ABSTRACT
The genus Coccidioides consists of two species: C. immitis and C. posadasii. Prior to 2000, all disease was thought to be caused by a single species, C. immitis. The organism grows in arid to semiarid alkaline soils throughout western North America and into Central and South America. Regions in the United States, with highest prevalence of disease, include California, Arizona, and Texas. The Mexican states of Baja California, Coahuila, Sonora, and Neuvo Leon currently have the highest skin test positive results. Central America contains isolated endemic areas in Guatemala and Honduras. South America has isolated regions of high endemicity including areas of Colombia, Venezuela, Argentina, Paraguay, and Brazil. Although approximately 15,000 cases per year are reported in the United States, actual disease burden is estimated to be in the hundreds of thousands, as only California and Arizona have dedicated public health outreach, and report and track disease reliably. In this review, we survey genomics, epidemiology, ecology, and summarize aspects of disease, diagnosis, prevention, and treatment.
Introduction
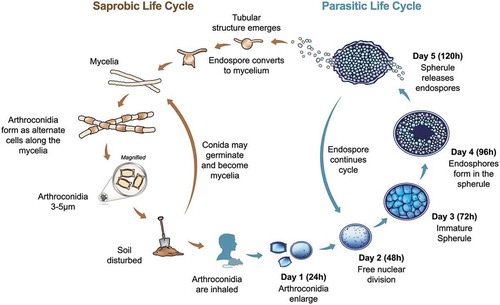
The disease coccidioidomycosis, which is commonly known as valley fever (VF), was first described in the late 1800s in Argentina by Dr Alejandro Posadas [Citation1]. The causative agent was first thought to be a protozoan that caused severe disease (thus, the etymology of Coccidioides immitis: Coccidia protozoan and immitis “not mild”) but was later identified as a dimorphic fungus, with most disease being asymptomatic or mild [Citation2–Citation4]. The unusual life cycle is defined by the large pathogenic structure called a spherule (). The initial spherule develops from an inhaled arthroconidia, which is the asexual propagule that develops in the environment. This environmental life stage consists of nondescript mycelia that mature into alternating arthroconidia as the fungus grows and ages. The exact conditions required for growth and maturation in the environment are unknown, but evidence suggests that keratin sources and precipitation play a role [Citation5,Citation6]. Once infection is established, the spherule life stage predominates in the host, with endospores developing internally and the outer cell wall rupturing to release mature endospores. Each endospore can develop into a new spherule, and endospores are likely recognized and engulfed by host immune cells [Citation7–Citation9]. Once the spherule matures and enlarges, it cannot be engulfed and can rupture the host cell. Thus, it appears the Coccidioides can be both an intracellular and extracellular pathogen. The sexual stage of the life cycle has yet to be discovered, but appears to occur with high frequency [Citation10,Citation11].
Figure 1. Life cycle of Coccidioides spp. During the saprobic phase (left) the organism grows as mycelia, which mature into arthroconidia. These asexual conidia can be inhaled by a susceptible host. If this occurs, the fungus undergoes a morphological shift to form a spherule (right). The spherule structure matures to contain endospores, which can potentially disseminate to other body sites in the host including skin, bones, or central nervous system.
Both organisms grow in arid to semiarid alkaline thermic soils throughout western North America and into Central and South America [Citation12]. Endemic regions in the United States with highest predicted prevalence of disease include the Central Valley of California, southern Arizona, southwestern Texas, but only Arizona and California track and report disease prevalence to the Centers for Disease Control and Prevention (CDC), along with about half of the US states. The Mexican states of Baja California, Coahuila, Sonora, and Neuvo Leon currently have the highest skin test positive results, although Mexico no longer tracks the disease (pers comm Laura Rosio Castañón). Isolated areas in Guatemala (Motagua Valley) and Honduras (Comayagua Valley) have documented cases [Citation13]. South America has several geographically isolated regions of endemicity including the northeastern area of Colombia; Lara and Falcon states in Venezuela; the Chaco region in Argentina/Paraguay; and Piaui, Maranhao, Ceara, and Bahai states of Brazil [Citation14,Citation15]. Disease prevalence in South America is not well characterized, possibly due to lower population densities and lower socioeconomic status in the regions of endemicity, but could also reflect the genotypes and phenotypes of both the pathogen and host found specifically in South America.
