Buruli ulcer (BU) is a chronic necrotizing skin disease caused by Mycobacterium ulcerans, which has been reported worldwide from more than 30 mainly tropical and subtropical countries,Citation1,2 with the highest burden in West-Africa.Citation3 In Australia, the majority of BU cases are found in 2 endemic areas, Far North Queensland,Citation4,5 and temperate coastal Victoria.Citation6-11 The macrolide toxin mycolactone is an essential virulence factor of M. ulcerans.Citation12 It causes apoptosis in mammalian cellsCitation13 and down-regulates local and systemic immune responses by interfering with the activation of immune cells.Citation14-21 BU presents with a wide clinical spectrum that includes pre-ulcerative forms (nodules, plaques, edema) and ulcerative lesions.Citation2,22,23 The dermis and epidermis overlying the initially closed M. ulcerans infection foci eventually degenerate, when mycolactone mediated destruction of the subcutaneous tissue spreads. This leads to the formation of ulcers with undermined edges and a necrotic slough in the base. So far the pathogenesis of BU has primarily been studied by histopathological analyses of advanced lesions. These have revealed contiguous coagulation necrosis of the deep dermis and subcutaneous fat tissue, with destruction of blood vessels and interstitial edema. Presence of clusters of extracellular acid fast bacilli (AFB) and relative lack of infiltrating leukocytes are regarded as further characteristic histopathological hallmarks of BU lesions.Citation1,24,25 African BU patients often seek health care late, which may have several reasons, including cultural beliefs, poor access to the formal health system and late recognition of the often painless BU lesions.Citation26 In contrast, most Australian patients seek treatment early with lesions of the World Health Organization (WHO) defined category 1.Citation4,11,27 The current WHO treatment recommendation is a combination chemotherapy with rifampicin and streptomycin for 8 weeks for all forms of the disease, combined with wound care and prevention of disability.Citation28 However, Australian guidelines to treat M. ulcerans infections recommend a different regimen that combines rifampicin with oral clarithromycin or an oral fluoroquinolone as first line therapy.Citation27,29-31 Furthermore, surgery is accepted as treatment of BU when antibiotics are declined, not tolerated or the patient's preference is excision and direct wound closure of small lesions.Citation4,29 For the present study we therefore could get access to early lesions of BU patients from Northern Queensland, which were excised without prior antibiotic treatment. Here we investigated whether the well described lack of infiltrates is a characteristic of M. ulcerans infection from the beginning of the infection.
Our study cohort consisted of 12 patients () with IS2404 quantitative real-time PCR confirmed early BU lesions. Patients were recruited between 2000 and 2015 in Far North Queensland; their lesions had persisted for only 3 to 12 weeks (mean 7.5 weeks) before excision. In all cases the surgical procedure consisted of a narrow elliptical excision which encompassed the ulcer plus the indurated surrounding skin and subcutaneous tissue. The excised specimens were bisected through the ulcer; one half was used for PCR, smear microscopy and culture, the other half for histopathology. Excisions were closed primarily with non-absorbable sutures which were removed after 7 to 10 d. The patients were reviewed at 4 weeks, 3 months and 12 months. The median age of the patients was 44.5 y (range 5 to 82 years), which is in line with the observation, that the median age of patient cohorts from Queensland and Victoria (45 and 61 years, respectivelyCitation11,27) is much higher than that of patients from West Africa.Citation32-34 Both in Africa and Australia, BU affects primarily the limbs, in particular the lower legs,Citation11,32 and also in our cohort 75% (8/12) of the lesions were located on the lower and the rest (25%; 4/12) on the upper extremities. While 3 of the analyzed lesions showed only a first very small opening, where the epidermal layer had focally broken in (), ulceration had progressed slightly more in the others (). As exemplified in more detail for patient 5 (), in all lesions the characteristic signs of BU were found, including epidermal hyperplasia (), large necrotic areas () containing fat cell ghosts () and extracellular clumps of AFB (). A strong TUNEL staining clearly marked the entire necrotic area of all lesions ( A, B1 (red star), B3). While the TUNEL assay has been primarily developed to detect apoptotic cells that undergo extensive DNA degradation during the late stages of apoptosis, the diffuse staining of the necrotic core of the BU lesions is most likely caused by residual DNA fragments still present in the destroyed tissue.
