ABSTRACT
Candida glabrata is the second most common pathogen of severe candidiasis in immunocompromised hosts, following C. albicans. Although C. glabrata and C. albicans belong to the same genus, they are phylogenetically distinct. C-type lectin receptors (CLRs), acting as pattern-recognition receptors (PRRs), play critical roles in host defense against C. albicans infections. However, our understanding of the specific roles of CLRs in host defense against C. glabrata is limited. Here, we explored the potential roles of the C-type lectins Dectin-1 and Dectin-2 in host defense against C. glabrata. We found that both Dectin-1-deficient mice (Dectin-1−/−) and Dectin-2-deficient mice (Dectin-2−/−) are more susceptible to C. glabrata infection. Dectin-1confers host higher sensitivity for sensing C. glabrata infections, while the effect of Dectin-2 in the host defense against C. glabrata is infection dose dependent. Dectin-1 is required for host myeloid cells recognition, killing of C. glabrata, and development of subsequent Th1 and Th17 cell-mediated adaptive immune response. Significantly impaired inflammatory responses such as inflammatory cells recruitment and cytokines release that were induced by C. glabrata were manifested in Dectin-1-deficient mice. Together, our study demonstrates that Dectin-1 plays an important role in host defense against systemic Candida glabrata infections, indicating a previous unknown control mechanism for this particular type of infection in host. Our study, therefore, provides new insights into the host defense against C. glabrata.
Introduction
Candida albicans is the commonest fungal species causing mucosal and systemic infections. There is an increasing number of candidiasis cases in the last decades, and a shift toward infections with non-albicans Candida spp., particularly C. glabrata.Citation1-3 C. glabrata is the second most common pathogen in candidiasis infections, accounting for about 15–25% of all Candida infections.Citation4,5 Similar to C. albicans, C. glabrata also colonizes the skin, genital mucosa, and intestinal mucosa.Citation6 C. glabrata can invade into the bloodstream and cause life-threatening systemic infection in immunocompromised patients. Compared to C. albicans, systemic C. glabrata infections lead to a higher mortality. In addition, C. glabrata is resistant to some antifungal drugs, particularly azoles, thereby making clinical treatment difficult.Citation7-9 Although C. albicans infections have been studied extensively, our knowledge on pathophysiology of C. glabrata infection is limited.
The development of systemic candidiasis is the result of an imbalance between pathogen invasion and host defense response.Citation10 Fungi are recognized by the innate immune system via pattern recognition receptors (PRRs) which are predominantly expressed on myeloid cells, such as Toll-like receptors (TLRs) and C-type lectin receptors (CLRs).Citation11 Although all of the PRRs are involved in antifungal immune recognition, only CLR pathway mutations are associated with the spontaneous human fungal infections development.Citation11 Previous clinical study demonstrated that patients with Dectin-1 mutation (Y238X) have an increased occurrence of mucosal C. albicans infections.Citation12
Several well-characterized CLRs, such as Dectin-1, Dectin-2, Mincle, mannose receptor (MR), SIGNR-1, and Galectin-3, are involved in the binding, uptake, and killing of C. albicans, as well as initiation and modulation of host immune responses.Citation13,14 Among the CLRs, Dectin-1 and Dectin-2 are well-studied in host defense against invasive C. albicans infections. Dectin-1 recognizes β-glucan by binding the yeast form of C. albicans, thereby triggering a series of cellular antifungal responses such as respiratory burst,Citation15 phagocytosis,Citation16 neutrophil extracellular traps,Citation17 and the production of various cytokines.Citation18,19 Host Dectin-2 preferentially binds to the hyphal form of C. albicans, which is essential for the development of Th17 cells mediated adaptive immune response, thereby coordinating the Th1 cells mediated immune response together with Dectin-1.Citation20,21 Previous study suggested that Dectin-2 plays a role in host defense against C. glabrata, which might link host innate immune response and adaptive Th cells mediated immune response.Citation22 However, to data, our understanding of the role of Dectin-1 in host immune response to C. glabrata is limited.
In the present study, we explored the effects of host Dectin-1 in the pathophysiology of systemic C. glabrata infection, and compared the roles of Dectin-1 and Dectin-2 in host defense against C. glabrata. We found that recognition of β-glucan by Dectin-1 is essential for host immunity against C. glabrata through triggering innate immune cells activation and priming the subsequent Th cell mediated adaptive immune response. Our studies also demonstrate that Dectin-1 plays a more important role in the induction of protective immune responses against C. glabrata compared with Dectin-2, providing new insights into host defense against this pathogenic fungus.
Results
Dectin-1 is required for myeloid cells recognizing C. glabrata
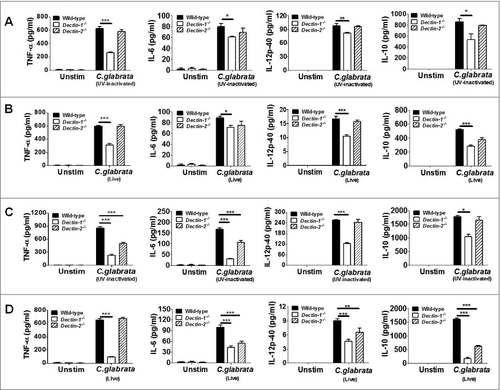
Macrophages play an essential role in triggering host innate immune recognition and the subsequent adaptive immune responses against fungus.Citation23 In the present study, we first investigated Dectin-1-deficient and Dectin-2-deficient macrophages recognition and its responses to C. glabrata using a macrophage-C. glabrata interaction model. We found that C. glabrata could activate NF-κB signaling, which including nuclear translocation of NF-κB (p65), Syk phosphorylation, IκBα phosphorylation, together with IκBα degradation in thioglycolate-elicited peritoneal macrophages (Figs. S1A and C). Furthermore, C. glabrata also induced the phosphorylation of ERK, p38, and JNK in macrophages, thereby suggesting the activation of the MAPK signaling pathway (Fig. S1B). Subsequently, C. glabrata (UV-inactivated and live C. glabrata) induced release of inflammatory cytokines, including TNF-α, IL-6, IL-12p40, and IL-10, in thioglycolate-elicited peritoneal macrophages (). Moreover, the above inflammatory cytokines release was significantly lower in C. glabrata-challenged Dectin-1-deficient macrophages compared with wild-type macrophages, while no significant differences in inflammatory cytokine production were detected between Dectin-2-deficient and wild-type macrophages at multiplicity of infection (MOI) = 1 (). When challenged by C. glabrata at higher dose (MOI = 5), not only Dectin-1-deficient, but also Dectin-2-deficient macrophages produced lower levels of inflammatory cytokines, compared with wild-type macrophages ( and ). The above results suggested that Dectin-1 is required for macrophages sensing C. glabrata infection. In addition, the effects of Dectin-2 for sensing C. glabrata is infection dose dependent.