The genus Coccidioides consists of two species: C. immitis and C. posadasii. Prior to 2000, all disease was thought to be caused by a single species, C. immitis. However, genetic analysis clearly supports two distinct species [Citation16]. Within each species, several populations have been proposed. For C. immitis, there are indications of population structure within the Central Valley, southern California/Baja California, Mexico, and a separate population in the newly identified endemic region of eastern Washington State [Citation17–Citation19]. For C. posadasii, a clear separation between isolates from Arizona and isolates from Mexico/Texas/South America has been consistently observed [Citation19]. Additionally, Arizona isolates from the Phoenix region and the higher elevation Tucson region may be distinct subpopulations [Citation17,Citation18]. The true population structure will remain an enigma until direct isolations from soil are made throughout the range of the organism. Despite the clear genotypic variation, no clinical differences have been defined among species or populations – although no published reports have ever assessed phenotypic variation in this context.
The disease caused by Coccidioides is highly variable among human patients. The majority (60%) of patients are asymptomatic after infection [Citation20]. For the remainder, symptoms can be mild, including pneumonia, and these infections normally resolve without intervention. However, if the infection become extrapulmonary, medical intervention may be necessary. Infections can disseminate to spleen, liver, brain, bone, and many other tissues in the body. Complications in diagnosis, treatment failure, and unusual presentations may result in severe disease progression, and even death. No vaccine for this disease is available, although efforts are underway to develop an effective vaccine. In this review, we summarize historical and recent developments in the study of Coccidioides and coccidioidomycosis.
The ecology of Coccidioides spp
Most ascomycete fungi are saprotrophic in the environment and have an association with plants, but Coccidioides spp. has evolved the ability to infect immunocompetent mammals including humans [Citation21]. Despite a dramatic increase in patients diagnosed with coccidioidomycosis in recent years, the ecology of the organism is poorly understood. Arid and semiarid soils of the southwestern United States, Mexico, Central, and South American are the natural reservoir for the fungus [Citation12]. The distribution of the fungus in soil is inconsistent and unpredictable even in the endemic region where there is high disease burden [Citation22]. There is evidence of an association between Coccidioides and animals (small desert mammals) due to greater detection of the fungus in close proximity to animal activity [Citation23–Citation28]. There is also evidence of climatic and seasonal variables that may influence growth and dispersion of the fungus leading to patterns of disease outbreaks [Citation29]. This section will synthesize the information known about the ecology of Coccidioides spp. and propose areas for future research.
Biotic factors: Soil
During the mycelial life cycle, Coccidioides spp. are thought to be a saprotrophic soil-dwelling fungi, although a preferred nutrient source is not described. The distribution in the soil is sporadic and irregular and may be driven by abiotic soil factors such as pH and electrical conductivity or possibly driven by biotic associations with small desert mammals such as rodents [Citation27]. Few studies have tried to identify the abiotic soil variables that may be driving the distribution of Coccidioides spp. with limited support for any one predictor variable. In the 1970s, Lacy and Swatek investigated C. immitis around California archeological sites and reveled that sandy-textured soils made up of 98% of positive samples and 96.7% of positive soils were alkaline [Citation30]. Elconin et al. also found a positive correlation between C. immitis isolation and increased soil salinity [Citation31]. Though these studies provided evidence for an association with C. immitis and soils with alkaline pH, the samples sizes were quite small and relied on culture-based methods for fungal identification. Fisher et al. analyzed more abiotic variables, such as soil temperature, soil texture, chemical characteristics, and water quantity, which could affect the distribution and growth of Coccidioides throughout the southwestern United States [Citation12]. The authors proposed that soils with low water content (water table is not near the surface) are more favorable for fungi, because as the soil dries microorganisms that can grow as filamentous hyphae may reach water pockets. Based on their assessment of laboratory and field studies, the optimal soil temperature range that promotes peak growth of the fungus is between 20 and 40°C and the soil texture (proportion of sand, silts, and clays in a given soil) in which Coccidioides is most commonly found is sandy loam (low water-holding capacity) with pH ranging from 6.1 to 8 with relatively low electrical conductivity. These data suggest that pH and texture are not limiting factors for the growth, but that temperature and water availability may be more important. The relatively few studies that have examined the role of abiotic factors do not provide strong evidentiary support for soil variables that can be used to predict the growth pattern and distribution of Coccidioides spp. in the environment [Citation27].
