ABSTRACT
Melioidosis is a severe infectious disease with a high mortality that is endemic in South-East Asia and Northern Australia. The causative pathogen, Burkholderia pseudomallei, is listed as potential bioterror weapon due to its high virulence and potential for easy dissemination. Currently, there is no licensed vaccine for prevention of melioidosis. Here, we explore the use of rapid plasmid DNA vaccination against B. pseudomallei flagellin for protection against respiratory challenge.
We tested three flagellin DNA vaccines with different subcellular targeting designs. C57BL/6 mice were vaccinated via skin tattoo on day 0, 3 and 6 before intranasal challenge with B. pseudomallei on day 21. Next, the most effective construct was used as single vaccination on day 0 by tattoo or intranasal formulation. Mice were sacrificed 72 hours post-challenge to assess bacterial loads, cytokine responses, inflammation and microscopic lesions.
A construct encoding a cellular secretion signal resulted in the most effective protection against melioidosis via tattooing, with a 10-fold reduction in bacterial loads in lungs and distant organs compared to the empty vector. Strikingly, a single intranasal administration of the same vaccine resulted in >1000-fold lower bacterial loads and increased survival. Pro-inflammatory cytokine responses were significantly diminished and strong reductions in markers for distant organ damage were observed.
A rapid vaccination scheme using flagellin DNA tattoo provides significant protection against intranasal challenge with B. pseudomallei, markedly improved by a single administration via airway mucosa. Hence intranasal vaccination with flagellin-encoding DNA may be applicable when acute mass vaccination is indicated and warrants further testing.
Introduction
Burkholderia pseudomallei is a facultative intracellular Gram negative bacterium that causes melioidosis and is endemic in Southeast Asia and Northern Australia.Citation1,2 Infection usually occurs through dermal lesions via rice paddies and infected soil. During severe weather events, when the soil is disturbed and B. pseudomallei becomes aerosolized, inhalation is thought to cause infection. Importantly, B. pseudomallei was recently upgraded to a Tier 1 select agent by the National Select Agent Registry due to its high associated morbidity and mortality, intrinsic resistance to standard antimicrobial agents and lack of a vaccine. Even when treated with adequate antibiotics, mortality remains high and varies between 14 to 40% depending on geographic regions.Citation1,2 Deliberate release in an aerosol form may infect large populations and poses a potential bioterrorist threat.
Despite ongoing efforts, no vaccine is currently available in humans. The requirements of a vaccination strategy for large populations that are at risk for B. pseudomallei bioterrorism differ from those for populations in endemic areas.Citation3 While the latter requires a cost-effective vaccine primarily targeted at risk groups such as diabetics, the former should be rapidly producible and applicable in large groups of people, ideally by self-administration. In addition, the different routes of infection (i.e. aerosol distribution in case of bioterrorism) should be accounted for in the vaccination approach.Citation3
Vaccination studies have focused on different methods, including live attenuated -, whole cell killed -, subunit -, recombinant - and plasmid DNA vaccines.Citation4,5 Live attenuated vaccination is currently regarded as the “gold standard” in mouse models, but will have limited value in humans given the potential risk of reversion into a virulent phenotype.Citation4 Vaccination with subunit and recombinant protein vaccines in Complete Freund's Adjuvant (CFA) is unattractive in humans, since the subcutaneously injected vaccine may induce painful granulomas.Citation6 DNA vaccines induce both humoral and cell-mediated immune responsesCitation7 and have the additional benefit of being rapidly producible and having a long shelf life without the need of a cold chain.Citation7
Flagellin is a suitable antigen candidate for vaccine development, as it is an important putative virulence factor of B. pseudomallei.Citation8 It signals via Toll like receptor-5, which is upregulated in granulocytes and monocytes of patients with melioidosis.Citation9 The only study so far on intramuscular DNA vaccination against B. pseudomallei flagellin (FliC) showed diminished bacterial loads and improved survival in mice.Citation10 In this study, flagellin-encoding plasmid DNA was injected intramuscularly in BALB/c mice on day 0, 7 and 14, followed by intravenous challenge with 105 CFU B. pseudomallei (mixture of 16 strains) eight weeks post-vaccination. In a follow-up study, using similar routes of immunization and infection, the authors reported induction of Th1-type responses as the underlying mechanism, which could be augmented by CpG oligodeoxynucleotides.Citation11
Application of DNA vaccines by dermal tattoo was previously shown to improve T-cell immunity.Citation12 When DNA is injected via thousands of skin perforations, an adjuvant inflammatory milieu is generated, making immune responses more robust and allowing for faster vaccination regimens.Citation13 We and others have shown that rapid immunization schedules with short times between immunizations with DNA vaccines can be effective against HPV-induced tumors, viruses and bacteria, and that rapid DNA vaccination by dermal tattoo is more potent than intramuscular administration.Citation12-15 Another recent development is mucosal immunization, directed at inducing tissue-resident memory T-cells.Citation16 DNA vaccination via the airway mucosa would theoretically be very well-suited to prevent infection by inhalation.Citation7,17 Several DNA vaccine formulations have been described in this context, one of which involving polymers such as polyethylenimine (PEI) that increase mucosal transfection by approximately thousand fold.Citation18-21
In this study, we have explored rapid application of B. pseudomallei flagellin DNA as a possible vaccine for biodefense use, comparing dermal tattooing and intranasal administration. First, we compared multiple FliC encoding DNA vaccine designs, applied in a rapid tattoo immunization schedule. Next, using the most efficient vaccine, we tested the efficacy of intranasal administration against intranasal melioidosis.
