ABSTRACT
Cyclosporin A (CsA) is widely used as an immunosuppressive agent for organ transplant recipients. CsA inhibits calcineurin, which is highly conserved in mammals and fungi, and thus affects both types of organism. In mammals, the immunosuppressive effect of CsA is via hampering T cell activation. In fungi, the growth inhibitory effect of CsA is via interference with hyphal growth. The aim of this study was to determine whether CsA renders mice susceptible to invasive pulmonary aspergillosis (IPA) and whether it can protect immunosuppressed mice from infection. We therefore examined both the antifungal and the immunosuppressive activity of CsA in immunosuppressed and in immunocompetent mice infected with Aspergillus fumigatus to model IPA. We found that daily injections of CsA could not produce an antifungal effect sufficient to rescue immunosuppressed mice from lethal IPA. However, a 100% survival rate was obtained in non-immunosuppressed mice receiving daily CsA, indicating that CsA did not render the mice vulnerable to IPA. The lymphocyte subset was significantly suppressed by CsA, while the myeloid subset was not. Therefore, we speculate that CsA does not impair the host defense against IPA since the myeloid cells are preserved.
Introduction
Cyclosporin A (CsA) is administered to organ transplant patients to prevent rejection by inhibiting the activation of T-cells through the inhibition of calcineurin.Citation1 By binding to cyclophilin A, the cyclosporin A-cyclophilin A complex binds to calcineurin, which is required for the dephosphorylation and activation of the nuclear factor of activated T-cells, which increases the transcription of interleukin 2.Citation1,2
Immunosuppression given to organ-transplant recipients also increases patients' susceptibility to opportunistic infections.Citation3 Invasive pulmonary aspergillosis (IPA) is among the major opportunistic invasive fungal infections in these populations, and the mortality rate of organ transplant recipients with invasive aspergillosis is high.Citation4-7 The risk factors of post-transplantation invasive fungal infections vary between different studies. For instance, John et al. showed that kidney transplant recipients receiving CsA were associated with higher risk of systemic mycoses within the first 6 months following kidney transplantation as compared with those receiving prednisolone and azathioprine therapy.Citation8 Paya et al. concluded that CsA did not significantly alter the prevalence or severity of fungal infections in solid organ transplantation recipients.Citation9 In contrast, Dummer et al. argued that invasive fungal infections developed more as a result of the type of transplantation than of the type of immunosuppression.Citation8-10 Collectively, the specific and mechanistic role of CsA treatment as a risk factor for opportunistic fungal infections among organ-transplant recipients, besides increasing the overall net immunosuppressive state, is uncertain.
The drug target of CsA, calcineurin, is conserved in mammals and other eukaryotes.Citation11 In yeasts and molds, calcineurin plays a role in growth and pathogenicity in vitro.Citation12 Although the gene encoding for calcineurin A (cnaA) is not essential in A. fumigatus, the ΔcnaA mutant displayed defective hyphal growth.Citation13 In humans, apart from causing T cell dysfunction, calcineurin inhibitors were also recently found to affect the host antimicrobial innate immunity. For example, the antifungal activity of neutrophils from allogenic haematopoietic stem cell transplant (HSCT) recipients, who received calcineurin inhibitors for the prevention of both graft reject and graft versus host disease, was impaired in one study.Citation14 The distinct functions of calcineurin in mammals and other eukaryotes provide calcineurin inhibitors with dual antifungal and immunosuppressive activities. Several animal and ex vivo studies have been previously conducted to investigate the effect of CsA against invasive fungal infection,Citation15-22 which could not produce a consistent conclusion. Moreover, these animal studies generally focus on one of the dual actions (antifungal and immunosuppression) of CsA.
Due to the unclear effect of CsA and conflicting reports on its activity, this study aimed to examine the effect of CsA on the antifungal activity vs. immunity in a murine model of invasive pulmonary aspergillosis. To our knowledge, this study is the first to explore the effect of CsA on immune cellularity, which would certainly be useful in elucidating its role in post-transplantation invasive fungal infections.
Results
In vitro effect of CsA against A. fumigatus
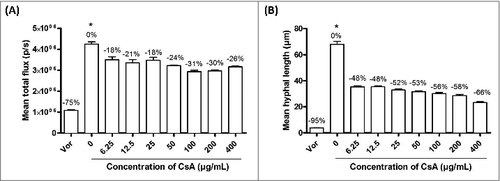
CsA inhibited growth and hyphal elongation of A. fumigatus (). After 10-h incubation with CsA (6.25 to 400 µg/mL), the growth of A. fumigatus resting conidia was inhibited by 18% to 31% as compared with the untreated control (). CsA exerted a profound effect on A. fumigatus hyphal elongation by inhibiting 48% to 66% of the mean hyphal length in the same range of concentration (). Voriconazole, a drug used to invasive fungal infections, was used as a control. This drug was able to achieve almost complete inhibition of growth and hyphal elongation at a concentration of 0.35 µg/mL ().
