment occurred from January 2012 to March 2014. A total of 101 HIV-infected subjects and 86 controls were enrolled into the study. By design, groups were similar in age, sex, and race (). The HIV-infected group had a statistically higher SBP (120 vs. 117 mm/Hg), lower high-density lipoprotein (HDL) cholesterol (46 vs. 54 mg/dL), and higher triglycerides (TG) (89 vs. 63 mg/dL) compared with the controls, but DBP, body mass index (BMI), waist-to-hip ratio (WHR), total cholesterol (TC), low-density lipoprotein (LDL) cholesterol, HOMA-IR, current smoking, current alcohol use, and current marijuana use were similar between groups. No subject had diabetes. Of note, none of the healthy controls were biologically-related to any of the HIV-infected subjects, and none had a history of in-utero HIV/ARV exposure. All but 4 of the controls had no known relationship with any of the HIV-infected subjects.
Table 1. Subject characteristics by study group.
HIV-related variables are shown in . As per the inclusion criteria, all subjects had a low number of HIV-1 RNA copies (< 1,000 copies/mL). Seventy-eight percent of subjects had “undetectable” levels based on that particular clinical laboratory's lower limit of detection. Of the 22 subjects who did not have an undetectable HIV-1 RNA at the time of entry, 1 subject had no additional documented detectable HIV-1 RNA levels over the year before enrollment, 9 subjects had at least one additional documented low-level HIV-1 RNA between 80–4233 copies/mL, 10 subjects had uncontrolled viremia at some point during the year before entry (15k-1.1 million copies/mL), and 2 subjects did not have available data.
Because there were several different assays used in various clinical laboratories to measure HIV-1 RNA levels, there were varying lower limits of detection (< 20, <40, <48, <79 and <80 copies/mL). Thus, we also considered an HIV-1 RNA level <80 copies/mL to define undetectable HIV-1 RNA. Using this cut-off, 89% of subjects had undetectable viremia. Of the 11 remaining subjects, 6 had an HIV-1 RNA level between 89–190 copies/mL, and 5 were between 520–920 copies/mL.
There was no median (Q1, Q3) difference in PWV between the HIV-infected subjects and the healthy controls (HIV+: 5.7 (5.2, 6.3) m/s vs. controls: 5.7 (4.9, 6.5) m/s; P = 0.81) (). As blood pressure is known to affect PWV, mean differences between groups were also considered for SBP-corrected PWV. Again, there were no statistically significant differences between groups (HIV+ mean = 5.7 m/s; control mean = 5.8 m/s; P = 0.26). On the other hand, CCA, ICA and bulb IMT were all statistically higher in the HIV-infected group compared with the healthy controls. Systolic-adjusted carotid IMT means were also compared between groups with no qualitative change in the differences between groups (mean CCA IMT HIV+: 0.57 vs. controls: 0.54 mm, P = 0.009; mean carotid bulb IMT HIV+: 0.62 vs. controls: 0.55 mm, P = 0.0004; mean ICA IMT HIV+: 0.54 vs. controls: 0.47 mm, P = 0.003).
Among the traditional CVD risk factors, male sex [male sex: 5.8 (5.4, 6.3) m/s vs. female sex: 5.2 (4.7, 6.1) m/s; P = 0.01] and a higher SBP (R = 0.31, P = 0.002) and DBP (R = 0.29, P = 0.004) were associated with an increased PWV in the HIV-infected group, but age, BMI, smoking status, race, WHR, TC, LDL, HDL, TG, and HOMA-IR were not (all P ≥ 0.05). Current alcohol use (any amount of alcohol consumption vs. no consumption) was also associated with increased PWV [alcohol consumption: 6.0 (5.6, 6.5) m/s vs. no consumption: 5.4 (4.8, 6.0) m/s; P = 0.0002], but current marijuana use was not.