Results
Designing and testing of FliC plasmid DNA vaccine candidates
In order to select a DNA vaccine that rapidly protects against intranasal B. pseudomallei infection, we designed three constructs with FliC sequences in plasmid vector pVAX (“pVAX-FliC”). All vaccines were codon-optimized for mouse tRNA and contained a Kozak sequence to optimize translation efficiency and ribosomal binding (). “pVAX-hTPA-FliC” contained an N-terminal signal peptide from human tissue plasminogen activator (hTPA), thus enabling protein secretion and augmenting MHC-II presentation by antigen presenting cells. “pVAX-FliC-KDEL” had a four-amino acid C-terminal KDEL sequence leading to FliC accumulation in the endoplasmatic reticulum (ER) of transfected cells, which via ER stress and improved MHC-I presentation may increase T-cell priming effectiveness of the vaccine. Mice were immunized by tattoo vaccination with 20 µg of plasmid DNA on day 0, 3 and 6, with a control group receiving an equal amount of empty pVAX vector via tattoo. After 20 days, all vaccine designs had induced significantly elevated anti-FliC IgG levels in plasma compared to the empty pVAX control (; pVAX-FliC: p = 0.020; pVAX-hTPA-FliC: p = 0.006; pVAX-FliC-KDEL: p = 0.007). No significant differences in antibody levels were observed between FliC vaccinated groups.
Figure 1. Decreased bacterial loads via rapid DNA tattooing during experimental intranasal melioidosis. (A) Schematic overview of DNA vaccine designs based on B. pseudomallei flagellin (FliC), cloned into the pVAX1 vector. All vaccines are codon optimized and contain a Kozak sequence to optimize ribosomal binding. hTPA-FliC contains an N-terminal cellular secretion signal, FliC-KDEL contains a C-terminal endoplasmatic reticulum retention signal. (B) FliC-specific IgG induction in plasma after rapid tattoo vaccination at t = 0, 3 and 6 days (indicated by arrows). Bars represent mean ± SEM (C-F) Three weeks after the first vaccination, mice were inoculated intranasally with 200 CFU B. pseudomallei and sacrificed 72 hours later. Bacterial loads in lung (C), blood (D), liver (E) and spleen homogenate (F) are depicted as scatter dot plots with a line at the median (n = 8 mice per group). Numbers in the box below (D) indicate the number of positive blood cultures/total number of mice. The vaccinated groups were compared to the empty pVAX control group using a Mann-Whitney test. #p < 0.05, ##p < 0.01. For pVAX-hTPA-FliC vs. empty pVAX blood CFU a Chi-square test was performed (p = 0.007)
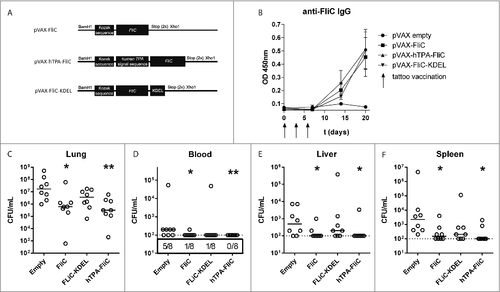
On day 21 post-vaccination, mice were challenged intranasally with 200 colony forming units (CFU) of B. pseudomallei 1026b followed by sacrifice 72 hours after infection, when all mice usually have symptoms of systemic infection. Bacterial loads were determined in lung, blood, liver and spleen (). Significantly reduced bacterial loads were found in both lung, blood and distant organs in mice vaccinated with pVAX-FliC and pVAX-hTPA-FliC, with the lowest bacterial concentrations in the lung observed in pVAX-hTPA-FliC vaccinated mice. In addition, all pVAX-hTPA-FliC vaccinated mice were blood-culture negative. Immunization with pVAX-FliC-KDEL was less effective than pVAX-FliC, with only a trend toward reduced bacterial loads compared to the empty pVAX control.
Since cytokines and chemokines are important regulators of the host response during melioidosis,Citation8,22 we next measured pulmonary and systemic levels of tumor necrosis factor-alpha (TNFα), interleukin (IL)-6, interferon (IFN)γ and chemokine ligand-1 (CXCL1, ). For lung cytokine production, pVAX-hTPA-FliC was the only vaccine that significantly reduced both TNFα, IL-6 and CXCL1 (). Plasma cytokine concentrations were significantly reduced as well, in particular IFNγ. In contrast with the observed bacterial loads, pVAX-FliC-KDEL vaccination resulted in reduced plasma levels of TNFα, IL-6 and IFNγ, whereas pVAX-FliC only significantly decreased IL-6 levels. Intranasal inoculation with B. pseudomallei is associated with profound lung and distant organ pathology.Citation9,23,24 A trend toward lower lung- and liver histopathologic scores was observed for all FliC vaccines, as well as a lower influx of neutrophils to the lungs as reflected by Ly6G staining, but this was not statistically significant (Fig. S1; see Supplementary Methods for histopathology scoring criteria). Combining these results, the cellular secretion signal of pVAX-hTPA-FliC appeared to improve the effectivity of the DNA vaccine. We therefore selected pVAX-hTPA-FliC as the vaccine design with the most potential for further testing.
