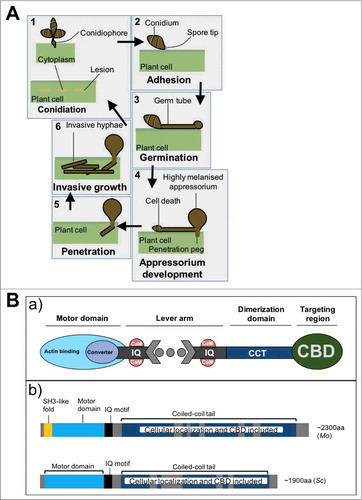
Magnaporthe oryzae adversely impacts cultivated rice and wheat in Asia, and is primarily considered economically and scientifically relevant.Citation1-5 This fungus develops intricate infection-related phenotypes that are almost exclusively observed during biotrophy, a symptomless infection strategy required for growth and feeding on living tissues.Citation6 Biotrophy begins when conidia attach to the leaf surface () and germinate into germ tubes (), which differentiate into appressoria (). These infection structures can become highly melanized and accumulate glycerol,Citation6-8 a combination which dramatically reduces appressorial wall pore sizes and blocks glycerol efflux.Citation6 In turn, this generates internal turgor and mechanical force required for breaching the leaf cuticle, consequently marking the beginning of invasive hyphal growth.Citation6-8 Key events, including the emergence of a penetration peg regulated by the transcription factor, MoGTI1,Citation9 and septin-mediated cytoskeleton remodeling controlled by NADPH oxidase ensue at the onset of invasion ().Citation10 Following this, conidiophores develop while discernable blast lesions emerge on the leaf surface (). This symptomatic stage (necrotrophy) allows M. oryzae to thrive even after localized host cell death has commenced. Effector proteins are deployed during infection to interfere with host immunity during biotrophy and activate events leading to host cell death, and establishing necrotrophy.Citation11-14 Because of its experimental tractability, M. oryzae is considered a model cereal pathogen important for the basic understanding of fungal-host interplay.
Figure 1. (A) Infection related morphogenesis in M. oryzae (details presented in the text). (B) (a) A typical myosin, drawn according to refCitation29. IQ, IQ motif responsible for binding of calmodium (CaM); CCT, coiled-coli tail; CBD, consignment-binding domain. (b) Domain design of myosin II from M. oryzae (Mo) and S. cerevisiae (Sc), as predicted by refCitation24; aa, amino acids.
To date, numerous studies are conducted by, and collections of M. oyzae mutants held in, various laboratories around the world.Citation15 The basis for using mutant collections is that they can be screened for various important phenotypes. Screens of mutants from various fungi have contributed immensely to the understanding of fungal-host interactions and mechanism-of-action of antifungal compounds.Citation16-22 These studies also add to the list of proteins which are future targets for new classes of antifungals.Citation11,23
In this issue of Virulence, Guo et al presents a mutant collection of 1,500 insertion mutants of M. oryzae Guy11.Citation24 One of these strains, severely reduced for virulence during screening using rice seedlings, represents the fungal myosin protein, herein named MoMyo2. Authors performed sequence-based analyses and found an insertion in the second exon of the target gene (MGG_03060) for this protein. This insertion caused virulence deficits in the mutant strain. This study has predicted that MGG_03060 is 7384-bp long and represents 2388 amino acids ().
Myosins are molecular motors involved in the shipment of consignments (e.g. Golgi stacks and secretory vesicles), from the cell to the site of polarized growth.Citation25 Particularly, second class myosins are the major contractile proteins required to organize actin filaments into contractile rings. They are found in animals and lower eukaryotes, and they are highly conserved between yeasts and fungi.Citation26,27 Successful shipment of microscale loads for myosins begins with actin-cytoskeleton interaction which generates actin patches, cables and rings.Citation28 A typical myosin has a motor and tail domain (). Consignment delivery depends on conformational changes in the motor domain, and is coupled with the sequential release of Adenosine triphosphate (ATP) hydrolysis. To accomplish this role, the motor domain comprises highly conserved ATP-binding elements and variable actin-binding motifs ().Citation29 As such, myosins have similar rudimentary actinomyosin ATPase cycles but vary in the rates of transition between these cycles, and this bestows specific myosin motor physiologic properties.Citation27 In contrast, the tail domain is probably the most variable region serving to specify myosin roles and the type of consignment they associate with (). Consignments are typically diverse but secretory vesicles primarily supply key enzymes essential for membrane expansion and cell wall biogenesis. Naturally for most eukaryotes, this is a role of class V myosins. Considering that M. oryzae exhibits highly polarized invasive hyphae (), studying how molecular motors export consignments is crucial to fundamentally understand the polarized growth machinery.
