ABSTRACT
Coccidioides immitis and C. posadasii are two highly pathogenic dimorphic fungal species that are endemic in the arid areas of the new world, including the region from west Texas to southern and central California in the USA that cause coccidioidomycosis (also known as Valley Fever). In highly endemic regions such as southern Arizona, up to 50% of long term residents have been infected. New information about fungal population genetics, ecology, epidemiology, and host-pathogen interactions is becoming available. However, our understanding of some aspects of coccidioidomycosis is still incomplete, including the extent of genetic variability of the fungus, the genes involved in virulence, and how the changes in gene expression during the organism’s dimorphic life cycle are related to the transformation from a free-living mold to a parasitic spherule. Unfortunately, efforts to develop an effective subunit vaccine have not yet been productive, although two potential live fungus vaccines have been developed.
Introduction
Coccidioidomycosis was first recognized as a fatal disseminated infection 120 years ago, but the fungal etiology was only determined two decades later, and the full clinical spectrum of the disease was not realized for another 40 years after that [Citation1]. In the last decade there have been advances in our understanding of the protective immune response in mice, and modest progress was made toward creating a protective vaccine. Advances in molecular methodology have been used to clarify the taxonomy of the pathogen, the epidemiology of these regionally important, dimorphic, endemic fungal pathogens, and to compare fungal gene expression in its different morphologies. It is still difficult to make precise, targeted mutations in these pathogens, which has limited our ability to identify virulence factors, and to fully understand how the fungus transforms from a mold into the complex and unique parasitic structure (spherule) which is the form it takes within the host. Some of our current knowledge of these issues will be discussed in this selective review.
Taxonomy
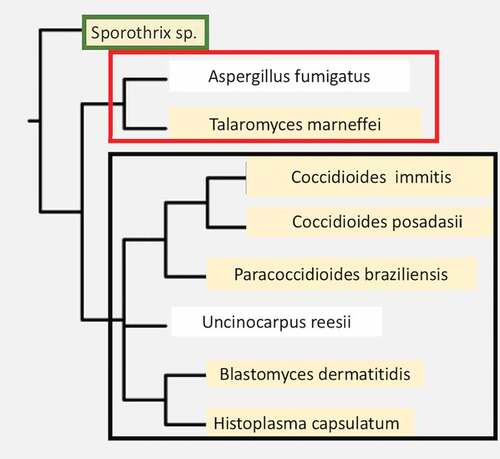
Coccidioides species are fungi within Ascomycete division, Eurotiomycetes class, Onygenales order [Citation2,Citation3]. This order includes a variety of dimorphic human pathogens capable of causing invasive disease in immunologically normal hosts, including Histoplasma capsulatum, Paracoccidioides spp., and Blastomyces spp. The monomorphic mold, Aspergillus fumigatus, which is an opportunistic pathogen, is also found in this order. Several non-pathogenic but closely related species are also found within this order (). Coccidioides immitis and C. posadasii are morphologically identical and their predicted proteins are more than 90% homologous [Citation2]. They cannot be distinguished by serologic tests, but the two species can be distinguished by genetic polymorphisms, and some differences in growth characteristics have been reported [Citation4,Citation5]. C. posadasii has a larger population size, which is more diverse than C. immitis [Citation6]. The biggest difference between the two species is their geographic distribution. C. immitis is primarily found in the desert regions of Central and Southern California (including Baja California), while C. posadasii is primarily found in desert regions of Nevada, Arizona, New Mexico, West Texas, Mexico, and Central and South America implying that were significant geographic barriers at the time the species diverged from a common ancestor [Citation6]. Some geographic overlap between the two species also occurs in Southern California and Baja California [Citation7]. Both species contain subpopulations that cluster within smaller geographic areas [Citation8].
Figure 1. A phylogenetic tree of dimorphic fungi that are human pathogens. A few close relatives that are not dimorphic primary pathogens are shown for comparison (not highlighted in tan). The Orders are shown to the right of the boxed names. Organisms within each Order are boxed together. The phylogenetic data was obtained using the NCBI taxonomy tool and the tree was constructed using Phylip-3.695.
Ecology
Coccidioides spp. grow in the arid, alkaline desert soil in California, but soil is very complex [Citation9] and the characteristics of contaminated soil may differ between endemic regions [Citation10]. So far, no one set of physical, biological, and chemical characteristic describes all the sites where the organism has been found, and even in one “site” the fungus is distributed unevenly in the soil for unclear reasons. Some evidence exists for the importance of the carcasses of infected small mammals for soil colonization [Citation7], which may explain the spotty recovery from soil samples even in places where small outbreaks have occurred [Citation11], but it is unlikely that infected animals are required for soil colonization [Citation12,Citation13]. In addition to animal carcasses in burrows, it is likely that there is left-over food that the animals brought into the burrows. The genomes of Coccidioides reveal an expansion of proteolytic enzymes, which has been interpreted as indirect evidence that the fungus uses proteins (carcasses) as carbon sources in its environmental niches, as it is known that small desert rodents such as kangaroo rats often have the coccidioidal granulomas in their lungs [Citation11].
Recovering the organism from the soil by culture can be difficult [Citation14]. Recovering the organism by mouse inoculation is more sensitive but that technique also has its limitations [Citation15]. Coccidioides DNA is found in only a small fraction of soil samples from endemic regions in Baja California and Arizona even by sensitive tests such as PCR [Citation12,Citation15], but a new methodology may increase the sensitivity and specificity of DNA detection [Citation16]. It has been easier to grow C. immitis from the soil in the San Joaquin Valley of California in proximity to sites where humans are known to have been infected [Citation7,Citation11,Citation14]. Detecting the organism from the atmosphere is even more difficult than finding it in the soil although recent development of new techniques may improve the sensitivity of detection of aerosolized fungal DNA [Citation17].