Figure 1. Dectin-1 and Dectin-2 is required for C. glabrata-induced immune responses in host macrophage. ELISA results for the production of TNF-α, IL-6, IL-12p40 and IL-10 in supernatants of thioglycolate-elicited macrophage. Wild-type, Dectin-1-deficient and Dectin-2-deficient macrophage were stimulated with UV-inactivated (A, MOI = 1; C, MOI = 5) or live (B, MOI = 1; D, MOI = 5) C. glabrata ATCC 28226 for 6 h (n = 5). Data are representative of 3 independent experiments and shown as means ± SD. #, P < 0.05; ##, P < 0.01; ###, P < 0.001 (Kruskal-Wallis nonparametric one-way ANOVA with Dunn's posttest)
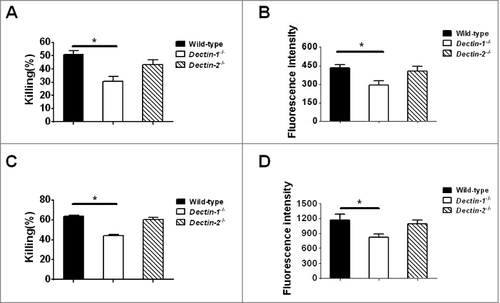
Neutrophils are the first abundant leukocytes, which is important for phagocytosis of invading fungus.Citation24 When challenged with unopsonized or opsonized live C. glabrata, we found that Dectin-1-deficientneutrophils showed attenuated killing ability accompanied with a defect in ROS production, compared with wild-type neutrophils (). However, there is no significant differences between Dectin-2-deficient and wild-type neutrophils when infected with live C. glabrata (). Thus, our results suggested Dectin-1, but not Dectin-2, mediates neutrophils recognition and killing of C. glabrata.
Figure 2. Impaired killing ability of C. glabrata with respiratory burst of Dectin-1-deficient neutrophils. (A, C) Neutrophils killing assay. Wild-type, Dectin-1-deficient neutrophils or Dectin-2-deficient neutrophils (6 × 105 cells) were incubated with 1 × 104 unopsonized cells (A) or opsonized cells (C) of C. glabrata ATCC 28226 for 1 h (n = 5). Then the suspension was plated on SDA agar for 48 h to quantify C. glabrata colonies. (B, D) Neutrophils respiratory burst assay. Peritoneal neutrophils were culture with unopsonized cells (B) or opsonized cells (D) of C. glabrata for 1 h (MOI = 1) (n = 5). The cellular hydrogen peroxide (H2O2) production of peritoneal neutrophils were measured by assessing the fluorescence of conversion of dihydrorhodamine 123 to rhodamine. Fluorescence intensity was used to assay the translation of dihydrorhodamine 123 to rhodamine. Data are representative of 3 independent experiments and shown as means ± SD. #, P < 0.05; ##, P < 0.01 (Student's t-test)
Dectin-1-deficient mice, but not Dectin-2-deficient mice, show attenuated inflammatory response when challenged by C. glabrata
As our host cell-C. glabrata interaction model showed impaired activation of innate immune cells in Dectin-1-deficient mice, we then explored how the absence of Dectin-1 affected inflammatory responses to C. glabrata in vivo through a peritoneal infection model.
We first explored whether deletion of Dectin-1 and Dectin-2 receptor influence the recruitment of immune cells in vivo. Dectin-1-deficient, Dectin-2-deficient and wild-type mice were intraperitoneally injected thioglycolate, and flow cytometry performed 4h later. The results demonstrated that there were no differences in terms of the recruitment of SSChighCD11b+ Ly-6C+Ly-6G+ neutrophils, SSChighCD11b+ Ly-6C+Ly-6G− monocyte-derived cells, and SSChighCD11b+Siglec-F+ eosinophils among the 3 mice strains (Figs. S2).
Subsequently, mice were intraperitoneally infected with C. glabrata, and flow cytometry performed 4 h later revealed that Dectin-1-deficientmice recruited a lower number of inflammatory cells in peritoneum than that in wild-type mice, including SSChighCD11b+ Ly-6C+Ly-6G+ neutrophils, SSChighCD11b+ Ly-6C+Ly-6G− monocyte-derived cells, and SSChighCD11b+Siglec-F+ eosinophils ( and ). Meanwhile, a few immune cells were detected in peritoneum of PBS intraperitoneally infected control mice, which was much less than C. glabrata infected mice (Fig S3).
Figure 3. Dectin-1 but not Dectin-2 is required for normal antifungal inflammatory response in vivo. Dectin-1-deficient, Dectin-2-deficient and wild-type mice were intraperitoneal infected with 1 × 106 live C. glabrata ATCC28226 for 4 h. (A) Flow cytometry SSChighCD11b+Ly-6C+Ly-6G+ neutrophils and SSChigh CD11b+ Ly-6C+ Ly-6G− monocyte-derived cells and SSChighCD11b+ Siglect-F+ eosinophils in the peritoneum of the indicated mice with intraperitoneal infection (the shown percentages refer to total cells) (n = 5). (B) Scatter plots of myeloid cell subsets in the peritoneum of the indicated mice with intraperitoneal infection (n = 5). (C) ELISAs for cytokines, chemokines and growth factors in lavage fluid from the peritoneal cavities. MCP-1, chemokine CCL2; GM-CSF, granulocyte-monocyte colony-stimulating factor (n = 6). Data are representative of 3 independent experiments and shown as means ± SD. #, P < 0.05; ##, P < 0.01(Mann-Whitney nonparametric t-test)
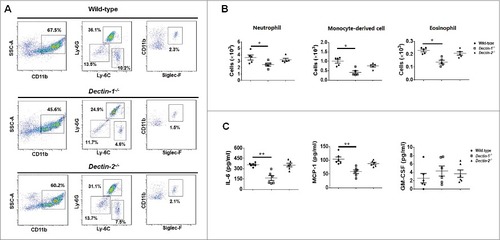
The observed lower inflammatory cells recruitment in Dectin-1-deficient mice was also associated with a decrease in the production of specific cytokines and chemokines including IL-6 and MCP-1, while not of granulocyte-monocyte colony-stimulating factor (GM-CSF) (). In contrast, no significant differences in inflammatory cells recruitment and specific cytokines or chemokines between Dectin-2-deficient and wild-type mice were observed ().
Thus, our results indicated that Dectin-1-deficient mice, but not Dectin-2-deficient mice have an intrinsic defect in the development of immune response to C. glabrata in vivo.
Dectin-1, but not Dectin-2, confers host a higher sensitivity for sensing C. glabrata infection
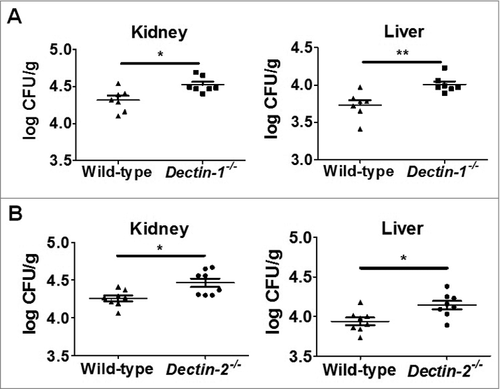
Based on our above findings that Dectin-1 is required for myeloid cell recognition and inflammatory response to C. glabrata, we next examined the effect of Dectin-1 deficiency on the susceptibility of C. glabrata infection in vivo. We intravenously infected mice with C. glabrata ATCC28226 or ATCC1182 (1 × 105 cells per mouse) as a model of systemic candidiasis. At day 7 post-infection, Dectin-1-deficient mice showed significantly higher fungal burden in kidney and liver compared with that in wild-type mice ( and ). In addition, the mice showed significantly lower levels of the inflammatory cytokines IL-1β, IL-6 and IFN-γ in kidneys, while not of IL-17 (). However, no significant differences in fungal burden of the kidney and liver between Dectin-2-deficientand wild-type mice were detected ( and ). Furthermore, the levels of inflammatory cytokines in the kidneys of C. glabrata-infected Dectin-2-deficient mice were also similar to that of wild-type mice (). We then further challenged the mice with a higher dose of C. glabrata (1 × 107 cells per mouse), which showed that both Dectin-1-and Dectin-2-deficientmice exhibited significantly higher fungal burdens than those of the wild-type mice (). Although both Dectin-1 and Dectin-2 are required for host to control C. glabrata infection, our results suggested that Dectin-1 renders host competent to sense C. glabrata invasion more sensitively, while the effect of Dectin-2 is infection dose dependent.
