ABSTRACT
Eicosanoids are bioactive lipid mediators generated in almost all mammalian cells from the oxidation of arachidonic acid and other related twenty-carbon polyunsaturated fatty acids (PUFA). Eicosanoids regulate various physiological functions, including cellular homoeostasis and modulation of inflammatory responses in mammals. The mode of action of these lipid mediators depend on their binding to different G-protein coupled receptors. The three main enzymatic pathways associated with their production are the COX pathway, LOX pathway and cytochrome P450 pathway. Interestingly, investigations have also revealed that several human pathogenic fungi are capable of producing these bioactive lipid mediators; however, the exact biosynthetic pathways and their function in pathogenicity are not yet extensively characterized. The aim of the current review is to summarize the recent discoveries pertaining to eicosanoid production by human pathogenic yeasts with a special focus on the opportunistic human fungal pathogen Candida parapsilosis.
Introduction
Oxylipins are oxidized lipid molecules generated from the oxidation of polyunsaturated fatty acids [Citation1]. Eicosanoids are oxylipin molecules and the main precursor for their production is the twenty-carbon chain fatty acid molecule, arachidonic acid. Prostaglandins, thromboxanes, prostacyclins, leukotrienes, lipoxins, hepoxilins, hydroxy fatty acids, hydroxylated fatty acids and epoxy derivatives all belong to the eicosanoid family [Citation2]. They are synthesized through enzymatic as well as non-enzymatic pathways (non-enzymatic free-radical-induced peroxidation of PUFAs) [Citation3–Citation5]. The majority of our knowledge available regarding eicosanoid biology derives from research performed on mammalian cells. Eicosanoids regulate various functions, mainly during inflammation and protective immune responses, and they also act as messengers in the central nervous system. Remarkably, they function as both pro-, as well as anti-inflammatory or pro-resolving mediators during immune responses against infections [Citation3]. Although bioactive eicosanoid production by yeasts has been acknowledged since the early 1990’s [Citation6], detailed descriptions of their biosynthetic pathways and function is still unavailable. Based upon the currently available studies, in this review, we provide an up-to-date and brief summary of eicosanoid production in pathogenic yeasts as well as their role in pathogenesis development, with a special focus on an emerging fungal pathogenic species, Candida parapsilosis.
Eicosanoid production by human pathogenic yeasts
In human pathogenic yeasts, the presence of fungal eicosanoids was first reported in the opportunistic fungal pathogen Candida albicans. In 2001, Deva et al. reported the production of 3,18-dihydroxy-5,8,11,14-eicosatetraenoic acid (3,18 di-HETE) by C. albicans from exogenous arachidonic acid, as determined by GC/MS analysis [Citation7]. In the same year, Noverr et al. showed that both C. albicans and Cryptococcus neoformans were able to generate immunomodulatory prostaglandin from exogenous arachidonic acid [Citation8,Citation9]. The authors referred to this molecule as PGEx due to its cross-reactivity with the “E” class of prostaglandin in ELISA, although mass spectroscopic analysis later revealed that the identified prostaglandin was PGE2 [Citation10]. Besides HETE and PGE2, these species are also able to produce PGD2 and PGF2α as well as leukotrienes (LTB4, cysteinyl leukotrienes) from exogenous arachidonic acid [Citation11]. Subsequently, C. albicans was also shown to produce the pro-resolving lipid mediator Resolvin E1 (RvE1), that is chemically identical to those produced by human cells and its biosynthetic precursors, 18-hydroxyeicosapentaenoic acid (HEPE), 15-HEPE and 5-HEPE [Citation12]. In recent years, investigations have shown that non-albicans Candida species are also capable of producing immunomodulatory prostaglandins. These species include C. dubliniensis, C. tropicalis and C. glabrata [Citation13,Citation14]. Interestingly, C. albicans planktonic cells and biofilms are able to produce PGE2 from exogenous arachidonic acid [Citation15–Citation17] and the production of 15-HETE by C. albicans biofilm has also been reported [Citation18]. It has also been reported that both the high and low virulent strains of the human pathogenic dimorphic fungus Paracoccidioides brasiliensis produce PGE2 and leukotriene B4 from the same substrate [Citation19–Citation21]. Pathogenic dimorphic fungi with an infectious yeast phase such as Histoplasma capsulatum, Blastomyces dermatitidis and Sporothrix schenckii can also produce a range of eicosanoids namely PGE2, PGD2, PGF2α and leukotrienes from exogenous arachidonic acid [Citation11]. Our current knowledge on eicosanoid production by pathogenic yeasts is summarized in . and .