Figure 1. Inhibition of A. fumigatus (A) growth and (B) hyphal length by Cyclosporin A (CsA) in vitro (6.25 – 400 µg/mL). Voriconazole (Vor) (0.35 µg/mL) was used as a positive control. The percentages of inhibition compared with the negative control (without CsA) are presented in the graph above the columns. # - The inhibition of CsA and voriconazole were significantly different from that of the negative control across all concentrations tested (p < 0.05). No significant differences were found between different concentrations of CsA. It should be noted that even the highest concentration of CsA tested did not result in a complete inhibition of growth in either (A) or (B), suggesting that the antifungal activity of CsA is limited
In vivo effect of CsA and cyclophosphamide (cyclo) in murine model of invasive pulmonary aspergillosis
The mean weight of all the mice receiving cyclophosphamide, with or without CsA (cyclo group and cyclo/CsA group), showed a similar constant decrease from D-4 until the end of the experimental period (). The weight drop in the pre-infection period (D-4 to D0) in these mice was due to the effect of cyclophosphamide, which was given to the mice on D-4 and D-1. The weight drop in the post-infection period (from D1 onwards) however was attributed to the infection. In contrast, the mean weight of the mice of the CsA and the control group remained largely constant throughout the experimental period (). The survival rate of the CsA and the control group mice was 100%, while the survival rates of the cyclo group mice and the cyclo/CsA group mice were both 0% (). The median survival of cyclo group mice and cyclo/CsA group mice was 4 d for both groups. The similar pattern of weight loss and survival rate between the cyclo group and the cyclo-CsA group indicated that CsA did not rescue the cyclophosphamide-immunosuppressed mice from lethal IPA. On the other hand, the administration of CsA to the non-immunosuppressed mice did not render the animals susceptible to IPA.
Figure 2. (A) Mean weight of mice. No significant weight loss was observed in the control group and the group receiving only CsA, suggesting an absence of infection. However, the mean weight of all mice receiving cyclophosphamide decreased from D-4 onwards. (B) Survival rate. The median survival for mice in the cyclo group and cyclo/CsA group was 4 d. The survival of the control group and the CsA group mice was 100% and was significantly higher than that of the other 2 groups. (C) Bioluminescence emission from representative mice on D3 after 5-min exposure. (D) Mean total photons flux (expressed in log scale). There was no significant difference in bioluminescence between the groups on D1 and D2. On D3, there was no significant difference in bioluminescence between the control group and the CsA group. The values of both the cyclo group and cyclo/CsA group were significantly higher than those of the CsA group and the control group. In addition, the bioluminescence of the cyclo/CsA group was significantly higher than that of the cyclo group. Each group contained 10 mice. #p < 0.05
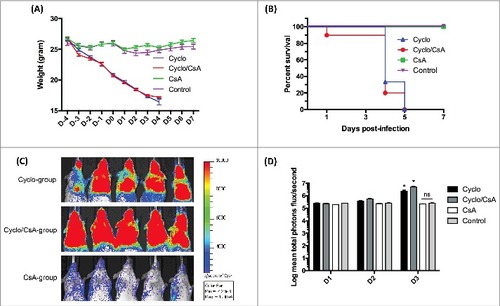
The bioluminescence emission acquired from the 4 groups (on D1, D2 and D3) further validated our findings. The images acquired on D3 are shown in . Low levels of bioluminescence were detected in all mice on D1 (). On D2, the bioluminescence from cyclo group and cyclo/CsA group mice started to increase and peaked on D3 (). The mean total flux of bioluminescence of the mice from each group was significantly different from each other on D3 (p < 0.05) (). It is noteworthy that the mean total flux of bioluminescence of the mice from cyclo/CsA group was significantly greater than that of the cyclo group. This suggests that CsA worsened the IPA. The level of bioluminescence from CsA and the control group mice remained consistently low from D1 to D3, indicating the absence of infection (). The bioluminescence acquisition ended on D3, as most of the immunosuppressed mice died on the following day.
Changes in leukocyte counts in peripheral blood of mice treated with immunosuppressants
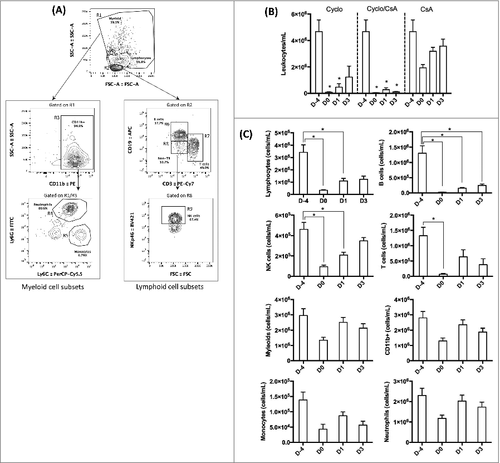
The cell populations from the peripheral blood samples were analyzed by flow cytometry. The gating strategy is shown in . The number of leukocytes including myeloid and lymphoid cells from the mice that received cyclophosphamide only decreased significantly on D0 and D1 when compared with D-4 (baseline) (). The number of leukocytes in the peripheral blood of the mice receiving both cyclophosphamide and CsA also decreased significantly on all days of sampling when compared with D-4 (baseline) indicating that cyclophosphamide suppressed all lymphoid and myeloid subsets. In contrast, CsA treatment alone did not significantly lower the number of total leukocytes. Since the number of leukocytes was too low, in the cyclophosphamide-treated groups, these 2 groups of mice were excluded from further analysis shown in , only the blood samples of the CsA-treated mice were analyzed. CsA treatment significantly decreased the number of T- and B-lymphocytes as well as natural killer (NK) cells but not the myeloid population (). Interestingly, although daily CsA injection induced a decrease in the myeloid population during the pre-infection period (from D-4 to D0), no statistical significance was obtained. In addition, during the post-infection period (from D1 onwards), the myeloid population rose to reach the level of the baseline (D-4).