Figure 2. Decreased proinflammatory pulmonary and systemic cytokine levels in rapid DNA tattoo-vaccinated mice during experimental intranasal melioidosis. Mice were given rapid tattoo vaccination at t = 0, 3 and 6 days followed by intranasal bacterial challenge (200 CFU B. pseudomallei) on day 21. Mice were sacrificed 72 hours after intranasal B. pseudomallei challenge and heparinized blood plasma and lung homogenates were obtained. TNFα (A), IL-6 (B) and CXCL1 (C) were measured in lung homogenate; TNFα (D), IL-6 (E) and IFNγ (F) were measured in plasma. Values are in pg/mL; data are presented as box- and whisker plots showing the smallest observation, lower quartile, median, upper quartile and largest observation. N = 8 mice per group. Vaccinated groups were compared to the control group (empty pVAX) using a Mann Whitney test. #p < 0.05, ##p < 0.01, ###p < 0.001
Single intranasal administration of pVAX-hTPA-FliC strongly reduces bacterial loads
Next, we were interested in the effects of a single vaccine delivery on preventing imminent intranasal B. pseudomallei infection. Therefore, we compared a single DNA vaccination on day 0, either via tattoo or intranasally, with recombinant FliC vaccination plus Complete Freund's Adjuvant (CFA, administered subcutaneously) as a positive control or empty pVAX tattoo as a negative control (). After a single pVAX-hTPA-FliC tattoo, the FliC-specific total IgG antibody response on day 20 was not as robust as after triple vaccination (). No evident skewing toward IgG1 or IgG2a was detected. Interestingly, intranasal DNA vaccination did not induce a FliC- specific IgG response at all, whereas recombinant FliC with CFA induced a strong humoral response. Serum IgA was below detection in all experimental groups (data not shown).
Figure 3. A single intranasal vaccination with pVAX-hTPA-FliC is more effective in lowering bacterial loads than single tattoo administration during experimental intranasal melioidosis. A single dose of pVAX-hTPA-FliC was administered on day 0 either via tattoo or intranasally and compared with recombinant FliC + CFA s.c. as a positive control or empty pVAX tattoo as a negative control. All mice were inoculated intranasally with 300 CFU B. pseudomallei on day 21. (A) FliC-specific IgG in plasma at day 0 and 20 after vaccination. Bars represent mean ± SEM (B-E) Bacterial loads in blood and organ homogenates 72 hours after infection, depicted as scatter dot plots with a line at the median. Groups were compared using a Kruskal Wallis test followed by Dunns multiple comparisons test; #p < 0.05, ##p < 0.01, ###p < 0.001 versus empty pVAX; # p<0.05 vs. pVAX-hTPA-FliC tattoo. N = 7 or 8 mice per group. s.c., subcutaneous; i.n., intranasal; tt, tattoo
On day 21, mice were infected with 300 CFU B. pseudomallei and sacrificed 72 hours later as in the previous experiment. A single tattoo vaccination with pVAX-hTPA-FliC did not lower bacterial loads, compared to the empty pVAX tattoo control group (). However, a single intranasal application of the same DNA vaccine very effectively diminished bacterial growth and dissemination to distant organs, i.e., more than 1000-fold compared to the empty pVAX tattoo control group. This equaled the efficacy of recombinant FliC with CFA. In a separate set of experiments we compared single intranasal vaccination with pVAX-hTPA-FliC with empty pVAX intranasally and a control group without any vaccine (Fig. S2). This experiment confirmed the effectiveness of a single intranasal vaccination with pVAX-hTPA-FliC in lowering bacterial burdens. The intranasal empty pVAX vaccine formulated in PEI did not alter bacterial loads in blood or organs compared to the non-vaccinated group, confirming the antigen dependency of the pVAX-hTPA-FliC induced protection.
Single intranasal vaccination with pVAX-hTPA-FliC reduces lung inflammation and damage
To further investigate the effect of vaccination on lung inflammation, we first assessed lung cytokine levels (). Pulmonary IL-6 and CXCL1 were strongly reduced by recombinant FliC with CFA, as well as by intranasal pVAX-hTPA-FliC (p<0.001 compared to tattoo with empty pVAX vector; p = 0.06 for IL-6 and p = 0.08 for CXCL1compared to recombinant FliC with CFA). Intranasal pVAX-hTPA-FliC in addition resulted in significantly lower levels of TNFα (p<0.01 compared to empty pVAX vector, ). A trend toward lower IL-6 and CXCL1 was observed for single tattoo vaccination with pVAX-hTPA-FliC, but this did not reach statistical significance. We also measured neutrophil influx by staining lung sections for Ly6G, an important marker of neutrophils. As expected, the percentage of surface positive for Ly6G was significantly reduced by both intranasal pVAX-hTPA-FliC and recombinant FliC with CFA (). Neutrophil degranulation, reflected by myeloperoxidase levels, was not different between the experimental groups ().
Figure 4. Decreased pulmonary cytokine levels and lung pathologic scores after single intranasal vaccination with pVAX-hTPA-FliC. All mice were given a single vaccination on day 0 (tattoo, subcutaneously or intranasally) and inoculated intranasally with 300 CFU B. pseudomallei on day 21. Lungs were harvested 72 hours after intranasal challenge with B. pseudomallei. TNFα (A), IL-6 (B) and CXCL1 (C) in lung homogenate; values are in pg/mL. Paraffin-embedded lung tissue sections were stained for Ly6G, a marker for neutrophil infiltration, and the percentage of the total lung surface positive for Ly6G was calculated digitally (D). As a representation for neutrophil degranulation, myeloperoxidase (MPO) was measured in lung homogenates (E). Lung tissue sections were stained with haematoxylin/eosin and scored on different parameters for pathology by a blinded pathologist (F). Representative images (with the median score) of the empty pVAX tattoo (G), rFliC + CFA (H), pVAX-hTPA-FliC tattoo (I) and pVAX-hTPA-FliC i.n. (J) groups (4x magnification). Data are presented as box- and whisker plots showing the smallest observation, lower quartile, median, upper quartile and largest observation. Groups were compared using a Kruskal Wallis test followed by Dunns multiple comparisons test; #p < 0.05, ##p < 0.01, ###p < 0.001 vs. empty pVAX; # p <0.05 vs. pVAX-hTPA-FliC tattoo. N = 7 or 8 mice per group. s.c., subcutaneous; i.n., intranasal; tt, tattoo
Lastly, HE stained sections were scored by a blinded pathologist for several parameters of inflammation, which were combined into a lung histopathologic score (; see Supplementary Methods for histopathology scoring criteria). All mice showed microscopic lesions in the lungs characterized by necrosis, interstitial inflammation, bronchitis, endothelialitis and edema 72 hours after inoculation with B. pseudomallei. Importantly, intranasal vaccination with pVAX-hTPA-FliC significantly reduced melioidosis- induced lung histopathology scores when compared to controls. Representative lung sections of each group are shown in .