Epidemiology
It is estimated that 30–50% of people in highly endemic areas have been infected, as detected by coccidioidin skin test [Citation18]. These estimates are based on old data as skin tests reagents were unavailable for decades and no large scale epidemiological studies have been done since the recent availability of a spherulin skin test reagent. It is important to remember that the endemic areas include the urban areas of Phoenix, Tucson, Los Angeles, and San Diego, so one does not have to travel to the open desert to contract coccidioidomycosis. Although urbanization probably reduces the risk of infection by decreasing the surface area of exposed contaminated soil, it also increases the numbers of people who can potentially be infected by airborne arthroconidia that are generated by activities that disturb soil, such as construction and earthquakes [Citation19,Citation20]. In addition, windborne spores from an endemic area can infect people and animals many miles from the endemic area [Citation21]. Coccidioides are salt tolerant so they may survive in coastal waters, and this could explain how they can infect marine mammals [Citation7,Citation22].
The incidence of reported human infections varies a good bit from year to year, perhaps because of weather patterns. Wet winters seem to correlate with larger numbers of infections in subsequent months [Citation23]. In addition, there has been an overall trend toward an increased number of symptomatic infections over the past 10 years [Citation23,Citation24]. Although the reasons for this increase in incidence are not completely established, in Arizona there are more susceptible people (including older people) moving from non-endemic areas to endemic areas, which is one possible explanation. While the population of the Central Valley of California is not increasing in the same way, the number of cases in suburban Los Angeles County has increased dramatically with urban expansion [Citation18]. In addition, several new prisons were built in the middle of the endemic area in the San Joaquin Valley, resulting in unacceptably high attack rates among the prisoners and guards [Citation25]. Recently a small number of C. immitis infections were diagnosed in an arid area in eastern Washington State. Organisms were also isolated from soil samples where the infections were acquired, thousands of miles north of the nearest known endemic areas in California [Citation26]. The soil and human isolates were identical but were genetically distinct from Central and Southern California isolates of C. immitis.
There are a number of recent reviews of the clinical manifestations of coccidioidomycosis that contain detailed clinical information [Citation18,Citation27,Citation28]. Pulmonary symptoms are the most common reasons that patients seek medical help, but it is estimated that only 30–50% of infections are symptomatic [Citation18]. Even though most infections are not diagnosed, a large fraction of cases of outpatient pneumonias in Arizona are due to coccidioidomycosis [Citation29]. Nearly all coccidioidal pneumonias are self-limited, even in cases where patients present with extra-pulmonary complications, but in some cases the pneumonia can persist for weeks to months [Citation27,Citation29]. In other cases there may be residual granulomas or thin walled cavities that remain long after the pneumonia is resolved.
Fewer than 5% of immunocompetent patients develop disseminated disease [Citation21,Citation30]. Aside from disease or drug-induced immunosuppression, the risk of disseminated disease is strongly influenced by host factors, such as the third trimester of pregnancy and old age [Citation30,Citation31]. Ethnicity is also a major risk factor for dissemination. In many studies, people who describe themselves as African-Americans are 2–10 times more likely to develop disseminated disease than people of European descent, even when there are no apparent differences in their exposures (such as occurs in prisons and on military bases) [Citation18,Citation25,Citation32]. Filipinos are also more likely to have disseminated infection [Citation33]. The genes and the mechanisms behind ethnic predisposition to dissemination have not been established.
Genomes
The DNA sequences of several of isolates of C. immitis and many C. posadasii isolates have been determined [Citation8,Citation34]. There is some hybridization between the two species [Citation6,Citation35]. Sequencing of a large number of clinical isolates in Arizona established that almost all were genetically diverse strains of C. posadasii [Citation8]. Groups of isolates from Tucson and Phoenix areas are genetically distinct. A study comparing soil isolates from Phoenix to isolates from patients in the same area found that the environmental isolates were more genetically diverse than the clinical isolates, but there was no evidence for a subset of more pathogenic strains that might explain the increasing incidence of infections in that area [Citation6,Citation34].
The genomes of Coccidioides spp. are 28–29 megabases (Mb). At least four chromosomes have been identified by contour-clamped homogeneous electric field gel electrophoresis [Citation36]. The genomes are haploid and no mating has been observed within or between species, although genes coding for mating functions are present [Citation2] and genetic recombination occurs [Citation35]. About 18% of the genome consists of repetitive DNA. Coccidioides spp. appear to have a repeat-induced mutation mechanism to control proliferation of transposons [Citation35]. Both DNA and long terminal repeat transposons are found; Gypsy, which is a retrovirus-like transposon that is common in fungi, is the most common type of transposon. Transposons are found more frequently in genomic regions that have few structural genes and are usually found in clusters [Citation37]. The majority of transposons have degenerated and do not have all the domains needed for transposition. There is some evidence that C. immitis transposons are preferentially associated with genes coding for protein phosphorylation. In addition, many C. immitis genes flanked by some transposon super families are poorly expressed [Citation37]. Of interest, a newly FDA-licensed PCR assay for identifying organisms in clinical specimens and a new modification of that assay that is both sensitive and specific for use on soil samples targets assay targets a copia-like retrotransposon that is present in high copy number in the genome [Citation16].
