ABSTRACT
Cryptococcus-macrophage interaction is crucial in the development of cryptococcocal diseases. C. neoformans and C. gattii are major pathogenic species that occupy different niches and cause different clinical manifestations. However, the differences of macrophage interaction among these species in affecting different disease outcomes and immune responses have not been clearly addressed. Here, we examined the macrophage uptake rates, intracellular loads and intracellular proliferation rates of C. neoformans and C. gattii clinical isolates from Thailand and analyzed the effect of those interactions on fungal burdens and host immune responses. C. neoformans isolates showed a higher phagocytosis rate but lower intracellular proliferation rate than C. gattii. Indeed, the high intracellular proliferation rate of C. gattii isolates did not influence the fungal burdens in lungs and brains of infected mice, whereas infection with high-uptake C. neoformans isolates resulted in significantly higher brain burdens that associated with reduced survival rate. Interestingly, alveolar macrophages of mice infected with high-uptake C. neoformans isolates showed distinct patterns of alternatively activated macrophage (M2) gene expressions with higher Arg1, Fizz1, Il13 and lower Nos2, Ifng, Il6, Tnfa, Mcp1, csf2 and Ip10 transcripts. Corresponding to this finding, infection with high-uptake C. neoformans resulted in enhanced arginase enzyme activity, elevated IL-4 and IL-13 and lowered IL-17 in the bronchoalveolar lavage. Thus, our data suggest that the macrophage interaction with C. neoformans and C. gattii may affect different disease outcomes and the high phagocytosis rates of C. neoformans influence the induction of type-2 immune responses that support fungal dissemination and disease progression.
Abbreviation: Arg1: Arginase 1; BAL: Bronchoalveolar lavage; CCL17: Chemokine (C-C motif) ligand 17; CNS: Central nervous system; CSF: Cerebrospinal fluid; Csf2: Colony-stimulating factor 2; Fizz1: Found in inflammatory zone 1; HIV: Human immunodeficiency virus; ICL: Intracellular cryptococcal load; Ifng: Interferon gamma; Ip10: IFN-g-inducible protein 10; IPR: Intracellular proliferation rate; Mcp1: Monocyte chemoattractant protein 1; Nos2: Nitric oxide synthase 2; PBS: Phosphate-Buffered Saline; Th: T helper cell; Tnfa: Tumor necrosis factor alpha.
Introduction
Cryptococcal disease is caused by fungal infection with two major pathogenic species, C. neoformans and C. gattii. Infection by these fungi is acquired by inhaling desiccated yeasts or basidiospores that result in pulmonary or disseminated cryptococcosis. Diseases caused by C. neoformans infection are often associated with immunocompromised hosts, while C. gattii infection has been reported to cause disease in immunocompetent individuals. In patients with immunosuppressed conditions, particularly HIV-positive patients, cryptococcal meningitis occurring by C. neoformans infection is one of the most common life-threatening diseases that have been documented. In contrast, patients infected with C. gattii often suffer from respiratory illness in addition to having meningoencephalitis [Citation1,Citation2]. The differences in primary target organs and patterns of lung and brain pathology resulting from the infection of these two species have been revealed [Citation3,Citation4]. Indeed, the divergence in the host immune responses to these species may determine disease outcomes and be responsible for the differences in disease pathogenesis of C. neoformans and C. gatii infections.
Studies in humans and murine cryptococcosis models have emphasized the importance of T helper cell-mediated immunity in controlling cryptococcal infection [Citation5–Citation7]. Infection with C. gattii was found to modulate host immune response by subverting the induction of protective Th1 and Th17 cells [Citation8]. During a chronic infection, C. neoformans elicited Th2 type-2 immune responses, including the induction of IL-4, IL-5 and IL-13 that provided a permissive environment for fungal proliferation and dissemination [Citation9,Citation10]. In addition to adaptive immunity, the collaborative function of innate immune response is required to stimulate fungal clearance. It has been suggested that alveolar macrophage and phagocytosis may play crucial roles in early control or exacerbation of cryptococcal infection [Citation11–Citation13]. Indeed, macrophages may alter their phenotypes to classical (M1) or alternatively activated (M2) macrophages in exposure to type-1 or type-2 cytokine environments, respectively. While M1 macrophages play a role in the early containment of C. neoformans, M2 macrophages promote fungal persistence [Citation14]. Recently, C. neoformans with high phagocytosis rate by macrophages was reported to be correlated with CNS fungal burden and poor clinical outcomes [Citation15]. Moreover, C. gattii strains from the Vancouver Island outbreak were found to exhibit the increased intracellular proliferative capability in macrophages that contribute to the virulence in murine cryptococcosis model [Citation16].
Although many studies have emphasized the roles of fungal uptake and proliferation in macrophages as critical virulence factors in cryptococcal disease, their involvement in the virulence of C. neoformans and C. gattii and the effect of this interaction on the induction of immune responses remain unclear. In this study, we showed that the phagocytosis rate of C. neoformans was higher than that of C. gattii, while intracellular proliferation rate of C. gattii was greater than that of C. neoformans. High fungal uptake of C. neoformans but not the intracellular proliferation rate of C. gattii was associated with the fungal loads in a mouse model of cryptococcosis. Interestingly, mice infected with high-uptake C. neoformans strains displayed enhanced M2 macrophages and type-2 immune responses. Our studies suggest that the initial phagocytosis of C. neoformans but not C. gattii by macrophages may determine poor clinical outcomes by modulating M2 macrophages and eliciting the unfavorable type-2 immune response.