Environmental detection
Detection of the pathogen in soil is a difficult task as culture methods are shown to be insensitive (thousands of soils with no/few cultured strains) and mouse inoculation is expensive and time consuming with variable results [Citation27,Citation28,Citation32–Citation34]. With the development of new molecular technologies, it is easier and faster to detect presence of the pathogen using PCR, DNA sequencing, and real-time qPCR methods. Several methods target regions of the ITS or rDNA, and often require additional sequencing to verify the target identity [Citation27,Citation35,Citation36]. A real-time PCR method targets a novel repetitive sequence that was first identified for use in a clinical diagnostic system [Citation37,Citation38]. The main benefit to molecular-based methods is the large number of soil samples that can be screened in a relatively short amount of time.
Wind, dust, and airborne conidia
The role of dust in the dispersion of Coccidioides spp. propagules has been posited for many years, but has not been experimentally validated because has never been isolated from ambient dust and molecular detection is difficult [Citation39–Citation48]. Chow et al. were able to detect airborne Coccidioides in a simulated dust storm with relative success, but detecting the fungus in actual dust storms is much more difficult [Citation41]. Climate models show a drying trend in the endemic areas for Coccidioides that increases the likelihood for dust storms [Citation49,Citation50]. Tong et al. showed that dust storms have increased 240%, from 1990s to the 2000s, in the southwestern United Stated and have a positive correlation with dust storm frequency and reported cases of coccidioidomycosis [Citation51]. It is proposed that with the increasing frequency of dust storms comes a higher risk of inhaling infectious propagules. With the increase in dust and wind activity comes a greater need for better air surveillance techniques. After the California Northridge Earthquake in 1994, there was an outbreak of coccidioidomycosis that included three deaths. This outbreak was attributed to many landslides that generated massive dust clouds that blew into nearby densely populated valleys [Citation44]. Stochastic events that can generate a large bolus of dust containing infectious propagules, such as earthquakes, may increase the possibility of outbreaks. There is also a risk for wind to disperse the fungus to “nonendemic” areas [Citation40,Citation42,Citation44]. Weil et al. showed that dust storms can transplant entire microbial communities hundreds of kilometers (Saharan desert to the Italian Alps) including pathogenic “black-mold,” and these communities have the potential to become established in new areas [Citation52]. Blowing dust has the potential to disperse infectious Coccidioides propagules to nonendemic areas, and should be monitored.
Animal associations
Most fungi cannot survive higher temperatures and acidic pH of the mammalian body. Enduring the unforgiving conditions of desert soil microenvironments, such as extreme temperatures, dramatic pH shifts, and microbial competition via secondary metabolites may have led to promotion pathogenesis and the ability to infect mammals via “ready-made” virulence factors [Citation53,Citation54].