Figure 3. Mean leukocyte count from peripheral blood. (A) Gating strategy of the lymphocyte and myeloid subsets. (B) The average total number of leukocytes per milliliter of peripheral blood from the mice. Cyclo – cyclophosphamide-treated mice; Cyclo/CsA – mice received cyclophosphamide and cyclosporin A; CsA – mice received only cyclopsporin A. The bars of D-4 are the same for the 3 groups, as they represented the baseline number of leukocytes of all mice (n = 30) before the start of drug administration. (C) Leukocyte population of peripheral blood obtained from mice received CsA only. # - significant (p < 0.05)
Discussion
CsA, an antifungal antibiotic extracted from the fungus Tolypocladium inflatum, was originally found to have a narrow spectrum of antifungal activity.Citation23 However, CsA was later discovered to be a potent immunosuppressant, and has since been used to prevent acute rejection in organ transplantation and also graft-vs.-host disease in organ transplantation in allogenic stem cell transplant recipient.Citation24
The antifungal activity of CsA was originally thought to be limited as no growth inhibition was observed on various fungi, including Candida albicans and Saccharomyces cerevisiae.Citation23 However, contrary to this original finding, the fungal calcineurin pathway was found to be involved in growth and pathogenicity in fungi, suggesting the great potential of calcineurin inhibitors, such as CsA and tacrolimus (FK506), as novel antifungal agents.Citation12 Calcineurin is essential for growth and virulence in a variety of fungi, including A. fumigatus,Citation13 C. albicansCitation25 and Cryptococcus neoformans.Citation26
Invasive aspergillosis (IA) is the leading major mold infection among HSCT recipients as well as solid organ-transplant recipients.Citation27 The mortality rate of IA in organ transplant recipients ranges from 63% to 100%.Citation6,28 There exists a discrepancy among epidemiological studies regarding the association of CsA and fungal infections. A study by John et al. concluded that CsA is associated with a 4-fold risk of systemic mycoses within the first 6 months of kidney transplantation as compared with prednisolone and azathioprine therapy.Citation8 In contrast, Dummer et al. concluded that fungal infections are only found in the group of patients who received a liver transplant with CsA treatment and argued that fungal infections is associated with the type of transplantation rather than the type of immunosuppression.Citation10 In addition, it has been found that CsA treatment did not significantly reduce the incidence of invasive fungal infections but rather of bacterial and viral infections among organ transplant recipients compared with other immunosuppressants.Citation29 Such a conflict also exists among various animal studies of the effect of CsA on fungal infections. Some studies have demonstrated that CsA-treated mice were susceptible to systemic fungal infections,Citation15-18 while others have shown otherwise.Citation19-22 The association between CsA treatment and fungal infections is therefore uncertain.
Drugs with a dual antifungal and anti-inflammatory activity have recently been proposed as novel therapeutic strategy for fungal infections.Citation30 As a result, we sought to examine the effect of CsA, which has that dual activity, in murine model of invasive pulmonary aspergillosis, the most common form of IA in organ-transplant recipients.Citation4,31
The growth and hyphal elongation of A. fumigatus were significantly inhibited in vitro by CsA (6.25 to 400 µg/mL) (). This is consistent with previous results, which showed that CsA delays and impairs hyphal growth.Citation13,32 Voriconazole, which is a fungicidal antifungal agent for A. fumigatus, was used as the positive control, at the concentration of 0.35 µg/mL. The inhibition of growth and hyphal elongation by voriconazole was much more pronounced than that of CsA. Moreover, there was no significant difference among the values obtained from different concentrations of CsA. No complete inhibition of fungal growth and hyphal elongation was observed with concentrations up to 400 μg/mL of CsA, suggesting that CsA has limited fungistatic activity against A. fumigatus.