Materials and methods
Mice
BALB/c mice, female, 6 to 8 weeks old, were obtained from Nomura Siam International Co., Ltd, Thailand. Mice were housed under specific pathogen-free conditions in the animal facility of Thammasat University. At the end of the experiment, mice were euthanized by controlled gradual displacement with CO2 using a flow meter in accordance with IACUC and the American Veterinary Medicine Association (AMVA) guidelines on euthanasia. All animal studies were approved by the Thammasat University Animal Care and Use Committee (008/2557).
Cryptococcus strain
All clinical isolates of Cryptococcus spp. (23 isolates of C. neoformans and 18 isolates of C. gattii) were obtained from the Department of Microbiology, Faculty of Medicine, Siriraj Hospital, Mahidol University. All the isolates were identified with the RapID™ Yeast Plus System (Thermo Fisher Scientific, MA, USA). Urease and melanin production test were also used to classify C. neoformans/C. gattii species. L-Canavanine–glycine–bromothymol blue (CGB) agar was used to differentiate C. neoformans from C. gattii [Citation17]. The reference strains of C. neoformans (H99) and C. gattii (R265) were used as control strains in all experiments. Cryptococcus strains were stored in 20% glycerol at −80°C until use. Cryptococcus was thawed and maintained in Sabouraud dextrose agar (SDA) (Thermo Fisher Scientific) at room temperature. A single colony of yeast cells was resuspended in Sabouraud dextrose broth (SDB) and cultured at 37°C then shaking at 200 rpm for 24 hours before in vitro and in vivo infection [Citation8,Citation15].
Macrophage culture
The murine macrophage cell line, J774 (ATCC® TIB-67TM) was cultured as previously described [Citation15]. Briefly, macrophages were cultured in complete Dulbecco’s Modified Eagle Medium (cDMEM), DMEM medium supplemented with 10% heat-inactivated fetal bovine serum (FBS), 1% L-glutamine and 1% penicillin and streptomycin (Invitrogen) in a 5% CO2 humidified atmosphere at 37°C. Before infection, 1.5 × 105 cells of J774 macrophages were cultured in 24-well culture plate (Corning) containing cDMEM for 24 hours at 37°C with 5% CO2.
In vitro phagocytosis and intracellular proliferation assay
The rate of phagocytosis (uptake) and intracellular proliferation (IPR) by macrophages of Cryptococcus spp. were determined as previously described [Citation15]. Briefly, J774 macrophages were cultured in serum-free medium for 2 hours followed by activating with 1 µg/ml phorbol myristate acetate (PMA) (Sigma-Aldrich) for 30 minutes at 37°C with 5% CO2 before infection. Cryptococci washed with 1x Dulbecco’s Phosphate-Buffered Saline (DPBS) (Invitrogen) were then opsonized with 1 µg/ml of anti-capsule mAb 18B7 (kindly provided by Arturo Casadevall, Albert Einstein College of Medicine, New York, USA). For in vitro infection, macrophages were exposed to opsonized cryptococci at 1:10 ratio and incubated at 37°C with 5% CO2. Cryptococcal uptake was determined by the number of intracellular cryptococci within the macrophage 2 hours after infection. After 2-hour infection, extracellular cryptococci were removed, macrophage cultures were lysed with sterile distilled water and intracellular cryptococci were counted using a hemocytometer and confirmed by plating on Sabouraud dextrose agar (SDA) (Thermo Fisher Scientific) for the analysis of CFU. To determine the intracellular cryptococci (ICL; intracellular cryptococcal load), the remaining wells were maintained and lysed at 18 and 24 hours as described above. The IPR of Cryptococcus at 18 and 24 hours postinfection was calculated from the number of intracellular cryptococci at 18 or 24 hours postinfection divided by the number of intracellular cryptococci at 2 hours postinfection.
Murine model of Cryptococcus infection
For in vivo infection, the 24-hour cryptococcal cultures were washed by PBS, counted using a hemocytometer, and resuspended in PBS at a concentration of 1 × 106 yeast cells/ml as previously described [Citation8]. After anesthetization with isoflurane, mice were treated with PBS or infected with Cryptococcus by intranasal inoculation of 50 µl of the yeast cell suspension (5.0 x 104 yeast cells/mouse). For survival analysis, BALB/c mice were infected with the high-uptake and the low-uptake C. neoformans strains as described above (8 mice/strain) and monitored for their survival by inspection twice daily for 60 d and euthanized if they appeared to be in pain or moribund [Citation8].
Organ isolation and CFU assay
Following euthanasia, the lungs and brains of Cryptococcus-infected mice were excised, weighed and homogenized in sterile PBS with 1% penicillin and streptomycin using a tissue homogenizer and plated at various dilutions on SDA (Thermo Fisher Scientific). CFU was calculated following incubation at 30°C for 48 hours.
Bronchoalveolar lavage collection
At the indicated time points, an incision was made below the jaw to expose the trachea. A 22-gauge catheter was inserted in the airway and secured in place by a string. A total of 0.5 ml ice-cold PBS containing with 5 mM EDTA was instilled through the catheter and the lung was subsequently washed 5 times. The bronchoalveolar lavage (BAL) fluid was collected after centrifugation at 3,000 rpm for 5 min, and the supernatants (BAL fluids) were stored at −80°C for cytokine analysis and arginase activity.