Gene families coding for phosphotransferase activity, protein kinases, and proteinases, including subtilases and keratinases, are expanded in Coccidioides spp. compared to closely related species [Citation3]. This suggests that Coccidioides spp. may be specialized for growth on proteins in addition to carbohydrates. There are almost 800 genes that are unique to Coccidioides spp. and some are preferentially transcribed in spherules (see below).
Dimorphism
All the primary fungal pathogens except Cryptococcus spp. share the characteristic of being thermally dimorphic, growing as molds in the soil and differentiating to a yeast or spherule within mammals. Coccidioides spp. grow as mycelia (mold) in the soil and form spores known as arthroconidia within the mycelium as they mature (). Arthroconidia are released when the contaminated soil is disturbed and each one has the potential to form a new mycelium if it lands in the soil, or a spherule if it infects susceptible animals. The ability to form spherules from arthroconidia is required for pathogenicity. Arthroconidia round up and become immature spherules by isotropic growth. The maturation of spherules involves circumferential swelling of the organism and the synchronous division of nuclei and the cytoplasm to eventually fill the spherule with hundreds of 2 – 4 micron endospores. When a mature spherule ruptures those endospores are released and each one of them has the potential to form another spherule. The differentiation of endospore into mature spherules takes about 4–6 days in vivo so the number of spherules can increase very quickly. The transformation from arthroconidia to spherules when grown in a defined medium requires a temperature shift from 25° to 37°, and an increase in atmospheric CO2 to 10–14% [Citation38]. It is assumed that similar conditions prompt spherule development in vivo, but there is also evidence that contact with neutrophils can stimulate arthroconidia to spherule conversion [Citation39]. In chronic coccidioidal lung cavities, where there are no neutrophils, one can sometimes see reversion to hyphal forms that contain swellings that appear to be abortive attempts to make spherules [Citation40].
Figure 2. Mycelia containing arthroconidia and a mature spherule with endospores. (a) Mature mycelia grown in vitro showing darkly stained arthroconidia alternating with a nucleate thin walled segments within mycelia (lacto-phenol cotton blue stain). (b) A spherule containing endospores in tissue (Periodic Acid Schiff (PAS)). The images were obtained from the CDC (http://phil.cdc.gov/phil/details.asp). This figure was previously published in the Journal of Fungi [Citation70].
![Figure 2. Mycelia containing arthroconidia and a mature spherule with endospores. (a) Mature mycelia grown in vitro showing darkly stained arthroconidia alternating with a nucleate thin walled segments within mycelia (lacto-phenol cotton blue stain). (b) A spherule containing endospores in tissue (Periodic Acid Schiff (PAS)). The images were obtained from the CDC (http://phil.cdc.gov/phil/details.asp). This figure was previously published in the Journal of Fungi [Citation70].](/cms/asset/01856579-11de-4ba7-afbe-6ab21791b8bd/kvir_a_1509667_f0002_oc.jpg)
Relatively little is known about the change in the transcriptional program that mediates the striking transformation from arthroconidia to spherules. There are only two published studies comparing the transcriptome of mycelia to that of spherules grown in vitro; one studied both C. immitis and C. posadasii using RNA-seq and the other studied only C. immitis, using a whole genome open reading frame microarray [Citation41,Citation42]. The RNA-seq study compared mycelia and day 4 spherules and concentrated on genes that were differentially expressed in both species. 13% of the genes were up-regulated more than two-fold in the spherules of both species, including chitin-associated proteins, β-(1,3) glucan synthase, and the spherule outer wall glycoprotein.
The microarray study compared C. immitis mycelia to day 2 (early in transformation process) and day 8 (endosporulating) spherules. The level of expression of 22% of genes was either up- or down-regulated more than two-fold in day 2 or day 8 spherules compared to mycelia. There were also significant differences between the transcriptomes of day 2 and day 8 spherules (). Oxireductases (including extracellular superoxide dismutase that could help protect against neutrophil killing) are up-regulated in day 2 spherules, as are sugar transporters, thioesterase, and amylase. About a third of genes that are up-regulated in vivo have no assigned function and 5% are only found in Coccidioides spp [Citation42]. One of the most interesting gene families that are down-regulated in C. immitis spherules is the protein kinase family; 28 of 184 predicted protein kinase genes were downregulated. Some of the down-regulated protein kinase genes coded for cell cycle, cell wall, and stress response related proteins. The relationship between down-regulation of these genes and spherule formation is not clear.
Figure 3. Numbers of genes upregulated in spherules of three different maturities. Venn diagram of C. immitis genes up-regulated more than 2-fold compared to mycelial gene expression. (a) The data for day 4 spherules are from [Citation41] and (b) the data for Day 2 and day 8 spherules are from [Citation42].
![Figure 3. Numbers of genes upregulated in spherules of three different maturities. Venn diagram of C. immitis genes up-regulated more than 2-fold compared to mycelial gene expression. (a) The data for day 4 spherules are from [Citation41] and (b) the data for Day 2 and day 8 spherules are from [Citation42].](/cms/asset/19202862-744a-4b38-847c-e85abee48d30/kvir_a_1509667_f0003_b.gif)
One of the upregulated genes found in both studies is 4-hydroxphenylpyruvate dioxygenase (4-HPPD, or HpdA). This enzyme is part of a complex of genes involved in tyrosine catabolism. 4-HPPD degrades 4-hydroxyphenylpyruvate homogentisate, which is toxic. Homogentisate is further catabolized or oxidized and polymerized to form pyomelanin [Citation43]. 4-HPPD has been found to be up-regulated in the yeast phase of all dimorphic primary pathogenic fungi [Citation44]. Disruption of the 4-HPPD gene in Talaromyces marneffei results in a mutant that cannot grow and differentiate into yeast inside macrophages [Citation45]. The authors of this study believe that this phenotype is unlikely to be directly related to the effect of 4-HPPD on tyrosine metabolism because other mutation in the tyrosine catabolism pathway did not have that phenotype so they suggest that 4-HPPD may have other undiscovered properties.