Table 1. Study cohort
Figure 1. Histopathological overview over 5 of the early ulcerative lesions. Sections were either stained with Haematoxylin-Eosin (HE; A, D, G, J, M) or Ziehl-Neelsen/Methyleneblue (ZN-M; B, C, E, F, H, I, K, L, N, O) according to the WHO standard protocol.Citation1 Pictures were either taken with a Leica DFC 420C camera or with an Aperio ScanScope XT. The border of the tissue area affected by the disease is indicated with a black line in the HE staining (A, D, G, J, M). For patients one and 2 cross sections through the entire lesions are shown (A, D), lesions of patients 3 to 5 (G, J, M) were too large to be entirely processed and therefore only half of the lesion is shown with the necrotic lesion core in the upper right corner. ZN staining (B, E, H, K, N) revealed that AFB were exclusively found in the necrotic areas (red stars). Predominantly clusters of extracellular bacteria were present (C, I, O), however close to the infiltration belt extra- as well as intracellular bacteria (F, L) were observed. Similar findings were made for all 12 lesions analyzed
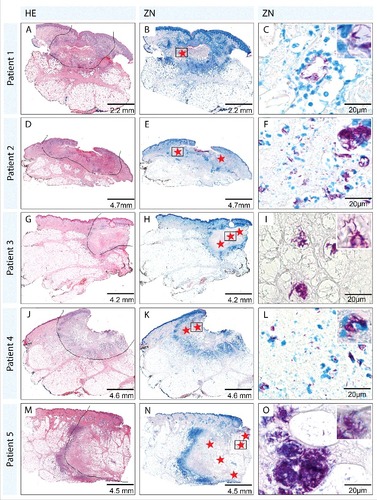
Figure 2. In depth analysis of the skin tissue in the necrotic core, the infiltrated area and the healthy tissue area (patient 5). Sections were either stained with Haematoxylin-Eosin (HE; A, F-Q) or Ziehl-Neelsen/Methyleneblue (ZN-M; B-E) according to the WHO standard protocol.Citation1 Pictures were either taken with a Leica DFC 420C camera or with an Aperio ScanScope XT. A: Overview of the histopathological section from patient 5, depicting the 3 regions (healthy tissue, infiltration, necrotic core) which were found in all early ulcerative lesions. Healthy tissue (F-I) presented with a normal epidermal layer (F), no sub- epidermal infiltration (G), healthy fat cells (H) and intact collagen fibers (I). The infiltrated area (J-M), separating the healthy tissue from the necrotic core, reflected a transition state with a slight thickening of the epidermal layer (J), some sub-epidermal infiltration (K), fat cells which started to round up and shrink (L) and the accumulation of large numbers of infiltrating cells (M). The necrotic core (N-O) presented with strongly elongated rete ridges and an epidermal layer which was more than 3 times thicker than the healthy epidermis (N), a strong sub-epidermal infiltration which was largest above the necrotic core (O), fat cell ghosts which displayed signs of cell death (rounding up, shrinkage, absence of nuclei) (P) and necrotic connective tissue without collagen fibers (Q). In the necrotic core focally clustered AFB (D, E, red stars in A) as well as a secondary infection (B, C; at the open ulcer surface (black box A)) could be observed