Among the HIV-related variables, current CD4 count (R = −0.23; P = 0.02), CD4/CD8 ratio (R = −0.31; P = 0.002), and cumulative duration of ART use (R = −0.21; P = 0.04) were negatively correlated with PWV, but cumulative duration of tenofovir (TDF) use (R = 0.27, P = 0.007) was positively correlated with PWV. Current TDF use [current TDF use: 5.8 (5.3, 6.4) m/s vs. no current TDF use: 4.9 (4.4, 5.7) m/s; P = 0.001] and a detectable HIV-1 RNA level [detectable RNA: 6.1 (5.6, 6.6) m/s vs. undetectable RNA: 5.6 (5.0, 6.2) m/s; P = 0.03] were both also associated with an increased PWV. Nadir CD4 count, HIV duration, and current use or cumulative duration of a protease inhibitor (PI), nucleoside reverse transcriptase inhibitor, non-nucleotide reverse transcriptase inhibitor, or efavirenz alone were not associated with PWV (all P ≥ 0.05).
In multivariable regression analysis investigating independent associations with PWV in the HIV-infected group, the only variables that were statistically significant in the final model were SBP and alcohol consumption, where any amount of current alcohol consumption was associated with increased PWV (). The multivariable regression analyses were repeated with the inclusion of the variable undetectable vs. detectable HIV-1 RNA level; in this instance, again, only SBP and current alcohol consumption were significantly associated with PWV in the final model (data not shown).
Table 2. Multivariable regression models for variables associated with pulse wave velocity.
Among the healthy controls, age (R = 0.50, P < 0.0001), BMI (R = 0.42, P < 0.0001), SBP (R = 0.22, P = 0.04), DBP (R = 0.32, P = 0.003), and current smoking [smoking: 6.6 (6.0, 7.7) m/s vs. no smoking: 5.6 (4.8, 6.3) m/s; P = 0.01], marijuana use [marijuana use: 6.2 (5.2, 7.4) m/s vs. no marijuana use: 5.6 (4.8, 6.3) m/s; P = 0.04], and alcohol use [alcohol use: 6.3 (5.2, 7.1) m/s vs. no alcohol use: 5.5 (4.8, 6.2) m/s; P = 0.005) were all positively associated with PWV, but male sex, race, TC, LDL, HDL, TG and HOMA-IR were not.
In this group, age, BMI, and current marijuana use were the only factors that remained significantly associated with PWV in the final multivariable regression model (). The analysis was repeated with SBP retained in the regression model at each step regardless of statistical significance, due to the strong association between PWV and blood pressure. Qualitatively, the results were unchanged, and only age, BMI, and current marijuana use were statistically significant in the final model (data not shown).
Multivariable regression analyses were then repeated with subjects and controls combined to investigate independent associations with PWV, including study arm (). Only age, BMI, and current alcohol use demonstrated a statistically significant association.
Table 3. Multivariable regression model for variables associated with pulse wave velocity for all subjects.
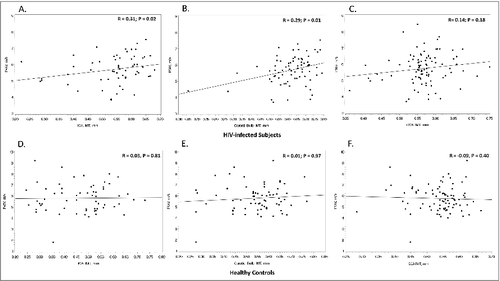
Ninety-three HIV-infected subjects had available CCA IMT measurements, and they were not correlated with PWV (R = 0.14; P = 0.18). Among these 93 subjects, ICA IMT and the carotid bulb IMT were accessible on the ultrasound images for measurement in 58 and 76 subjects, respectively. Mean ICA IMT (R = 0.31; P = 0.02) and carotid bulb IMT (R = 0.29; P = 0.01) were both positively associated with PWV (). Eighty-five healthy controls had available CCA IMT measurements. Among these, ICA and bulb IMT were accessible on the ultrasound images for measurement in 61 and 71 subjects, respectively. None of these IMT measurements were correlated with PWV (CCA: R = −0.09, P = 0.40; ICA: R = 0.03, P = 0.81; bulb: R = 0.01, P = 0.97) ().