Despite the differences in experimental design and data analysis, there are 152 genes that were up-regulated in spherules in both studies, regardless of the stage of maturation (). Many of these upregulated genes are found in enzymatic pathways of complex carbohydrate metabolism (). The enrichment of genes in complex carbohydrate pathways seems plausible since extensive remodeling and synthesis of new cell walls must be required for transformation into and growth of spherules and endospores. Twenty-six of the common genes up-regulated in spherules were also up-regulated in the process of Histoplasma capsulatum differentiation into yeast [Citation46]. In addition to 4-HPPD, these include several sugar transporters, a sulfite transporter, amylase, keto-reductases, a protein kinase, and a polyketide synthase gene. There have been no published transcriptome studies of Coccidioides spherules in vivo.
Table 1. Some enriched metabolic pathways among the 152 genes up-regulated in day 2, 4 and 8 spherules.
Proteomics is another approach that can identify expressed genes in spherules and validate so-called hypothetical proteins. There is one published study using this approach that identified proteins in C. posadasii mycelia and spherules [Citation47]. They detected 837 proteins (8% of the total predicted from DNA sequence), 88% of which corresponded to the proteins predicted by genome analysis. They also identified 172 novel proteins, most of which could be attributed to differential spicing of the genes. A transposon protein was expressed, indicating that at least one transposon is still transcriptionally active. Studies like this will undoubtedly improve the annotation of the genome.
While this transcriptomic data can identify genes that are preferentially expressed in spherules, they do not tell us what genes are necessary for arthroconidia to differentiate into spherules and what changes in expression are a consequence of the transformation [Citation46]. The most conclusive way to identify the essential genes for spherule formation is to knock them out or to down-regulate their expression with a regulatory RNA and observe the phenotype.
Only a few deletion mutants have been made due to the difficulty of making targeted mutations. Chitinase 2 and 3 genes are expressed at higher levels in spherules than in mycelia, and they were hypothesized to be important for remodeling the cell wall of maturing spherules [Citation48]. A double knockout of those genes resulted in a mutant that does not produce mature spherules with endospores and is avirulent in mice. This mutant was an effective live attenuated vaccine in mice. The CPS1 gene is also important for virulence as deletion of the gene resulted in spherules that grew more slowly than wildtype, could not endosporulate, and the mutant was avirulent even in highly immunocompromised mice [Citation49]. The CPS1 gene product is not well characterized. The gene was chosen as a target because its homologue is associated with pathogenicity in Cochiobolus heterostrophus. In mutant spherules, 33 genes were either up or down-regulated compared to wildtype spherules [Citation49]. It is difficult to infer the function of CPS1 from the up- and down-regulated genes. This mutant is also an effective live vaccine in mice.
Deletion of the gene coding for a spherule outer wall glycoprotein also decreased virulence significantly [Citation50]. The outer wall glycoprotein is produced exclusively in spherules and forms the outermost layer that comes in contact with host cells. This proline-rich protein is also an adhesin that is known to bind to laminin.
Enzymes involved in ammonia metabolism are also important for virulence. Infected tissues in mice are quite alkaline and Coccidioides synthesizes both a urease enzyme and ureidoglycolate hydrolase, an enzyme that degrades ureidoglycolate to ammonia and glyoxylate (UGH). A urease and UGH double knockout mutant produces less ammonia and is highly attenuated even in genetically susceptible BALB/c mice, suggesting that the ability to alkalinize their environment is important for pathogenicity [Citation51]. However, because the glyoxylate shunt is also a metabolic pathway in fungi for energy generation in the absence of glucose, the UGH mutation may have deleterious effects other than reduced ammonia production.
Immunology
There is a strong correlation between the type of immune response that people make and the prognosis of acute pulmonary coccidioidomycosis. The majority of people have self-limited pulmonary infections, and they develop delayed type hypersensitivity (DTH) as measured by positive skin tests to coccidiodin or spherulin, but they make only low titers of complement fixing (CF) antibodies. In contrast, patients who go on to dissemination of the infection make high titers of CF antibody and do not develop DTH [Citation30,Citation52]. Although this has been known for many decades, there is little or no understanding of what drives the immune response toward a TH1 (DTH, IFNγ) pathway in people who do well. Clearly CD4 T cells are required since patients with low CD4 counts because of untreated AIDS are at high risk for disseminated infections [Citation53] Patients treat with tumor necrosis factor inhibitors are also at higher risk [Citation54]. We also know from rare human genetic mutations that inability to make or respond to IFNɣ is a risk for dissemination of infection [Citation55,Citation56]. A gain of function mutation in Stat1 also predisposes to disseminated coccidioidomycosis and histoplasmosis, but the mechanism(s) behind this increased susceptibility to invasive dimorphic fungi is not clear [Citation57].