M. oryzae MYO2 shows striking similarities with other class II myosin-encoding genes from yeasts () and distantly related fungi.Citation24 This is confirmed by the encoded protein clustering with other class II myosins. The roles that these myosins play in contractile ring development and polarized growth are generally well established for yeasts.Citation25,30 while in virulence of filamentous fungi and fungal-host interactions they are relatively elusive. In breaching this knowledge crack, Guo et al explores a range of important processes that are regulated by M. oryzae MoMyo2, including development, adaptation to stress and pathogenicity.Citation24 To do this elegantly, genetic manipulations were conducted to generate MoMYO2 deletion (∆momyo2) and comlement (∆momyo2-c) strains. Along with M. oryzae Guy 11, these strains were assessed for morphology and adaptive responses using artificial media and a series of other tests.Citation24 The screen identified substantial deficits in the development of mutants, which included radial growth deficits, aberrant hyphae with unusually more septa and shorter sub-apical cells, as well as undifferentiated conidia, when compared with Guy11 and ∆momyo2-c. Conidial germination in the mutant strains also appeared as delayed; it became clear only when growth time was markedly extended. Under stress-inducing cues (e.g., hydrogen peroxide), conidia and mycelia of mutants were highly sensitive. The wild type and complement strains were resistant under the same conditions. These data indicate that MoMyo2 controls multiple developmental and adaptive response pathways in M. oryzae.
For species such as Candida albicans, a prominent human pathogenic yeast, unicellular budding cells can switch to the filamentous state, or hyphae.Citation31-33 Hyphal formation involves yeast cells initially differentiating into germ tubes marked by the actomyosin ring (actin and class II myosin). Actin can polymerize into patches (e.g., F-actin cables) required for myosin to load and transport consignments to the site of polarized growth. Guo et al's findings using M. oryzae mutants suggest that a similar system of events may underlie germ tube differentiation into appressoria.Citation24 This process may involve septum formation driven by MoMyo2, which potentially separates appressoria from the germ tube and further into invasive hyphae. Additionally, authors demonstrated that the roles of MoMyo2 could be more diverse. According to their observation, appressoria for momyo2 were significantly fewer and demelanized, and in addition, displayed thinner and leaky cell wall compared with the appressoria of the wild type and momyo2-c strains.Citation24 A general expectation was that these attributes could collapse appressorial wall. This was however not the case in this study as mutant cells recuperated from collapsing in just an hour,Citation24 likely the cause of thin and porous cell wall allowing glycerol (used in the cytorrhysis assay) to diffuse into cells causing inflation. Nonetheless, the leaky and demelanized appressorial wall may well contribute to highly reduced pathogenicity in MoMYO2 isolates toward barley and rice,Citation24 suggesting that MoMyo2 controls host pathogenicity in M. oryzae.