Alveolar macrophage isolation
Alveolar macrophages were recovered from bronchoalveolar lavage cells. Cells were washed and cultured in serum-free medium at 37°C, 5% CO2 for 2 hours in a 24-well plate. After incubating for 2 hours, nonadherent cells were removed and alveolar macrophages (adherent cells) were washed and harvested with a rubber scraper for further RNA extraction. Cell viability was determined using trypan blue exclusion and purity was analyzed with CD11b staining by FACS analysis [Citation18].
Quantitative RT-PCR
Alveolar macrophage RNA was extracted using TRIzol® reagent according to the manufacturer’s instructions. RNA was served as a template for cDNA synthesis using oligo-dT, random hexamers and MMLV reverse transcriptase (Invitrogen). To quantify macrophage polarization markers, cDNA samples were amplified using iTaqTM universal SYRB® Green Supermix (Biorad Laboratories). Expression levels of target genes were normalized to endogenous actin (Actb) transcript levels, and relative quantification of samples was compared with PBS control serving as the baseline.
Antigen-specific cytokine production
Lung draining lymph nodes harvested from PBS-treated and Cryptococcus-infected mice were minced in RPMI medium supplemented with 10% heat-inactivated fetal bovine serum (FBS), 1% L-glutamine, and 1% penicillin and streptomycin through a 100 μm sterile nylon mesh to prepare cell suspension solution. Single cell suspensions were plated in a 24-well plate and stimulated with live or heat-killed Cryptococcus cells (at a ratio of 2:1 Cryptococcus cells to leukocytes). Following 72 hour stimulation at 37°C with 5% CO2, the culture supernatant was collected and kept at −80°C for cytokine analysis [Citation8].
Cytokines measurement
BAL fluids and lung draining lymph node culture supernatants were used to determine the concentration of cytokines. IL-4, IFN-γ and IL-17 were analyzed using the antibody pairs from BD Pharmingen and IL-13 was assessed using ELISA kits obtained from R&D Systems. All cytokine assays were performed according to the manufacturer instructions.
Arginase activity assay
The activity of arginase enzyme was determined as previous described [Citation19]. Briefly, 100 µg/ml BAL fluids were added to the activation solution (50 mmol Tris-HCl and 10 mmol MnCl2, pH 7.5). After incubating at 56°C for 10 minutes, 0.5 M L-arginine (pH 9.7) was added to the activated mixture, incubated at 37°C for 2 hours, followed by adding acid stop solution (H2SO4:H3PO4:H2O, 1:3:7). For the colorimetric determination of urea, 9% α-isonitrosopropiophenone (ISPF) (Sigma-Aldrich) in ethanol was added, and the mixture was heated at 100°C for 45 min. Urea concentration was determined spectrophotometrically by measuring absorbance at 490 nm. The activity of arginase enzyme was determined as a concentration of produced urea (µg/ml).
Statistical analysis
Each experiment was performed two to three times. Data are presented as mean value ± SD. Data were analyzed using unpaired t test (2-tailed) as well as the one-way ANOVA with Turkey’s post hoc analysis. Correlation study was analyzed using Spearman’s rank correlation coefficient. Survival curves were evaluated for statistical significance with Kaplan-Meier survival curves, and P values were obtained from a log-rank test. Statistical analysis was performed with GraphPad Prism 5 Software. A value of p <0.05 was considered significant.
Results
Differences in phagocytosis rate and intracellular proliferation rate of C. neoformans and C. gattii by macrophages
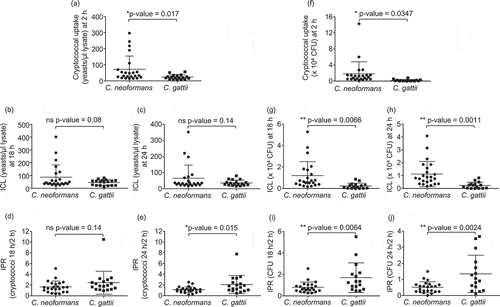
Differences between the interaction of macrophages with C. neoformans and C. gattii have not been clearly investigated. Therefore, to determine the effect of C. neoformans and C. gattii infection on host macrophages, we first compared the phagocytosis rate of 23 isolates of C. neoformans and 18 isolates of C. gattii using the established murine macrophage–like cell line J774 [Citation16]. By counting cells using a hemocytometer or enumerating the colony forming unit (CFU), the phagocytic uptake of C. neoformans was significantly greater than those of C. gattii (, P = 0.017 and , P = 0.0347, respectively). We next evaluated whether C. neoformans and C. gattii may differ in the intracellular fungal load and intracellular proliferation rate because they have been known to be associated with the virulence of C. neoformans and C. gattii, respectively. Although we observed no difference between the intracellular load of C. neoformans and C. gattii by counting the number of intracellular cryptococci at 18 or 24 hours (), the intracellular load determined as CFU of C. neoformans was higher than those of C. gattii (, P = 0.0066 and = 0.0011). However, we found that the intracellular proliferation rate at 18 and 24 hours was higher in macrophages infected with C. gattii than those infected with C. neoformans (, P = 0.14, , P = 0.015, , P = 0.0064 and , P = 0.0024). These data suggest that although C. neoformans is more readily engulfed by macrophages than C. gattii, C. neoformans is able to proliferate in macrophages less than C. gattii.
Figure 1. Phagocytic uptake, intracellular load and proliferation rate of C. neoformans and C. gatii clinical isolates by macrophages.