Although an animal reservoir has not been identified for Coccidioides spp., there is strong evidence of mammalian associations with pathogenic and nonpathogenic relatives. Paracoccidioides brasiliensis, a close pathogenic relative of Coccidioides, has been isolated from the feces of bats (Artibeus lituratus) and from the internal organs of the nine-banded armadillo [Citation55,Citation56]. There is evidence of animal association with another close pathogenic cousin of Coccidioides, Blastomyces spp. The fungus has been isolated from the feces of bats and from other various animal manures as well as from beaver dams [Citation57–Citation59]. There also is a strong association with prairie dog burrows which, like many other burrowing mammals, create designated latrine areas to store their waste that the fungus seems to prefer [Citation60]. There is indication that fungal pathogens within the order Onygenales are associated with wild animals, either in vivo or in situ, and this close relationship may be an indication of how they evolved to become pathogenic in humans and other animals.
Coccidioides spp. as well as other fungi in the order Onygenales have the ability to degrade keratin and utilize it as a source of carbon, nitrogen, phosphorus, sulfur, amino acids, and other minerals [Citation61,Citation62]. The Coccidioides genome has a significantly reduced fungal-cellulose binding domain gene family that gives the fungus the ability to break down plant material suggesting that Coccidioides has reduced this capability [Citation21]. The subtilisin N domain-containing family is highly expanded in the Coccidioides genome as well as in close relatives. This gene family contains the peptidase S8 family domain that encodes several keratinolytic subtilases (keratinases) and this gene family is three times larger in Coccidioides than in other taxa [Citation21]. This genomic information suggests that unlike other fungal taxa in the sister order Eurotiales, which are often associated with plants and plant materials, Coccidioides and other Onygenales utilize animal-derived substrates, and may have lost ability to thrive on a vegetarian diet.
The ability to metabolize animal derived material may restrict where the fungus is growing in the environment. There is a large amount of animal material, such as keratin, in desert rodent burrows suggesting a suitable habitat for Coccidioides. Multiple studies have shown that most soils containing the pathogen are extracted from or in the vicinity to rodent burrows, and infected animals buried in soil can establish and grow [Citation27,Citation34,Citation63]. In a recent study from the endemic area in Mexico, 82% of soils containing Coccidioides were taken from rodent burrows indicating a strong correlation to the burrow microhabitat [Citation64]. The abundance of desert rodents inhabiting the endemic region of the fungus suggests a possible connection. In early studies Coccidioides was isolated from deer mice, pocket mice, ground squirrels, grasshopper mice, kangaroo rats, and pack rats [Citation34]. Although these early studies were culture and morphology based, they provide evidence that desert rodents could be natural reservoirs that harbor and disperse the fungus in the environment. However, there is recent evidence that Coccidioides spp. is harbored in nonrodent animals such as bats and armadillos, and in some cases animals in captivity such as otters, kangaroos, and nonhuman primates have developed severe disease and had the fungus isolated from tissue, so specific reservoirs reamin to be defined [Citation65–Citation68].
This association differs from an opportunistic fungal pathogen like Aspergillus fumigatus which is an environmental saprobe that releases a large quantity of conidia into the air [Citation69] A. fumigatus is a ubiquitous environmental fungus that is usually associated with plants, decaying organic material, marine and aquatic systems that typically infect humans when they are immunocompromised [Citation70]. Animals are constantly being exposed to Aspergillus conidia, estimated few hundred per day, which does not lead to disease unless the immune system is compromised [Citation71,Citation72].
Climate/seasonality
Changes in the environment can influence dispersal patterns of arthroconidia into the atmosphere that can lead to fluctuations in reported cases of VF [Citation73]. There may be an association with increased incidence of reported disease with precipitation patterns based on the life cycle of the fungus. It is hypothesized that Coccidioides responds to soil moisture, so that when moisture is abundant the fungus grows as mycelium in soil and when the soil dries out specific viable hyphal cells mature into arthroconidia, which are released into the air [Citation5,Citation22,Citation29,Citation74–Citation76]. This is the time when humans are at greater risk for inhaling infectious coccidioidal propagules. In Arizona, low precipitation in early summer correlates to higher incidences of coccidioidomycosis in the later summer (July, August, and September); but, when there is increased monsoonal activity in the early summer, there are lower incidences of VF in the later summer [Citation22]. The authors show that there is also a positive correlation of increased incidence when there are high precipitation levels in the winter and spring months, and increased cases of VF in the summer months after heavy winter rains. These saturation events may give the fungus enough moisture to proliferate and create greater fungal biomass in the soil and when the soil dries out release more spores into the air. A complicating factor for all climate models to date is the reliance on human case report data, which may be months after the exposure event.