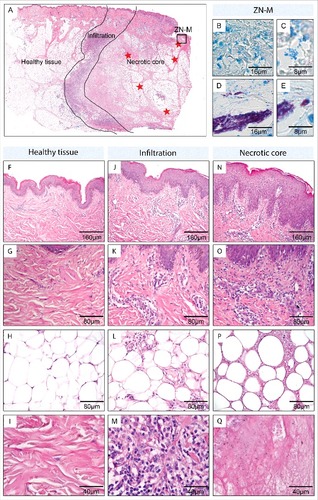
Figure 3. TUNEL staining. The TUNEL protocol allows the staining of low molecular weight DNA fragments typically occurring during apoptosis (brown staining). Sections of patients one to 5 were stained with the “In Situ Cell Death Detection Kit, POD” (Cat. No. 11684817910) from Roche according to the manufacturer's protocol (protocol used was for tissue sections with high unspecific background). Counter stain was done with Haematoxylin. Pictures were either taken with a Leica DFC 420C camera or with an Aperio ScanScope XT. Overview over tissue sections reveals a diffuse staining of the necrotic lesion core (A1-A4, B1) and no staining of the healthy tissue area. Increased magnification (B2, B3) showed a distinct staining of single cells in the belt of infiltrating cells (B1 green star, B2) representing cells currently undergoing apoptosis and a more dispersed staining inside the necrotic core (B1 red star, B3) representing leftover DNA fragments from the initial infiltration. Similar findings were made for all 12 lesions analyzed
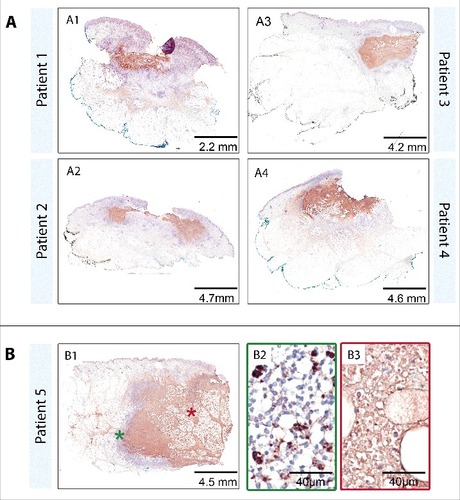
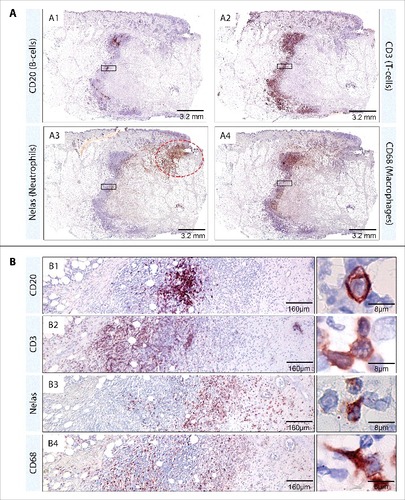
In all lesions, a belt of infiltrating leukocytes was found, which separated the highly confined necrotic core from the intact tissue which surrounded the lesions (; ). In the infiltration belt, TUNEL staining identified individual apoptotic cells dispersed in the infiltrate ( (green star), B2). In contrast, inside the necrotic area staining was diffuse and not cell-associated ( (red star), B3). The analysis of serial sections from the lesions revealed that CD20 positive B-cells were present in small and large clusters throughout the infiltrated area (). CD3 positive T-cells, neutrophils and CD68 positive macrophages appeared in a more dispersed manner all over the infiltration belts (). The distribution of different cell types was not homogeneous within the belt: while CD3 T-cells () were primarily located close to the healthy tissue, neutrophils () were mainly present close to the necrotic core.Citation35 Macrophages () were distributed all over the infiltrated area with preponderance to the inner layers toward the lesion core. Besides intact neutrophils in the infiltration belt, neutrophilic debris was observed inside the lesion core, which appears to be the aftermath of a massive early neutrophil infiltration, which is also observed in an experimental M. ulcerans pig infection model.Citation36,Citation37 Furthermore, in areas with secondary infection (), accumulations of neutrophils were found ().