The J774 macrophage was infected by clinical isolates of C. neoformans and C gattii at a 1:10 ratio followed by lysis at 2, 18 and 24 h postinfection to determine cryptococcal uptake at 2 h, intracellular loads (ICL) at 18 h and 24 h and intracellular proliferation rate (IPR) at 18 h and 24 h by (a – e) counting with hemocytometer and (f-j) enumerating using CFU assay. Each dot represents one clinical isolate. Results were expressed as the mean of 3 to 6 experimental repeats. Error bars denote mean ± SD. Significance was determined using unpaired t test (2-tailed) * p <0.05, ** p <0.01, ns = not significantly different.
The high uptake rate of C. neoformans but not high intracellular proliferation rate of C. gattii correlated with the cryptococcal virulence in a murine model of cryptococcosis
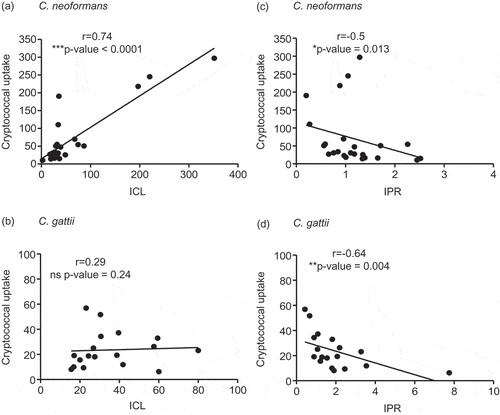
The high uptake rate of C. neoformans by macrophages was shown to be positively correlated to the intracellular fungal load and inversely associated with the intracellular proliferation rate [Citation15]. We further determined whether these relationships could be applied in clinical isolates of both C neoformans and C. gattii. Interestingly, we found a profound positive correlation between the fungal uptake and intracellular fungal load at 24 hours of C. neoformans (r =0.74, P <0.0001) (), but not that of C. gattii (r =0.29, P = 0.24) (). The relationship between the fungal uptake and intracellular proliferation rate was found to be inversely correlated for both C. neoformans (r =−0.5, P = 0.013) () and C. gattii (r =−0.64, P = 0.004) ().
Figure 2. Association of the cryptococcal uptake of C. neoformans and C. gattii clinical isolate with intracellular load and proliferation within macrophages.
C. neoformans and C. gattii clinical isolates were independently exposed to J774 macrophages and followed by determining the uptake rate, intracellular loads (ICL) and intracellular proliferation rate (IPR) at 24 h postinfection. The correlation between uptake rate and ICL of C. neoformans (a) or of C. gattii (b) clinical isolates was evaluated. The uptake rate of C. neoformans (c) or of C. gattii (d) was also assessed for the correlation to IPR. Spearman’s rank test was used to determine the correlation between the uptake rate, ICL and IPR. Correlation coefficients (r) and p-values were given for each correlation. * p <0.05, ** p <0.01, *** p <0.001 and ns = not significantly correlated.
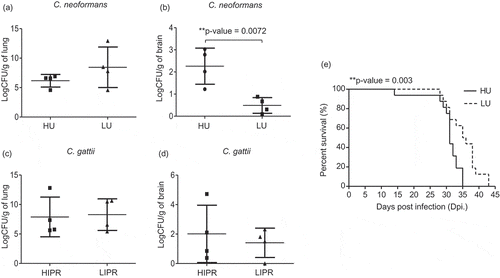
Previously, the fungal uptake of C. neoformans was found to be correlated with the clinical severity of cryptococcal disease in humans [Citation15]. Because we found that C. neoformans had greater ability than C. gattii to be engulfed by macrophages and the fungal uptake rate was strongly associated with the intracellular fungal load in macrophages, we next tested whether the fungal uptake rate of C. neoformans could predict the level of the organ fungal burdens. We selected 4 strains of C. neoformans with a high phagocytosis rate (approximately 200 cryptococci/µl lysate) and 4 strains of those with a low phagocytosis rate (<50 cryptococci/µl lysate) to evaluate the lung and brain fungal burdens in a murine cryptococcosis model (Supplementary table 1). Although mice infected with high and low fungal uptake isolates of C. neoformans exhibited comparable lung fungal burdens (), mice infected with high-uptake C. neoformans showed significantly higher brain fungal burdens than those infected with low-uptake strains (). Because the intracellular proliferation rate of C. gattii was higher than that of C. neoformans, we speculated that the high IPR might correlate to lung fungal burdens. Indeed, we did not detect any differences in the lung () or brain () fungal burdens of mice infected C. gattii isolates with high IPR (approximately 3) and low IPR (<1) (Supplementary table 1). Because high-uptake C. neoformans showed higher tendency to dissemination to the brain, we further investigated mouse survival following infection with those high and low fungal uptake isolates of C. neoformans. Consistent with our fungal burdens findings, we found that mice infected with high-uptake C. neoformans had significantly reduced survival compared with those infected with low-uptake strains (, P = 0.003). Altogether, these data emphasize the roles of high fungal uptake in macrophages associated with the ability of C. neoformans to disseminate to the brain in promoting disease progression.
Figure 3. Mice infected with high-uptake C. neoformans isolates exhibited higher brain fungal burdens and had reduced survival rate.