Since there are no pharmacodynamic or pharmacokinetic studies of CsA in mice, the maximum concentration of CsA in vivo could only be calculated by assuming that the bioavailability of CsA via the intraperitoneal route is 100%, and that CsA is evenly distributed within the mice.Citation33 Under these assumptions, the in vivo dose of 100 mg/kg could be equated to the in vitro concentration of 100 mg/L or 100 µg/mL. Therefore, 100 µg/mL of CsA in vitro is comparable to the amount of CsA administered to our murine model. At this concentration, the amount and mean hyphal length of A. fumigatus was reduced by 31% and 56%, respectively, when compared with that of the control (). This suggested that the dose of CsA for the mice, besides the immunosuppressive effect, might have an antifungal effect as well.
Our well-established cyclophosphamide-immunosuppressed murine model of IPA was used to investigate the in vivo antifungal activity of CsA. Since cyclophosphamide causes neutropenia and reduces the number of lymphocytes in mice,Citation34,35 the influence of host immunity could be eliminated. Mice in 2 of the groups were immunosuppressed by 2 injections of cyclophosphamide on D-4 and D-1, followed by an intranasal inoculation on D0, with a lethal dose of 5 × 105 conidia. CsA was given daily intraperitoneally to the mice (test group) from D-4. The mean weight and survival of the control group and test group were similar throughout the experimental period. All mice, from both cyclosphosphamide-containing groups, consistently lost weight and died by D4 ( and ). The similarity between the 2 groups suggested that the antifungal activity of CsA, even in this high dosage, was insufficient to rescue the mice. It is unclear if the dose of CsA was instead too high and therefore contributed to a stronger immunosuppressed state. However, the mice did not die at an accelerated rate compared with the cyclophosphamide-only suppressed mice.
Interestingly, the bioluminescence of the mice receiving both cyclophosphamide and CsA (cyclo/CsA group) was significantly higher than that from of the mice receiving only cyclophosphamide on D3 post-infection (). The significantly higher fungal burden suggested that the infection was not alleviated, but on the contrary, was worsened by CsA. CsA did not display any antifungal activity in vivo in our study. In addition, it seems likely that the immunosuppressive – antifungal balance was tipped to favor exaggerated immunosuppression, resulting in a higher fungal burden.
Infected mice receiving daily CsA injections and no cyclophosphamide had a 100% survival rate (), indicating that CsA-treated mice retained their resistance to IPA. A 100% survival rate was also seen among immunocompetent mice (control group) challenged with the same inoculum size of 5 × 105. Previous studies of the in vivo effect of CsA showed contradictory results and failed to suggest a definitive conclusion.Citation15-22 The contradiction could have arisen from a difference in protocols (dose of inoculation, dose of CsA, infection route, etc.). Moreover, some studies that found CsA treatment to be a risk factor of fungal infections also used another immunosuppressant in their animal model,Citation18 whereas in studies that used CsA alone, the CsA did not exacerbate the fungal infections.Citation20,22
On the cellular level, daily administration of CsA significantly suppressed the lymphoid subsets but not the myeloid subsets in the peripheral blood of the mice (). Both lymphoid and myeloid cells play a role in the immunity against A. fumigatus infection, but the myeloid subsets, and especially neutrophils, act as the first line of defense,Citation36-38 which explained the resistance against IPA in CsA-treated mice. This is consistent with the previous finding that CsA does not affect the production of neutrophils,Citation39 and that it does not affect the antifungal activity of phagocytes against A. fumigatus at relevant therapeutic concentrations.Citation40 However, Greenblatt et al. found that CsA-treated neutrophils are unable to kill C. albicans ex vivo, although no abnormality was observed in several effector responses, such as phagocytosis.Citation17 Another ex vivo study also showed that the antifungal activity of neutrophils from neutrophil extracellular traps with previous CsA treatment was impaired, which was likely due to the reduced production of neutrophil extracellular traps.Citation14 Collectively, these findings suggest that CsA moderately impairs the antifungal activity of neutrophils, as shown in ex vivo studies, but that the effect is not sufficient to render the host susceptible in vivo.
Although CsA is not a potent antifungal, it has synergistic effect with echinocandin and azoles in vitro against C. albicans and A. fumigatus.Citation32,41 Antifungal prophylaxis and immunosuppressants are commonly administrated concomitantly to transplant recipients. Our result, which indicated that CsA does not impair the host immune defense against IPA, further suggested that CsA is an ideal immunosuppressant for populations who are otherwise prone to invasive fungal infections. Future study of the in vivo synergism between antifungals and CsA would be of great clinical interest.
Furthermore, it would be of interest to investigate the antifungal activity of CsA using different knockout mice known to be susceptible to A. fumigatus infection without immunosuppression. For instance, the gp91/Phox strain (X-CGD mice) and the CXCR2 knockout mice are susceptible to IPA due to defect in phagocyte oxidative killing mechanism and impaired neutrophils recruitment, respectively.Citation38,42 In addition, a murine model immunosuppressed by corticosteroids, which impairs the pro-inflammatory response of the phagocytes by inhibiting NF-κB pathway, could also be used.Citation43,44
In conclusion CsA, despite its in vitro antifungal activity, could not rescue the mice from a lethal challenge of IPA under lymphopenia and neutropenia caused by cyclophosphamide. It is possible that the dose of CsA was too high, tipping the balance of action toward additional immunosuppression and therefore limiting the antifungal activity. To clarify this, multiple doses of CsA would have to be used to define the precise balance. On the other hand, this study is the first to discover that, although CsA suppressed the lymphoid subset in mice, the myeloid subset was largely intact for host defense against lethal challenge of IPA.