Figure 4. Composition of the immune infiltrate which surrounds the necrotic core. Serial sections from the lesion of patient 5 were stained by immunohistochemistry. The following antibodies were used according to the manufacturer's protocol: Elastase (polymorphonuclear neutrophils, NP57, Dako, M0752), CD3 (T lymphocytes, Dako, A0452), CD68 (macrophages/monocytes, KP1, Dako, M0814), and CD20 (B lymphocytes, 7D1, Novocastra, NCL-CD20–7D1). Pictures were either taken with a Leica DFC 420C camera or with an Aperio ScanScope XT. CD20 positive B-cells (A1, B1) were present in clusters while CD3 positive T-cells (A2, B2), N-elastase positive Neutrophils (A3, B3) and CD68 positive macrophages (A4, B4) were present throughout the infiltration belt in a layered manner. Accumulations of neutrophils were additionally present close to the secondary infection (A3, red dotted circle)
Early immune responses may in many cases help to contain M. ulcerans infections and prevent clinical BU disease, as indicated by the development of a serological response against M. ulcerans in many healthy individuals living in African BU endemic areas.Citation38–40 We assume that for the development of a chronic infection after inoculation with M. ulcerans it may be critical that a large enough bacterial cluster can develop that generates a protective cloud of mycolactone. After intracellular multiplication and killing of the host cell, globi-like bacterial accumulations may represent a starting point for the development of large extracellular clusters of M. ulcerans.Citation41–43 In early but nonetheless established lesions, as analyzed here, the infiltrating immune cells can no longer reach the extracellular mycobacteria in the necrotic center, despite massive extravasation of leukocytes. On the other hand the layer of infiltrating cells around the lesion core may – together with other factors, such as the formation of an abundant extracellular matrixCitation44- hinder spread of the mycobacteria into the lymph and bloodstream. This may contribute to the fact that most BU patients present with a single lesion.Citation2
The mode of transmission of M. ulcerans is currently not clear.Citation45 Both insect bites and direct inoculation of bacteria into the skin from an environmental reservoir after skin trauma have been implicated. After inoculation, an early intra-macrophage growth phase may play a role in disease development.Citation15,Citation46 In the early lesions analyzed here, AFB were exclusively found in the necrotic core of the lesions (red stars in ) and appeared mainly as extracellular cluster (). However, when present close to the infiltration belt, some mycobacteria appeared to be intracellularly located (). Like in more advanced lesions,Citation47 AFB were typically found in the subcutaneous tissue and only rarely in the dermis. Preferential location of M. ulcerans in the deeper subcutis may either reflect the inoculation route or a tropism related to nutritional needs only provided by the destroyed fat tissue.Citation47
Taken together histopathological data from our Australian patients clearly show that infection by M. ulcerans is accompanied by massive leukocyte infiltration. The apparent lack of infiltration described as a hallmark of BU is obviously restricted to the lesion core, where mycolactone causes death of all resident and infiltrating cells by apoptosis. If diagnostic punch biopsies are not taken from the center of the lesion, but more from its periphery, massive infiltration may thus be found. Together with an apparent lack of AFBs, which are not evenly spread and often missed in small tissue biopsies of BU patientsCitation47 this may mislead histopathological diagnosis. However these results should be reconfirmed by analyzing also African early BU lesions (plaque, nodules). M. ulcerans strains from Australia primarily produce mycolactone C while a mixture of mycolactone C and the more potent mycolactone A/BCitation48 is found in lipid extracts from African strains.Citation49 Despite these differences, the pathogenesis of BU seems to be comparable in patients from both continents and also in Australian patients large ulcers develop when treatment is delayed. In line with this, the typical histopathological features found in the lesions of our Australian patients were indistinguishable from those observed in samples from African patients.
When early immune responses do not eliminate a M. ulcerans inoculum, a chronic infection may develop and the extracellular clusters of the mycobacteria can no longer be reached by the infiltrating cells. However, the formation of the infiltration belt around the necrotic core of the lesion described here, most likely helps to prevent systemic spread of the infection; but it cannot prevent local multiplication of the bacteria followed by progressive ulceration.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We would like to thank Dr. Lindsay Wyndham, Pathology Queensland, Cairns and Dr Jan Kencian, Sullivan & Nicolaides Pathology Pty Ltd for slide preparation and verification. Additionally we thank Vincent Romanet, Caroline Stork, Ernesta Dammassa and Patricia Barzaghi Rinaudo from Novartis Basel for excellent technical support and providing access to laboratory equipment for histopathology.
Funding
This work was supported by the Medicor Foundation.