C. neoformans isolates were selected to represent the top 4 high-uptake and bottom 4 low-uptake strains and C. gattii isolates were selected to represent the top 4 high IPR and bottom 4 low IPR strains based on J774 macrophage uptake and IPR profiles. (a-d) BALB/c mice were intranasal infected with high- (HU) and low- (LU) uptake C. neoformans isolates or high (HIPR) and low IPR (LIPR) C. gattii isolates. Lung and brain fungal burdens of mice infected with C. neoformans (a, b) and C. gattii (c, d) were enumerated at 14 d postinfection. Graphs depict mean ± SD and are representative of 3 experiments with 3 to 5 mice per group. Significance was determined using unpaired t test (2-tailed) ** p <0.01, ns = not significantly different. (e) For survival analysis, BALB/c mice were infected with the high- (HU, solid line) or low- (LU, dot line) uptake strain of C. neoformans and were monitored for their survival daily for 60 d. Survival curves were generated from the results obtained with 8 mice per strain and evaluated for statistical significance with Kaplan-Meier survival curves, and P values were obtained from a log-rank test, ** p <0.01.
Infection with high-uptake C. neoformans potentiated the induction of alternatively activated macrophages
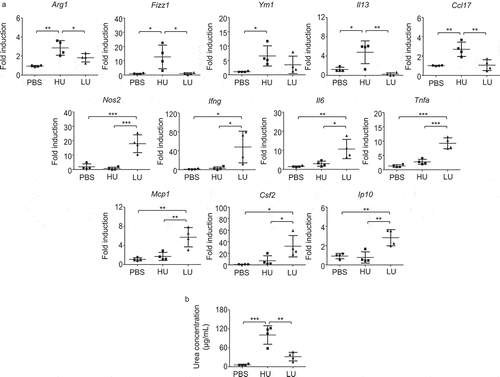
The polarization of macrophages to classical (M1) and alternatively (M2) activated macrophages plays a role in controlling and exacerbating cryptococcal diseases, respectively [Citation20,Citation21]. Although M2 macrophages have been shown to possess high phagocytosis capacity, they promote fungal persistence rather than contribute to fungal clearance [Citation22]. The high uptake rate of C. neoformans coupled with the enhanced intracellular loads in macrophages and fungal dissemination to the brain might be associated with the induction of M2 macrophages. To test this possibility, we examined the phenotypes of alveolar macrophages isolated from mice infected with high- and low-uptake C. neoformans as described in the above experiment. Mice were infected with high- and low-uptake C. neoformans and alveolar macrophages were isolated from the bronchoalveolar lavage following 14 d postinfection. Compared with alveolar macrophages of PBS-treated mice and low-uptake C. neoformans-infected mice, those of high-uptake C. neoformans-infected mice showed significantly increased expression of genes associated with M2 macrophage, including Arg1, Fizz1, Il13 and Ccl17 (). Interestingly, we observed only the low-but not high-uptake C. neoformans induced significantly higher levels of genes related to M1 markers and inflammatory cytokines and chemokines, including Nos2, Ifng, Il6, Tnfa, Mcp1, Csf2 and Ip10 (). Because arginase 1 is the prototypic marker for M2 macrophages and alveolar macrophages of high-uptake C. neoformans infected mice showed significantly higher Arg1 mRNA expression than those infected with low-uptake strains, we further compared the arginase enzyme activity between high-and low-uptake strains. The arginase enzyme activity of high-uptake strains in BAL fluids was significantly higher than those of low-uptake strains (). These data suggested that infection with low-and high-uptake C. neoformans results in altered macrophage phenotypes. While low-uptake C. neoformans macrophages may drive more inflammatory macrophages, high-uptake C. neoformans potentiates the induction of M2-like macrophages.
Figure 4. Infection with high-uptake C. neoformans strains induced alveolar macrophages with higher expression of genes related to M2 macrophage.
BALB/c mice received PBS or were infected with the top 4 high-and the top 4 low-uptake C. neoformans isolates. (a) Alveolar macrophages were isolated from the bronchoalveolar lavage of each mouse at 14 d postinfection and total RNA was isolated and subjected to cDNA synthesis, followed by real-time PCR analysis of gene for M1 and M2 including Arg1, Fizz1, Ym1, Il13, Ccl17, Nos2, Ifng, Il6, Tnfa, Mcp1, Csf2 and Ip10. Data were expressed as fold induction over actin (Actb) expression, with the mRNA levels in the PBS control set as 1. (b) Bronchoalveolar lavages of PBS-treated mice and high-uptake and low-uptake C. neoformans-infected mice were collected and the arginase enzyme activity was determined by photometric measurement of produced urea concentration. Graphs depict mean ± SD and are representative of 3 experiments with 3 to 5 mice per group. Significance was determined by one-way ANOVA with Tukey’s post hoc analysis * p <0.05, ** p <0.01, ***p <0.001.