Figure 4. Host Dectin-1 but not Dectin-2 is required for sensing lower dose of C. glabrata induced systemic infection. Dectin-1-deficient, Dectin-2-deficient, and wild-type mice were intravenously infected with lower dose (1 × 105 cells per mouse) of viable C. glabrata ATCC28226 (A, B, E, F) or C. glabrata ATCC1182 (C, D), respectively. (A, B, C, D) Quantification of the fungal burden in kidneys and livers of the indicated mice which were infected with C. glabrata at day 7 (n = 8). Data are representative of 3 independent experiments. (E, F) ELISA assays for IL-1β, IL-6, INF-γ, and IL-17 in kidneys of indicated mice with C. glabrata ATCC28226 infection (n = 8). Data are representative of 3 independent experiments and shown as means ± SD. #, P < 0.05; ###, P < 0.001 (Mann-Whitney nonparametric t-test)
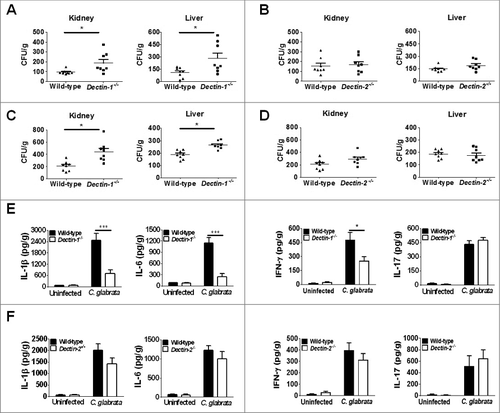
Figure 5. Both host Dectin-1 and Dectin-2 are required for sensing higher dose of C. glabrata systemic induced infection. Quantification of the fungal burden in kidneys and livers of Dectin-1-deficient mice (A) or Dectin-2-deficient mice (B) infected with higher dose of C. glabrata ATCC28226 (1 × 107 cells per mouse) at day 7 compared with wild-type mice (A, n = 7; B, n = 8). Data are representative of 3 independent experiments and shown as means ± SD. #, P < 0.05; ##, P < 0.01 (Mann-Whitney nonparametric t-test)
Dectin-1 directs Th cell responses to C. glabrata infection
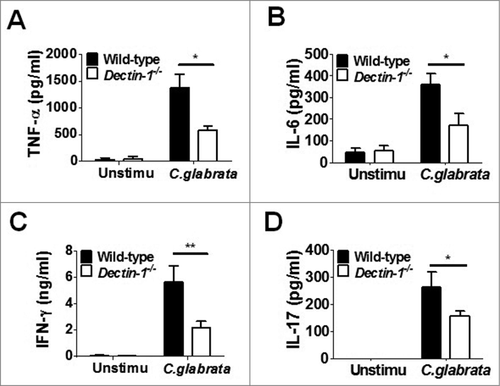
Engagement of PRRs on cell surface of innate immune cells could render them competent to prime T cells and then trigger the subsequently adaptive immune response.Citation25 Our findings have indicated that Dectin-1 is required for host innate immune response to C. glabrata. Thus, we further explored the contribution of Dectin-1 to adaptive immunity against C. glabrata. We collected splenocytes from C. glabrata-infected Dectin-1-deficient and wild-type mice, which were then restimulated with UV-inactivated C. glabrata for 2 or 5 d. Th1 and Th17 responses were monitored by measuring TNF-α, IL-6, IFN-γ and IL-17 in the cell supernatant. The levels of TNF-α, IL-6, IFN-γ and IL-17 production of splenocytes from C. glabrata-infected Dectin-1-deficienct mice were significantly lower than those from wild-type mice (), suggesting lower Th1 and Th17 responses in Dectin-1-deficient mice during C. glabrata systemic infection. In addition, we also analyzed Th1 and Th17 response in Dectin-2-deficient splenocytes restimulated with C. glabrata. We found that significantly lower TNF-α and IL-17 production, while similar IL-6 and IFN-γ production compared with wild-type splenocytes, suggesting that Dectin-2 are also required for host Th cell responses against C. glabrata (Fig. S4).
Figure 6. Dectin-1 is required for host Th cells response during systemic C. glabrata infection (splenocytes recall response assay). Dectin-1-deficient and wild-type mice were intravenously infected with 1 × 105 cells of C. glabrata ATCC28226 for 7 days, respectively. Then the splenocytes were collected and restimulated with UV-inactived C. glabrata for 48 h or 5days (MOI = 0.02). Accumulation of TNF-α (A), IL-6 (B), IFN-γ (C) for 48 h and IL-17 (D) for 5 d in the supernatants were measured by ELISA (n = 5). Data are representative of 3 independent experiments and shown as means ± SD. #, P < 0.05; ##, P < 0.01 (Mann-Whitney nonparametric t test)
β-(1,3)-glucan is exposed in C. glabrata yeast, but not in C. albicans yeast
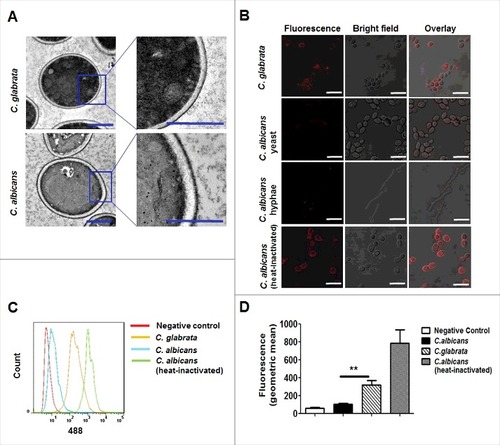
β-(1,3)-glucan is a well-characterized PAMP of C. albicans.Citation13 However, it is buried underneath the outer layer of the cell wall with highly glycosylated mannoproteins.Citation26 The present study examined the ultrastructure of C. glabrata cell wall by transmission electron microscopy (TEM), as well as cell surface β-(1,3)-glucans by confocal microscopy and flow cytometry. TEM observation indicated that the inner cell wall layer of C. albicans was surrounded by an external layer of dense mannosylated proteins, whereas that of C. glabrata was enclosed by a relatively loose outer cell wall layer (). We thus deduced that β-glucans are exposed on the cell surface of C. glabrata. To confirm this hypothesis, we detected cell surface β-(1, 3)-glucan using an anti-β-(1, 3)-glucan primary antibody, and then observed by confocal microscopy and quantitated by flow cytometry. The results suggested that β-(1, 3)-glucan was remarkably exposed on the cell surface of C. glabrata compared with that of C. albicans including yeast and filament forms (). Flow cytometry showed that C. glabrata had statistically greater reactivity with the anti-β-(1, 3)-glucan antibody than C. albicans ( and ). Heat-inactivated C. albicans, which showed marked β-glucan expose on cells surface, was used as a positive control in above experments.Citation27,28 Furthermore, we got similar results about β-(1, 3)-glucan exposure on UV-inactivated C. glabrata and C. albicans cells (Fig. S5). In addition, we found modest lower content of chitin and mannan in cell wall of C. glabrata compared with that of C. albicans (Fig. S6).