References
- WHO | Laboratory diagnosis of buruli ulcer [Internet]. WHO [cited 2014 Jul 16]; Available from: http://www.who.int/buruli/laboratory_diagnosis/en/
- Junghanss T, Johnson C, Pluschke G. Mycobacterium ulcerans disease. In: Manson's tropical diseases. Edinburgh: Saunders Ltd; 2014. p. 519-31.
- Pluschke G, Röltgen K. Epidemiology and disease burden of Buruli ulcer: A review. Res Rep Trop Med. 2015;6:59-73. doi:https://doi.org/10.2147/RRTM.S62026
- Steffen CM, Smith M, McBride WJH. Mycobacterium ulcerans infection in North Queensland: the “Daintree ulcer.” ANZ J Surg. 2010;80:732-6. doi:https://doi.org/10.1111/j.1445-2197.2010.05338.x. PMID:21040335
- Steffen CM, Freeborn H. Mycobacterium ulcerans in the Daintree 2009–2015 and the mini-epidemic of 2011. ANZ J Surg. 2016; PMID:27804194
- Flood P, Street A, O'Brien P, Hayman J. Mycobacterium ulcerans infection on Phillip Island, Victoria. Med J Aust. 1994;160:160. PMID:8295586
- Hayman JA, Huygens HJ. Mycobacterium ulcerans infection across Lake Victoria. Med J Aust. 1982;1:138. PMID:7132854
- Hayman J. Mycobacterium ulcerans infection in Victoria: celebration of a golden jubilee? Australas J Dermatol. 1987;28:99-105. doi:https://doi.org/10.1111/j.1440-0960.1987.tb00346.x. PMID:3504150
- Johnson PDR, Lavender CJ. Correlation between Buruli ulcer and vector-borne notifiable diseases, Victoria, Australia. Emerging Infect Dis. 2009;15:614-5. doi:https://doi.org/10.3201/eid1504.081162. PMID:19331750
- van Ravensway J, Benbow ME, Tsonis AA, Pierce SJ, Campbell LP, Fyfe JAM, Hayman JA, Johnson PDR, Wallace JR, Qi J. Climate and landscape factors associated with Buruli ulcer incidence in Victoria, Australia. PLoS ONE. 2012;7:e51074. doi:https://doi.org/10.1371/journal.pone.0051074. PMID:23251425
- Boyd SC, Athan E, Friedman ND, Hughes A, Walton A, Callan P, McDonald A, O'Brien DP. Epidemiology, clinical features and diagnosis of Mycobacterium ulcerans in an Australian population. Med J Aust. 2012;196:341-4. doi:https://doi.org/10.5694/mja12.10087. PMID:22432674
- George KM, Chatterjee D, Gunawardana G, Welty D, Hayman J, Lee R, Small PL. Mycolactone: a polyketide toxin from Mycobacterium ulcerans required for virulence. Science. 1999;283:854-7. doi:https://doi.org/10.1126/science.283.5403.854. PMID:9933171
- Bieri R, Scherr N, Ruf M-T, Dangy J-P, Gersbach P, Gehringer M, Altmann K-H, Pluschke G. The Macrolide Toxin Mycolactone Promotes Bim-Dependent Apoptosis in Buruli Ulcer through Inhibition of mTOR. ACS Chem Biol. 2017;12:1297-1307. doi:https://doi.org/10.1021/acschembio.7b00053. PMID:28294596.