Materials and methods
A. fumigatus strain
The bioluminescent A. fumigatus strain 2/7/1 was used in this study for both in vitro and in vivo assays. Strain 2/7/1, contains the Photinus pyralis luciferase gene lucOpt, was generated from the background of wild-type A. fumigatus strain CBS144.89.Citation45,46
In vitro effect of CsA against A. fumigatus
Each well of a 24-well plate contained 5 × 104 resting conidia of A. fumigatus strain 2/7/1 in 500 µL RPMI containing 10% of fetal bovine serum, 5% penicillin and streptomycin, sodium pyruvate and HEPES (All from Gibco). CsA (Abcam) of concentrations ranged 6.25 – 400 µg/mL was added to the wells in triplicate. The control wells contained no CsA. Voriconazole (0.35 µg/mL) was used as a positive control. The plate was incubated at 37°C for 10 h before bioluminescence acquirement and hyphal length measurement. The fungal growth is represented by the mean total flux detected from the fungi. The mean hyphal length was determined by measuring the length of 100 A. fumigatus hyphae in each well using ImageJ. Both assays were repeated for 3 times. The percentage inhibition of growth and hyphal length by CsA was expressed by the ratio of mean growth or hyphal length between the test and the negative control (without CsA). The equation used in this calculation was (test – control)/control x 100%. The methodology was previously defined as congruent with EUCAST antifungal susceptibility testing.Citation47
Mice and ethics statement
Eight-week old male BALB/c mice of approximately 25 g (Janvier, France) were used in this study. All procedures were performed in accordance with Institut Pasteur guidelines in compliance with European guidelines. This study was approved by the ethical committee for animal experimentation CETEA (Comité d’éthique en experimentation animale, Project license number 2013-0020).
Immunosuppression of mice by cyclophosphamide and cyclosporin A (CsA) treatment
Four groups of mice (n = 10) were used in this study: cyclo group (mice receiving cyclophosphamide only), cyclo/CsA group (mice receiving cyclophosphamide and CsA), CsA group (mice receiving CsA only) and control group (immunocompetent mice that received neither cyclophosphamide nor CsA). Cyclophosphamide (Sigma-Aldrich) was dissolved in sterile distilled water and given to the mice on day -4 (D-4) and day -1 (D-1) by intraperitoneal injection (200 mg/kg).Citation45 CsA (Abcam) was dissolved in ethanol in a concentration of 500 mg/mL. An intraperitoneal injection containing 2.5 mg of CsA in emulsion of 15% v/v ethanol/castor oil was given to the mice daily from D-4 onwards.
Establishment of invasive pulmonary aspergillosis in murine model
Invasive pulmonary aspergillosis was established in a murine model, as described previously, in each of the 3 groups of mice on D0.Citation38 Bioluminescent A. fumigatus strain 2/7/1 was subcultured on 2% malt agar for 8 d at room temperature. Conidial suspension was prepared in 0.1% Tween 20 and PBS and mycelia were filtered with 40-µm cell strainer (BD Falcon). First, the mice were anaesthetized with an intramuscular injection of 150 µL containing 10 mg/mL ketamine and 10 mg/mL xylazine. The mice were inoculated intranasally with 5 × 105 conidia in a volume of 25 µL. The control group mice were inoculated with 25 µL PBS. Weight and survival of the mice were monitored daily. The bioluminescence measurement was started one day post-infection and was repeated every day thereafter. Luciferin was injected intraperitoneally before each measurement. Then, mice were anaesthetized by 2.5% isoflurane for 5 min using the XGI-8 gas anesthesia system.Citation46 The bioluminescence was recorded after 5-min exposure by the IVIS 100 system (PerkinElmer, Boston, MA). The mice were killed on D7 using CO2.
Leukocyte counts in murine peripheral blood by flow cytometry
Blood samples were collected from the submandibular vein on D-4, D0, D1 and D3 for leukocytes quantification by flow cytometry (MACSQuant, Miltenyi Biotec). Blood was collected in 500 µL Eppendorf tubes with 50 µL EDTA and incubated with 1 mL of red blood cell lysis buffer for 5 min at 4°C. Cells were transferred to a 15 mL tube, topped up to 10 mL in wash buffer (PBS with 0.5% fetal calf serum and 2 µM EDTA) and centrifuged for 7 min at 1,400 rpm. The supernatant was removed and the pellet was suspended in 100 µL wash buffer with fluorescent antibodies at the recommended concentrations (NKp46 – BV421 (BB Bioscience), Ly6G – FITC, CD11b – PE, Ly6C – PerCP-Cy5.5, CD3 – PE-Cy7, CD19 – APC (eBioscience)) and transferred to a 5 mL tube for 15 min at 4°C. The cells were washed in PBS and labeled with live/dead dye (eBioscience). Following another centrifugation in wash buffer the cells were resuspended in 200 µL of wash buffer for acquirement. Fluorescent labeling and cell counts were performed simultaneously using total events for cell counts and live cells for fluorescent label analysis.