Table 1. Eicosanoids produced by human pathogenic yeasts and genes identified for PGE2 production in C. albicans, C. parapsilosis and C. neoformans.
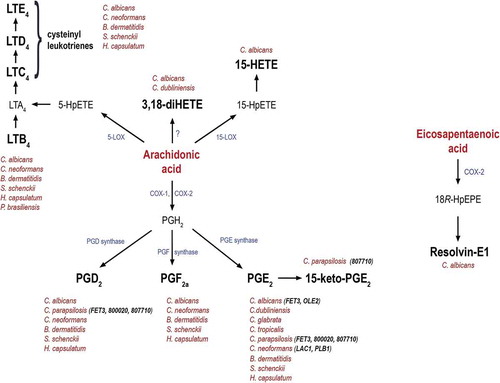
Figure 1. Schematic representation of eicosanoid biosynthesis, their production by fungi and the corresponding genes involved in their production.
Eicosanoid production from the precursor arachidonic acid or eicosapentaenoic acid. Besides mammals, cysteinyl leukotrienes, 3,18-diHETE, 15-HETE, PGD2, PGF2a, PGE2, 15-keto-PGE2 and resolvin-E1 are also produced by different pathogenic fungi. Although the exact biosynthetic pathways remain unknown, several fungal genes have been proposed to regulate their synthesis. These include FET3 and OLE2 in C. albicans, FET3 (CPAR2_603600), CPAR2_800020 and CPAR2_807710 in C. parapsilosis, and LAC1 and PLB1 in C. neoformans.
Fungal eicosanoids in pathogenesis and immune regulation
Eicosanoid signaling regulates the mammalian immune system similarly to cytokine signaling [Citation3]. They function during both the generation and the resolution of inflammatory reactions, and also participate in cellular homoeostasis [Citation3] . Fungal infections can also induce the production of eicosanoids in different host cells, which contributes to the generation of an antifungal immune response [Citation22–Citation29]. Fungal eicosanoids modulate host immune responses as well as pathogenesis [Citation30,Citation31]. For example, the PGE2 produced by C. albicans induces yeast to hyphal transition, which is an important virulence trait of pathogenic fungi [Citation32,33]. However, it has been shown previously that the null mutant strain of FET3 did not alter pathogenicity compared with the wild-type strain in the mouse model of systemic candidiasis [Citation34]. In contrast, C. neoformans deletion mutants of phospholipase B (PLB) or laccase (LAC) enzymes are less virulent in mice compared to the wild type strain, indicating the role of these genes in pathogenesis [33,Citation35], albeit their functions impact more than eicosanoid biology. Fungal prostaglandins produced by these two species have also been confirmed to have immunomodulatory functions as they alter host cytokine responses by down-regulating chemokine (IL-8) and pro-inflammatory cytokine (e.g. TNFα) production, while up-regulating anti-inflammatory responses by promoting IL-10 release [Citation8]. In the presence of human keratinocytes, C. albicans, C. tropicalis as well as C. glabrata produced 10-fold more PGE2 [Citation14]. Taken together, these observations indicate the importance of fungal eicosanoids in host-pathogen interactions during fungal infection.