Figure 7. β-(1, 3)-glucan is markedly exposed on cell surface of C. glabrata compared with C. albicans. (A) Representative cell wall ultrastructures of C. glabrata ATCC28226 and C. albicans SC5314 were imaged by transmission electron microscopy (scale bar = 1 μm). (B) Representative fluorescence micrographs of C. glabrata ATCC28226 and C. albicans SC5314. The cells were stained with β-glucan antibody and Cy3-labeled secondary antibody for visualizing cell surface of β-(1, 3)-glucan (scale bar = 10 μm). (C, D) The cells stained with β-(1, 3)-glucan were subsequently stained with the secondary antibody (Alexa-488-labeled) at 30°C for 1 h. A flow cytometry was used to quantify the fluorescence. Heat-inactivated yeast C. albicans SC5314 was presented as a positive control for β-(1,3)-glucan detection. Data are representative of 3 independent experiments and shown as means ± SD. ##, P < 0.01 (one-way ANOVA with Bonferroni's posttest)
Overall, our results indicated β-(1, 3)-glucans are exposed on the surface of C. glabrata, which may explain the critical roles of Dectin-1 in host recognition of C. glabrata.
Discussion
CLRs, mainly Dectin-1 and Dectin-2, are involved in the recognition of β-glucans and α-mannan on fungal cell walls. Previous studies have demonstrated that Dectin-1 and Dectin-2 play critical roles in host immune response to C. albicans.Citation29,30 C. albicans is commonly used as a model organism in investigations on immune responses to Candida. However, there is evidence of a variety of immune responses to different speciessuch as that in C. albicans and C. glabrata.Citation29,31 Our understanding of the role of CLRs in host immune response to C. glabrata is limited. In the present study, we comparatively studied the roles of Dectin-1 and Dectin-2 in host defense against systemic C. glabrata infections. We initially determined that Dectin-1 renders host a higher sensitivity for sensing C. glabrata invasion, suggesting new insights into CLRs in the immune responses to this fungal pathogen.
Neutrophils provide the first line of fungal killing, which are especially essential in neutropenic and immunosuppressed patients.Citation23 A reduction in neutrophil response during intra-abdominal C. glabrata infection is associated with an increase in peritoneal fluid fungal burden.Citation32 CLRs induce signaling and ROS formation via the NADPH oxidase system, which result in neutrophil-mediated killing of C. albicans.Citation33 Our results showed that neutrophils also killed C. glabrata through an augmented respiratory burst (). On the other hand, we found the killing ability of Dectin-1-deficient neutrophils was markedly impaired, whereas that of Dectin-2-deficient neutrophils was similar to that of wild-type neutrophils (). These findings suggest that the recognition of exposed β-(1,3)-glucans by the Dectin-1 receptor, as well as ROS formation, contribute to the neutrophils responses against C. glabrata. However, Ifrim et al. reported that Dectin-2-deficient neutrophils exhibited defection in augmented respiratory burst when challenged by C. glabrata.Citation22 The different results may be attributed to variations in experimental protocols. Ifrim et al. challenged peritoneal neutrophils with opsonized C. glabrata at MOI = 10,Citation22 whereas we used C. glabrata at MOI = 1. The findings of our study suggest that Dectin-1, but not Dectin-2, confers host higher neutrophils sensitivity to C. glabrata infections.
Innate immune recognition could render antigen-presenting cells competency to trigger T cells differentiation, and then drive Th cells dependent adaptive immune responses.Citation26 Host innate immune cells could secrete several cytokines when encounter pathogens, thereby leading to trigger Th cell differentiation.Citation34,35 We demonstrated that C. glabrata could be recognized by macrophages, which is associated with the activation of the NF-κB and MAPK pathways (Fig. S1), as well as production of inflammatory cytokines, including TNF-α, IL-6, IL-1β, and IL-12p40 (). TNF-α was involved in the host innate immune responses against fungus by promoting neutrophil production and activation.Citation36 IL-23 (consisting of IL-12p40 and p19) contribute to Th17 differentiation, and IL-1β is an essential pro-inflammatory regulator of Th17 cells both at the priming and effect phases.Citation37 We demonstrated that Dectin-1 deficiency, but not Dectin-2 deficiency, affects cytokine production in host macrophages response to lower dose of C. glabrata challenge (MOI = 1) ( and ). However, both Dectin-1 and Dectin-2 are required for sensing higher dose of C. glabrata infection (MOI = 5) ( and ). Therefore, our study demonstrated that Dectin-1 is required for host macrophages detecting C. glabrata infections, while the effect of Dectin-2 for sensing C. glabrata is infection dose dependent. These results suggested that Dectin-1 is likely to render host more vigilant to C. glabrata infection compared with Dectin-2.
We then performed further experiment to confirm that Dectin-1 was required for host sensing C. glabrata infection in vivo. We demonstrated that both Dectin-1 and Dectin-2 are required for controlling high-dose C. glabrata infections in systemic candidiasis mouse model (). However, only Dectin-1 was required for low-dose C. glabrata infections (), thereby suggesting that Dectin-1, but not Dectin-2, renders host competent to detect C. glabrata infections with higher sensitivity. Our results indicated that the effect of Dectin-2 in host immune responses to C. glabrata is infection dose dependent. Dectin-2 is inclined to recognize the hyphae form of fungi.Citation20 Without pseudo- or true hyphae formation in C. glabrata might prevent it from being recognized by host Dectin-2.
It is well known that both Th1 and Th17 cells dependent adaptive immune response could mediate protection against fungal infections.Citation25,38 IFN-γ and IL-17 are the most important cytokines released by Th1 and Th17 cells, respectively. IFN-γ downregulation in kidneys (), and the decreased splenocyte recall responses of C. glabrata-infected Dectin-1-deficienct mice ( and ) indicated that Dectin-1 is required for C. glabrata inducing host Th1 and Th17 responses. To our knowledge, the variations of cytokines responses to candidiasis in the kidneys of animal model were associated with the time after infection. Although Dectin-1 deficient mice infected intravenously with C. glabrata did not show lower levels of IL-17 in kidney in our study (), we think it cannot exclude the importance of IL-17 during Dectin-1 mediated immune response against C. glabrata in vivo. The downregulation of pro-inflammatory cytokines in the kidneys of C. glabrata-infected Dectin-1-deficienct mice might result in an impairment in innate immune cells recruitment, as well as defection in triggering the subsequent adaptive immune response to C.glabrata.
Our peritoneal infection model demonstrated that a markedly decrease in the production of cytokines and chemokines, such as IL-6 and MCP-1, in the peritoneal cavity of Dectin-1-deficient mice, which results in reduction of neutrophils and monocyte-derived cells recruitment (). In addition to priming neutrophils production and activation, IL-6 also plays an important role in triggering Th17 cells differentiation.Citation30 MCP-1 is a critical factor for monocyte-derived cells recruitment.Citation39 Dectin-2-deficient mice did not show an attenuated inflammatory response when peritoneally infected with C. glabrata (), suggesting that Dectin-1, but not Dectin-2, is required for host inflammatory responses induced by C. glabrata in vivo.