Single intranasal vaccination with pVAX-hTPA-FliC reduces systemic inflammation and organ damage
Systemic cytokine production followed a similar pattern as in the lungs ( A-D). TNFα, IL-6, IFNγ and MCP1 in plasma were all significantly lower in the intranasally pVAX-hTPA-FliC vaccinated group compared to the empty pVAX vector tattoo group, whereas for recombinant FliC in CFA this was only true for IL-6 and MCP1. A liver histopathologic score was generated in a similar way as for the lungs (; see Supplementary Methods for histopathology scoring criteria). Intranasal pVAX-hTPA-FliC protected against B. pseudomallei induced liver pathology as reflected by strongly reduced liver histopathologic scores (). In line with this finding, plasma levels of markers for hepatocellular damage alanine aminotransferase (ALT) and aspartate aminotransferase (AST) remained unaffected upon infection, in sharp contrast to the controls (). Finally, lactate dehydrogenase (LDH) was measured in plasma as a marker for general cellular damage (). Notably, this was only decreased in the intranasal pVAX-hTPA-FliC group.
Figure 5. Diminished plasma cytokine levels and distant organ injury after single intranasal vaccination with pVAX-hTPA-FliC. All mice were given a single vaccination on day 0 (tattoo, subcutaneously or intranasally) and inoculated intranasally with 300 CFU B. pseudomallei on day 21. Plasma TNFα (A), IL-6 (B), IFNγ (C) and MCP-1 (D); values are in pg/mL. Liver tissue sections were stained with haematoxylin/eosin and scored on different parameters by a blinded pathologist, combined in a pathology score (E). ALT (F), AST (G) and LDH (H) were measured in plasma as markers for liver- and general cellular damage. Data are presented as box- and whisker plots showing the smallest observation, lower quartile, median, upper quartile and largest observation. N = 6–8 samples per group. Groups were compared using a Kruskal Wallis test followed by Dunns multiple comparisons test; #p < 0.05, ##p < 0.01, ###p < 0.001 vs. empty pVAX; # p < 0.05 vs. rFliC + CFA s.c. s.c., subcutaneous; i.n., intranasal; tt, tattoo
Single intranasal vaccination with pVAX-hTPA-FliC increases survival
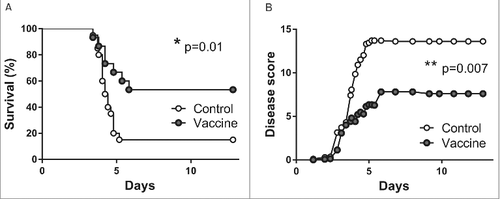
To investigate whether the observed beneficial effects of intranasal vaccination would also affect outcome after intranasal infection with B. pseudomallei, we performed a survival experiment of 14 days. Twenty mice per group were vaccinated intranasally once with pVAX-hTPA-FliC and compared to twenty control mice that did not receive any vaccine. Unfortunately, the intranasally administered DNA vaccine led to undesired weight loss in some of the mice during this experiment, due to which four mice died and one was euthanized because of reaching a humane end point (>20% weight loss; all five were lost before the start of the survival experiment and are therefore not included in the statistics). At the moment of bacterial challenge, vaccinated- and control mice had similar weights. Intranasally vaccinated mice had significantly increased survival (53% in the vaccinated group vs. 15% in the unvaccinated control group, ). In addition, a clinical observation score reflects the severity of disease over time in both groups. In accordance with the survival curves, disease severity is significantly lower in the vaccinated group ().
Figure 6. Increased survival after single intranasal vaccination with pVAX-hTPA-FliC. Survival (A) and clinical observation score (B) of control (open dots, n = 20) and intranasally vaccinated mice (gray dots, n = 15). All mice in the vaccinated group were given a single intranasal vaccination on day 0 and inoculated intranasally with 500 CFU B. pseudomallei on day 21. Controls did not receive any vaccine. Survival was monitored for 14 days. Data are presented as Kaplan-Meier survival curves. #p < 0.05, ##p < 0.01
Discussion
To our knowledge, this study is the first to investigate rapid vaccination against B. pseudomallei flagellin using plasmid DNA administered via tattoo or intranasally. A pVAX vector construct encoding secreted flagellin proved most effective at inducing a balanced immune response against B. pseudomallei, reducing bacterial burdens and improving survival without resulting in severe cytokine-induced damage. Although IFNγ is believed to play a protective role in the host defense against melioidosis,Citation1,25,26 levels measured in both the systemic and pulmonary compartment during experimental melioidosis are also a reflection of disease severity; lower levels are observed in subjects with a relatively mild disease.Citation23,27 As a result, the observed lower levels of IFNγ in vaccinated mice after infection with B. pseudomallei are most probably a reflection of a decreased inflammatory state. A single intranasal administration of the aforementioned vaccine was equally effective as subcutaneous injection of recombinant protein in CFA, strongly diminishing bacterial loads and organ damage. Intranasal DNA vaccination was the only vaccine approach that significantly diminished lung and liver pathology after a single dose. Survival of intranasally vaccinated mice was significantly increased compared to unvaccinated controls.