The immune response to infection and vaccination have been studied extensively in mice. It is generally accepted that that the innate immune response determines the nature of the adaptive immune response, and that is also true in experimental coccidioidomycosis. DBA/2, a highly resistant mouse strain makes more IL-12p70, IL-23p19, IFNγ, Stat1, and IL-17a and less IL-10 after infection compared to highly susceptible strains, both in vivo and in vitro [Citation58,Citation59]. In mice, increased IL-10 production results in increased susceptibility, and conversely, IL-10 KO mice are more resistant than the susceptible parental strain, closely resembling DBA/2, the most resistant inbred strain [Citation60]. The only known driver of those differences in mice is Dectin-1, the β glucan receptor, which is expressed on dendritic and other myeloid cells [Citation61]. There is a difference in the structure and function of Dectin-1 in susceptible B6 mice and resistant DBA/2 mice that is based on alternative splicing of the encoding gene, Clec7a [Citation59]. That gene is also alternatively spliced in human cells [Citation62,Citation63], but there are no studies on how that differential splicing affects the human response to spherules.
It is likely that Dectin-1 is also at least partially responsible for generating a TH17 immune response as spherules interact strongly with that C type lectin receptor [Citation59]. A mutation of the IL-17 receptor impairs the ability of mice to develop immunity after vaccination by a live avirulent strain of C. posadasii [Citation64]. A patient with a STAT-3 mutation, which affect CD4 TH17 differentiation, developed coccidioidal meningitis [Citation65,Citation66]. Since STAT-3 also is involved in many other signaling pathways it is possible that the low levels of IL-17a found in these patients may not be the only explanation for her susceptibility.
The mechanism whereby spherules and/or endospores are killed in vivo is also unknown but must depend indirectly on CD4+ T cells as there is an inverse correlation between the total CD4 count and the risk of disseminated coccidioidomycosis in patients with AIDS [Citation67]. The effector molecules that actually kill or inhibit multiplication of the fungus in vitro are unknown. People with chronic granulomatous disease are apparently not more susceptible to these pathogens, nor are mice with an orthologous genetic defect [Citation68], so the NADPH oxidase is not needed. There is some uncertainly about the role of iNOS. A broad-spectrum NOS inhibitor increased the susceptibility of DBA/2 mice to C. immitis [Citation69]. In contrast, a Nos2 mutant mouse strain was not more susceptible [Citation70]. However, that mutation was made in C57BL/6 mice, which themselves make very little NO in response this infection [Citation69].
Vaccine efforts
Vaccine-induced immunity against coccidioidomycosis seems feasible because symptomatic second infections with Coccidioides spp. are extraordinarily rare (one cannot exclude subclinical infections that boost immunity in highly endemic areas) [Citation71]. Successful vaccination requires a T-cell mediated immune response with both TH1 and TH17 immune responses playing a role in vaccine mediated protection [Citation64]. There is no evidence that an antibody response is protective in humans as there is no association between inherited or acquired immunoglobulin deficiencies and disseminated coccidioidomycosis, but in mouse models there is conflicting evidence about whether B cells are needed for vaccine-induced immunity [Citation72,Citation73]. Ideally, a vaccine would prevent or drastically reduce the incidence of infection, but one that could prevent symptomatic infection and protect those at high-risk for disseminated disease would be considered successful from a clinical standpoint.
Vaccine candidates were all tested initially in genetically susceptible inbred mice, and many recombinant protein vaccines protect them against small but not against large inocula of arthroconidia. It is now feasible to identify and produce recombinant cloned proteins that can be tested as antigens for a vaccine because the genomes of both C. immitis and C. posadasii are available, and there are enough completly sequenced genomes so that one can choose highly conserved proteins. Unfortunately, there currently is no way to predict which spherule proteins will elicit protective immunity. It was initially believed that highly expressed surface proteins would make the best vaccines, but not all cell-surface proteins are protective (unpublished observations, TNK). shows some of the recombinant proteins that have been tested as vaccines [Citation71]. Protection was assessed by decreases in fungal colony counts from quantitative culture of lungs and spleen, and in some cases by survival. The exact criteria varied from one study to another. Single antigens tend to be modestly protective at best [Citation74–Citation76]. The multiple protein/epitope approach seemed to produce the most promising subunit vaccines [Citation77–Citation79]. One dual protein vaccine was tested in cynomolgus macaques and there was a reduction in the burden of disease but the vaccination but did not produce sterilizing immunity [Citation79]. Despite the promising results in mice and monkeys, the development of a recombinant protein vaccine requires important decisions about the what are the best proteins, the amount of those proteins, adjuvant, formulation, and how many doses will be needed to make an effective vaccine. Perhaps most importantly, a pharmaceutical partner willing to manufacture the product is required, given the relatively small population at risk for infection. Careful planning of a clinical trial is also critical. For all these reasons, it is unrealistic to expect a vaccine to be ready for clinical evaluation in the near future.
Table 2. Experimental Recombinant Vaccines.
Summary
The study of Coccidioides spp. has made significant advances over the past decade. Our understanding of ecology and population biology of these organisms is dramatically improved. The availability of genomic sequence of a number of strains has been invaluable for many aspects of research, including creation of several knockout strains that are highly attenuated and provide important knowledge about pathogenesis. The immunology of coccidioidomycosis and Coccidioides spp. vaccines is better understood. Unfortunately, there are significant gaps in our knowledge. More information about ecology of this organism is still needed. Understanding of the biology of transformation from mycelium to spherules is limited at best. More information about the human immune response to the infection is also needed. Despite cloning, expression and testing of a number proteins, a subunit vaccine has not been developed. These and other topics provide many challenges for the future.