C. neoformans strains with high uptake rate promotes type 2 immune responses
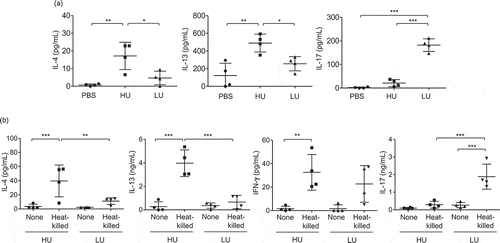
While type-1 immune responses have been shown to drive protective immunity against cryptococcal infection, type 2-skewed immune responses defined by the production of IL-4, IL-5, and IL-13 play deleterious roles in infection with C. neoformans [Citation23]. Moreover, type-2 cytokine environment has been known to promote the induction of alternative activation of macrophages [Citation24].We further investigated whether the high-uptake C. neoformans strain that induced greater disease severity might be involved in inducing type-2 biased immune responses. Mice were infected with high-and low-uptake C. neoformans strains as described above and the bronchoalveolar lavage (BAL) cytokines after 14 d post infection were evaluated. We did not detect IFN-γ secretion in the BAL fluids of mice infected with these clinical isolates; however, significantly increased levels of Th2 cytokine IL-4 and IL-13 and Th17 cytokine IL-17 were revealed (). Interestingly, the production of IL-4 and IL-13 in mice infected with high-uptake isolates were potently elevated more than those infected with low-uptake isolates (). In contrast, infection with low-uptake isolates induced a significantly greater extent of IL-17 cytokine than those infected with high-uptake isolates (P < 0.001) (). To further investigate whether the high-and low-phagocytosis rate of C. neoformans by macrophages may influence T helper cell differentiation, we next examined the antigen-specific cytokine responses by draining lymph node cells of mice infected with high-and low-uptake isolates co-pulsed with heat-killed Cryptococcus cells (). Although we did not detect the significant difference of antigen-specific IFN-γ production in the draining lymph node cells of mice infected high and low-uptake infection, the high-uptake-infected groups showed a significantly higher cryptococcal antigen-specific IL-4 and IL-13 production and lower IL-17 production than low uptake-infected groups (). We also found the consistent data of antigen-specific cytokine production in the draining lymph node cells stimulated with live Cryptococcus cells (Supplementary Figure 1). Altogether, these data indicate that C. neoformans with high uptake rate potentiate the induction of type-2 immune responses and the activation of alternative macrophage. These changes in host immune responses driven by high uptake rate C. neoformans may promote the enhanced intracellular load in macrophages that contributes to the CNS fungal dissemination and disease progression.
Figure 5. High-uptake C. neoformans potentiated the induction of type 2 immune responses and reduced type-17 immune responses in the lungs.
BALB/c mice received PBS or were infected with the top 4 high-and the top 4 low-uptake C. neoformans isolates. (a) The bronchoalveolar lavages were collected and assessed for cytokine production at 14 d postinfection. (b) Lung draining lymph nodes were harvested and cell suspensions were prepared to analyze antigen-specific cytokine production. After lymph node cells were stimulated with heat-killed Cryptococcus cells for 72 h, supernatant was collected and subjected to analyze cytokines by ELISA. Graphs depict mean ± SD and are representative of 3 experiments with 3 to 5 mice per group. Significance was determined by one-way ANOVA with Tukey’s post hoc analysis * p <0.05, ** p <0.01 and *** p <0.001.
Discussion
The contribution of macrophage uptake and intracellular proliferation rate in the pathogenesis of Cryptococcus infection indicate the influence of early host-pathogen interaction in disease development. Although C. neoformans and C. gattii infection were shown to have pathological differences, the effect of macrophage interaction with these two pathogenic species has not been clearly investigated. Moreover, the influence of macrophage uptake in modulating phenotypes of macrophages and immune responses has not yet been studied. In the present study, we examined the fungal uptake and intracellular proliferation rate of C. neoformans and C. gattii isolates. Infection with C. neoformans showed a higher fungal uptake but lower intracellular proliferation rate than infection with C. gattii. C. gattii strains exhibiting high intracellular proliferation rates caused infection with comparable organ burdens, while C. neoformans strains exhibiting high-uptake resulted in infection with higher brain fungal burden. Moreover, infection with the high-uptake strains of C. neoformans induced distinct alveolar macrophages expressing higher M2 marker and potentiated type-2 immune response. Thus, our findings revealed different interactions between macrophages and C. neoformans and C. gattii and how higher uptake strains of C. neoformans might affect host immune responses that promote the fungal dissemination and disease progression.
While high-uptake C. neoformans was known to be associated with patient CSF fungal burdens and the hypervirulent C. gattii strains that exhibited enhanced intracellular proliferation rate was correlated with mouse survival [Citation15,Citation16], the differences of macrophage uptake and proliferation between infections with these two species are less clear. Indeed, we detected that C. neoformans strains had higher macrophage uptake rates than C. gattii strains. In contrast, the intracellular proliferation rate describing as the ratio of intracellular cryptococci at 24 hours and the number of intracellular cryptococci at 2 hours postinfection was higher in macrophages infected with C. gattii than C. neoformans. C. gattii exhibiting lower uptake ability than C. neoformans is likely because of having a higher capsule size. One related study showed that C. gattii capsules were larger than those of C. neoformans [Citation25]. Moreover, macrophages were shown to preferentially phagocytose C. neoformans strains that had smaller capsules [Citation26]. In contrast to C. gattii, our data showed a strong association between the uptake and intracellular loads of C. neoformans. Mice infected with high-uptake C. neoformans strains showed higher brain fungal burdens that associated with the reduced survival compared with those infected with low-uptake strains. Consistent with a related study [Citation15], the high-uptake C. neoformans isolates may be more capable of retaining and surviving inside macrophages. Unlike C. neoformans, we did not detect the differences in lung and brain fungal burdens in C. gattii with high-and low-intracellular proliferation rates. Previous study suggested that the intracellular proliferation rate of C. gattii can predict the survival rate of mice infected with C. gattii. Quite possibly, organ fungal burdens may not reflect the survival rate of C. gattii infection. Recent study using bone marrow-derived macrophage have suggested that the efficacy of phagocytosis of reference strains of C. neoformans (H99) and C. gattii (R265, WM161, WM179) may be qualitatively similar [Citation27]. Larger studies including strains from other geographical regions with different types of macrophage should be carried out to support our findings. Moreover, further studies on detailed characterization of other virulent factors between these two species and the their associations with macrophage uptake and intracellular proliferation rates may provide further understanding on the underlying mechanisms involved in the role of macrophage for the pathogenesis of C. neoformans and C. gattii infection.