Statistical analysis
Statistical significance of the data was analyzed with Prism 6 (GraphPad Software). Two-way ANOVA with Bonferroni's correction was performed for multiple comparison. A statistical significance is achieved if p-value is less than 0.05. Error bars in graphs denote standard error of the mean.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Schreiber SL, Crabtree GR. The mechanism of action of cyclosporin A and FK506. Immunol Today 1992; 13:136-42; PMID:1374612; https://doi.org/https://doi.org/10.1016/0167-5699(92)90111-J
- Liu J, Farmer JD, Lane WS, Friedman J, Weissman I, Schreiber SL. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell 1991; 66:807-15; PMID:1715244; https://doi.org/https://doi.org/10.1016/0092-8674(91)90124-H
- Fishman JA. Infection in solid-organ transplant recipients. N Engl J Med 2007; 357:2601-14; PMID:18094380; https://doi.org/https://doi.org/10.1056/NEJMra064928
- Neofytos D, Fishman JA, Horn D, Anaissie E, Chang CH, Olyaei A, Pfaller M, Steinbach WJ, Webster KM, Marr KA. Epidemiology and outcome of invasive fungal infections in solid organ transplant recipients. Transpl Infect Dis 2010; 12:220-9; PMID:20113459; https://doi.org/https://doi.org/10.1111/j.1399-3062.2010.00492.x
- Marr KA, Carter RA, Boeckh M, Martin P, Corey L. Invasive aspergillosis in allogeneic stem cell transplant recipients: changes in epidemiology and risk factors. Blood 2002; 100:4358-66; PMID:12393425; https://doi.org/https://doi.org/10.1182/blood-2002-05-1496
- Marr KA, Carter RA, Crippa F, Wald A, Corey L. Epidemiology and outcome of mould infections in hematopoietic stem cell transplant recipients. Clin Infect Dis 2002; 34:909-17; PMID:11880955; https://doi.org/https://doi.org/10.1086/339202
- Singh G, Imai J, Clemons KV, Stevens DA. Efficacy of caspofungin against central nervous system Aspergillus fumigatus infection in mice determined by TaqMan PCR and CFU methods. Antimicrob Agents Chemother 2005; 49:1369-76; PMID:15793114; https://doi.org/https://doi.org/10.1128/AAC.49.4.1369-1376.2005
- John GT, Shankar V, Talaulikar G, Mathews MS, Abraham MA, Thomas PP, Jacob CK. Epidemiology of systemic mycoses among renal-transplant recipients in India. Transplantation 2003; 75:1544-51; PMID:12792512; https://doi.org/https://doi.org/10.1097/01.TP.0000061610.34110.04
- Paya CV. Fungal infections in solid-organ transplantation. Clin Infect Dis 1993; 16:677-88; PMID:8507760; https://doi.org/https://doi.org/10.1093/clind/16.5.677
- Dummer JS, Hardy A, Poorsattar A, Ho M. Early infections in kidney, heart, and liver transplant recipients on cyclosporine. Transplantation 1983; 36:259-67; PMID:6310832; https://doi.org/https://doi.org/10.1097/00007890-198309000-00007
- Stewart AA, Ingebritsen TS, Manalan A, Klee CB, Cohen P. Discovery of A Ca2+-and calmodulin-dependent protein phosphatase. FEBS Letters 1982; 137:80-4; PMID:6279434; https://doi.org/https://doi.org/10.1016/0014-5793(82)80319-0
- Steinbach WJ, Reedy JL, Cramer RA, Perfect JR, Heitman J. Harnessing calcineurin as a novel anti-infective agent against invasive fungal infections. Nat Rev Micro 2007; 5:418-30; https://doi.org/https://doi.org/10.1038/nrmicro1680
- Steinbach WJ, Cramer RA, Perfect BZ, Asfaw YG, Sauer TC, Najvar LK, Kirkpatrick WR, Patterson TF, Benjamin DK, Heitman J. Calcineurin controls growth, morphology, and pathogenicity in Aspergillus fumigatus. Eukaryotic Cell 2006; 5:1091-103; PMID:16835453; https://doi.org/https://doi.org/10.1128/EC.00139-06
- Imbert S, Bresler P, Boissonnas A, Gauthier L, Souchet L, Uzunov M, Leblond V, Mazier D, Nguyen S, Fekkar A. Calcineurin inhibitors impair neutrophil activity against Aspergillus fumigatus in allogeneic hematopoietic stem cell transplant recipients. J Allergy Clin Immunol 2016; 138:860-8; PMID:27132218; https://doi.org/https://doi.org/10.1016/j.jaci.2016.02.026
- High KP, Washburn RG. Invasive aspergillosis in mice immunosuppressed with cyclosporin A, tacrolimus (FK506), or sirolimus (rapamycin). J Infect Dis 1997; 175:222-5; PMID:8985226; https://doi.org/https://doi.org/10.1093/infdis/175.1.222