The cell wall plays a key role in host-fungus interactions that facilitate the development of invasive infections.Citation34 The structure of the cell wall of C. albicans has been extensively characterized, whereas information on that of C. glabrata is limited. The organization of the cell wall of C. glabrata, which is apparently similar to that of C. albicans, is a complex dynamic structure that consists of a core structure of β-(1,3)-glucans that are covalently linked to β-(1,6)-glucan, chitin, and an outer layer of the matrix composed of mannoproteins.Citation40 Our TEM observation revealed that the cell wall of C. glabrata consists of a dynamic bilayered structure that includes an electron-dense outer layer surrounding a semitransparent inner layer (). It has been reported that the recognition of β-(1,3)-glucan by Dectin-1 is key for host defense against invasive fungal infection.Citation29 However, the cell wall outer glycosylated mannoproteins shielded β-(1,3)-glucan, preventing C. albicans from being recognized by Dectin-1 expressed on innate immune cells.Citation26,28 Interestingly, this seems not to be the case for C. glabrata, of which cell wall β-(1, 3)-glucans are markedly exposed (, , and ). These findings might explain the important role of Dectin-1 in host recognition of C. glabrata.
C. albicans has the ability to switch morphologies from that of the yeast to the filamentous form, and filamentous form is a key virulence factor in the development of invasive infection. α-mannans are fungal ligands recognized by Dectin-2.Citation30 Previous reports have highlighted that Dectin-2 recognizes the hyphal form but not the yeast form of C. albicans.Citation20 The haploid yeast C. glabrata only develop pseudohyphae under some specific conditions in vitro.Citation41 There is no evidence for C. glabrata pseudo- or true hyphae formation during colonization or tissue infection.Citation40 The exposure of cell walls with β-(1,3)-glucan and defects in filamentous formation may explain Dectin-1 confers host higher sensitivity in detecting C. glabrata infections, while the effect of Dectin-2 is infection dose dependent.
In conclusion, our study reveals an important role of β-(1,3)-glucan recognition by Dectin-1 in host defense against C. glabrata. The immune response triggered by Dectin-1 links myeloid cell recognition, killing of C. glabrata and the subsequent adaptive immune response. We also demonstrated that Dectin-1confers host higher sensitivity for sensing C. glabrata infections, while the effect of Dectin-2 in the host defense against C. glabrata is dose dependent, thereby elucidating a previously unknown mechanism in controlling further proliferation of this pathogen in vivo. Our study, therefore, provides new insights into host defense against C. glabrata.
Materials and methods
Ethics statement
All of the animal experiment conformed to the Regulations for the Administration of Affairs Concerning Experimental Animals as approved by the State Council of People's Republic of China. Institutional Animal Care and Use Committee of Tongji University validated the protocol of animal experiment.
Mice
C57BL/6 female mice were obtained from Shanghai Laboratory Animal Center of the Chinese Academy of Sciences. Dr. Gordon D. Brown generously provided C57BL/6 background Dectin-1-deficient (Clec7a−/−) mice.Citation42 And Dr. Yoichiro Iwakura generously provided C57BL/6 background Dectin-2-deficient (Clec4n−/−) mice.Citation30
Antibodies
Antibodies for PCNA, p65, phospho-IκBα, phospho-ERK, JNK, phospho-JNK, p38, phospho-p38, Syk, phospho-Syk, were obtained from Cell Signaling Technologies. Antibodies for ERK IκBα and ERK were obtained from Santa Cruz Biotechnology. Antibody for β-(1,3)-glucan was obtained from Biosupplies Inc. Antibody for β-actin were purchased from Abmart. Cy3-labeled goat anti-mouse antibodies and Alexa-488-labeled antibodies were obtained from Life Technologies. For flow cytometry analysis, the following antibodies, together with their parallel isotype controls were used: peridinin-chlorophyll-protein-complex anti-Ly-6C (clone HK1.4, Biolegend), phycoerythrin-conjugated anti-Ly-6G (clone 1A8, Biolegend), phycoerythrin-Cy7-conjugated anti-CD11b (clone M1/70, Biolegend), Alexa Fluor 647 Siglec-F (clone E50–2440, BD PharMingen), Fixable Viability Dye eFluor 450 (eBioscience).
Candida strains and growth conditions
C. glabrata ATCC28226, C. glabrata ATCC1182 and C. albicans SC5314 strains were routinely cultured on sabouraud dextrose agar (SDA) plates (1% peptone, 4% dextrose, and 1.8% agar) for isolation of individual clone, and culture in yeast peptone dextrose (YPD) liquid medium (1% yeast extract, 2% peptone, and 2% dextrose) at 30°C in a shaking incubator for yeast cells growth. For hyphal formation, C. albicans SC5314 were culture in RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum (FBS) for 4h at 37°C. For yeast opsonization, exponentially growing C. glabrata ATCC28226 (1 × 107 cells) were culture in RPMI-1640 containing 10% fresh mouse serum for 30 min at 30°C. For C. albicans heat-inactivation, C. albicans SC5314 (2.5 × 107/ml) were boiled in PBS for 10 min.Citation28
Peritoneal macrophages and neutrophils isolation
C57BL/6 female mice were intraperitoneally injected with 2 mL of 3% (weight/volume) thioglycollate (Merck). For macrophages isolation, the cells in the abdominal cavity were harvested by washing with PBS supplemented with 0.5 mM EDTA. Three days later and then cultured in RPMI-1640 supplemented with 10% FBS for 2 d. Flow cytometry analysis suggested that the harvested cells contained 90–95% CD11b+ F4/80+ macrophages (Fig. S7A).
For neutrophils isolation, the peritoneal cells of mice were collected after 14 h of thioglycollate injection and then were purified by Percoll gradient (1.090 and 1.070 g/ml) centrifugation (2,000rpm, 30 min).Citation43 The purified neutrophils were cultured in RPMI-1640 contained 10% FBS. Flow cytometry analysis indicated that the purified cells contained 89–96% CD11b+Ly-6C+Ly-6G+ neutrophils (Figs. S7B and C).
Macrophage-C. glabrata interactions
C. glabrata ATCC28226 cells were collected and washed with PBS. Subsequently, yeasts were adjusted to a cell density of 2.5 × 107/mL and exposed to a CL−1000 UV crosslinker (UVP) for 5 doses of 100,000 μJ/cm2. The cells were agitated between each dose to make them evenly.Citation39 The UV-inactivated or live C. glabrata were added to the plates with thioglycollate-elicited macrophages (MOI = 1,5) for the indicated time.
For nuclear extraction, 8 × 106 peritoneal macrophages were cultured with the C. glabrata ATCC28226 (UV-inactivated) in 6-cm plates for the indicated time at MOIs of 0.1, 1. For total cell lysate preparation, 3 × 106 peritoneal macrophages were incubated with C. glabrata ATCC28226 in 12-well plates at MOIs of 0.1 or 1 for the indicated time. For the cytokine production assay, 2 × 105 peritoneal macrophage cells were culture with live or UV-inactivated C. glabrata ATCC28226 for 6 h in 48-wells plates, followed by collection of cell supernatants for analysis.