Figure 1. Correlations between PWV and carotid IMT. Scatter plots depict the bivariate relationship between PWV and (A) ICA IMT, (B) carotid bulb IMT, and (C) CCA IMT in the HIV-infected subjects and (D) ICA IMT, (E) carotid bulb IMT, and (F) CCA IMT in the healthy control group. R = Spearman correlation coefficient. PWV, pulse wave velocity; ICA, internal carotid artery; CCA, common carotid artery; IMT, intima-media thickness.
Discussion
In this study, we compared PWV in young HIV-infected subjects with virologic suppression or low-level viremia and healthy controls; we further evaluated variables associated with PWV in subjects and controls and investigated the correlation of PWV with carotid IMT, a well-known marker of sub-clinical atherosclerosis. We did not find a difference in PWV between the HIV-infected youths and their comparable healthy controls, in contrast with a previous study of adult subjectsCitation12 and HIV-infected and uninfected youth.Citation5 Both groups of investigators found a difference in PWV between HIV-infected and uninfected subjects. Our findings instead are consistent with a recent analysisCitation13 demonstrating no difference in PWV by HIV serostatus. These discrepant results could be due to a statistical type 1 error (i.e small sample sizes), the use of different arterial segments (carotid-radial vs carotid-femoral), the subjects' age being too young in the study by Charakida et al.Citation5 to permit a clear separation of risk by PWV measurements, or the actual absence of a difference between HIV infected and uninfected subjects.
In a recent meta-analysis,Citation8 HIV-infected adults overall were shown to have higher arterial stiffness as measured by PWV compared with HIV-uninfected subjects. However, when analyzed by age brackets, there was no longer a difference in PWV for those in the youngest age group, similar to the findings in our study. The investigators also performed a meta-analysis of studies of carotid IMT in HIV-infected subjects. Unlike the PWV findings, IMT was still higher in the younger HIV-infected patients compared with the HIV-uninfected subjects. It is impossible to know if the different association of IMT and PWV with age in this meta-analysis was due to the small number of studies available to analyze, the limitations within each study, or if one modality is more accurate than the other for assessing CVD risk in this younger age group. Importantly, the studies with median ages similar to our study that were included in the meta-analysis used varying anatomic sites to measure both IMT and PWV, combined HIV-infected subjects receiving and not receiving ART, and did not directly compare ART-treated HIV-infected subjects vs. HIV-uninfected subjects.Citation5,14-17 These inconsistencies emphasize the challenge of assessing CVD risk in HIV-infected individuals, particularly in the younger population and highlight the current equipoise regarding the information provided by PWV measurements in patients with HIV.
