The incidence of Crohn’s disease (CD) in industrialized countries has increased steadily due to improvements in the standard of living since the Second World War[Citation1]. CD is characterized by an uncontrolled immune response within the intestinal mucosa. The activation and/or chronicity of the inflammatory response depends on genetic susceptibility to various environmental stimuli, including the intestinal flora [Citation2]. The development of CD is associated with the sequential appearance of antibodies against microbial molecular motifs, the levels of which remain stable once the disease has become established. These antibodies, initially used for diagnostic purposes, are now used for CD clinical management. The number of antigenic targets and the magnitude of the response, are indicative of disease severity [Citation3], are now taken into account when defining the nature of initial treatment[Citation4]. Similarly, their pre-existence to CD onset is increasingly considered in surveys of high risk populations[Citation5]. Despite their widespread use, the mechanisms of generation and persistence of these antibodies remain unknown.
Anti-Saccharomyces cerevisiae antibodies (ASCA) are the most widely used of these antibodies. A recent paper involving murine models reported that the inability of S. cerevisiae to catabolize purines affects host metabolism through uric acid (UA) production and that this may negatively affect the course of inflammatory bowel disease (IBD) [Citation6]. As an argument in favour of this hypothesis, a correlation between ASCA and UA was reported in healthy subjects. Because of the impact of this conclusion, which suggests that S. cerevisiae is potentially pathogenic in human beings, especially those susceptible to developing CD, we carried out experiments to investigate this correlation. We measured UA and ASCA levels in our cohorts of IBD patients and controls. Our conclusion was that this correlation does not exist and we think it important to publish these data and to extend the discussion to the significance of ASCA, the role of S. cerevisiae and, more generally, the role of yeasts in CD.
Our investigations involved two cohorts of IBD patients included in previous studies comprising ASCA levels determination. Sera collected from four groups of patients were analyzed. The first serum repository (CD1) consisted of 28 sera collected from 28 patients (nine male/19 female; mean age: 27.5 years; age at diagnosis: 21 years; location of disease: ileal (L1; n = 22), colonic (L2; n = 0), ileocolonic (L3; n = 6) selected on the basis of high ASCA levels associated with CD complications needing ileocecal resection. The sera were taken the day before surgery [Effect of Fluconazole on the Levels of Anti-Saccharomyces cerevisiae Antibodies (ASCA) After Surgical Resection for Crohn’s Disease. Multicenter, Randomized, and Controlled in Two Parallel Groups Versus Placebo. ClinicalTrialsgov ID: NCT02997059]. The second serum repository (CD2) came from genetic and functional studies of IBD patients [French Ministry for Health, Programme Hospitalier de Recherche Clinique, Etudes génétique et fonctionnelle des patients atteints de maladies inflammatoires chroniques de l’intestin; Grant 2006-Lille19-01]. This consisted of 85 sera from 49 CD patients (14 male/35 female; mean age: 26 years; age at diagnosis: 20 years; location of disease: L1 (n = 11), L2 (n = 4), L3 (n = 34). The third serum repository (UC) was collected from 36 patients with ulcerative colitis (male/female: 20/16; mean age: 34 years, age at diagnosis: 31 years) with the following phenotypes at diagnosis: proctitis (E1; n = 11), left colitis under splenic flexure (E2; n = 15) and colitis above splenic flexure (E3; n = 10). Finally, the fourth group (Controls) consisted in 30 sera from healthy blood donors (14 male/16 female; mean age: 37 years) obtained from Etablissement Français du Sang, Nord de France, Lille, France. ASCA levels were determined by ELISA (IBDX gASCA; Glycominds) [Citation7] . Serum UA was measured by enzymatic colorimetric assay using a commercially available kit from Roche Diagnostics (reference ranges for serum UA in healthy adults are 25–70 mg/L).