Single dermal DNA vaccination induced inferior IgG levels compared to the rapid immunization schedule of three immunizations within six days. Interestingly, single intranasal DNA vaccination did not induce an IgG response at all, while being more effective in lowering histopathologic scores and cytokine levels compared to recombinant protein vaccination, which induced a strong IgG response. Because intranasal DNA vaccination against B. pseudomallei has not been previously described, further studies are required to provide better insight into the immunological characteristics needed for such vaccines to optimally protect against melioidosis.
In the context of bioterrorism by deliberate release of aerosolized B. pseudomallei, a rapidly working vaccine aimed at preventing a B. pseudomallei pneumonia that can easily be administered can be of paramount importance. It has been described previously in mouse models of melioidosis that administration of both vaccine and bacteria via the same route induces stronger protection.Citation4 Intraperitoneally administered vaccine candidates had less protective efficacy after inhalation of B. pseudomallei than after intraperitoneal challenge; likewise, intranasal vaccination was more effective than intraperitoneal vaccination when bacteria were administered via the nose.Citation28-30 Our results support the notion that vaccination via the airways is a suitable vaccination route to protect against aerosolized B. pseudomallei infection, as was suggested by a number of studies.Citation28,31,32 Overall, many vaccine candidates are still either considered unsafe for use in humans (live attenuated) or only induce humoral responses (purified or recombinant antigens, heat-killed whole cell vaccines) that may be insufficient to combat intracellular B. pseudomallei infection. DNA vaccines were shown to be effective against intracellular bacteria as well, allegedly through inducing a protective cellular immune response.Citation17,33,34 The results of our study suggest that a strong cellular immune response was elicited, as we observed a strong reduction in bacterial loads through a single intranasal vaccination, with almost no detectable antibody response after the intranasal pVAX-hTPA-FliC vaccination. One important drawback of intranasal DNA vaccines is the possibility of vaccination related morbidity, manifesting as weight loss after vaccination, similar to weight loss observed after intranasal influenza infection.Citation14 In young mice with a low body weight this can be life threatening. We experienced this in one of the four experiments presented in this paper (the survival experiment). Importantly, all mice - vaccinated and control mice - had similar weights at the time of bacterial challenge.
We did not include the impact of this vaccine on cell-mediated immunity in our study. Also, we did not test our vaccine in other mouse strains or in a diabetic mouse model, nor with a second strain of B. pseudomallei. Neither did we compare our vaccine candidate with the current “gold standard” (i.e., a live attenuated vaccine). These experiments would abide the consensus criteria for the development of melioidosis vaccines that have been published recently.Citation3 However, these criteria are of special relevance in the development of a vaccine for the general population in endemic areas, as illustrated by the requirement that bacterial challenge should take place no sooner than four weeks after vaccination. Clearly, earlier challenge is specifically suitable to test the effectiveness of vaccines in an acute bioterrorism setting.
Future research should focus on understanding the underlying mechanisms of the immunity induced by intranasal DNA vaccination. In addition, it will be of interest to see whether the tattoo-administered vaccine has superior effectivity when bacteria are inoculated intradermally. The intradermal route might be more applicable to at risk populations in endemic areas, where the majority of infections are thought to occur via the cutaneous route.Citation1,2 Using different vaccination regimens and routes of administration, a cost effective and safe DNA vaccine could be extremely valuable for both people at risk in endemic areas and for preparedness planning for potential deliberate release of B. pseudomallei.
Material and methods
Plasmid DNA sequences, experimental infection, sample harvesting, determination of bacterial loads and assays are described in more detail in the online supplement.
Mice
Seven-week old female specific pathogen-free C57BL/6 mice were purchased from Charles River. After one week of acclimatization, vaccination was performed at eight weeks of age. Infection was induced at eleven weeks of age. Animals were housed in IVC cages in rooms with a controlled temperature and light cyclus and received standard rodent chow and water ad libitum. The Institutional Animal Care and Use Committee of the Academic Medical Center approved all experiments.
DNA vaccines
Three DNA vaccine inserts were designed based on the FliC gene of B. pseudomallei 1026b (Genbank accession U73848.1) and synthetized (Biobasic inc.). The FliC sequence was codon-optimized to murine codon usage, using JCAT web-based software.Citation35 A Kozak sequence was added to all vaccines, which consists of a GCCACC before the start codon and an additional GAC triplet immediately following the start codon, leading to increased ribosomal binding. Also, a double stop codon was added. The inserts, flanked by a BamH1 and Xho1 restriction site, were cloned into pVAX1 and amplified using the Nucleobond Xtra EF kit (Macherey-Nagel) to form construct “pVAX-FliC.” Construct “pVAX-FliC-KDEL” was similarly generated with an additional 5′end AAGGACGAGCTG sequence (amino acid code: KDEL) immediately before the stop codon, thus adding an endoplasmatic reticulum retention signal. For construct “pVAX-hTPA-FliC” the start codon was immediately followed by the codon-optimized hTPA signal sequence (GenBank accession AAA61213.1), thus targeting the gene product to secretory vesicles. Full insert sequences are described in the Supplemental Material.