- Adusumilli S, Mve-Obiang A, Sparer T, Meyers W, Hayman J, Small PLC. Mycobacterium ulcerans toxic macrolide, mycolactone modulates the host immune response and cellular location of M. ulcerans in vitro and in vivo. Cell Microbiol. 2005;7:1295-304. doi:https://doi.org/10.1111/j.1462-5822.2005.00557.x. PMID:16098217
- Coutanceau E, Marsollier L, Brosch R, Perret E, Goossens P, Tanguy M, Cole ST, Small PLC, Demangel C. Modulation of the host immune response by a transient intracellular stage of Mycobacterium ulcerans: the contribution of endogenous mycolactone toxin. Cell Microbiol. 2005;7:1187-96. doi:https://doi.org/10.1111/j.1462-5822.2005.00546.x. PMID:16008585
- Simmonds RE, Lali FV, Smallie T, Small PLC, Foxwell BM. Mycolactone inhibits monocyte cytokine production by a posttranscriptional mechanism. J Immunol. 2009;182:2194-202. doi:https://doi.org/10.4049/jimmunol.0802294. PMID:19201873
- Pahlevan AA, Wright DJ, Andrews C, George KM, Small PL, Foxwell BM. The inhibitory action of Mycobacterium ulcerans soluble factor on monocyte/T cell cytokine production and NF-kappa B function. J Immunol. 1999;163:3928-35. PMID:10490994
- Coutanceau E, Decalf J, Martino A, Babon A, Winter N, Cole ST, Albert ML, Demangel C. Selective suppression of dendritic cell functions by Mycobacterium ulcerans toxin mycolactone. J Exp Med. 2007;204:1395-403. doi:https://doi.org/10.1084/jem.20070234. PMID:17517970
- Boulkroun S, Guenin-Macé L, Thoulouze M-I, Monot M, Merckx A, Langsley G, Bismuth G, Di Bartolo V, Demangel C. Mycolactone suppresses T cell responsiveness by altering both early signaling and posttranslational events. J Immunol. 2010;184:1436-44. doi:https://doi.org/10.4049/jimmunol.0902854. PMID:20042571
- Torrado E, Adusumilli S, Fraga AG, Small PLC, Castro AG, Pedrosa J. Mycolactone-mediated inhibition of tumor necrosis factor production by macrophages infected with Mycobacterium ulcerans has implications for the control of infection. Infect Immun. 2007;75:3979-88. doi:https://doi.org/10.1128/IAI.00290-07. PMID:17517872
- Guenin-Macé L, Carrette F, Asperti-Boursin F, Le Bon A, Caleechurn L, Di Bartolo V, Fontanet A, Bismuth G, Demangel C. Mycolactone impairs T cell homing by suppressing microRNA control of L-selectin expression. Proc Natl Acad Sci USA. 2011;108:12833-8. doi:https://doi.org/10.1073/pnas.1016496108. PMID:21768364
- Asiedu K, Scherpbier, R, Raviglione, M. Buruli ulcer: Mycobacterium ulcerans infection. Geneva: World Health Organization. 2000.
- van der Werf TS, van der Graaf WT, Tappero JW, Asiedu K. Mycobacterium ulcerans infection. Lancet. 1999;354:1013-8. doi:https://doi.org/10.1016/S0140-6736(99)01156-3. PMID:10501380
- Hayman J. Out of Africa: observations on the histopathology of Mycobacterium ulcerans infection. J Clin Pathol. 1993;46:5-9. doi:https://doi.org/10.1136/jcp.46.1.5. PMID:8432888
- Hayman J, McQueen A. The pathology of Mycobacterium ulcerans infection. Pathology. 1985;17:594-600. doi:https://doi.org/10.3109/00313028509084759. PMID:4094789
- Stienstra Y, van der Graaf WTA, Asamoa K, van der Werf TS. Beliefs and attitudes toward Buruli ulcer in Ghana. Am J Trop Med Hyg. 2002;67:207-13. doi:https://doi.org/10.4269/ajtmh.2002.67.207. PMID:12389949
- O'Brien DP, Friedman ND, Cowan R, Pollard J, McDonald A, Callan P, Hughes A, Athan E. Mycobacterium ulcerans in the Elderly: More Severe Disease and Suboptimal Outcomes. PLoS Negl Trop Dis. 2015;9:e0004253. doi:https://doi.org/10.1371/journal.pntd.0004253. PMID:26630648
- WHO | Treatment of Mycobacterium ulcerans disease (Buruli Ulcer) [Internet]. WHO [cited 2016 Apr 29]; Available from: http://www.who.int/buruli/treatment/en/.