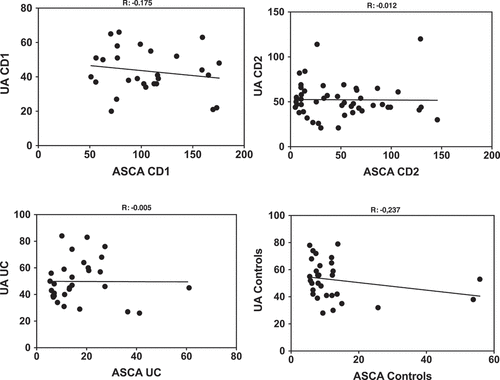
The levels of ASCA and UA determined in the different populations are shown in . ASCA levels in the CD1 cohort were all above the limit of significance (50 AU), due to the mode of selection, with some of them exhibiting very high values typically associated with complicated disease needing surgery[Citation3] . ASCA values in patients from the CD2 cohort were widely distributed with lower values corresponding to patients with a colonic location and/or older age at onset. Among the UC patients, only one was at the limit of significance of the test, in coherence with its initial indication for the differential diagnosis of the two forms of IBD [Citation8]. Finally, the presence of ASCA in the non-IBD population (in the order of 6–7%) is consistent with that described previously[Citation9]. These results, revealing very significant differences between the two IBD forms and controls, validated the choice of our cohorts. In terms of UA levels ()), a striking homogeneity was observed between the IBD groups and controls. With few exceptions (i.e. not belonging to the high ASCA levels group (CD1), all subjects were within the normal range of UA values and no significant difference was found between the groups. When investigating correlations between ASCA and UA to explore the previous statement that host immune reaction to “commensal yeasts” positively correlated with UA production [Citation6], we did not find any correlations in any of our groups (). The correlation was close to zero in the CD2 and UC groups and even presented a trend towards negativity in the CD1 group and controls.
Figure 1. ASCA (a) and Uric acid levels (b) in the different populations examined. Dotted lines represent the limit of significance for ASCA and the reference ranges in healthy subjects for UA.
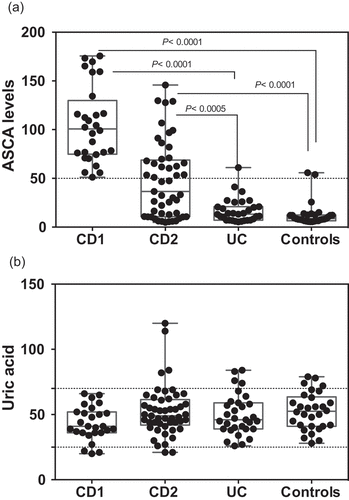
Figure 2. Correlations between ASCA and Uric acid levels in the CD patients groups (CD1 and CD2), Ulcerative colitis patients (UC) and Controls.
ASCA remain the most widely used test for the diagnosis of CD and have led to more than 400 publications on this subject. An ELISA test using mannan from S. cerevisiae as an antigen to detect antibodies in CD patients sera was first described by our group in 1996[Citation10]; sequential depolymerization of the mannan molecular complex showed that the discriminating epitopes consisted of α-1,3 Man at the non-reducing end of tri or di α–1,2 mannosides [Citation10].
In a second paper, we reported the interest of this test when combined with the detection of anti-neutrophil cytoplasmic antibodies (ANCA) for the differential diagnosis of CD vs. UC and designated the antibodies detected as ASCA [Citation8] in order to confer homogeneity between acronyms of antibodies for differentiating the two forms of IBD. This was the first mention of the term ASCA in the literature. In retrospect, it was probably an unfortunate choice since it cast suspicion on S. cerevisiaewhose merite resides in being able to synthesize and assemble an extremely vast repertoire of oligomannosides and oligomannosidic epitopes in mannan. In this respect, it is interesting to note that most of our current knowledge about genes controlling the early steps of glycosylation of human molecules originates from studies on mannan mutants of S. cerevisiae [Citation11]. With hindsight and from an immunochemical point of view it would probably have been more rational to name these antibodies, whose epitope has been confirmed by others [Citation12], anti-oligomannose antibodies.