Eicosanoid biosynthesis genes identified in human pathogenic yeasts
The three main enzymatic pathways involved in eicosanoid production in mammals include cyclooxygenases (COX), lipoxygenases (LOX) and cytochrome P450 enzymes [Citation4]. In silico analysis of the recently available whole genome sequences of pathogenic fungi did not identify homologues of the corresponding mammalian genes. This indicated the presence of novel fungal eicosanoid biosynthetic pathways that may differ from the previously described mechanisms in mammals [Citation36]. The use of different enzyme inhibitors against COX, such as acetylsalicylic acid (ASA) and other non-steroidal anti-inflammatory drugs (NSAIDs), as well as LOX inhibitors was inconclusive [Citation8,Citation12,Citation22,Citation23] as the addition of these inhibitors reduced eicosanoid production as well as concomitantly reducing the viability of the fungi.
After the discovery of prostaglandin molecules in C. albicans, two non-COX/LOX-related enzymes were reported to be involved in PGE2 production in this species: a fatty acid desaturase, Ole2p, and a multicopper oxidase, Fet3p [Citation10]. The homozygous deletion mutant strains of the corresponding genes showed a significant reduction in PGE2 levels. The fact that PGE2 production was still detectable in both ole2Δ/Δ and fet3Δ/Δ strains, indicated the presence of additional enzymes that could also be involved in the biosynthesis of this eicosanoid. Using a specific inhibitor 6-(2-propargyloxyphenyl)hexanoic acid (PPOH) against cytochrome P450, the involvement of these enzymes was confirmed in PGE2 production in both C. albicans and C. dubliniensis biofilms [Citation16]. The biosynthesis of RvE1 in C. albicans is also sensitive to lipoxygenase and cytochrome P450 monooxygenase inhibitors [Citation12]. In C. neoformans, the LAC1 laccase, another multicopper oxidase, was further identified as a regulator of PGE2 production, as the lac1Δ/Δ deletion mutant strain showed a reduction in PGE2 production. Additionally, the recombinant cryptococcal laccase enzyme is efficient in converting PGG2 to PGE2 but did not generate any new prostaglandins when incubated with only arachidonic acid or PGH2 [Citation24]. The deletion of the C. neoformans phospholipase (PLB1) gene also resulted in a reduction in PGE2 production [Citation25].
The inclusion of COX like enzymes in PGE2 biosynthesis has been implicated in P. brasiliensis, although the corresponding biosynthetic pathway is yet unexplored [Citation19,Citation20]. The significant reduction of LTB4 production by both selective or non-selective LOX inhibitors (MK886 or nordihydroguaiaretic acid) in P. brasiliensis indicated that the fungus produces LTB4 by using the LOX pathway or with a biochemically similar enzyme [Citation21].
Eicosanoid production by Candida parapsilosis
Candida species remain the most prevalent cause of invasive fungal infections, exceeding invasive aspergillosis and mucormycosis [Citation37,Citation38] and other infections by pathogenic fungi. Although, C. albicans is still the most common cause of invasive candidiasis, bloodstream infections caused by non-albicans Candida species such as C. glabrata, C. krusei, C. auris, C. parapsilosis, and C. tropicalis, altogether have risen to account for approximately one-half of all candidemia cases [Citation39].C. parapsilosis is a commensal of the skin and it is also frequently isolated from the gastrointestinal tract [Citation40]. This species is one of the major causes of invasive fungal infections in premature infants [Citation41]. The incidence of C. parapsilosis is increasing in this particular patient group and it outnumbers C. albicans infections in some geographic regions [Citation42]. C. parapsilosis is known for its ability to form biofilms on catheters and other implanted devices [Citation43,Citation44]. Different risk factors that are associated with C. parapsilosis driven neonatal candidiasis include low birth weight (<1500 g), prematurity, prior colonization, the use of parenteral nutrition, intravascular catheters and prolonged treatment with antibiotics or steroids [Citation45].