Our study, restricted to HIV-infected children and young adults on stable ART, showed a significant correlation between PWV and ICA and carotid bulb IMT. Not surprisingly, there was no significant correlation of PWV with CCA IMT; in fact, studies in the general population and in HIV-infected adults have shown that atherosclerosis and IMT progress more rapidly in the bulb and ICA compared with the CCA and that the association with HIV is stronger with ICA IMT (including the bulb region) than with CCA IMT.Citation18 Ironically, although atherosclerosis in the ICA and bulb region progresses more rapidly, these segments are more difficult to visualize by ultrasound, and limiting IMT measurements to the far wall of the CCA is the preferred strategy for clinical testing.Citation11
One potential advantage of using PWV over IMT to assess CVD risk in this population is that it may be more reliable for longitudinal assessment in HIV-infected children and young adults. As Fernhall, et al.Citation19 pointed out in a thorough review of the literature, discrepancies among various studies of IMT in children may be due to the fact that IMT changes very little during childhood, and, as it changes, so does arterial size and luminal diameter.Citation20,21 These facts likely make measuring carotid IMT longitudinally in children more difficult than in adults. We previously measured carotid IMT in HIV-infected children followed prospectively for 144 weeks. Despite relatively large yearly IMT changes, both CCA and ICA IMT measurements at 144 weeks were approximately equivalent to baseline.Citation22 Furthermore, IMT changes seen from year to year in HIV-infected children are likely insignificant, and only extended longitudinal trials spanning over a decade or more would be clinically meaningful. Pulse wave velocity, on the other hand, may offer a better method to assess CVD risk in this younger HIV population, as it can change quickly with effective interventions. For example, PWV decreased in subjects with mild hypertension after 1 y of treatment after statistical adjustment for blood pressure.Citation23 Likewise, even after 3 and 12 months of tocilizumab and rituximab treatment, respectively, PVW decreased significantly in patients with rheumatoid arthritis.Citation24 Longitudinal studies have also been successfully conducted in children and adolescents.Citation25,26
One additional important finding in our study is that reported current alcohol use, regardless of the quantity consumed, was associated with arterial stiffness. Interestingly, this was the only significant variable associated with PWV besides systolic blood pressure in the final multivariable regression model in the HIV-infected subjects. An inverse association between light alcohol consumption and arterial stiffness is well-documented in the general population.Citation27-29 Many studies suggested that light-moderate, non-binge consumption of alcoholic beverages, especially red wine, significantly reduces the risk of coronary heart disease. Laboratory experiments have shown that moderate alcohol consumption promotes anti-inflammatory processes along with several other proposed mechanisms that may induce cardioprotection.Citation30 However, while moderate drinking may be beneficial, both heavy drinkers and non-drinkers have a higher risk of CVD, resulting in a U-shaped association. Although few studies have specifically investigated alcohol consumption and CVD in HIV, a recent systematic review summarized 13 studies that assessed alcohol use as a covariate.Citation31 While methods and results varied considerably among studies, the authors found that the weighted pooled crude effect sizes were 1.75 (95% confidence interval (CI) 1.06, 3.17) for general and 1.78 (95% CI 1.09, 2.93) for heavy alcohol use on CVD.
Our study had some limitations worth mentioning: we were unable to collect exact information on the number and type of drinks the subjects consumed, including binge drinking. However, given that heavy drinking and alcohol use disorders are common in HIV-infected patientsCitation32 and the effects of alcohol are likely different in this population that is already at risk for cardiovascular, metabolic, liver, coagulation, and inflammatory disorders, our finding is of interest and warrants further exploration. Other limitations include the cross-sectional design, the small sample size on which we measured ICA and bulb IMT, the heterogeneous nature of the study group with a wide age range, HIV-infected subjects were not all vertically-infected, they were at 2–14 y since HIV diagnosis and some were receiving ART for only 6 months before inclusion.
Nonetheless, we believe that our study offers some useful information. The association of PWV with IMT in HIV-infected youth revealed its potential ability to identify patients with markers of clinical risk. As HIV-infected children and young adults will likely live for many decades with chronic infection on combination ART, accurate, easy, and inexpensive modalities to assess CVD risk are urgently needed. Whether PWV may become a useful method to assess cardiovascular risk in HIV infected youth deserves further investigation.
Disclosure of potential conflicts of interest
ARE has received research funding from Bristol-Myers Squibb, Cubist Pharmaceuticals, and GlaxoSmithKline and has served as an advisor and speaker for Gilead. GAM serves as a consultant for Bristol-Myers Squibb, ViiV/GlaxoSmithKline, Gilead, Pfizer, and ICON, and has received grant funding from Bristol-Myers Squibb, ViiV/GlaxoSmithKline, and Gilead. CTL has received research funding from Medtronic Philanthropy, the Wolf Family Foundation, and Bristol-Myers Squibb. All other authors declare no conflicts of interest.