To date, the anatomy and mechanisms of contractile ring formation remain underrepresented in the M. oryzae research arena. Much of the background stems from work conducted in yeasts, deemed ‘humble servants’ for understanding biologic processes in fungi. However, it is crucial that fundamental processes are directly analyzed in fungi given noticeable distinctions between the 2 eukaryotic groups. These include differences in host occupancy and phenotypic features exclusively displayed by filamentous fungi (e.g., appressoria). Guo et al has provided vital insights pertaining to contractile ring organization in the model fungal pathogen of plants, and have further detailed MoMyo2 roles that are unique to this pathogen.Citation24 Therefore, their work suggests that mechanisms governing invasive polarized growth might be more complex in filamentous fungi than they are in yeasts, and henceforth more informative. The same laboratory for which this work originates, also reports characterization of MoMyo5 (class V myosin).Citation34 Unlike MoMyo2 which localizes to hyphal septation site to power contractile ring assembly,Citation24 MoMyo5 localizes to the tip during polarized growth, consistent with other class V myosins. Therefore, it is thrilling that the composition of an articulate ‘molecular motor tool box’ is set in motion for M. oryzae. Much in the same temperament, the prospect for integrating this molecular tool box into therapeutic goals should be recognized immediately given the economic eminence of M. oryzae. Already, a defined selective inhibitor (CK-2018571) of smooth muscle myosin motor domain which allosterically prevents binding of smooth muscle myosin to actin and allows smooth muscles to relax exists.Citation35 Indeed, dissecting pharmacological properties of CK-2018571 will improve treatment of smooth muscle diseases in humans.Citation36 Owing to the conserved status of motor domains in myosin proteins (), this inhibitor could be customized for fungal myosins. Additionally, Tamoxifen, an estrogen receptor antagonist used to treat breast cancer, has been shown to regularly restrict binding of class V myosin to calmodulin in yeast, fundamentally preventing hyphal growth.Citation37 Therefore, blocking plant-related mycoses using the molecular motor tool box is achievable.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
TEM is supported by the National Research Foundation (NRF) of South Africa (Grant no. 104914). The financial assistance of the NRF is hereby acknowledged. Opinions expressed and conclusions arrived at, are those of the authors and not necessarily attributed to the NRF.
References
- Callaway E. Devastating wheat fungus appears in Asia for first time. Nature 2016; 532:421-2; PMID:27121815; https://doi.org/10.1038/532421a
- Islam MT, Croll D, Gladieux P, Soanes DM, Persoons A, Bhattacharjee P, Hossain MS, Gupta DR, Rahman MM, Mahboob MG, et al. Emergence of wheat blast in Bangladesh was caused by a South American lineage of Magnaporthe oryzae. BMC Biol 2016; 14:84; PMID:27716181; https://doi.org/10.1186/s12915-016-0309-7
- Toriyama K. Rice is life: Scientific perspectives for the 21st century. International Rice Research Inst., Los Banos (Philippines) engWorld Rice Research Conference eng 4–7 Nov 2004 Tsukuba (Japan) Heong KL and Hardy B (eds.)
- Dean R, Van Kan JA, Pretorius ZA, Hammond-Kosack KE, Di Pietro A, Spanu PD, Rudd JJ, Dickman M, Kahmann R, Ellis J, et al. The top 10 fungal pathogens in molecular plant pathology. Mol Plant Pathol 2012; 13:414-30; PMID:22471698; https://doi.org/10.1111/j.1364-3703.2011.00783.x