Immunization
In the first experiment, vaccination was performed at day 0, 3 and 6. Eight mice per group were anesthetized using 2–4% isoflurane and their abdomens were shaved followed by additional hair removal using cream (VEET, Reckitt Benckiser). 20 µg of DNA vaccine or a control vaccine (empty pVAX) in 10 μL sterile water was administered to the naked skin and vaccination was performed for 45 seconds at 100 Hz using a tattoo machine as described previously.Citation13 In the following experiments, 20 μg pVAX-hTPA-FliC or empty pVAX was administered at day 0 either via tattoo or intranasally. To optimize stability and transfection efficiency in the intranasally vaccinated group, the pVAX-hTPA-FliC was administered in 50 uL sterile, endotoxin free water with 6,5 vol/vol% polyethylenimine (PEI, PolyPlus Transfection Inc.) and 5% glucose. In the positive control group, an emulsion of 50 μg recombinant flagellin of B. pseudomallei 1026b (kindly provided by professor Donald E. Woods, University of Calgary, Alberta, Canada) with 50 μL Complete Freund's Adjuvant (CFA) was injected subcutaneously at two different sites at the back of the mouse.
Induction of melioidosis and sample harvesting
Plasma was collected by tail vein bleeds at day 0, 7, 14 and 20. On day 21, experimental melioidosis was induced by intranasal inoculation with 200–500 colony forming units (CFU; approximately LD50) of B. pseudomallei strain 1026b in 50 µL sterile saline as described.Citation9,23,24 At 72 hours post-infection, mice were euthanized (ketamine 75mg/kg and medetomedine 1,0 mg/kg intraperitoneally) and sacrificed by bleeding from the heart, after which organs were harvested.Citation9,23,24 Tissues were homogenized, serially diluted and plated to assess bacterial loads (for full methods see Supplemental Material).
Antibody responses
Induction of FliC-specific IgG was measured by coating 1 µg/mL rFliC on high-binding ELISA plates (Greiner Bio-one) overnight at 4°C, followed by blocking with 1% bovine serum albumin (BSA) in phosphate buffered saline (PBS) for 2 hours, washing with PBS-0.05%Tween, and incubation with 1:100 diluted mouse sera in 1% BSA/PBS for 1 hour. Next, plates were washed and incubated with 1:2500 anti-mouse IgG-HRP (Cell signaling) in 1% BSA-PBS for 30 minutes. After washing, plates were developed using 3,3′,5,5′-Tetramethylbenzidine substrate and read at 450 nm (Biotek).
Survival and clinical observation score
Mice were observed for 14 days after intranasal inoculation to study survival. Clinical signs were scored as previously described:Citation36 solitude (0 absent, 1 present), posture (0 normal, 1 sphere), fur (0 normal, 1 pilo-erection), eyes (0 open, 1 closed, 2 dirty), alertness (0 normal, 1 slow, 2 apathic, 3 non-responsive), pace (0 normal, 1 shaky, 2 collapse), respiration (0 normal, 1 heavy, 2 slow, 3 intermittent) and time to ascent when laid down (0 normal, 1 <5 seconds, 2 >5 seconds, 3 unresponsive); resulting in a maximum score of 16, which was also given to deceased mice. Four humane end points were enforced: if the animal was non-responsive; if time to ascent was >5 seconds, or if the animal collapsed.
Statistical analysis
Differences between groups were analyzed by one-way ANOVA followed by Dunns multiple comparisons test or Mann Whitney test as indicated. For survival analysis, Kaplan-Meier analysis followed by log-rank test was performed. Clinical disease scores were analyzed by matched two-way ANOVA (all using GraphPad Prism 5, GraphPad Software). Values of p < 0.05 were considered statistically significant.
Abbreviations
| ALT | = | alanine aminotransferase |
| AST | = | aspartate aminotransferase |
| BSA | = | bovine serum albumin |
| CFA | = | Complete Freund's Adjuvant |
| CFU | = | colony forming units |
| FliC | = | flagellin |
| HE | = | haematoxylin/eosin |
| IFN | = | interferon |
| IL | = | interleukin |
| LDH | = | lactate dehydrogenase |
| PBS | = | phosphate-buffered saline |
| PEI | = | polyethylenimine |
| TNF | = | tumor necrosis factor |
| TPA | = | tissue plasminogen activator |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
KVIR_S_1307485.doc
Download MS Word (1.3 MB)Acknowledgments
The authors would like to thank Marieke ten Brink, Joost Daalhuisen and Regina de Beer for their indispensable technical assistance in these experiments. Onno J. de Boer, pathologist, was of great help in the analysis of the neutrophil stainings.
Funding
This work was supported by the Netherlands Organization for Scientific Research (NWO; VIDI grant tot WJW, grant number: 91716475; VENI grant to JWH, grant number 91611065; and VENI grant to ADB, grant number 91610005). The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
References
- Wiersinga WJ, Currie BJ, Peacock SJ. Melioidosis. The N Engl J Med 2012; 367:1035-44.