Western blotting analysis
For total cell lysate collection, peritoneal macrophages were lysed in the total protein lysis buffer (1 mM EDTA, 50 mM HEPES, 250 mM NaCl, 1% Nonidet P-40, pH 7.4) supplemented with protease inhibitor. Cells were lysed in another lysis buffer [0.5 mM dithiothreitol (DTT), 1.5 mM MgCl2, 10 mM KCl, 10 mM HEPES, 0.05% Nonidet P-40, pH 7.9] supplemented with protease inhibitor for nuclear extracts. The extraction buffer (0.2 mM EDTA, 5 mM HEPES, 1.5 mM MgCl2, 300 mM NaCl, pH 7.9) was added to the collected nuclear pellets for nuclear protein extraction and vortexed for 30min at 4°C. Equal amounts of extracts were loaded for SDS-PAGE and blotted using indicated antibodies. Image J software were used for quantifying the densitometry of indicated blot.
Cytokine production assay
The production of IFN-γ, IL-17, IL-12p40, IL-10, IL-6, TNF-α, IL-1β, and granulocyte-monocyte colony-stimulating factors (GM-CSF), chemokine monocyte chemotactic protein-1 (MCP-1), in murine kidney homogenates, cell culture supernatant or peritoneal lavage were measured by commercial Ready-Set-Go cytokine kits (eBioscience).
In vitro neutrophils killing assay
Thioglycollate-elicited peritoneal neutrophils (6 × 105 cells)were collected and co-cultured with unopsonized or opsonized live C. glabrata ATCC28226 (1 × 104 cells), in a 48-well plate at 4°C for 1h to make the cells settle, and then transferred to 37°C for another 1h. During the incubation, control plates were placed at 4°C in parallel. Then, the cells were scraped and subsequently plated on SDA agar. Viable C. glabrata were calculated after 48 h incubation for 30°C as described previously.Citation44
Respiration burst assay
To evaluate the generation of hydrogen peroxide (H2O2), neutrophils were incubated with live C. glabrata ATCC28226 with 10 μM dihydrorhodamine 123. After 1h incubation at 37°C, a Multiscan Spectrum (485nm excitation, 538nm emission) was used to assay the conversion of dihydrorhodamine 123 for H2O2 production determination.Citation45 Cells cultured with dihydrorhodamine 123 without C. glabrata were used for normalization.
Peritoneal infection model of mice
Female wild-type, Dectin-1and Dectin-2-deficient mice were intraperitoneally infected with live C. glabrata ATCC28226 cells (1 × 106 cells per mouse) and killed after 4 h. The mice which intraperitoneal infection PBS as control group of animals. For inflammatory cells collection, mice were intraperitoneally injected 5mM EDTA in PBS for lavage. Subsequently, the cells were counted and blocked with PBS supplemented with 5% heat-inactivated FBS and 1 mM sodium azide at 4°C before primary antibodies addition. After fixing with PBS containing 1% formaldehyde, the leukocyte composition were analyzed by flow cytometer (BD FACS Verse™) as described elsewhere.Citation29 To measure cytokine and chemokines production, mice were intraperitoneally injected 5mM EDTA in PBS for lavage to collect the inflammatory cells after being intraperitoneally infected with live C. glabrata ATCC28226 (5 × 106 cells per mouse). The amount of GM-CSF, MCP-1 and IL-6 from the lavage fluid were measure as describe above.
Murine systemic candidiasis model
Six-eight weeks old wild-type, Dectin-1 and Dectin-2-deficient mice were administered with 200 μL of 1 × 105 or 1 × 107 live C. glabrata ATCC28226 or C. glabrata ATCC1182 cells in sterile saline via lateral tail veins. The subgroups of mice were killed at 7 d after infection. The kidneys and livers were weighed, and homogenized in 500 μL of sterile PBS for determination of fungal burden. The supernatants of homogenized kidneys were collected and kept at −80°C until measurement.
Splenocyte recall response assay
Female wild-type, Dectin-1, and Dectin-2-deficient mice were intravenously infected with 1 × 105 live C. glabrata ATCC28226 cells. Mice were killed at 7 d post infection, spleens were removed and passed gently through a sterile 200-μm filter chamber. Cells were seeded in 48-well plates (5 × 106 cells/well) and co-cultured in RPMI-1640 supplemented with 10% (vol/vol) FBS with UV-inactivated C. glabrata at MOI = 0.02. The supernatants of splenocytes were harvested and kept at −80°C until cytokine measurement.
Transmission electron microscopy
C. glabrata ATCC28226 or C. albicans SC5314 cells were collected after 14h growth in YPD medium. The cells were fixed at 4°C for 24h in 4 ml fixative solution (pH 7.2) supplemented with 3.6% glutaraldehyde and 3% paraformaldehyde washed with sterile water after 2 h fixed with 1% phosphotungstic acid. Then the cells were dehydrated through a graded series of alcohol after block-stained with uranyl acetate. After that the cells were submerged in propylenoxide and glycide-ether. Thin sections were imaged with Hitachi H-800 transmission electron microscope.
Fluorescence microscopy
For β-glucan staining, exponentially growing C. glabrata ATCC28226 yeast cells or C. albicans SC5314 yeast or hyphal cells were blocked with PBS supplemented with in PBS at 30°C for 1 h. After overnight incubated with β-(1,3)-glucan antibody at 4 °C, the cells were incubated with Cy3-labeled secondary antibody at 30°C for 1 h. Heat-inactivated yeast C. albicans SC5314 was presented as a positive control in the experiment.
The cells were incubated with PBS supplyment with 50 μg/ml Concanavalin A or 30 μg/ml Calcofluor white for 30 min to stain α-mannopyranosyl or chitin respectively. The stained cells were fixed on a slide after being washed and imaged with a laser scanning confocal microscope (TCS SP5; Leica).
To quantify the stained cells, the above stained cells were fixed with PBS containing 1% formaldehyde and quantified fluorescence by flow cytometry (BD FACSVerse). And heat-inactivated yeast C. albicans SC5314 was presented as a control in the experiment.
Statistical analysis
All experiments were performed at least 3 biologic replicates. Two groups were analyzed using 2-tailed student's t-test, with paired analysis when appropriate. For multiple groups analysis, one-way ANOVA with Bonferroni post-test was used. The Kruskal-Wallis test or Mann-Whitney test was used for nonparametrically distributed data. Statistical significance were considered when P < 0.05.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
KVIR_S_1346756.zip
Download Zip (700.4 KB)Acknowledgments
We thank Dr. Gordon D. Brown (University of Aberdeen) for providing Dectin-1 KO mice and Dr. Yoichiro Iwakura (Tokyo University of Science) for Dectin-2 KO mice.
Funding
This study was supported by the National Natural Science Foundation of China (81471924, 81330083, 81671989, 81202563), the National Key Basic Research Program of China (2013CB531602), the Fundamental Research Funds for the Central Universities.