C. parapsilosis is capable of producing a variety of eicosanoids. The prostaglandin profile of C. parapsilosis is quite similar to that of C. albicans, with PGE2 and PGD2 being predominantly produced in the presence of arachidonic acid as a sole carbon source. Interestingly, unlike in case of C. albicans, the fatty acid desaturase homologous gene OLE2 does not play a role in their synthesis [Citation46]. Recently, CPAR2_603600 (homologous of CaFET3), CPAR2_807710 (homologue of the acyl-coenzyme A oxidase, ScPOX1-3) and CPAR2_800020 (homologue of 3-ketoacyl-CoA thiolase, ScPOT1) have been demonstrated to be involved in the generation of fungal eicosanoids in C. parapsilosis [Citation47]. LC/MS analysis showed that the disruption of each gene led to a decrease in the production of PGE2, PGD2 and 15-keto-PGE2. The deletion mutant strains of CPAR2_603600, CPAR2_800020 and CPAR2_807710 produced less prostaglandin D2 (PGD2) and also had a significant decrease in PGE2 production. However, only the deletion mutant strain of CPAR2_807710 has a reduction in 15-keto-prostaglandin E2 (15-keto-PGE2) production. This study also reported the presence of fungal 5-D2-isoprostane in C. parapsilosis by LC/MS analysis. The eicosanoid mutant strains were also shown to induce more pro-inflammatory cytokines by human peripheral blood derived macrophages and they were less virulent in a mouse model of systemic candidiasis compared to the wild type strain [Citation47], which indicates the importance of these fungal derived eicosanoids in C. parapsilosis virulence and pathogenicity mechanisms.
Future perspectives
The significance of fungal eicosanoid lipid mediators in pathogenesis is increasingly validated through interesting published and ongoing research, although their biosynthetic pathways and exact function in pathobiology is not yet fully explored. Furthermore, it is also unclear whether these pathogenic yeasts contain specific receptors for their recognition, such as G-protein coupled receptors (GPCRs) in mammalian cells. These immunomodulatory lipid molecules are produced by not only pathogenic yeasts, but also filamentous fungi such as Aspergillus nidulans and A. fumigatus [Citation48]. Interestingly, eukaryotic parasites, such as Plasmodium falciparum [Citation49] and Trypanosoma brucei [Citation50], have also recently been shown to produce prostaglandin like compounds. It is possible that eicosanoids secreted by eukaryotic pathogenic organisms can vary in function, and influence microbial growth and maturation, or effect host interactions, by modulating immune responses. Although it remains to be fully elucidated whether microbial eicosanoids are also virulence factors and why the pathogenic fungi belong to a different family evolved with mechanisms for producing these eicosanoid molecules that are structurally similar to the bioactive lipid mediators generated by human hosts.
Acknowledgments
Critical reading of the manuscript by Joshua D. Nosanchuk is gratefully acknowledged.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Smith WL. The eicosanoids and their biochemical mechanisms of action. Biochem J. 1989;259:315–324.
- Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294:1871–1875.
- Dennis EA, Norris PC. Eicosanoid storm in infection and inflammation. Nat Rev Immunol. 2015;15:511–523.
- Buczynski MW, Dumlao DS, Dennis EA. Thematic review series: Proteomics. an integrated omics analysis of eicosanoid biology. J Lipid Res. 2009;50:1015–1038.
- Vigor C, Bertrand-Michel J, Pinot E, et al. Non-enzymatic lipid oxidation products in biological systems: assessment of the metabolites from polyunsaturated fatty acids. J Chromatogr B Anal Technol Biomed Life Sci. 2014;964:65–78.
- van Dyk MS, Kock JL, Coetzee DJ, et al. Isolation of a novel arachidonic acid metabolite 3-hydroxy-5,8,11,14-eicosatetraenoic acid (3-HETE) from the yeast Dipodascopsis uninucleata UOFs-Y128. FEBS Lett. 1991;283:195–198.
- Deva R, Ciccoli R, Kock L, et al. Involvement of aspirin-sensitive oxylipins in vulvovaginal candidiasis. FEMS Microbiol Lett. 2001;198:37–43.
- Noverr MC, Phare SM, Toews GB, et al. Pathogenic yeasts Cryptococcus neoformans and Candida albicans produce immunomodulatory prostaglandins. Infect Immun. 2001;69:2957–2963.