- Sharma R. Wheat blast research: Status and imperatives. Afr J Agri Res 2017; 12:377-81; https://doi.org/10.5897/AJAR2016.11860
- Yan X, Talbot NJ. Investigating the cell biology of plant infection by the rice blast fungus Magnaporthe oryzae. Curr Opin Microbiol 2016; 34:147-53; PMID:27816794; https://doi.org/10.1016/j.mib.2016.10.001
- de Jong JC, McCormack BJ, Smirnoff N, Talbot NJ. Glycerol generates turgor in rice blast. Nature 1997; 389:244-244; https://doi.org/10.1038/38418
- Foster AJ, Ryder LS, Kershaw MJ, Talbot NJ. The role of glycerol in the pathogenic lifestyle of the rice blast fungus Magnaporthe oryzae. Environ Microbiol 2017; 19:1008-16; PMID:28165657; https://doi.org/10.1111/1462-2920.13688
- Li Y, Wang G, Xu JR, Jiang C. Penetration peg formation and invasive hyphae development require stage-specific activation of MoGTI1 in Magnaporthe oryzae. Mol Plant Microbe Interact 2016; 29:36-45; PMID:26441323; https://doi.org/10.1094/MPMI-06-15-0142-R
- Ryder LS, Dagdas YF, Mentlak TA, Kershaw MJ, Thornton CR, Schuster M, Chen J, Wang Z, Talbot NJ. NADPH oxidases regulate septin-mediated cytoskeletal remodeling during plant infection by the rice blast fungus. Proc Natl Acad Sci U S A 2013; 110:3179-84; PMID:23382235; https://doi.org/10.1073/pnas.1217470110
- Shipman EN, Jones K, Jenkinson CB, Kim DW, Zhu J, Khang CH. Nuclear and structural dynamics during the establishment of a specialized effector-secreting cell by Magnaporthe oryzae in living rice cells. BMC Cell Biol 2017; 18:11; PMID:28125974; https://doi.org/10.1186/s12860-017-0126-z
- Oliveira-Garcia E, Valent B. How eukaryotic filamentous pathogens evade plant recognition. Curr Opin Microbiol 2015; 26:92-101; PMID:26162502; https://doi.org/10.1016/j.mib.2015.06.012
- Chen S, Songkumarn P, Venu RC, Gowda M, Bellizzi M, Hu J, Liu W, Ebbole D, Meyers B, Mitchell T, et al. Identification and characterization of in planta-expressed secreted effector proteins from Magnaporthe oryzae that induce cell death in rice. Mol Plant Microbe Interact 2013; 26:191-202; PMID:23035914; https://doi.org/10.1094/MPMI-05-12-0117-R
- Wang Y, Wu J, Kim SG, Tsuda K, Gupta R, Park SY, Kim ST, Kang KY. Magnaporthe oryzae-secreted protein MSP1 induces cell death and elicits defense responses in rice. Mol Plant Microbe Interact 2016; 29:299-312; PMID:26780420; https://doi.org/10.1094/MPMI-12-15-0266-R
- Motaung TE, Saitoh H, Tsilo TJ. Large-scale molecular genetic analysis in plant pathogenic fungi: A decade of genome-wide functional analysis. Mol Plant Pathol 2016; 18:754-64.
- Homann OR, Dea J, Noble SM, Johnson AD. A phenotypic profile of the Candida albicans regulatory network. PLoS Genet 2009; 5:e1000783; PMID:20041210; https://doi.org/10.1371/journal.pgen.1000783
- Liu OW, Chun CD, Chow ED, Chen C, Madhani HD, Noble SM. Systematic genetic analysis of virulence in the human fungal pathogen Cryptococcus neoformans. Cell 2008; 135:174-88; PMID:18854164; https://doi.org/10.1016/j.cell.2008.07.046
- Noble SM, French S, Kohn LA, Chen V, Johnson AD. Systematic screens of a Candida albicans homozygous deletion library decouple morphogenetic switching and pathogenicity. Nat Genet 2010; 42:590-8; PMID:20543849; https://doi.org/10.1038/ng.605
- De Souza CP, Hashmi SB, Osmani AH, Andrews P, Ringelberg CS, Dunlap JC, Osmani SA. Functional analysis of the Aspergillus nidulans kinome. PLoS One 2013; 8:e58008; PMID:23505451; https://doi.org/10.1371/journal.pone.0058008
- Jung KW, Yang DH, Maeng S, Lee KT, So YS, Hong J, Choi J, Byun HJ, Kim H, Bang S, et al. Systematic functional profiling of transcription factor networks in Cryptococcus neoformans. Nat Commun 2015; 6:6757; PMID:25849373; https://doi.org/10.1038/ncomms7757