References
- Bassetti M, Righi E, Costa A, Fasce R, Molinari MP, Rosso R, Pallavicini FB, Viscoli C. Epidemiological trends in nosocomial candidemia in intensive care. BMC Infect Dis 2006; 6:21; PMID:16472387; https://doi.org/https://doi.org/10.1186/1471-2334-6-21
- Colombo AL, Guimarães T, Silva LR, de Almeida Monfardini LP, Cunha AK, Rady P, Alves T, Rosas RC. Prospective observational study of candidemia in São Paulo, Brazil: incidence rate, epidemiology, and predictors of mortality. Infect Control Hosp Epidemiol 2007; 28:570-76; PMID:17464917; https://doi.org/https://doi.org/10.1086/513615
- Chakrabarti A, Chatterjee SS, Rao KL, Zameer MM, Shivaprakash MR, Singhi S, Singh R, Varma SC. Recent experience with fungaemia: change in species distribution and azole resistance. Scand. J. Infect. Dis 2009; 41:275-84; PMID:19229762; https://doi.org/https://doi.org/10.1080/00365540902777105
- Rodrigues CF, Silva S, Henriques M. Candida glabrata: a review of its features and resistance. Eur J Clin Microbiol Infect Dis 2014; 33:673-88; PMID:24249283; https://doi.org/https://doi.org/10.1007/s10096-013-2009-3
- Pfaller MA, Diekema DJ. Twelve years of fluconazole inclinical practice: global trends in species distribution and fluconazole susceptibility of bloodstream isolates of Candida. Clin Microbiol Infect 2004; 10(Suppl. 1):11-23; PMID:14748799; https://doi.org/https://doi.org/10.1111/j.1470-9465.2004.t01-1-00844.x
- Kaur R, Domergue R, Zupancic ML, Cormack BP. A yeast by any other name: Candida glabrata and its interaction with the host. Curr opin in microbial 2005; 8:378-84; https://doi.org/https://doi.org/10.1016/j.mib.2005.06.012
- Almeida AA, Mesquita CS, Svidzinski TI, Oliveira KM. Antifungal susceptibility and distribution of Candida spp. isolates from the University Hospital in the municipality of Dourados, State of Mato Grosso do Sul, Brazil. Rev Soc Bras Med Trop 2013; 46:335-39; PMID:23856873; https://doi.org/https://doi.org/10.1590/0037-8682-0074-2012
- Lee I, Morales KH, Zaoutis TE, Fishman NO, Nachamkin I, Lautenbach E. Clinical and economic outcomes of decreased fluconazole susceptibility in patients with Candida glabrata bloodstream infections. Am J Infect Control 2010; 38:740-5; PMID:20542354; https://doi.org/https://doi.org/10.1016/j.ajic.2010.02.016
- Vermitsky JP1, Edlind TD. Azole Resistance in Candida glabrata: Coordinate Upregulation of Multidrug Transporters and Evidence for a Pdr1-Like Transcription Factor. Antimicrob Agents Chemother 2004; 48:3773-81; PMID:15388433; https://doi.org/https://doi.org/10.1128/AAC.48.10.3773-3781.2004
- Choo ZW, Chakravarthi S, Wong SF, Nagaraja HS, Thanikachalam PM, Mak JW, Radhakrishnan A, Tay A. A comparative histopathological study of systemic candidiasis in association with experimentally induced breast cancer. Oncol Lett 2010; 1:215-22; PMID:22966285
- Drummond RA, Lionakis MS. Mechanistic Insights into the Role of C-Type Lectin Receptor/CARD9 Signaling in Human Antifungal Immunity. Front Cell Infect Microbiol 2016; 6:39; PMID:27092298; https://doi.org/https://doi.org/10.3389/fcimb.2016.00039
- Ferwerda B, Ferwerda G, Plantinga TS, Willment JA, van Spriel AB, Venselaar H, Elbers CC, Johnson MD, Cambi A, Huysamen C, et al. Human dectin-1 deficiency and mucocutaneous fungal infections. N Engl J Med 2009; 361:1760-7; PMID:19864674; https://doi.org/https://doi.org/10.1056/NEJMoa0901053
- Hardison SE, Brown GD. C-type lectin receptors orchestrate antifungal immunity. Nat Immunol 2012; 13:817-22; PMID:22910394; https://doi.org/https://doi.org/10.1038/ni.2369
- Netea MG, Joosten LA, van der Meer JW, Kullberg BJ, van de Veerdonk FL. Immune defence against Candida fungal infections. Nat Rev Immunol 2015; 15:630-42; PMID:26388329; https://doi.org/https://doi.org/10.1038/nri3897
- Underhill DM, Rossnagle E, Lowell CA, Simmons RM. Dectin-1 activates Syk tyrosine kinase in a dynamic subset of macrophages for reactive oxygen production. Blood 2005; 106:2543-50; PMID:15956283; https://doi.org/https://doi.org/10.1182/blood-2005-03-1239
- Goodridge HS, Reyes CN, Becker CA, Katsumoto TR, Ma J, Wolf AJ, Bose N, Chan AS, Magee AS, Danielson ME, et al. Activation of the innate immune receptor Dectin-1 upon formation of a ‘phagocytic synapse’. Nature 2011; 472:471-5; PMID:21525931; https://doi.org/https://doi.org/10.1038/nature10071
- Branzk N, Lubojemska A, Hardison SE, Wang Q, Gutierrez MG, Brown GD, Papayannopoulos V. Neutrophils sense microbe size and selectively release neutrophil extracellular traps in response to large pathogens. Nat Immunol 2014; 15:1017-25; PMID:25217981; https://doi.org/https://doi.org/10.1038/ni.2987
- Xu S, Huo J, Lee KG, Kurosaki T, Lam KP. Phospholipase Cgamma2 is critical for Dectin-1-mediated Ca2+ flux and cytokine production in dendritic cells. J Biol Chem 2009; 284:7038-46; PMID:19136564; https://doi.org/https://doi.org/10.1074/jbc.M806650200
- Cheng SC, van de Veerdonk FL, Lenardon M, Stoffels M, Plantinga T, Smeekens S, Rizzetto L, Mukaremera L, Preechasuth K, Cavalieri D, et al. The dectin-1/inflammasome pathway is responsible for the induction of protective T-helper 17 responses that discriminate between yeasts and hyphae of Candida albicans. J Leukoc Biol 2011; 90:357-66; PMID:21531876; https://doi.org/https://doi.org/10.1189/jlb.1210702
- Sato K, Yang XL, Yudate T, Chung JS, Wu J, Luby-Phelps K, Kimberly RP, Underhill D, Cruz PD, Jr, Ariizumi K. Dectin-2 is a pattern recognition receptor for fungi that couples with the Fc receptor gamma chain to induce innate immune responses. J Biol Chem 2006; 281:38854-66; PMID:17050534; https://doi.org/https://doi.org/10.1074/jbc.M606542200
- Robinson MJ, Osorio F, Rosas M, Freitas RP, Schweighoffer E, Gross O, Verbeek JS, Ruland J, Tybulewicz V, Brown GD, et al. Dectin-2 is a Syk-coupled pattern recognition receptor crucial for Th17 responses to fungal infection. J Exp Med 2009; 206:2037-51; PMID:19703985; https://doi.org/https://doi.org/10.1084/jem.20082818
- Ifrim DC, Bain JM, Reid DM, Oosting M, Verschueren I, Gow NA, van Krieken JH, Brown GD, Kullberg BJ, Joosten LA, et al. Role of Dectin-2 for host defense against systemic infection with Candida glabrata. Infect Immun 2014; 82:1064-73; PMID:24343653; https://doi.org/https://doi.org/10.1128/IAI.01189-13
- Romani L. Immunity to fungal infections. Nat Rev Immunol 2011; 11:275-88; PMID:21394104; https://doi.org/https://doi.org/10.1038/nri2939
- Low CY, Rotstein C. Emerging fungal infections in immunocompromised patients. F1000 Med Rep 2011; 3:14; PMID:21876720; https://doi.org/https://doi.org/10.3410/M3-14
- Wuthrich M, Deepe GS, Jr., Klein B. Adaptive immunity to fungi. Annu Rev Immunol 2012; 30:115-48; PMID:22224780; https://doi.org/https://doi.org/10.1146/annurev-immunol-020711-074958
- Gow NA, van de Veerdonk FL, Brown AJ, Netea MG. Candida albicans morphogenesis and host defence: discriminating invasion from colonization. Nat Rev Microbiol 2012; 10:112-22.