- Currie BJ. Melioidosis: evolving concepts in epidemiology, pathogenesis, and treatment. Semin Respir Crit Care Med 2015; 36:111-25; PMID:25643275; https://doi.org/https://doi.org/10.1055/s-0034-1398389
- Limmathurotsakul D, Funnell SG, Torres AG, Morici LA, Brett PJ, Dunachie S, Atkins T, Altmann DM, Bancroft G, Peacock SJ, Steering Group on Melioidosis Vaccine Development. Consensus on the development of vaccines against naturally acquired melioidosis. Emerg Infect Dis 2015; 21(6); PMID:25992835; https://doi.org/https://doi.org/10.3201/eid2106.141480
- Peacock SJ, Limmathurotsakul D, Lubell Y, Koh GC, White LJ, Day NP, Titball RW. Melioidosis vaccines: a systematic review and appraisal of the potential to exploit biodefense vaccines for public health purposes. PLoS Negl Tropical Dis 2012; 6:e1488; PMID:22303489; https://doi.org/https://doi.org/10.1371/journal.pntd.0001488
- Bondi SK, Goldberg JB. Strategies toward vaccines against Burkholderia mallei and Burkholderia pseudomallei. Expert Rev Vaccines 2008; 7:1357-65; PMID:18980539; https://doi.org/https://doi.org/10.1586/14760584.7.9.1357
- Bennett B, Check IJ, Olsen MR, Hunter RL. A comparison of commercially available adjuvants for use in research. J Immunol Methods 1992; 153:31-40; PMID:1517600
- Gurunathan S, Klinman DM, Seder RA. DNA vaccines: immunology, application, and optimization. Annu Rev Immunol 2000; 18:927-74; PMID:10837079; https://doi.org/https://doi.org/10.1146/annurev.immunol.18.1.927
- Wiersinga WJ, van der Poll T, White NJ, Day NP, Peacock SJ. Melioidosis: insights into the pathogenicity of Burkholderia pseudomallei. Nat Rev Microbiol 2006; 4:272-82; PMID:16541135; https://doi.org/https://doi.org/10.1038/nrmicro1385
- Wiersinga WJ, Wieland CW, Dessing MC, Chantratita N, Cheng AC, Limmathurotsakul D, Chierakul W, Leendertse M, Florquin S, de Vos AF, et al. Toll-like receptor 2 impairs host defense in gram-negative sepsis caused by Burkholderia pseudomallei (Melioidosis). PLoS Med 2007; 4:e248; PMID:17676990; https://doi.org/https://doi.org/10.1371/journal.pmed.0040248
- Chen YS, Hsiao YS, Lin HH, Yen CM, Chen SC, Chen YL. Immunogenicity and anti-Burkholderia pseudomallei activity in Balb/c mice immunized with plasmid DNA encoding flagellin. Vaccine 2006; 24:750-8; PMID:16169637; https://doi.org/https://doi.org/10.1016/j.vaccine.2005.08.069
- Chen YS, Hsiao YS, Lin HH, Liu Y, Chen YL. CpG-modified plasmid DNA encoding flagellin improves immunogenicity and provides protection against Burkholderia pseudomallei infection in BALB/c mice. Infect Immunity 2006; 74:1699-705; PMID:16495541; https://doi.org/https://doi.org/10.1128/IAI.74.3.1699-1705.2006
- van den Berg JH, Oosterhuis K, Schumacher TN, Haanen JB, Bins AD. Intradermal vaccination by DNA tattooing. Methods Mol Biol 2014; 1143:131-40; PMID:24715286; https://doi.org/https://doi.org/10.1007/978-1-4939-0410-5_9
- Wagemakers A, Mason LM, Oei A, de Wever B, van der Poll T, Bins AD, Hovius JW. Rapid outer-surface protein C DNA tattoo vaccination protects against Borrelia afzelii infection. Gene Ther 2014; 21:1051-7; PMID:25273355; https://doi.org/https://doi.org/10.1038/gt.2014.87
- Bins AD, Jorritsma A, Wolkers MC, Hung CF, Wu TC, Schumacher TN, Haanen JB. A rapid and potent DNA vaccination strategy defined by in vivo monitoring of antigen expression. Nat Med 2005; 11:899-904; PMID:15965482; https://doi.org/https://doi.org/10.1038/nm1264
- van de Wall S, Walczak M, van Rooij N, Hoogeboom BN, Meijerhof T, Nijman HW, Daemen T. Tattoo delivery of a Semliki Forest Virus-Based Vaccine Encoding Human Papillomavirus E6 and E7. Vaccines (Basel) 2015; 3:221-38; PMID:26343186; https://doi.org/https://doi.org/10.3390/vaccines3020221
- Stary G, Olive A, Radovic-Moreno AF, Gondek D, Alvarez D, Basto PA, Perro M, Vrbanac VD, Tager AM, Shi J, et al. VACCINES. A mucosal vaccine against Chlamydia trachomatis generates two waves of protective memory T cells. Science 2015; 348:aaa8205; PMID:26089520; https://doi.org/https://doi.org/10.1126/science.aaa8205
- Ingolotti M, Kawalekar O, Shedlock DJ, Muthumani K, Weiner DB. DNA vaccines for targeting bacterial infections. Expert Rev Vaccines 2010; 9:747-63; PMID:20624048; https://doi.org/https://doi.org/10.1586/erv.10.57
- Xu Y, Yuen PW, Lam JK. Intranasal DNA Vaccine for protection against respiratory infectious diseases: The delivery perspectives. Pharmaceutics 2014; 6:378-415; PMID:25014738; https://doi.org/https://doi.org/10.3390/pharmaceutics6030378
- Wegmann F, Gartlan KH, Harandi AM, Brinckmann SA, Coccia M, Hillson WR, Kok WL, Cole S, Ho LP, Lambe T, et al. Polyethyleneimine is a potent mucosal adjuvant for viral glycoprotein antigens. Nat Biotechnol 2012; 30:883-8; PMID:22922673; https://doi.org/https://doi.org/10.1038/nbt.2344