In the present study, we once again confirmed what has been established over several decades about the prevalence and evolution of ASCA and associated phenotypes in CD, UC and healthy controls [Citation3,Citation8,Citation13]. Interestingly, it has recently been shown that, besides CD, ASCA may also be associated with other diseases such as autoimmune diseases, including celiac disease [Citation14] or physiological disorders[Citation15] . Regarding UA, the levels encountered in our cohorts were well within the normal range for males and females, and no differences were found between IBD patients and controls. Regarding the correlation between ASCA and UA reported by Chiaro et al. [Citation6], we were unable to confirm the correlations in any of the groups examined, and even found a trend towards a negative correlation in the control population. The reasons for these differences are unknown, but an explanation may reside in an unforeseen correlation in their control population which was unrelated to S. cerevisiae. High levels of UA are regularly reported in patients with metabolic disorders associated with diabetes or obesity [Citation16] and the presence of ASCA has been reported several times in the same patients, particularly in association with obesity [Citation17]. Thus, if the cohort studied by Chiaro et al. [Citation6] included subjects with excess of weight it is possible that this could be the origin of the correlations they observed. By contrast in a recent study investigating ASCA and the presence of S. cerevisiae in the diet, no association was found between yeast-containing foods and ASCA IgG-positivity, or between yeast-containing foods and fat mass [Citation15]. Thus, our study does not support the ASCA-based hypothesis derived from studies in mice that S. cerevisiae could worsen colitis by affecting host purine metabolism leading to increased UA production. Regarding the contrast between the conclusion of Chiaro’s study presenting S. cerevisiae as an important pathogen and scientific and clinical evidence gathered so far in basic and medical mycology, this lack of confirmation is not surprising. First, S. cerevisiae cannot be qualified as an “endosaprophyte”, which would mean that the normal condition of life for this species is the gut environment in which it is able to reproduce and disseminate[Citation18]. This niche has been colonized by a limited number of fungal species which can survive and reproduce in the human gut environment, including Candida albicans and C. glabrata. These yeasts have been studied extensively in experimental models for their adaptation to commensalism and their status as an opportunistic pathogen in relation to iatrogenic or intrinsic disturbances of host homeostasis[Citation19].
Genetically, despite sharing homologies with C. albicans and more particularly with C. glabrata, S. cerevisiae lacks most of the traits required to develop in the human gut and be an opportunistic pathogen [Citation19]. Accordingly, isolation of live S. cerevisiae in the medical mycology laboratory is rare. Additionally, confusion has recently arisen from metagenomic analyses which regularly detect S. cerevisiae DNA in stools. S. cerevisiae is as a very common component of human food (bread, beer or food additives) and the presence of S. cerevisiae DNA in stools reflects its transit rather than its development inside the host. This was demonstrated by recent convincing experiments addressing the important question of discrepancies between conventional mycological methods and NGS analysis which, among other points, clarified the status of S. cerevisiae [Citation20]. As stated in an excellent review, the ability of some S. cerevisiae strains to grow at 37°C and the opportunities for repeated introduction render it among the most commonly detected fungal species in faecal samples[Citation20]. Thus, in this setting it likely contributes to gut microbial ecology. However, casting suspicion on the safety of an ancestral ingredient of human food worldwide and generating suspicion for billions of human beings deserves debate.