- Gantner BN, Simmons RM, Underhill DM. Dectin-1 mediates macrophage recognition of Candida albicans yeast but not filaments. EMBO J 2005; 24:1277-1286; PMID:15729357; https://doi.org/https://doi.org/10.1038/sj.emboj.7600594
- Wheeler RT, Fink GR. A drug-sensitive genetic network masks fungi from the immune system. PLoS Pathog 2006; 2:e35; PMID:16652171; https://doi.org/https://doi.org/10.1371/journal.ppat.0020035
- Taylor PR, Tsoni SV, Willment JA, Dennehy KM, Rosas M, Findon H, Haynes K, Steele C, Botto M, Gordon S, Brown GD. Dectin-1 is required for beta-glucan recognition and control of fungal infection. Nat Immunol 2007; 8:31-38; PMID:17159984; https://doi.org/https://doi.org/10.1038/ni1408
- Saijo S, Ikeda S, Yamabe K, Kakuta S, Ishigame H, Akitsu A, Fujikado N, Kusaka T, Kubo S, Chung SH, et al. Dectin-2 recognition of alpha-mannans and induction of Th17 cell differentiation is essential for host defense against Candida albicans. Immunity 2010; 32:681-91; PMID:20493731; https://doi.org/https://doi.org/10.1016/j.immuni.2010.05.001
- Saijo S, Fujikado N, Furuta T, Chung SH, Kotaki H, Seki K, Sudo K, Akira S, Adachi Y, Ohno N, et al. Dectin-1 is required for host defense against Pneumocystis carinii but not against Candida albicans. Nat Immunol 2007; 8:39-46; PMID:17159982; https://doi.org/https://doi.org/10.1038/ni1425
- Shaoji Cheng, Cornelius J. Clancy, Douglas J. Hartman, Binghua Hao, Nguyen M Hong. Candida glabrata Intra-Abdominal Candidiasis Is Characterized by Persistence within the Peritoneal Cavity and Abscesses. Infect Immun 2014; 82:3015-22; PMID:24799629; https://doi.org/https://doi.org/10.1128/IAI.00062-14
- Gazendam RP, van Hamme JL, Tool AT, van Houdt M, Verkuijlen PJ, Herbst M, Liese JG, van de Veerdonk FL, Roos D, van den Berg TK, et al. Two independent killing mechanisms of Candida albicans by human neutrophils: evidence from innate immunity defects. Blood 2014; 124:590-7; PMID:24948657; https://doi.org/https://doi.org/10.1182/blood-2014-01-551473
- Netea MG, Brown GD, Kullberg BJ, Gow NA. An integrated model of the recognition of Candida albicans by the innate immune system. Nat.Rev. Microbiol 2008; 6:67-78; PMID:18079743; https://doi.org/https://doi.org/10.1038/nrmicro1815
- LeibundGut-Landmann S, Gross O, Robinson MJ, Osorio F, Slack EC, Tsoni SV, Schweighoffer E, Tybulewicz V, Brown GD, Ruland J, et al. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat Immunol 2007; 8:630-8; PMID:17450144; https://doi.org/https://doi.org/10.1038/ni1460
- Netea MG, van Tits LJ, Curfs JH, Amiot F, Meis JF, van der Meer JW, Kullberg BJ. Increased susceptibility of TNF-lymphotoxin-double knockout mice to systemic candidiasis through impaired recruitment of neutrophils and phagocytosis of Candida albicans. J Immunol 1999; 163:1498-505; PMID:10415052
- Zielinski CE, Mele F, Aschenbrenner D, Jarrossay D, Ronchi F, Gattorno M, Monticelli S, Lanzavecchia A, Sallusto F. Pathogen-induced human TH17 cells produce IFN-gamma or IL-10 and are regulated by IL-1beta. Nature 2012; 484:514-8; PMID:22466287; https://doi.org/https://doi.org/10.1038/nature10957
- Hernandez-Santos N, Gaffen SL. Th17 cells in immunity to Candida albicans. Cell Host Microbe 2012; 11:425-35; PMID:22607796; https://doi.org/https://doi.org/10.1016/j.chom.2012.04.008
- Wenzel U, Schneider A, Valente AJ, Abboud HE, Thaiss F, Helmchen UM, Stahl RA. Monocyte chemoattractant protein-1 mediates monocyte/macrophage influx in anti-thymocyte antibody-induced glomerulonephritis. Kidney Int 1997; 51:770-6; PMID:9067909; https://doi.org/https://doi.org/10.1038/ki.1997.108
- de Groot PW, Kraneveld EA, Yin QY, Dekker HL, Gross U, Crielaard W, de Koster CG, Bader O, Klis FM, Weig M. The cell wall of the human pathogen Candida glabrata: differential incorporation of novel adhesin-like wall proteins. Eukaryot Cell 2008; 7:1951-64; PMID:18806209; https://doi.org/https://doi.org/10.1128/EC.00284-08
- Csank C, Haynes K. Candida glabrata displays pseudohyphal growth. FEMS Microbiol. Lett 2000; 189:115-20; PMID:10913876; https://doi.org/https://doi.org/10.1111/j.1574-6968.2000.tb09216.x
- Vautier S, Drummond RA, Redelinghuys P, Murray GI, MacCallum DM, Brown GD. Dectin-1 is not required for controlling Candida albicans colonization of the gastrointestinal tract. Infect Immun 2012; 80:4216-22; PMID:22988015; https://doi.org/https://doi.org/10.1128/IAI.00559-12
- Yamane H, Sugimoto Y, Tanaka S, Ichikawa A. Prostaglandin E2 Receptors, EP2 and EP4, differentially modulate TNF-a and IL-6 production induced by lipopolysaccharide in mouse peritoneal neutrophils. Biochem Biophys Res Commun 2000; 278:224-8; PMID:11071876; https://doi.org/https://doi.org/10.1006/bbrc.2000.3779
- Zhang SQ, Zou Z, Shen H, Shen SS, Miao Q, Huang X, Liu W, Li LP, Chen SM, Yan L, et al. Mnn10 Maintains Pathogenicity in Candida albicans by Extending alpha-1,6-Mannose Backbone to Evade Host Dectin-1 Mediated Antifungal Immunity. PLoS pathog 2016; 12:e1005617; PMID:27144456; https://doi.org/https://doi.org/10.1371/journal.ppat.1005617
- Shen H, Chen SM, Liu W, Zhu F, He LJ, Zhang JD, Zhang SQ, Yan L, Xu Z, Xu GT, et al. Abolishing Cell Wall Glycosylphosphatidylinositol-Anchored Proteins in Candida albicans Enhances Recognition by Host Dectin-1. Infect immun 2015; 83:2694-704; PMID:25895969; https://doi.org/https://doi.org/10.1128/IAI.00097-15