- Vecchiarelli A, Cenci E, Marconi P, Rossi R, Riccardi C, Bistoni F. Immunosuppressive effect of cyclosporin A on resistance to systemic infection with Candida albicans. J Med Microbiol 1989; 30:183-92; PMID:2511321; https://doi.org/https://doi.org/10.1099/00222615-30-3-183
- Greenblatt MB, Aliprantis A, Hu B, Glimcher LH. Calcineurin regulates innate antifungal immunity in neutrophils. J Exp Med 2010; 207:923-31; PMID:20421389; https://doi.org/https://doi.org/10.1084/jem.20092531
- Herbst S, Shah A, Mazon Moya M, Marzola V, Jensen B, Reed A, Birrell MA, Saijo S, Mostowy S, Shaunak S, et al. Phagocytosis-dependent activation of a TLR9-BTK-calcineurin-NFAT pathway co-ordinates innate immunity to Aspergillus fumigatus. EMBO Mol Med 2015; 7:240-58; PMID:25637383; https://doi.org/https://doi.org/10.15252/emmm.201404556
- Krause MW, Schaffner A. Comparison of immunosuppressive effects of cyclosporine A in a murine model of systemic candidiasis and of localized thrushlike lesions. Infect Immun 1989; 57:3472-8; PMID:2807532
- Ito E, Tanaka Y. Influences of immunosuppressive agents, FK506 and cyclosporin on systemic Candida albicans infection in mice. Mycopathologia 1997; 138:57-64; PMID:9433807; https://doi.org/https://doi.org/10.1023/A:1006827828838
- Mody CH, Toews GB, Lipscomb MF. Treatment of murine cryptococcosis with cyclosporin-A in normal and athymic mice. Ann Arbor 1989; 1001:48109-0360
- Mody C, Toews G, Lipscomb M. Cyclosporin A inhibits the growth of Cryptococcus neoformans in a murine model. Infect Immun 1988; 56:7-12; PMID:3275587
- Dreyfuss M, Härri E, Hofmann Hea, Kobel H, Pache W, Tscherter H. Cyclosporin A and C. Eur J Applied Microbiol Biotechnol 1976; 3:125-33; https://doi.org/https://doi.org/10.1007/BF00928431
- Cohen D, Loertscher R, Rubin M, Tilney N, Carpenter C, Strom T. Cyclosporine: a new immunosuppressive agent for organ transplantation. Annals Internal Med 1984; 101:667-82; PMID:6385799; https://doi.org/https://doi.org/10.7326/0003-4819-101-5-667
- Bader T, Bodendorfer B, Schröppel K, Morschhäuser J. Calcineurin is essential for virulence in Candida albicans. Infect Immun 2003; 71:5344-54; PMID:12933882; https://doi.org/https://doi.org/10.1128/IAI.71.9.5344-5354.2003
- Odom A, Muir S, Lim E, Toffaletti DL, Perfect J, Heitman J. Calcineurin is required for virulence of Cryptococcus neoformans. EMBO J 1997; 16:2576-89; PMID:9184205; https://doi.org/https://doi.org/10.1093/emboj/16.10.2576
- Upton A, Kirby KA, Carpenter P, Boeckh M, Marr KA. Invasive aspergillosis following hematopoietic cell transplantation: outcomes and prognostic factors associated with mortality. Clin Infect Dis 2007; 44:531-40; PMID:17243056; https://doi.org/https://doi.org/10.1086/510592
- Singh N, Paterson DL. Aspergillus infections in transplant recipients. Clin Microbiol Rev 2005; 18:44-69; PMID:15653818; https://doi.org/https://doi.org/10.1128/CMR.18.1.44-69.2005
- Kim JH, Perfect JR. Infection and cyclosporine. Rev Infect Dis 1989; 11:677-90; PMID:2682942; https://doi.org/https://doi.org/10.1093/clinids/11.5.677
- Borghi E, Morace G, Borgo F, Rajendran R, Sherry L, Nile C, Ramage G. New strategic insights into managing fungal biofilms. Frontiers Microbiol 2015; 6:1077; PMID:26500623; https://doi.org/https://doi.org/10.3389/fmicb.2015.01077
- Clancy CJ, Jaber RA, Leather HL, Wingard JR, Staley B, Wheat LJ, Cline CL, Rand KH, Schain D, Baz M, et al. Bronchoalveolar lavage galactomannan in diagnosis of invasive pulmonary aspergillosis among solid-organ transplant recipients. J Clin Microbiol 2007; 45:1759-65; PMID:17428933; https://doi.org/https://doi.org/10.1128/JCM.00077-07
- Steinbach WJ, Schell WA, Blankenship JR, Onyewu C, Heitman J, Perfect JR. In vitro interactions between antifungals and immunosuppressants against Aspergillus fumigatus. Antimicrob Agents Chemother 2004; 48:1664-9; PMID:15105118; https://doi.org/https://doi.org/10.1128/AAC.48.5.1664-1669.2004