Abbreviations
| PCR | = | polymerase chain reaction |
| INF | = | interferon |
| TNF | = | tumor necrosis factor |
| IL | = | interleukin |
| Stat | = | signal transducer and activator of transcription |
| KO | = | knocked out |
| THT | = | helper cell |
| NOS | = | nitric oxide synthetase |
| NADPH | = | nicotine adenine dinucleotide phosphate oxidase |
Disclosure statement
No potential conflict of interest was reported by the author.
Additional information
Funding
References
- Hirschmann JV. The early history of coccidioidomycosis: 1892–1945. Clin Infect Dis. 2007;44(9):1202–1207.
- Sharpton TJ, Rounsley SD, Gardner MJ, et al. Comparative genomic analyses of the human fungal pathogens Coccidioides and their relatives. Genome Res. 2009;19(10):1722–1731.
- Whiston E, Jw T. Comparative phylogenomics of pathogenic and nonpathogenic species. G3(Bethesda). 2016;6(2):235–244.
- Fisher MC, Koenig G, White TJ, et al. A test for concordance between the multilocus genealogies of genes and microsatellites in the pathogenic fungus Coccidioides immitis. Mol Biol Evol. 2000;17(8):1164–1174.
- Fisher MC, Rannala B, Chaturvedi V, et al. Disease surveillance in recombining pathogens: multilocus genotypes identify sources of human Coccidioides infections. Proc Natl Acad Sci U S A. 2002;99(13):9067–9071.
- Teixeria MM, Barker BM. Use of population genetics to assess the ecology, evolution, and population structure of Coccidioides. Emerg Inf Dis. 2016;22(6):1022–1030.
- Del Rocio-Reyes-Montes M, Perez-Huitron MA, Ocana-Monroy JL, et al. The habitat of Coccidioides spp and the role of animals as reservoirs in disseminators to nature. BMC Infect Dis. 2016;16(1):550–558.
- Engelthaler DM, Roe CC, Hepp CM, et al. Local population structure and patterns of western hemisphere dispersal for Coccidioides spp., the fungal cause of valley fever. mBio. 2016;7(2):e00550–16.
- Lauer A, Talamantes J, Olivares LRC, et al. Combining forces - the use of landsat TM satellite imagery, soil parameter information, and multiplex PCR to Detect Coccidioides immitis growth sites in Kern County, California. PLoS One. 2014;9(11):e111921.
- Brown J, Benedict K, Park BJ, et al. Coccidioidomycosis: epidemiology. Clin Epidemiol. 2013;5:185–197.
- Taylor JW, Barker BM. The endozoan, small mammal-reservoir hypothesis and the life cycle Coccidioides species. Med Mycol. 2018:1–5.
- Vargas-Gastelum L, Romero-Olivares AL, Escalante AE, et al. Impact of seasonal changes on fungal diversity of a semi-arid ecosystem revealed by 454 pyrosequencing. FEMS Microbiol Ecol. 2015;91:fiv044.
- Lacy GH, Swatek EE. Soil ecology of Coccidioides immitis at Amerindian middens in California. Appl Microbiol. 1974;27:379–388.
- Greene DR. Soil isolation and molecular identification of Coccidioides immitis. Mycologia. 2000;92:406–410.
- Barker BM, Tabor JA, Shubitz LF, et al. Detection and phylogenetic analysis of Coccidioides posadasii in Arizona soil samples. Fungal Ecol. 2012;5(2):163–176.
- Bowers JR, Parise KL, Kelley EJ, et al. Direct detection of Coccidioides from Arizona soils using CocciENV, a highly sensitive and specific real-time PCR assay. Med Mycol. 2018;56.
- Chow NA, Griffin DW, Barker BM, et al. Molecular detection of airborne Coccidioides in Tuscon, Arizona. Med Mycol. 2016;54(6):584–592.
- Nguyen C, Barker BM, Hoover S, et al. Recent advances in our uderstanding of the environmental, epidemiological, immunological, and clinical dimnsions of coccidioidomycosis. Clin Microbiol Rev 2013;26(3):505–525.
- Guevara RE, Motala T, Terashita D. The changing epidemiology of coccidioidomycosis in Los Angeles (LA) County, California, 1973–2011. PLoS One. 2015;10(8):e0136753.
- Schneider E, Hajjeh R, Jibson R, et al. A coccidioidomycosis outbreak following the Northridge, California earthquake. JAMA. 1997;277(11):904–908.
- Flynn NM, Hoeprich PD, Kawachi MM, et al. An unusual outbreak of windborne coccidioidomycosis. N Engl J Med. 1979;301(7):358–362.
- Elconin AF, Egeberg RO, Egeberg MC. Significance of soil salinity in the ecology of Coccidioides immitis. J Bacteriol. 1964;87(3):500–503.
- Park BJ, Sigel K, Vaz V, et al. An epidemic of coccidioidomycosis in Arizona associated with climatic changes, 1998-2001. J Infect Dis. 2005;191(11):1981–1987.
- Cooksey GS, Nguyen A, Knutson K, et al. Notes from the Field: increase in coccidioidomycosis — California, 2016. MMWR Morb Mortal Wkly Report. 2017;66(31):833.
- Wheeler C, Lucas KD, Mohle-Boetani JC. Rates and risk factors for Coccidioidomycosis among prison inmates, California, USA, 2011. Emerg Infect Dis. 2015;21(1):70–75.