- Qin T, Yin Y, Huang L, Yu Q, Yang Q. H9N2 influenza whole inactivated virus combined with polyethyleneimine strongly enhances mucosal and systemic immunity after intranasal immunization in mice. Clin Vaccine Immunol 2015; 22:421-9; PMID:25673304; https://doi.org/https://doi.org/10.1128/CVI.00778-14
- Torrieri-Dramard L, Lambrecht B, Ferreira HL, Van den Berg T, Klatzmann D, Bellier B. Intranasal DNA vaccination induces potent mucosal and systemic immune responses and cross-protective immunity against influenza viruses. Mol Ther 2011; 19:602-11; PMID:20959813; https://doi.org/https://doi.org/10.1038/mt.2010.222
- Strieter RM, Belperio JA, Keane MP. Cytokines in innate host defense in the lung. J Clin Invest 2002; 109:699-705; PMID:11901175; https://doi.org/https://doi.org/10.1172/JCI15277
- Kager LM, Weehuizen TA, Wiersinga WJ, Roelofs JJ, Meijers JC, Dondorp AM, van ′t Veer C, van der Poll T. Endogenous alpha2-antiplasmin is protective during severe gram-negative sepsis (melioidosis). Am J Resp Critical Care Med 2013; 188:967-75; PMID:23992406; https://doi.org/https://doi.org/10.1164/rccm.201307-1344OC
- Weehuizen TA, Hommes TJ, Lankelma JM, de Jong HK, Roelofs JJ, de Vos AF, Colonna M, van der Poll T, Wiersinga WJ. Triggering receptor expressed on Myeloid Cells (TREM)-2 impairs host defense in experimental melioidosis. PLoS Negl Trop Dis 2016; 10:e0004747; PMID: 27253382; https://doi.org/https://doi.org/10.1371/journal.pntd.0004747
- Conejero L, Potempa K, Graham CM, Spink N, Blankley S, Salguero FJ, Pankla-Sranujit R, Khaenam P, Banchereau JF, Pascual V, et al. The blood transcriptome of experimental melioidosis reflects disease severity and shows considerable similarity with the human disease. J Immunol 2015; 195:3248-61; PMID:26311902; https://doi.org/https://doi.org/10.4049/jimmunol.1500641
- Koh GC, Schreiber MF, Bautista R, Maude RR, Dunachie S, Limmathurotsakul D, Day NP, Dougan G, Peacock SJ. Host responses to melioidosis and tuberculosis are both dominated by interferon-mediated signaling. PloS One 2013; 8:e54961; PMID:23383015; https://doi.org/https://doi.org/10.1371/journal.pone.0054961
- Weehuizen TA, Lankelma JM, De Jong HK, De Boer OJ, Roelofs JJ, Day NP, Gram H, De Vos AF, Wiersinga WJ. Therapeutic Administration of a Monoclonal Anti-Il-1beta antibody protects against experimental melioidosis. Shock 2016; 46(5):566-74; PMID:27219859; https://doi.org/https://doi.org/10.1097/SHK.0000000000000625
- Easton A, Haque A, Chu K, Patel N, Lukaszewski RA, Krieg AM, Titball RW, Bancroft GJ. Combining vaccination and postexposure CpG therapy provides optimal protection against lethal sepsis in a biodefense model of human melioidosis. J Infect Dis 2011; 204:636-44; PMID:21791666; https://doi.org/https://doi.org/10.1093/infdis/jir301
- Nelson M, Prior JL, Lever MS, Jones HE, Atkins TP, Titball RW. Evaluation of lipopolysaccharide and capsular polysaccharide as subunit vaccines against experimental melioidosis. J Med Microbiol 2004; 53:1177-82; PMID:15585494; https://doi.org/https://doi.org/10.1099/jmm.0.45766-0
- Sarkar-Tyson M, Smither SJ, Harding SV, Atkins TP, Titball RW. Protective efficacy of heat-inactivated B. thailandensis, B. mallei or B. pseudomallei against experimental melioidosis and glanders. Vaccine 2009; 27:4447-51; PMID:19490962; https://doi.org/https://doi.org/10.1016/j.vaccine.2009.05.040
- Mott TM, Vijayakumar S, Sbrana E, Endsley JJ, Torres AG. Characterization of the Burkholderia mallei tonB mutant and its potential as a backbone strain for vaccine development. PLoS Negl Tropical Dis 2015; 9:e0003863; PMID:26114445; https://doi.org/https://doi.org/10.1371/journal.pntd.0003863
- Aschenbroich SA, Lafontaine ER, Hogan RJ. Melioidosis and glanders modulation of the innate immune system: barriers to current and future vaccine approaches. Expert Rev Vaccines 2016; 15:1163-81; PMID:27010618; https://doi.org/https://doi.org/10.1586/14760584.2016.1170598
- Cornell KA, Bouwer HG, Hinrichs DJ, Barry RA. Genetic immunization of mice against Listeria monocytogenes using plasmid DNA encoding listeriolysin O. J Immunol 1999; 163:322-9; PMID:10384131
- Ferraz JC, Stavropoulos E, Yang M, Coade S, Espitia C, Lowrie DB, Colston MJ, Tascon RE. A heterologous DNA priming-Mycobacterium bovis BCG boosting immunization strategy using mycobacterial Hsp70, Hsp65, and Apa antigens improves protection against tuberculosis in mice. Infect Immunity 2004; 72:6945-50; PMID:15557616; https://doi.org/https://doi.org/10.1128/IAI.72.12.6945-6950.2004
- Grote A, Hiller K, Scheer M, Munch R, Nortemann B, Hempel DC, Jahn D. JCat: a novel tool to adapt codon usage of a target gene to its potential expression host. Nucleic Acids Res 2005; 33:W526-31; PMID:15980527; https://doi.org/https://doi.org/10.1093/nar/gki376
- de Stoppelaar SF, van ′t Veer C, Claushuis TA, Albersen BJ, Roelofs JJ, van der Poll T. Thrombocytopenia impairs host defense in gram-negative pneumonia-derived sepsis in mice. Blood 2014; 124:3781-90; PMID:25301709; https://doi.org/https://doi.org/10.1182/blood-2014-05-573915