Funding
This work was made possible by the National Institute of Child Health and Development at the National Institutes of Health [K23 HD069199 to ARE; R01 HD070490 to GAM; K23 HL123341 to CTL], Case Western Reserve University's Center for AIDS Research (P30 AI36219), Emory University's Center for AIDS Research (P30 AI050409), Emory+Children's Pediatric Research Center, Immunology Research Core, Cardiovascular Imaging Research Cores, Children's Healthcare of Atlanta, and the Clinical and Translational Science Collaborative of Cleveland (UL1TR000439) from the National Center for Advancing Translational Sciences (NCATS) component of the National Institutes of Health and NIH roadmap for Medical Research, and the National Center for Advancing Translational Sciences of the National Institutes of Health (UL1TR000454). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- Hanna DB, Ramaswamy C, Kaplan RC, Kizer JR, Anastos K, Daskalakis D, Zimmerman R, Braunstein SL. Trends in Cardiovascular disease mortality among persons with HIV in New York City, 2001–2012. Clin Infect Dis 2016; 63:1122-9; PMID:27444412; http://dx.doi.org/10.1093/cid/ciw470
- Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab 2007; 92:2506-12; PMID:17456578; http://dx.doi.org/10.1210/jc.2006-2190
- Freiberg MS, Chang CC, Kuller LH, Skanderson M, Lowy E, Kraemer KL, Butt AA, Bidwell Goetz M, Leaf D, Oursler KA, et al. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med 2013; 173:614-22; PMID:23459863; http://dx.doi.org/10.1001/jamainternmed.2013.3728
- McComsey GA, O'Riordan M, Hazen SL, El-Bejjani D, Bhatt S, Brennan ML, Storer N, Adell J, Nakamoto DA, Dogra V. Increased carotid intima media thickness and cardiac biomarkers in HIV infected children. AIDS 2007; 21:921-7; PMID:17457085; http://dx.doi.org/10.1097/QAD.0b013e328133f29c
- Charakida M, Donald AE, Green H, Storry C, Clapson M, Caslake M, Dunn DT, Halcox JP, Gibb DM, Klein NJ, et al. Early structural and functional changes of the vasculature in HIV-infected children: impact of disease and antiretroviral therapy. Circulation 2005; 112:103-9; PMID:15983247; http://dx.doi.org/10.1161/CIRCULATIONAHA.104.517144
- Choo J, Shin C, Barinas-Mitchell E, Masaki K, Willcox BJ, Seto TB, Ueshima H, Lee S, Miura K, Venkitachalam L, et al. Regional pulse wave velocities and their cardiovascular risk factors among healthy middle-aged men: a cross-sectional population-based study. BMC Cardiovasc Disord 2014; 14:5; PMID:24410766; http://dx.doi.org/10.1186/1471-2261-14-5
- O'Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK, Jr. Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med 1999; 340:14-22
- Sun D, Wu Y, Yuan Y, Wang Y, Liu W, Yang J. Is the atherosclerotic process accentuated under conditions of HIV infection, antiretroviral therapy, and protease inhibitor exposure? Meta-analysis of the markers of arterial structure and function. Atherosclerosis 2015; 242:109-16; PMID:26188532; http://dx.doi.org/10.1016/j.atherosclerosis.2015.06.059
- Ross AC, Storer N, O'Riordan MA, Dogra V, McComsey GA. Longitudinal changes in carotid intima-media thickness and cardiovascular risk factors in human immunodeficiency virus-infected children and young adults compared with healthy controls. Pediatr Infect Dis J 2010; 29:634-8; PMID:20589981; http://dx.doi.org/10.1097/INF.0b013e3181d770c4