- Erb-Downward JR, Huffnagle GB. Cryptococcus neoformans produces authentic prostaglandin E2 without a cyclooxygenase. Eukaryot Cell. 2007;6:346–350.
- Erb-Downward JR, Noverr MC. Characterization of prostaglandin E2 production by Candida albicans. Infect Immun. 2007;75:3498–3505.
- Noverr MC, Toews GB, Huffnagle GB. Production of prostaglandins and leukotrienes by pathogenic fungi. Infect Immun. 2002;70:400–402.
- Haas-Stapleton EJ, Lu Y, Hong S, et al. Candida albicans modulates host defense by biosynthesizing the pro-resolving mediator resolvin E1. PLoS One. 2007;2:e1316.
- Noverr MC, Erb-Downward JR, Huffnagle GB. Production of eicosanoids and other oxylipins by pathogenic eukaryotic microbes. Clin Microbiol Rev. 2003;16:517–533.
- Shiraki Y, Ishibashi Y, Hiruma M, et al. Candida albicans abrogates the expression of interferon-gamma-inducible protein-10 in human keratinocytes. FEMS Immunol Med Microbiol. 2008;54:122–128.
- Alem MAS, Douglas LJ. Prostaglandin production during growth of Candida albicans biofilms. J Med Microbiol. 2005;54:1001–1005.
- Ells R, Kock JLF, Albertyn J, et al. Effect of inhibitors of arachidonic acid metabolism on prostaglandin E(2) production by Candida albicans and Candida dubliniensis biofilms. Med Microbiol Immunol. 2011;200:23–28.
- Ells R, Kemp G, Albertyn J, et al. Phenothiazine is a potent inhibitor of prostaglandin E2 production by Candida albicans biofilms. FEMS Yeast Res. 2013;13:849–855.
- Fourie R, Ells R, Swart CW, et al. Candida albicans and Pseudomonas aeruginosa Interaction, with Focus on the Role of Eicosanoids. Front Physiol. 2016;7:64.
- Bordon AP, Dias-Melicio LA, Acorci MJ, et al. Prostaglandin E(2) production by high and low virulent strains of Paracoccidioides brasiliensis. Mycopathologia. 2007;163:129–135.
- Biondo GA, Dias-Melicio LA, Bordon-Graciani AP, et al. Paracoccidioides brasiliensis uses endogenous and exogenous arachidonic acid for PGE x production. Mycopathologia. 2010;170:123–130.
- Biondo GA, Dias-Melicio LA, Bordon-Graciani AP, et al. de Campos Soares AMV. Production of leukotriene B4 by Paracoccidioides brasiliensis. Yeast. 2012;29:201–208.
- Ells R, Kock JL, Albertyn J, et al. Arachidonic acid metabolites in pathogenic yeasts. Lipids Health Dis. 2012;11:100.
- Alem MAS, Douglas LJ. Effects of aspirin and other nonsteroidal anti-inflammatory drugs on biofilms and planktonic cells of Candida albicans. Antimicrob Agents Chemother. 2004;48:41–47.
- Erb-Downward JR, Noggle RM, Williamson PR, et al. The role of laccase in prostaglandin production by Cryptococcus neoformans. Mol Microbiol. 2008;68:1428–1437.
- Noverr MC, Cox GM, Perfect JR, et al. Role of PLB1 in pulmonary inflammation and cryptococcal eicosanoid production. Infect Immun. 2003;71:1538–1547.
- Harris SG, Padilla J, Koumas L, et al. Prostaglandins as modulators of immunity. Trends Immunol. 2002;23:144–150.
- Suram S, Gangelhoff TA, Taylor PR, et al. Pathways regulating cytosolic phospholipase A2 activation and eicosanoid production in macrophages by Candida albicans. J Biol Chem. 2010;285:30676–30685.
- Suram S, Brown GD, Ghosh M, et al. Regulation of cytosolic phospholipase A2 activation and cyclooxygenase 2 expression in macrophages by the β-glucan receptor. J Biol Chem. 2006;281:5506–5514.