In the middle of the last century, attention was drawn to the threat of invasive fungal infections (IFIs) in the hospital environment. Due to longer periods of immunosuppression, the morbidity and mortality from IFIs continue to increase despite an impressive therapeutic arsenal. In parallel, the spectrum of fungi involved has moved from well-known opportunistic pathogens with characterized virulence factors to other species previously categorized as harmless but finding, in these debilitated patients, conditions favourable to in vivo growth [Citation21]. S. cerevisiae is among the species which are characterized as “Generally Recognized as Safe (GRAS)” microorganisms. Numerous studies have revisited the virulence status of S. cerevisiae strains distributed in clinical isolates, industrial strains (wine, beer, bakery) and probiotic strains (var. boulardii). Such studies have included in vitro analysis of physiological traits (i.e. the ability to grow at body temperature, pseudo-hyphal growth, protease and phospholipase secretion) [Citation22] and in vivo analysis in experimental models of infection/colonization [Citation23,Citation24]. These studies demonstrated that there are obvious differences in pathogenic potential of strains although the reasons for this are not yet understood. A comprehensive review concluded that due to high genotypic and phenotypic heterogeneity a polyphasic approach combining genetic, in vitro and in vivo exposure studies is needed to characterize the pathogenic potential of S. cerevisiae strains [Citation25]. Furthermore, host dependence is another trait revealed in convincing in vivo experiments showing differences in responses depending on the S. cerevisiae strain and origin of macrophages [Citation26]. To complete this very complex picture, it has been shown that, like C. albicans [Citation27], microevolution of probiotic or food strains occurs in human hosts which does not necessarily impose a selective pressure toward higher virulence/pathogenicity [Citation26]. Since all S. cerevisiae strains present in human environments are obviously not identical, strict characterization of the strains involved in experiments is required. Unfortunately, the single S. cerevisiae strain used in the study of Chiaro et al. was poorly defined. The same concern applies to the strains/species used as controls. These consisted of Rhodotorula aurantiaca qualified as an “emerging pathogen”, but never reported as a pathogen to date, whereas C. albicans was, for undefined reasons, a 50:50 mixture of a wild strain and mutant for WOR1, a master regulator of white-opaque switching associated with C. albicans commensalism and pathogenesis[Citation28].
Regarding the experimental model, although ASCA prompted the study, the presence of these antibodies was surprisingly not investigated in germ-free mice treated with DSS and producing UA following S. cerevisiae colonization by gavage. This contrasts with the results from at least two independent groups that demonstrated ASCA generation in mice colonized with C. albicans [Citation29,Citation30]. Indeed considering the relation between CD and anti-yeast mannan antibodies, it appears that according to the data accumulated over decades in basic and medical mycology C. albicans would be a more convincing suspect for CD triggering and/or maintenance than S. cerevisiae. In humans, we demonstrated that, through its well-known ability to modulate its oligomannose repertoire under pathogenic conditions, C. albicans was an immunogen for ASCA[Citation31]. When investigating a large number of CD families we showed that CD and their first-degree healthy relatives (FDR) were more frequently colonized with C. albicans than control populations and in FDR the so far unexplained higher prevalence of ASCA with regard to control populations (30%) was associated with the carriage of C. albicans [Citation31] In these studies, C. albicans represented 76–81% of the yeast species isolated compared to only 2–4% for S. cerevisiae. This increase in Candida abundance during CD in contrast with a reduction in S. cerevisiae revealed by culture methods was recently confirmed through NGS micro-mycobiome analysis by two independent groups [Citation4,Citation5]. Thus, the involvement of S. cerevisiae in CD is not supported by epidemiological, pathophysiological or immunological arguments, nor by the unconfirmed correlation between ASCA and UA. Concerning UA, its involvement in the pathophysiology of IBD is not supported by any data and neither is the hypothesis that an improvement of CD patients on allopurinol could result from “preventive yeast-induced UA build-up in the intestine”. The adjunctive role of allopurinol therapy in patients receiving thiopurines probably results from its role as a xanthine oxidase inhibitor shifting the metabolism of azathioprine or mercaptopurine from the inactive metabolite 6-methylmercaptopurine to the pharmacologically active metabolite, 6-thioguanine nucleotide [Citation32].
In conclusion, we failed to confirm the clinical link between UA levels and antibodies against S. cerevisiae (ASCA) reported by Chiaro et al. [Citation6], that this species negatively affected the course of CD. It is clear that much has still to be learnt about the involvement of the fungome in IBD and the complex metabolic interactions between yeasts and mammals.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2018 Dec 23;390(10114):2769–2778. PubMed PMID: 29050646.
- Sartor RB, Wu GD. Roles for intestinal bacteria, viruses, and fungi in pathogenesis of Inflammatory Bowel diseases and therapeutic approaches. Gastroenterology. 2017 Feb;152(2):327–339 e4. PubMed PMID: 27769810; PubMed Central PMCID: PMCPMC5511756.