The interaction with macrophages is a critical step in the development of cryptococcal pathogenesis. Indeed, macrophages can polarize to classical (M1) or alternatively (M2) activated macrophages, depending on the cytokine environment. Related studies have indicated that M1 macrophage stimulated by IFN-γ is involved in the fungal clearance; however, M2 macrophages polarized by IL-4 promoted fungal proliferation [Citation12,Citation20]. C. neoformans, with its high uptake rate may modulate macrophages to the phenotypes that support the fungal retention within cells. Indeed, the alveolar macrophages isolated from mice infected with high-uptake strains exhibited the phenotype of M2-like macrophages by revealing the distinct Arg1, Fizz1, Ym1 and Il13 gene expressions when compared with those infected with low-uptake strains. The expressions of Arg1, Fizz1 and IL-13 are usually related to the alternative activation of macrophages. Fizz1 was known to be highly induced in M2 macrophages by IL-4 and IL-13 [Citation28]. IL-13 was known to be expressed by not only Th2 cells but also human M2 macrophages among patients with pulmonary fibrosis as well as in mouse alveolar macrophages exposed to instilled particles [Citation29,Citation30]. Moreover, we observed a higher arginase enzyme activity in bronchoalveolar lavages of mice infected with high-uptake strains. Altogether, these data indicate that infection with high-uptake C. neoformans may promote the activation of alternatively activated macrophages that could enhance the retention of cryptococci within macrophage cells.
The type-2 immune response is known to induce the activation of M2 macrophages. Compared with infections with low-uptake strains that secreted higher IL-17, infection with high-uptake C. neoformans strains induced higher secretions of bronchoalveolar lavage IL-4 and IL-13, the major cytokine profiles of type-2 immune responses. While the induction of type-1 and type-17 cytokine has been shown to promote the protective immunity against C. neoformans, cytokines related to type-2 immunity have been known to promote the persistence and progression of C. neoformans infection [Citation8,Citation31–Citation34].
In conclusion, we provided the evidence that cryptococcal-phagocyte interactions that play important roles in mediating disease pathogenesis may modulate types of macrophages and immune responses. C. neoformans and C. gattii exhibited different capacities in macrophage phagocytosis, fungal retention and proliferation. The high-uptake strains of C. neoformans promoted the induction of M2-like macrophages, enhanced arginase enzyme activity and elevated levels of type-2 cytokines that may support the progression of cryptococcal diseases and brain dissemination. Future studies on more clinical isolates of C. neoformans and C. gattii interaction with macrophages will be instrumental for the understanding of the complex interactions between cryptococci and host macrophages that contribute to cryptococcal diseases.
Supplemental Material
Download MS Word (72.4 KB)Acknowledgments
We thank the Faculty of Allied Health Sciences, Center of Scientific Equipment for Advanced Research, Thammasat University for their support. This work was supported by the fiscal year budget of the office of the National Research Council of Thailand to Thammasat University. A.H. was supported by the Royal Golden Jubilee Ph.D. (RGJ-PHD) Program Scholarship (Grant No. PHD/0016/2558).
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Huston SM, Mody CH. Cryptococcosis: an emerging respiratory mycosis. Clin Chest Med. 2009;30:253–64, vi.
- Smith RM, Mba-Jonas A, Tourdjman M, et al. Treatment and outcomes among patients with Cryptococcus gattii infections in the United States Pacific Northwest. PLoS One. 2014;9:e88875.
- Ngamskulrungroj P, Chang Y, Sionov E, et al. The primary target organ of cryptococcus gattii is different from that of Cryptococcus neoformans in a murine model. MBio. 2012;3.
- Speed B, Dunt D. Clinical and host differences between infections with the two varieties of Cryptococcus neoformans. Clin Infect Dis. 1995;21:28–34. discussion 5–6.
- Buchanan KL, Doyle HA. Requirement for CD4(+) T lymphocytes in host resistance against Cryptococcus neoformans in the central nervous system of immunized mice. Infect Immun. 2000;68:456–462.
- Uicker WC, McCracken JP, Buchanan KL. Role of CD4+ T cells in a protective immune response against Cryptococcus neoformans in the central nervous system. Med Mycol. 2006;44:1–11.
- Huffnagle GB, Yates JL, Lipscomb MF. Immunity to a pulmonary Cryptococcus neoformans infection requires both CD4+ and CD8+ T cells. J Exp Med. 1991;173:793–800.
- Angkasekwinai P, Sringkarin N, Supasorn O, et al. Cryptococcus gattii infection dampens Th1 and Th17 responses by attenuating dendritic cell function and pulmonary chemokine expression in the immunocompetent hosts. Infect Immun. 2014;82:3880–3890.