- Valdez PA, Vithayathil PJ, Janelsins BM, et al. Prostaglandin E2 suppresses antifungal immunity by inhibiting interferon regulatory factor 4 function and interleukin-17 expression in T cells. Immunity. 2012;36:668–679.
- Gagliardi MC, Teloni R, Mariotti S, et al. Endogenous PGE2 promotes the induction of human Th17 responses by fungal β-glucan. J Leukoc Biol. 2010;88:947–954.
- Smeekens SP, van de Veerdonk FL, van der Meer JWM, et al. The Candida Th17 response is dependent on mannan- and β-glucan-induced prostaglandin E2. Int Immunol. 2010;22:889–895.
- Noverr MC, Huffnagle GB. Regulation of Candida albicans morphogenesis by fatty acid metabolites. Infect Immun. 2004;72:6206–6210.
- Tsitsigiannis DI, Keller NP. Oxylipins as developmental and host-fungal communication signals. Trends Microbiol. 2007;15:109–118.
- Eck R, Hundt S, Hartl A, et al. A multicopper oxidase gene from Candida albicans: cloning, characterization and disruption. Microbiology. 1999;145(Pt 9):2415–2422.
- Erb-Downward JR, Huffnagle GB. Role of oxylipins and other lipid mediators in fungal pathogenesis. Future Microbiol. 2006;1:219–227.
- Brodhun F, Feussner I. Oxylipins in fungi. FEBS J. 2011;278:1047–1063.
- Guinea J. Global trends in the distribution of Candida species causing candidemia. Clin Microbiol Infect. 2014;20(Suppl 6):5–10.
- Pfaller MA, Diekema DJ. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev. 2007;20:133–163.
- Quindos G. Epidemiology of candidaemia and invasive candidiasis. A changing face. Rev Iberoam Micol. 2014;31:42–48.
- el-Mohandes AE, Johnson-Robbins L, Keiser JF, et al. Incidence of Candida parapsilosis colonization in an intensive care nursery population and its association with invasive fungal disease. Pediatr Infect Dis J. 1994;13:520–524.
- Pammi M, Holland L, Butler G, et al. Candida parapsilosis is a significant neonatal pathogen: a systematic review and meta-analysis. Pediatr Infect Dis J. 2013;32:e206–e216.
- Trofa D, Gacser A, Nosanchuk JD. Candida parapsilosis, an emerging fungal pathogen. Clin Microbiol Rev. 2008;21:606–625.
- Lupetti A, Tavanti A, Davini P, et al. Horizontal transmission of Candida parapsilosis candidemia in a neonatal intensive care unit. J Clin Microbiol. 2002;40:2363–2369.
- Bonassoli LA, Bertoli M, Svidzinski TIE. High frequency of Candida parapsilosis on the hands of healthy hosts. J Hosp Infect. 2005;59:159–162.
- Clerihew L, Lamagni TL, Brocklehurst P, et al. Candida parapsilosis infection in very low birthweight infants. Arch Dis Child Fetal Neonatal Ed. 2007;92:F127–F129.
- Grózer Z, Tóth A, Tóth R, et al. Candida parapsilosis produces prostaglandins from exogenous arachidonic acid and OLE2 is not required for their synthesis. Virulence. 2015;6:85–92.
- Chakraborty T, Thuer E, Heijink M, et al. Eicosanoid biosynthesis influences the virulence of Candida parapsilosis. Virulence. 2018;9:1019–1035.
- Tsitsigiannis DI, Bok J-W, Andes D, et al. Aspergillus cyclooxygenase-like enzymes are associated with prostaglandin production and virulence. Infect Immun. 2005;73:4548–4559.
- Kilunga Kubata B, Eguchi N, Urade Y, et al. Plasmodium falciparum produces prostaglandins that are pyrogenic, somnogenic, and immunosuppressive substances in humans. J Exp Med. 1998;188:1197–1202.
- Kubata BK, Duszenko M, Kabututu Z, et al. Identification of a novel prostaglandin f(2alpha) synthase in Trypanosoma brucei. J Exp Med. 2000;192:1327–1338.