- O'Brien DP, Jenkin G, Buntine J, Steffen CM, McDonald A, Horne S, Friedman ND, Athan E, Hughes A, Callan PP, et al. Treatment and prevention of Mycobacterium ulcerans infection (Buruli ulcer) in Australia: guideline update. Med J Aust. 2014;200:267-70. doi:https://doi.org/10.5694/mja13.11331. PMID:24641151
- Friedman ND, Athan E, Hughes AJ, Khajehnoori M, McDonald A, Callan P, Rahdon R, O'Brien DP. Mycobacterium ulcerans disease: experience with primary oral medical therapy in an Australian cohort. PLoS Negl Trop Dis. 2013;7:e2315. doi:https://doi.org/10.1371/journal.pntd.0002315. PMID:23875050
- Friedman ND, Athan E, Walton AL, O'Brien DP. Increasing Experience with Primary Oral Medical Therapy for Mycobacterium ulcerans Disease in an Australian Cohort. Antimicrob Agents Chemother. 2016;60:2692-5. doi:https://doi.org/10.1128/AAC.02853-15. PMID:26883709
- Bratschi MW, Bolz M, Minyem JC, Grize L, Wantong FG, Kerber S, Njih Tabah E, Ruf M-T, Mou F, Noumen D, et al. Geographic distribution, age pattern and sites of lesions in a cohort of Buruli ulcer patients from the Mapé Basin of Cameroon. PLoS Negl Trop Dis. 2013;7:e2252. doi:https://doi.org/10.1371/journal.pntd.0002252. PMID:23785529
- Capela C, Sopoh GE, Houezo JG, Fiodessihoué R, Dossou AD, Costa P, Fraga AG, Menino JF, Silva-Gomes R, Ouendo EM, et al. Clinical Epidemiology of Buruli Ulcer from Benin (2005-2013): Effect of Time-Delay to Diagnosis on Clinical Forms and Severe Phenotypes. PLoS Negl Trop Dis. 2015;9:e0004005. doi:https://doi.org/10.1371/journal.pntd.0004005. PMID:26355838
- Debacker M, Aguiar J, Steunou C, Zinsou C, Meyers WM, Scott JT, Dramaix M, Portaels F. Mycobacterium ulcerans disease: role of age and gender in incidence and morbidity. Trop Med Int Health. 2004;9:1297-304. doi:https://doi.org/10.1111/j.1365-3156.2004.01339.x. PMID:15598261
- Ruf M-T, Sopoh GE, Brun LV, Dossou AD, Barogui YT, Johnson RC, Pluschke G. Histopathological changes and clinical responses of Buruli ulcer plaque lesions during chemotherapy: a role for surgical removal of necrotic tissue? PLoS Negl Trop Dis. 2011;5:e1334. doi:https://doi.org/10.1371/journal.pntd.0001334. PMID:21980547
- Bolz M, Ruggli N, Borel N, Pluschke G, Ruf M-T. Local Cellular Immune Responses and Pathogenesis of Buruli Ulcer Lesions in the Experimental Mycobacterium Ulcerans Pig Infection Model. PLoS Negl Trop Dis. 2016;10:e0004678. doi:https://doi.org/10.1371/journal.pntd.0004678. PMID:27128097
- Bolz M, Ruggli N, Ruf M-T, Ricklin ME, Zimmer G, Pluschke G. Experimental infection of the pig with Mycobacterium ulcerans: A novel model for studying the pathogenesis of Buruli ulcer disease. PLoS Negl Trop Dis. 2014;8:e2968. doi:https://doi.org/10.1371/journal.pntd.0002968. PMID:25010421
- Diaz D, Dobeli H, Yeboah-Manu D, Mensah-Quainoo E, Friedlein A, Soder N, Rondini S, Bodmer T, Pluschke G. Use of the Immunodominant 18-Kilodalton Small Heat Shock Protein as a Serological Marker for Exposure to Mycobacterium ulcerans. Clin Vaccine Immunol. 2006;13:1314-21. doi:https://doi.org/10.1128/CVI.00254-06. PMID:17021247