- Rieder F, Hahn P, Finsterhoelzl L, et al. Clinical utility of anti-glycan antibodies in pediatric Crohn’s disease in comparison with an adult cohort. Inflamm Bowel Dis. 2012 Jul;18(7):1221–1231. PubMed PMID: 22147427.
- Siegel CA, Horton H, Siegel LS, et al. A validated web-based tool to display individualised Crohn’s disease predicted outcomes based on clinical, serologic and genetic variables. Aliment Pharmacol Ther. 2016 Jan;43(2):262–271. PubMed PMID: 26567467.
- Choung RS, Princen F, Stockfisch TP, et al. Serologic microbial associated markers can predict Crohn’s disease behaviour years before disease diagnosis. Aliment Pharmacol Ther. 2016 Jun;43(12):1300–1310. PubMed PMID: 27117843.
- Chiaro TR, Soto R, Zac Stephens W, et al. A member of the gut mycobiota modulates host purine metabolism exacerbating colitis in mice. Sci Transl Med. 2017 Mar 08;9(380). PubMed PMID: 28275154.
- Dotan I, Fishman S, Dgani Y, et al. Antibodies against laminaribioside and chitobioside are novel serologic markers in Crohn’s disease. Gastroenterology. 2006 Aug;131(2):366–378. PubMed PMID: 16890590.
- Quinton JF, Sendid B, Reumaux D, et al. Anti-Saccharomyces cerevisiae mannan antibodies combined with antineutrophil cytoplasmic autoantibodies in inflammatory bowel disease: prevalence and diagnostic role. Gut. 1998 Jun;42(6):788–791. PubMed PMID: 9691915; eng.
- Poulain D, Sendid B, Fajardy I, et al. Mother to child transmission of anti-Saccharomyces cerevisiae mannan antibodies (ASCA) in non-IBD families. Gut. 2000;47(6):870–871. PubMed PMID: 11186432.
- Sendid B, Colombel JF, Jacquinot PM, et al. Specific antibody response to oligomannosidic epitopes in Crohn’s disease. Clin Diagn Lab Immunol. 1996 Mar;3(2):219–226. PubMed PMID: 8991640; eng.
- Wild R, Kowal J, Eyring J, et al. Structure of the yeast oligosaccharyltransferase complex gives insight into eukaryotic N-glycosylation. Science. 2018 Feb 2;359(6375):545–550. PubMed PMID: 29301962.
- Dotan N, Altstock RT, Schwarz M, et al. Anti-glycan antibodies as biomarkers for diagnosis and prognosis. Lupus. 2006;15(7):442–450. PubMed PMID: 16898180.
- Poulain D, Sendid B, Standaert-Vitse A, et al. Yeasts: neglected pathogens. Dig Dis. 2009;27 Suppl 1:104–110. PubMed PMID: 20203505.
- Barta Z, Csipo I, Szabo GG, et al. Seroreactivity against Saccharomyces cerevisiae in patients with Crohn’s disease and celiac disease. World J Gastroenterol. 2003 Oct;9(10):2308–2312. PubMed PMID: 14562398; eng.
- Kvehaugen AS, Aasbrenn M, Farup PG. Anti-Saccharomyces cerevisiae antibodies (ASCA) are associated with body fat mass and systemic inflammation, but not with dietary yeast consumption: a cross-sectional study. BMC Obes. 2017;4:28. PubMed PMID: 28725447; PubMed Central PMCID: PMCPMC5512824.
- Tsushima Y, Nishizawa H, Tochino Y, et al. Uric acid secretion from adipose tissue and its increase in obesity. J Biol Chem. 2013 Sep 20;288(38):27138–27149. PubMed PMID: 23913681; PubMed Central PMCID: PMCPMC3779712.
- Salamati S, Martins C, Kulseng BB. yeast (Saccharomycescerevisiae) antigen in obese and normal weight subjects. Clin Obes. 2015 Feb;5(1):42–47. PubMed PMID: 25611585.
- Hallen-Adams HE, Suhr MJ. Fungi in the healthy human gastrointestinal tract. Virulence. 2017 Apr 3;8(3):352–358. PubMed PMID: 27736307; PubMed Central PMCID: PMCPMC5411236.