- Chaturvedi PR, Decker CJ, Odinecs A. Prediction of pharmacokinetic properties using experimental approaches during early drug discovery. Curr Opin Chem Biol 2001; 5:452-63; PMID:11470610; https://doi.org/https://doi.org/10.1016/S1367-5931(00)00228-3
- Huyan XH, Lin YP, Gao T, Chen RY, Fan YM. Immunosuppressive effect of cyclophosphamide on white blood cells and lymphocyte subpopulations from peripheral blood of Balb/c mice. Int Immunopharmacol 2011; 11:1293-7; PMID:21530682; https://doi.org/https://doi.org/10.1016/j.intimp.2011.04.011
- Lewis RE, Wiederhold NP. Murine model of invasive aspergillosis. Antifungal Agents: Methods Protocols 2005; 118:129-42
- Romani L. Immunity to fungal infections. Nat Rev Immunol 2004; 4:1-23; PMID:14661066; https://doi.org/https://doi.org/10.1038/nri1255
- Mircescu MM, Lipuma L, van Rooijen N, Pamer EG, Hohl TM. Essential role for neutrophils but not alveolar macrophages at early time points following Aspergillus fumigatus infection. J Infect Dis 2009; 200:647-56; PMID:19591573; https://doi.org/https://doi.org/10.1086/600380
- Ibrahim-Granet O, Jouvion G, Hohl T, Droin-Bergere S, Philippart F, Kim O, Adib-Conquy M, Schwendener R, Cavaillon JM, Brock M. In vivo bioluminescence imaging and histopathopathologic analysis reveal distinct roles for resident and recruited immune effector cells in defense against invasive aspergillosis. BMC Microbiol 2010; 10:105; PMID:20377900; https://doi.org/https://doi.org/10.1186/1471-2180-10-105
- Janco RL, English D. Cyclosporine and human neutrophil function. Transplantation 1983; 35:501-3; PMID:6302959; https://doi.org/https://doi.org/10.1097/00007890-198305000-00023
- Roilides E, Robinson T, Sein T, Pizzo PA, Walsh TJ. In vitro and ex vivo effects of cyclosporin A on phagocytic host defenses against Aspergillus fumigatus. Antimicrob Agents Chemother 1994; 38:2883-8; PMID:7695277; https://doi.org/https://doi.org/10.1128/AAC.38.12.2883
- Marchetti O, Moreillon P, Glauser MP, Bille J, Sanglard D. Potent synergism of the combination of fluconazole and cyclosporine in Candida albicans. Antimicrob Agents Chemother 2000; 44:2373-81; PMID:10952582; https://doi.org/https://doi.org/10.1128/AAC.44.9.2373-2381.2000
- Morgenstern DE, Gifford MA, Li LL, Doerschuk CM, Dinauer MC. Absence of respiratory burst in X-linked chronic granulomatous disease mice leads to abnormalities in both host defense and inflammatory response to Aspergillus fumigatus. J Exp Med 1997; 185:207-18; PMID:9016870; https://doi.org/https://doi.org/10.1084/jem.185.2.207
- Yamamoto Y, Gaynor RB. Therapeutic potential of inhibition of the NF-kappaB pathway in the treatment of inflammation and cancer. J Clin Invest 2001; 107:135-42; PMID:11160126; https://doi.org/https://doi.org/10.1172/JCI11914
- Balloy V, Huerre M, Latgé J-P, Chignard M. Differences in patterns of infection and inflammation for corticosteroid treatment and chemotherapy in experimental invasive pulmonary aspergillosis. Infect Immunity 2005; 73:494-503; PMID:15618189; https://doi.org/https://doi.org/10.1128/IAI.73.1.494-503.2005
- Galiger C, Brock M, Jouvion G, Savers A, Parlato M, Ibrahim-Granet O. Assessment of efficacy of antifungals against Aspergillus fumigatus: value of real-time bioluminescence imaging. Antimicrob Agents Chemother 2013; 57:3046-59; PMID:23587947; https://doi.org/https://doi.org/10.1128/AAC.01660-12
- Brock M, Jouvion G, Droin-Bergere S, Dussurget O, Nicola MA, Ibrahim-Granet O. Bioluminescent Aspergillus fumigatus, a new tool for drug efficiency testing and in vivo monitoring of invasive aspergillosis. Applied Environmental Microbiol 2008; 74:7023-35; PMID:18820063; https://doi.org/https://doi.org/10.1128/AEM.01288-08
- Laskaris P, Atrouni A, Calera JA, d'Enfert C, Munier-Lehmann H, Cavaillon JM, Latgé JP, Ibrahim-Granet O. Administration of zinc chelators improves survival of mice infected with Aspergillus fumigatus both in monotherapy and in combination with caspofungin. Antimicrob Agents Chemother 2016; 60:5631-9; PMID:27401578; https://doi.org/https://doi.org/10.1128/AAC.00324-16