Temperature is another variable that may influence the growth of arthroconidia and lead to changed patterns of incidence in the endemic region. There is a hypothesis that the soil becomes sterilized by extreme high temperatures but Coccidioides survives by growing into deeper soil horizons, and then when there is rain the fungus can grow back to the soil surface [Citation77]. Recent studies have shown that annual mean surface temperature is a significant driver of coccidioidomycosis cases. No counties in the endemic area have a mean surface temperature lower than 10°C and incidence rates higher than six cases per 100,000 people, whereas the counties with the highest incidence rate in California and Arizona (70 cases per 100,000 people) have a mean temperature that is greater than 16°C [Citation49]. This suggests that temperature may be an important predictor variable of where the fungus prefers to grow in the environment.
The changing climate may create suitable habitat for Coccidioides spp. outside of the endemic region, although it is clear that there are areas of transient endemicity that suggest that the true area is larger than proposed [Citation78]. Temperatures in the southwestern United States are expected to rise by 2°C, with the greatest increases expected during the summer and autumn months [Citation79]. Previous work indicates that Coccidioides may prefer to grow in areas with higher surface temperatures; therefore, this warming trend might shift the endemic regions farther north into areas that may not have been suitable environment for the fungus before [Citation49]. Drought projections show an intensification of drought throughout the current endemic area which can lead increased dust and dust events that will increase the rates of VF cases [Citation49,Citation80]. We propose that the changing climate may allow the pathogen to occupy new areas, expose more naïve hosts to the disease, and increase disease burden in already established endemic regions.
Vaccines
A vaccine for VF was proposed by several groups [Citation81–Citation83]. It was observed that a primary infection seemed to protect individuals from subsequent infection, and most (60%) infections are not symptomatic [Citation20]. Early vaccine tests were first assessed in guinea pigs, but without success [Citation82]. After these frustrating starts, effective vaccines were developed for use in mice, monkeys, and dogs [Citation84–Citation86]. These early vaccine candidates relied on inactive whole cell and fungal components, both from the parasitic (spherule) phase and well as the environmental phase (conidia/mycelia) [Citation87]. In fact, a formalin killed spherule vaccine went as far as Phase 3 human clinical trials. Nearly 3000 people received the vaccination, and 18 vaccinated individuals developed or were suspected to have developed mild VF, whereas 25 unvaccinated individuals developed or were suspected to have developed mild VF [Citation88]. Based on these results, work aggressively shifted to development of specific antigen vaccines and attenuated strains. One of the most promising antigen-based vaccines was based on the development of a recombinant protein of antigen2 and a proline-rich antigen (Ag2/PRA), and the Coccidioides specific antigen (CSA) [Citation89–Citation92]. However, protection was still only 50–60% of mice surviving a challenge of ~200 conidia.
Single and multiple gene deletions in Coccidioides have resulted in attenuation or abolition of virulence. Some of these avirulent strains have been proposed to be used as a vaccine. In particular, the deletion of 2 chitinase genes was shown to protect a very susceptible mouse model; however, T-cell based immune response was indicated as critical for protection, and the authors suggested that the vaccine would be less effective in HIV/AIDS patients [Citation93]. A current live attenuated vaccine is in development, and has shown highly protection in a mouse model of VF [Citation94,Citation95].