- Calderone R, Clancy C. Candida and Candidiasis. 2nd ed. Washington DC: ASM Press; 2012.
- Huseyin CE, Rubio RC, O’Sullivan O, et al. The fungal frontier: a comparative analysis of methods used in the study of the human Gut mycobiome. Front Microbiol. 2017;8:1432. PubMed PMID: 28824566; PubMed Central PMCID: PMCPMC5534473.
- Kohler JR, Casadevall A, Perfect J. The spectrum of fungi that infects humans. Cold Spring Harb Perspect Med. 2014 Nov 3;5(1):a019273. PubMed PMID: 25367975; PubMed Central PMCID: PMCPMC4292074.
- de Llanos R, Fernandez-Espinar MT, Querol A. A comparison of clinical and food Saccharomyces cerevisiae isolates on the basis of potential virulence factors. Antonie Van Leeuwenhoek. 2006 Oct;90(3):221–231. PubMed PMID: 16871421.
- de Llanos R, Llopis S, Molero G, et al. In vivo virulence of commercial Saccharomyces cerevisiae strains with pathogenicity-associated phenotypical traits. Int J Food Microbiol. 2011 Jan 5;144(3):393–399. PubMed PMID: 21081253.
- Jawhara S, Habib K, Maggiotto F, et al. Modulation of intestinal inflammation by yeasts and cell wall extracts: strain dependence and unexpected anti-inflammatory role of glucan fractions. PLOS ONE. 2012;7(7):e40648.
- Anoop V, Rotaru S, Ps S, et al. Review of current methods for characterizing virulence and pathogenicity potential of industrial Saccharomyces cerevisiae strains towards humans. FEMS Yeast Res. 2015 Sep;15(6). PubMed PMID: 26195617. DOI:10.1093/femsyr/fov057.
- Pfliegler WP, Boros E, Pazmandi K, et al. Commercial strain-derived clinical Saccharomyces cerevisiae can evolve new phenotypes without higher pathogenicity. Mol Nutr Food Res. 2017 Nov;61(11). PubMed PMID: 28731263. DOI: 10.1002/mnfr.201601099.
- Bougnoux ME, Diogo D, Francois N, et al. Multilocus sequence typing reveals intrafamilial transmission and microevolutions of Candida albicans isolates from the human digestive tract. J Clin Microbiol. 2006 May;44(5):1810–1820. PubMed PMID: 16672411; PubMed Central PMCID: PMCPMC1479199.
- Huang G, Wang H, Chou S, et al. Bistable expression of WOR1, a master regulator of white-opaque switching in Candida albicans. Proc Natl Acad Sci U S A. 2006 Aug 22 103(34):12813–12818. PubMed PMID: 16905649; PubMed Central PMCID: PMCPMC1540355
- Jawhara S, Thuru X, Standaert-Vitse A, et al. Colonization of mice by Candida albicans is promoted by chemically induced colitis and augments inflammatory responses through galectin-3. J Infect Dis. 2008 Apr 1;197(7):972–980. PubMed PMID: 18419533.
- Schaffer T, Muller S, Flogerzi B, et al. Anti-Saccharomyces cerevisiae mannan antibodies (ASCA) of Crohn’s patients crossreact with mannan from other yeast strains, and murine ASCA IgM can be experimentally induced with Candida albicans. Inflamm Bowel Dis. 2007 Nov;13(11):1339–1346. PubMed PMID: 17636567.
- Standaert-Vitse A, Jouault T, Vandewalle P, et al. Candida albicans is an immunogen for anti-Saccharomyces cerevisiae antibody markers of Crohn’s disease. Gastroenterology. 2006 May;130(6):1764–1775. PubMed PMID: 16697740.
- Hoentjen F, Seinen ML, Hanauer SB, et al. Safety and effectiveness of long-term allopurinol-thiopurine maintenance treatment in inflammatory bowel disease. Inflamm Bowel Dis. 2013 Feb;19(2):363–369. PubMed PMID: 22605661.