- Ampah KA, Nickel B, Asare P, Ross A, De-Graft D, Kerber S, Spallek R, Singh M, Pluschke G, Yeboah-Manu D, et al. A Sero-epidemiological Approach to Explore Transmission of Mycobacterium ulcerans. PLoS Negl Trop Dis. 2016;10:e0004387. doi:https://doi.org/10.1371/journal.pntd.0004387. PMID:26808978
- Röltgen K, Bratschi MW, Ross A, Aboagye SY, Ampah KA, Bolz M, Andreoli A, Pritchard J, Minyem JC, Noumen D, et al. Late onset of the serological response against the 18 kDa small heat shock protein of Mycobacterium ulcerans in children. PLoS Negl Trop Dis. 2014;8:e2904. doi:https://doi.org/10.1371/journal.pntd.0002904. PMID:24853088
- Schütte D, Umboock A, Pluschke G. Phagocytosis of Mycobacterium ulcerans in the course of rifampicin and streptomycin chemotherapy in Buruli ulcer lesions. Br J Dermatol. 2009;160:273-83. doi:https://doi.org/10.1111/j.1365-2133.2008.08879.x. PMID:19016694
- Ruf M-T, Chauty A, Adeye A, Ardant M-F, Koussemou H, Johnson RC, Pluschke G. Secondary Buruli ulcer skin lesions emerging several months after completion of chemotherapy: paradoxical reaction or evidence for immune protection? PLoS Negl Trop Dis. 2011;5:e1252. doi:https://doi.org/10.1371/journal.pntd.0001252. PMID:21829740
- Ruf M-T, Schütte D, Chauffour A, Jarlier V, Ji B, Pluschke G. Chemotherapy associated changes of histopathological features of Mycobacterium ulcerans lesions in a Buruli ulcer mouse model. Antimicrob Agents Chemother. 2012;56:687-96. doi:https://doi.org/10.1128/AAC.05543-11
- Marsollier L, Brodin P, Jackson M, Korduláková J, Tafelmeyer P, Carbonnelle E, Aubry J, Milon G, Legras P, André J-PS, et al. Impact of Mycobacterium ulcerans biofilm on transmissibility to ecological niches and Buruli ulcer pathogenesis. PLoS Pathog. 2007;3:e62. doi:https://doi.org/10.1371/journal.ppat.0030062. PMID:17480118
- Merritt RW, Walker ED, Small PLC, Wallace JR, Johnson PDR, Benbow ME, Boakye DA. Ecology and transmission of Buruli ulcer disease: a systematic review. PLoS Negl Trop Dis. 2010;4:e911. doi:https://doi.org/10.1371/journal.pntd.0000911. PMID:21179505
- Torrado E, Fraga AG, Castro AG, Stragier P, Meyers WM, Portaels F, Silva MT, Pedrosa J. Evidence for an intramacrophage growth phase of Mycobacterium ulcerans. Infect Immun. 2007;75:977-87. doi:https://doi.org/10.1128/IAI.00889-06. PMID:17145944
- Ruf M-T, Bolz M, Vogel M, Bayi PF, Bratschi MW, Sopho GE, Yeboah-Manu D, Um Boock A, Junghanss T, Pluschke G. Spatial Distribution of Mycobacterium ulcerans in Buruli Ulcer Lesions: Implications for Laboratory Diagnosis. PLoS Negl Trop Dis. 2016;10:e0004767. doi:https://doi.org/10.1371/journal.pntd.0004767. PMID:27253422
- Scherr N, Gersbach P, Dangy J-P, Bomio C, Li J, Altmann K-H, Pluschke G. Structure-activity relationship studies on the macrolide exotoxin mycolactone of Mycobacterium ulcerans. PLoS Negl Trop Dis. 2013;7:e2143. doi:https://doi.org/10.1371/journal.pntd.0002143. PMID:23556027
- Mve-Obiang A, Lee RE, Portaels F, Small PLC. Heterogeneity of mycolactones produced by clinical isolates of Mycobacterium ulcerans: implications for virulence. Infect Immun. 2003;71:774-83. doi:https://doi.org/10.1128/IAI.71.2.774-783.2003. PMID:12540557