Epidemiology
Steady increases in reported VF have been observed in the United States as regular reporting began in the 1990s [Citation96,Citation97]. In general, reported VF cases are highest in specific regions, primarily southern Arizona and the Central Valley of California. However, it is important to note that VF is only reported nationally in the United States, and is reported by only 24 states (and District of Colombia), and surprisingly known and suspected endemic states do not report disease including Texas, Oklahoma, Washington, Colorado, and Idaho, according to the CDC Morbidity and Mortality Weekly Report (https://www.cdc.gov/mmwr/index.html). No other countries in the endemic regions nationally report this disease, and therefore beyond the United States, there is not reliable data to understand if these increases are universal.
Delayed type hypersensitivity skin testing was used in early epidemiological surveys to determine regions of endemicity [Citation98,Citation99]. It was also used to determine the rate of infection among military personnel in California, and the first antigen used was called coccidioidin, which was administered intradermally [Citation48]. Antigens derived from spherules (spherulin) rather than mycelia seemed to improve the sensitivity of the reaction, but not all confirmed VF cases reveal a positive skin test [Citation100]. Importantly, patients with erythema nodosum should not receive the skin test due to potential tissue necrosis at the site of injection. Skin testing for determination of prior exposure may be useful to ascertain risk for certain occupations or among prison populations. Additionally, new epidemiological studies in novel endemic regions, such as eastern Washington State, are warranted.
Observed increases in disease could be attributed to improved reporting, diagnosis, and awareness [Citation101]. Alternatively, these increases could be the result of changing climate, increased construction, and soil disturbance, as discussed above [Citation5,Citation36,Citation40,Citation73,Citation102–Citation104]. Predictions on the effect of changing climate on the incidence of VF in the southwestern US suggest that disease incidence will increase in endemic regions under warming and changing precipitation patterns [Citation49]. The potential for expansion of the endemic region is a concern, and greater efforts regarding awareness of disease among clinicians and public health officials is critical for improving diagnosis and reporting
Certain patient populations have been shown to be at greater risk for severe disease. African Americans, Filipinos, pregnant women, and those with immunosuppressed conditions are well-documented groups at higher risk for dissemination [Citation105–Citation115]. Occupational exposures, such as working in construction, farm work, outdoor filing, solar farms, or archeological digs, have been associated with larger outbreaks among these workers [Citation43,Citation116–Citation122]. Additionally, prison inmates and guards/workers in the endemic regions have high rates of exposure and disease [Citation106,Citation107,Citation123–Citation126].
There have been no studies that conclude that canines are more susceptible to coccidioidomycosis compared to humans, but infection may be more prevalent in canines due to behavioral tendency to disrupt soil [Citation127]. As stated previously, infection is asymptomatic in 60% of human hosts and these rates in canines are comparable [Citation128]. Early symptoms for both species include coughing, fever, weight loss, lack of appetite, and lack of energy. Most commonly, canines may not express symptoms of a lung infection but at the minimum, will show signs of an active disseminated disease such as lameness and seizures, which will allow early detection of valley fever. Due to the ambiguity of the symptoms, diagnosis depends on specific tests (summarized below) in addition to the clinical symptoms.
Diagnosis
Several methods have been developed to diagnose VF. In addition to clinical diagnosis of symptoms, direct culture or histopathological evidence of the organism, and radiographic findings; diagnostics that have been used include tube precipitin (TP), complement fixation (CF), immunodiffusion, agar gel precipitin-inhibition, latex particle agglutination (LPA), and enzyme-linked immunosorbent assays (ELISA). There has been recent work suggesting the use of a peptide microarray based on immunosignatures to diagnose VF. These proposed peptide diagnostics were shown to be extremely sensitive, and cross react with other related infections [Citation129]. Charles Smith and colleagues developed the TP and CF tests in the 1950s [Citation130,Citation131]. Interestingly, the authors observed that TP positive reactions occurred within weeks of infection, whereas CF positivity occurred 2–3 months after infection, and CF titers could increase if infection was not controlled. In 2015, a new delayed-type hypersensitivity skin test was development and showed promise as a noninvasive diagnostic for VF with no cross-reactivity to other related infections such as histoplasmosis; but may also miss positive reactors and underestimate disease [Citation132]. It is now known that this reflects immunoglobulin M (IgM/TP) and immunoglobulin G (IgG/CF). IgM-positive reactions likely occur in the first few weeks of illness, whereas the IgG reaction becomes positive later in disease, and titers may increase if infection is uncontrolled [Citation133]. Similar to TP, LPA testing detects primarily IgM [Citation134]. In asymptomatic cases, IgM and/or IgG may be detected, but titers may become nondetectable after the resolution of infection. A serological ELISA method based on the detection of both IgM and IgG show high specificity and sensitivity, 98.5 and 95.5%, respectively, and is commonly used for diagnostics [Citation135].