- Milam JE, Herring-Palmer AC, Pandrangi R, et al. Modulation of the pulmonary type 2 T-cell response to Cryptococcus neoformans by intratracheal delivery of a tumor necrosis factor alpha-expressing adenoviral vector. Infect Immun. 2007;75:4951–4958.
- Muller U, Piehler D, Stenzel W, et al. Lack of IL-4 receptor expression on T helper cells reduces T helper 2 cell polyfunctionality and confers resistance in allergic bronchopulmonary mycosis. Mucosal Immunol. 2012;5:299–310.
- Kechichian TB, Shea J, Del Poeta M. Depletion of alveolar macrophages decreases the dissemination of a glucosylceramide-deficient mutant of Cryptococcus neoformans in immunodeficient mice. Infect Immun. 2007;75:4792–4798.
- Leopold Wager CM, Hole CR, Wozniak KL, et al. Cryptococcus and phagocytes: complex interactions that influence disease outcome. Front Microbiol. 2016;7:105.
- Charlier C, Nielsen K, Daou S, et al. Evidence of a role for monocytes in dissemination and brain invasion by Cryptococcus neoformans. Infect Immun. 2009;77:120–127.
- Arora S, Olszewski MA, Tsang TM, et al. Effect of cytokine interplay on macrophage polarization during chronic pulmonary infection with Cryptococcus neoformans. Infect Immun. 2011;79:1915–1926.
- Sabiiti W, Robertson E, Beale MA, et al. Efficient phagocytosis and laccase activity affect the outcome of HIV-associated cryptococcosis. J Clin Invest. 2014;124:2000–2008.
- Ma H, Hagen F, Stekel DJ, et al. The fatal fungal outbreak on Vancouver Island is characterized by enhanced intracellular parasitism driven by mitochondrial regulation. Proc Natl Acad Sci U S A. 2009;106:12980–12985.
- Hatthakaroon C, Pharkjaksu S, Chongtrakool P, et al. Molecular epidemiology of cryptococcal genotype VNIc/ST5 in Siriraj Hospital, Thailand. PLoS One. 2017;12:e0173744.
- Supasorn O, Sringkarin N, Srimanote P, et al. Matrix metalloproteinases contribute to the regulation of chemokine expression and pulmonary inflammation in Cryptococcus infection. Clin Exp Immunol. 2016;183:431–440.
- Cho JS, Oh YJ, Kim OS, et al. The effects of arginase inhibitor on lung oxidative stress and inflammation caused by pneumoperitoneum in rats. BMC Anesthesiol. 2015;15:129.
- Voelz K, Lammas DA, May RC. Cytokine signaling regulates the outcome of intracellular macrophage parasitism by Cryptococcus neoformans. Infect Immun. 2009;77:3450–3457.
- Davis MJ, Tsang TM, Qiu Y, et al. Macrophage M1/M2 polarization dynamically adapts to changes in cytokine microenvironments in Cryptococcus neoformans infection. MBio. 2013;4:e00264–13.
- Leopold Wager CM, Wormley FL Jr. Classical versus alternative macrophage activation: the Ying and the Yang in host defense against pulmonary fungal infections. Mucosal Immunol. 2014;7:1023–1035.
- Price MS, Perfect JR. Host defenses against cryptococcosis. Immunol Invest. 2011;40:786–808.
- Stenzel W, Muller U, Kohler G, et al. IL-4/IL-13-dependent alternative activation of macrophages but not microglial cells is associated with uncontrolled cerebral cryptococcosis. Am J Pathol. 2009;174:486–496.
- Thompson GR 3rd, Albert N, Hodge G, et al. Phenotypic differences of Cryptococcus molecular types and their implications for virulence in a Drosophila model of infection. Infect Immun. 2014;82:3058–3065.
- Bojarczuk A, Miller KA, Hotham R, et al. Cryptococcus neoformans intracellular proliferation and capsule size determines early macrophage control of infection. Sci Rep. 2016;6:21489.
- Freij JB, Fu MS, De Leon Rodriguez CM, et al. Conservation of intracellular pathogenic strategy among distantly related cryptococcal species. Infect Immun. 2018;86.
- Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32:593–604.
- Hancock A, Armstrong L, Gama R, et al. Production of interleukin 13 by alveolar macrophages from normal and fibrotic lung. Am J Respir Cell Mol Biol. 1998;18:60–65.
- Kang CM, Jang AS, Ahn MH, et al. Interleukin-25 and interleukin-13 production by alveolar macrophages in response to particles. Am J Respir Cell Mol Biol. 2005;33:290–296.
- Blackstock R, Murphy JW. Role of interleukin-4 in resistance to Cryptococcus neoformans infection. Am J Respir Cell Mol Biol. 2004;30:109–117.
- Muller U, Stenzel W, Kohler G, et al. IL-13 induces disease-promoting type 2 cytokines, alternatively activated macrophages and allergic inflammation during pulmonary infection of mice with Cryptococcus neoformans. J Immunol. 2007;179:5367–5377.
- Zhang Y, Wang F, Tompkins KC, et al. Robust Th1 and Th17 immunity supports pulmonary clearance but cannot prevent systemic dissemination of highly virulent Cryptococcus neoformans H99. Am J Pathol. 2009;175:2489–2500.
- Murdock BJ, Teitz-Tennenbaum S, Chen GH, et al. Early or late IL-10 blockade enhances Th1 and Th17 effector responses and promotes fungal clearance in mice with cryptococcal lung infection. J Immunol. 2014;193:4107–4116.