- Lee JA, Robbins N, Xie JL, Ketela T, Cowen LE. Functional genomic analysis of Candida albicans adherence reveals a key role for the Arp2/3 complex in cell wall remodelling and biofilm formation. PLoS Genet 2016; 12:e1006452; PMID:27870871; https://doi.org/10.1371/journal.pgen.1006452
- Lee KT, So YS, Yang DH, Jung KW, Choi J, Lee DG, Kwon H, Jang J, Wang LL, Cha S, et al. Systematic functional analysis of kinases in the fungal pathogen Cryptococcus neoformans. Nat Commun 2016; 7:12766; PMID:27677328; https://doi.org/10.1038/ncomms12766
- Motaung TE, Ells R, Pohl CH, Albertyn J, Tsilo TJ. Genome-wide functional analysis in Candida albicans. Virulence 2017; 8:1-17; PMID:27410461; https://doi.org/10.1080/21505594.2017.1292198
- Guo M, Tan L, Nie X, Zhang Z. A class-II myosin is required for growth, conidiation, cell wall integrity and pathogenicity of Magnaporthe oryzae. Virulence 2017; 1-20; PMID:28448785; https://doi.org/10.1080/21505594.2017.1323156
- Juanes MA, Piatti S. The final cut: Cell polarity meets cytokinesis at the bud neck in S. cerevisiae. Cell Mol Life Sci 2016; 73:3115-36; PMID:27085703; https://doi.org/10.1007/s00018-016-2220-3
- Guertin DA, Trautmann S, McCollum D. Cytokinesis in eukaryotes. Microbiol Mol Biol Rev 2002; 66:155-78; PMID:12040122; https://doi.org/10.1128/MMBR.66.2.155-178.2002
- Pollard TD. Mechanics of cytokinesis in eukaryotes. Curr Opin Cell Biol 2010; 22:50-6; PMID:20031383; https://doi.org/10.1016/j.ceb.2009.11.010
- Berepiki A, Lichius A, Read ND. Actin organization and dynamics in filamentous fungi. Nat Rev Microbiol 2011; 9:876-87; PMID:22048737; https://doi.org/10.1038/nrmicro2666
- Sweeney HL, Houdusse A. Structural and functional insights into the myosin motor mechanism. Annu Rev Biophys 2010; 39:539-57; PMID:20192767; https://doi.org/10.1146/annurev.biophys.050708.133751
- De La Cruz EM, Ostap EM. Relating biochemistry and function in the myosin superfamily. Curr Opin Cell Biol 2004; 16:61-67; PMID:15037306; https://doi.org/10.1016/j.ceb.2003.11.011
- Schmidt M, Bowers B, Varma A, Roh DH, Cabib E. In budding yeast, contraction of the actomyosin ring and formation of the primary septum at cytokinesis depend on each other. J Cell Sci 2002; 115:293-302; PMID:11839781
- Sudbery PE. Growth of Candida albicans hyphae. Nat Rev Microbiol 2011; 9:737-48; PMID:21844880; https://doi.org/10.1038/nrmicro2636
- Sudbery P, Gow N, Berman J. The distinct morphogenic states of Candida albicans. Trends Microbiol 2004; 12:317-24; PMID:15223059; https://doi.org/10.1016/j.tim.2004.05.008
- Tang W, Gao C, Wang J, Yin Z, Zhang J, Ji J, Zhang H, Zheng X, Zhang Z, Wang P. Disruption of actin motor function due to MoMyo5 mutation impairs host penetration and pathogenicity in Magnaporthe oryzae. Mol Plant Pathol 2017; PMID:28378891; https://doi.org/10.1111/mpp.12554
- Sirigu S, Hartman JJ, Planelles-Herrero VJ, Ropars V, Clancy S, Wang X, Chuang G, Qian X, Lu PP, Barrett E, et al. Highly selective inhibition of myosin motors provides the basis of potential therapeutic application. Proc Natl Acad Sci 2016; 113:E7448-55; PMID:27815532; https://doi.org/10.1073/pnas.1609342113
- Brozovich FV, Nicholson CJ, Degen CV, Gao YZ, Aggarwal M, Morgan KG. Mechanisms of vascular smooth muscle contraction and the basis for pharmacologic treatment of smooth muscle disorders. Pharmacol Rev 2016; 68:476-532; PMID:27037223; https://doi.org/10.1124/pr.115.010652
- Dolan K, Montgomery S, Buchheit B, Didone L, Wellington M, Krysan DJ. Antifungal activity of tamoxifen: In vitro and in vivo activities and mechanistic characterization. Antimicrob Agents Chemother 2009; 53:3337-46; PMID:19487443; https://doi.org/10.1128/AAC.01564-08