- Litvintseva AP, Marsden-Haug N, Hurst S, et al. Valley fever: finding new places for an old disease: Coccidioides immitis found in Washington State soil associated with recent human infection. Clin Inf Dis. 2015;30(1):e1–3.
- Kirkland TN, Fierer J. Coccidioidomycosis: a reemerging infectious disease. Emerg Infect Dis. 1996;2(3):192–199.
- Crum NF, Lederman ER, Stafford CM, et al. Coccidioidomycosis: a descriptive survey of a reemerging disease. Clinical characteristics and current controversies. Medicine. 2004;83:149–175.
- Valdivia L, Nix D, Wright M, et al. Coccidioidomycosis as a common cause of community-acquired pneumonia. Emerg Infect Dis. 2006;12(6):958–962.
- Pappagianis D. Epidemiology of coccidioidomycosis. Curr Top Med Mycol. 1988;2:199–238.
- Sondermeyer G, Lee L, Gilliss D, et al. Coccidioidomycosis-associated hospitalizations, California, USA, 2000-2011. Emerg Infect Dis. 2013;19(10):1590–1597.
- Smith CE, Beard RR. Varieties of coccidioidal infection in relation to the epidemiology and control of the diseases. Am J Public Health Nations Health. 1946;36(12):1394–1402.
- Mease L. Pulmonary and extrapulmonary coccidioidomycosis, active component, U.S. Armed Forces, 1999-2011. MSMR. 2012;9(12):2–4.
- Jewell K, Cheshier R, Cage GD. Genetic diversity among clinical Coccidioides spp. isolates in Arizona. Med Mycol. 2008;46(5):449–455.
- Neafsey DE, Barker BM, Sharpton TJ, et al. Population genomic sequencing of Coccidioides fungi reveals recent hybridization and transposon control. Genome Res. 2010;20(7):938–946.
- Pan S, Cole GT. Electrophoretic karyotypes of clinical isolates of Coccidioides immitis. Infect Immun. 1992;60(11):4872–4880.
- Kirkland TN, Muszewska A, Stajich JE. Analysis of transposable elements in Coccidioides species. J Fungi. 2018;4(1):13.
- Converse JL. Effect of physico-chemical environment of spherulation of Coccidioides immitis in a chemically defined medium. J Bacteriol. 1956;72(6):784–792.
- Galgiani JN, Payne CM. Leukocyte effects on the dimorphism of Coccidioides immitis. J Infect Dis. 1982;146(1):56–63.
- Munoz-Hernande B, Palma-Cortes G, Cabello-Gutierrez C, et al. Parasitic polymorphism of Coccidioides spp. BMC Infect Dis. 2014;14(213):1–9.
- Whiston E, Zhang Wise H, Sharpton TJ, et al. Comparative transcriptomics of the saprobic and parasitic growth phases in Coccidioides spp. PLoS One. 2012;7(7):e41034.
- Viriyakosol S, Singhania A, Fierer J, et al. Gene expression in human fungal pathogen Coccidioides immitis changes as arthroconidia differentiate into spherules and mature. BMC Microbiol. 2013;13(1):121.
- Schmaler-Ripcke J, Sugareva V, Gebhardt P, et al. Production of pyomelanin, a second type of melanin, via the tyrosine degradation pathway in Aspergillus fumigatus. Appl Environ Microbiol. 2009;75(2):493–503.
- Sil A, Andrianopoulos A. Thermally dimorphic human fungal pathogens-polypheletic pathogens with a convergent pathogenicity trait. Cold Springs Harb Perspect Med. 2014;58:e019794.
- Boyce KJ, McLauchlan A, Schreider L, et al. Intracellular growth is dependent on tyrosine catabolism in the dimorphic fungal pathogen Penicillium marneffei. PLoS Pathog. 2015;11(3):e1004790.
- Kirkland TN. A few shared up-regulated genes may influence conidia to yeast transformation in dimorphic fungal pathogens. Med Mycol. 2016;54(6):648–653.
- Mitchell NM, Sherrard AL, Dasari S, et al. Proteogenomic re-annotation of Coccidioides posadasii strain Silveira. Proteomics. 2018;18(1):1700173.
- Xue J, Chen X, Selby D, et al. A genetically engineered live attenuated vaccine of Coccidioides posadasii protects BALB/c mice against coccidioidomycosis. Infect Immun. 2009;77(8):3196–3208.
- Narra HP, Shubitz LF, Mandel MA, et al. A Coccidioides posadasii CPS1 deletion mutant is avirulent and protects mice from lethal injection. Infect Immun. 2016;84(10):3007–3016.
- Hung CY, Yu JJ, Seshan KR, et al. A parasitic phase specific adhesin of Coccidioides immitis contributes to the virulence of this respiratory fungi pathogens. Infect Immun. 2002;70(7):3443–3456.
- Wise HZ, Hung CY, Whiston E, et al. Extracellular ammonia at sites of pulmonary infection with Coccidioides posadasii contributes to severity of the respiratory disease. Microb Pathog. 2013;59-60:19–28.
- Smith CE, Saito MT, Simons SA. Patterns of 39,500 serologic tests in coccidioidomycosis. JAMA. 1956;160(7):546–552.
- Fish DG, Ampel NM, Galgiani JN, et al. Coccidioidomycosis during human immunodeficiency virus infection. A review of 77 patients. Medicine. 1990;69(6):384–391.
- Bergstrom L, Yocum DE, Ampel NM, et al. Increased risks of coccidioidomycosis in patients treated with tumor necrosis factor alpha antagonists. Arthritis Rheum. 2004;60:1959–1966.