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28:412-9; PMID:3899825; http://dx.doi.org/10.1007/BF00280883
- Stein JH, Korcarz CE, Hurst RT, Lonn E, Kendall CB, Mohler ER, Najjar SS, Rembold CM, Post WS. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima-Media thickness task force. Endorsed by the society for vascular medicine. J Am Soc Echocardiogr 2008; 21:93-111; quiz 189–190; PMID:18261694; http://dx.doi.org/10.1016/j.echo.2007.11.011
- Rider OJ, Asaad M, Ntusi N, Wainwright E, Clutton G, Hancock G, Banerjee R, Pitcher A, Samaras K, Clarke K, et al. HIV is an independent predictor of aortic stiffness. J Cardiovasc Magn Reson 2014; 16:57; PMID:25187084; http://dx.doi.org/10.1186/s12968-014-0057-1
- Echeverria P, Bonjoch A, Molto J, Jou A, Puig J, Ornelas A, Pérez-Álvarez N, Clotet B, Negredo E. Pulse wave velocity as index of arterial stiffness in HIV-infected patients compared with a healthy population. J Acquir Immune Defic Syndr 2014; 65:50-6; PMID:23982659; http://dx.doi.org/10.1097/QAI.0b013e3182a97c17
- Bonnet D, Aggoun Y, Szezepanski I, Bellal N, Blanche S. Arterial stiffness and endothelial dysfunction in HIV-infected children. AIDS 2004; 18:1037-41; PMID:15096807; http://dx.doi.org/10.1097/00002030-200404300-00012
- Di Biagio A, Rosso R, Maggi P, Mazzei D, Bernardini C, Nulvesu L, Parisini A, Nicco E, De Carli F, Rodriguez G, et al. Inflammation markers correlate with common carotid intima-media thickness in patients perinatally infected with human immunodeficiency virus 1. J Ultrasound Med 2013; 32:763-8; PMID:23620317; http://dx.doi.org/10.7863/ultra.32.5.763
- Charakida M, Loukogeorgakis SP, Okorie MI, Masi S, Halcox JP, Deanfield JE, Klein NJ. Increased arterial stiffness in HIV-infected children: risk factors and antiretroviral therapy. Antivir Ther 2009; 14:1075-9; PMID:20032537; http://dx.doi.org/10.3851/IMP1437
- Sainz T, Diaz L, Navarro ML, Rojo P, Blazquez D, Ramos JT, de José MI, Álvarez-Fuente M, Serrano-Villar S, Mellado MJ, et al. Cardiovascular biomarkers in vertically HIV-infected children without metabolic abnormalities. Atherosclerosis 2014; 233:410-4; PMID:24530771; http://dx.doi.org/10.1016/j.atherosclerosis.2014.01.025
- Grunfeld C, Delaney JA, Wanke C, Currier JS, Scherzer R, Biggs ML, Tien PC, Shlipak MG, Sidney S, Polak JF, et al. Preclinical atherosclerosis due to HIV infection: carotid intima-medial thickness measurements from the FRAM study. AIDS 2009; 23:1841-9; PMID:19455012; http://dx.doi.org/10.1097/QAD.0b013e32832d3b85
- Fernhall B, Agiovlasitis S. Arterial function in youth: window into cardiovascular risk. J Appl Physiol 2008; 105:325-33; PMID:18450990; http://dx.doi.org/10.1152/japplphysiol.00001.2008
- Sass C, Herbeth B, Chapet O, Siest G, Visvikis S, Zannad F. Intima-media thickness and diameter of carotid and femoral arteries in children, adolescents and adults from the Stanislas cohort: effect of age, sex, anthropometry and blood pressure. J Hypertens 1998; 16:1593-602; PMID:9856359; http://dx.doi.org/10.1097/00004872-199816110-00005
- Jourdan C, Wuhl E, Litwin M, Fahr K, Trelewicz J, Jobs K, Schenk JP, Grenda R, Mehls O, Tröger J, et al. Normative values for intima-media thickness and distensibility of large arteries in healthy adolescents. J Hypertens 2005; 23:1707-15; PMID:16093916; http://dx.doi.org/10.1097/01.hjh.0000178834.26353.d5
- Ross Eckard A, O'Riordan MA, Storer N, McComsey GA. Long-term changes in carotid intima-media thickness among HIV-infected children and young adults. Antivir Ther 2014; 19:61-8; PMID:23985545; http://dx.doi.org/10.3851/IMP2678
- Rodilla Sala E, Millasseau S, Escriva M, Garcia J, Costa JA, Pascual JM. 4d.10: changes in Pwv in previously untreated Mild Hypertensives are related to reduction of blood pressure by treatment. J Hypertens 2015; 33(Suppl 1):e63; PMID:26102880; http://dx.doi.org/10.1097/01.hjh.0000467514.06016.52
- Provan SA, Berg IJ, Hammer HB, Mathiessen A, Kvien TK, Semb AG. The impact of newer biological disease Modifying Anti-Rheumatic drugs on Cardiovascular Risk Factors: A 12-month longitudinal study in Rheumatoid Arthritis patients treated with Rituximab, Abatacept and Tociliziumab. PLoS One 2015; 10:e0130709; PMID:26114946; http://dx.doi.org/10.1371/journal.pone.0130709
- Kudo U, Takahashi I, Matsuzaka M, Umeda T, Kitagawa N, Kudo H, Chiba Y, Sasaki E, Nishimura M, Nakaji S. Influence of obesity on blood pressure and arterial stiffness in the early teens. Obes Res Clin Pract 2013; 7:e211-217; PMID:23697590; http://dx.doi.org/10.1016/j.orcp.2011.12.005
- Hvidt KN, Olsen MH, Ibsen H, Holm JC. Weight reduction and aortic stiffness in obese children and adolescents: a 1-year follow-up study. J Hum Hypertens 2015; 29:535-40; PMID:25589213; http://dx.doi.org/10.1038/jhh.2014.127
- O'Rourke MF, Hashimoto J. Mechanical factors in arterial aging: a clinical perspective. J Am Coll Cardiol 2007; 50:1-13; PMID:17601538; http://dx.doi.org/10.1016/j.jacc.2006.12.050
- Mattace-Raso FU, van der Cammen TJ, van den Elzen AP, Schalekamp MA, Asmar R, Reneman RS, Hoeks AP, Hofman A, Witteman JC. Moderate alcohol consumption is associated with reduced arterial stiffness in older adults: the Rotterdam study. J Gerontol A Biol Sci Med Sci 2005; 60:1479-83; PMID:16339338; http://dx.doi.org/10.1093/gerona/60.11.1479
- Tyrovolas S, Haro JM, Polychronopoulos E, Mariolis A, Piscopo S, Valacchi G, Makri K, Zeimbekis A, Tyrovola D, Bountziouka V, et al. Factors associated with components of arterial pressure among older individuals (the multinational MEDIS study): the role of the Mediterranean diet and alcohol consumption. J Clin Hypertens (Greenwich) 2014; 16:645-51; PMID:25056587; http://dx.doi.org/10.1111/jch.12370
- Collins MA, Neafsey EJ, Mukamal KJ, Gray MO, Parks DA, Das DK, Korthuis RJ. Alcohol in moderation, cardioprotection, and neuroprotection: epidemiological considerations and mechanistic studies. Alcohol Clin Exp Res 2009; 33:206-19; PMID:19032583; http://dx.doi.org/10.1111/j.1530-0277.2008.00828.x
- Kelso NE, Sheps DS, Cook RL. The association between alcohol use and cardiovascular disease among people living with HIV: a systematic review. Am J Drug Alcohol Abuse 2015; 41:479-88; PMID:26286352
- Freiberg MS, Kraemer KL. Focus on the heart: alcohol consumption, HIV infection, and cardiovascular disease. Alcohol Res Health 2010; 33:237-46; PMID:23584065