- Odio CD, Marciano BE, Galgiani JN, et al. Risk factors for disseminated coccidioidomycosis, United States. Emerg Inf Dis. 2017;23(2):306–311.
- Vinh DC, Schwartz B, Hsu AP, et al. Interleukin-12 receptor β1 deficiency predisposing to disseminated Coccidioidomycosis. Clin Infect Dis. 2011;52(4):e99–102.
- Sampaio EP, Hus AP, Pechacek J, et al. Signal transducer and activator of transcription 1 (STAT1) gain-of-function mutations and disseminated coccidioidomycosis and histoplasmosis. J Allergy Clin Immunol. 2013;13(6):1624–1634.
- Woelk CH, Zhang JX, Viriyakosol S, et al. Factors regulated by interferon gamma and hypoxia-inducible factor 1A contribute to responses that protect mice from Coccidioides immitis infection. BMC Microbiol. 2012;12(1):218.
- Del Pilar Jiménez-A M, Viriyakosol S, Walls L, et al. Susceptibility to Coccidioides species in C57BL/6 mice is associated with expression of a truncated splice variant of Dectin-1 (Clec7a). Genes Immun. 2008;9(4):338–348.
- Fierer J, Walls L, Eckmann L, et al. Importance of interleukin-10 in genetic susceptibility of mice to Coccidioides immitis. Infect Immun. 1998;66(9):4397–4402.
- Viriyakosol S, MdP J, Gurney M, et al. Dectin-1 is required for resistance to coccidioidomycosis in mice. mBio. 2013;4(1):e00597–612.
- Willment JA, Marshall ASJ, Reid DM, et al. The human beta-glucan receptor is widely expressed and functionally equivalent to murine Dectin-1 on primary cells. Eur J Immunol. 2005;35(5):1539–1547.
- Willment JA, Brown GS. Characterization of the human beta -glucan receptor and its alternatively spliced isoforms. J Biol Chem. 2001;276(47):43818–43823.
- Hung CY, Gonzalez A, Wuthrich M, et al. Vaccine immunity to coccidioidomycosis occurs by early activation of three signal pathways of T helper cell response (Th1, Th2, and Th17). Infect Immun. 2011;79(11):4511–45222.
- Odio CD, Milligan KL, McGowan K, et al. Endemic mycoses in patients with STAT3-mutated hyper-IgE (Job) syndrome. J Allergy Clin Immunol. 2015;136(5):1411–1413.
- Chandresris MO, Melki I, Natividad A, et al. Autosomal dominant STAT3 deficiency and hyper-IgE syndrome: molecular, cellular, and clinical features from a French national survey. Medicine (Baltimore). 2015;91(4):e1–e10.
- Ampel NM, Dols CL, Galgiani JN. Coccidioidomycosis during human immunodeficiency virus infection: results of a prospective study in a coccidioidal endemic area. Am J Med. 1993;94(3):235–240.
- Margolis D, Viriyakosol S, Fierer J, et al. The role of reactive oxygen intermediates in experimental coccidioidomycosis in mice. BMC Microbiol. 2011;11:71.
- Gonzalez A, Hung CY, Cole GT. Nitric oxide synthase activity has limited influence on the control of Coccidioides infection in mice. Microb Pathog. 2011;51(3):161–168.
- Jimenez M, Walls L, Fierer J. High levels of interleukin-10 impair resistance to pulmonary coccidioidomycosis in mice in part through control of nitric oxide synthase 2 expression. Infect Immun. 2006;74(6):3387–3395.
- Kirkland TN. A quest for a vaccine against coccidioidomycosis: a neglected disease in America. J Fungi (Basel). 2016;2(4). doi: 10.3390/jof2040034
- Fierer J, Waters C, Walls L. CD4+ and CD8+ T cells can mediate vaccine-induced protection against Coccidioides immitis infections in mice. J Infect Dis. 2006;193(9):1323–1331.
- Magee DM, Friedberg RL, Woltaske MD, et al. Role of B cells in vaccine-induced immunity against coccidioidomycosis. Infect Immun. 2005;73(10):7011–7013.
- Abuodeh RO, Shubitz LF, Siegel E, et al. Resistance to Coccidioides immitis in mice after immunization with recombinant protein or a DNA vaccine of a proline-rich antigen. Infect Immun. 1999;67(6):2935–2940.
- Delgado N, Xue J, Yu JJ, et al. A recombinant Beta-1,3-glucanosyltransferase homologue of Coccidioides posadasii protects mice against coccidioidomycosis. Infect Immun. 2003;71(6):3010–3019.
- Tarcha EJ, Basrur V, Hung CY, et al. A recombinant aspartyl protease of Coccidioides posadasii induces protection against pulmonary coccidioidomycosis in mice. Infect Immun. 2006;74(1):516–527.
- Hartgen BJ, Hung CY, Ostroff GR, et al. Construction and evaluation of a novel recombinant T cell epitope-based vaccine against coccidioidomycosis. Infect Immun. 2012;81(11):3960–3974.
- Shubitz LF, Yu JJ, Hung CY, et al. Improved protection of mice against lethal respiratory infection with Coccidioides posadasii using two recombinant antigens expressed as a single protein. Vaccine. 2006;24(31–32):5904–5911.
- Wüthrich M, Brandhorst TT, Sullivan TD, et al. Calnexin induces expansion of antigen-specific CD4(+) T cells that confer immunity to fungal ascomycetes via conserved epitopes. Cell Host Microb. 2015;17(4):452–465.