Some studies have discussed difficulties in antibody detection during early time-points of the infection, as well as in immunosuppressed patients [Citation136]. An alternative approach is the detection of fungal antigens in biofluids (typically sera) via antigen enzyme immunoassay [Citation137]. For example, antibodies against fungal galactomannan could improve detection of coccidioidomycosis [Citation138]. Cross-reactivity with other mycosis was shown in this report, so multiple diagnostic tests may be required and interpretation of results should consider the possibility of infection with other etiologic agents.
Molecular assays have been developed starting in the 90s, based on DNA hybridization and PCR/qPCR based methods, some mentioned above have been used in both clinical and environmental detection schemes. Detection and genotyping commonly relies on sequencing rDNA (both 18S and ITS1-5.8S-ITS2 have been targets). The ITS2 region in particular can be targeted for species specific detection [Citation139]. A rapid and specific real-time qPCR that uses a Taq-Man probe to target a unique LTR retrotransposon detected both C. posadasii and C. immitis and is commercially available [Citation38,Citation140]. Many fungal genomes have been sequenced to date, and are available for development of sophisticated molecular tools to detect Coccidioides biomarkers [Citation17,Citation141].
Antifungal drugs/treatment
Antifungal treatment recommendations for coccidioidomycosis are dependent on the clinical severity. The duration of treatment can range from 3–12 months to lifelong treatment. The deadliest infections include meningitis or the dissemination to the central nervous system and it is recommended that these cases are given lifelong antifungal medication [Citation142]. Studies investigating the effects of ceasing azole therapy for C. immitis have further demonstrated that with any level of infection, long-term azole or triazole therapy is suggested to prevent relapse that could lead to a more serious Coccidioides infection, especially if one is immunocompromised [Citation143].
Amphotericin B was introduced in the 1950s and quickly became the antifungal drug of choice due to its efficiency in clearing systemic fungal infections [Citation144]. Although its use as an antifungal drug was life-saving, the nephrotoxicity was underestimated and renal failure, mortality rate, and additional financial costs were not trivial [Citation145]. This drug became a “Gold Standard” but was only used when other antifungal treatments failed due to side effects. This led to the development of other antifungal drugs for the treatment of VF, such as fluconazole in the early 90s that had fewer side effects and toxicity [Citation146,Citation147].
The most common class of antifungal treatments for VF are the azoles that target ergosterol biosynthesis, and the polyenes that bind ergosterol. Ergosterol is a component of the fungal cell membrane, and these drugs often cease fungal growth but are not fungicidal. Common drugs that are administered for the treatment of coccidioidomycosis are fluconazole, itraconazole (azoles), and amphotericin B (polyene) [Citation148]. Both species of Coccidioides have shown variable resistance to the listed antifungal medications [Citation149–Citation151]. Further work is needed to determine the mechanism of fluconazole resistance in Coccidioides. It is currently unclear whether emerging resistance is concerning in a clinical setting, and the conditions under which antifungal resistance needs to be monitored.
Conclusions
Coccidioidomycosis is a potentially severe and understudied fungal infection. The regions of endemicity are often associated with lower socioeconomic status, and infections may be exacerbated by health disparities and other comorbidities. Both species are found in association with animals in the desert environment, but a lack of specific knowledge of the ecology and effects of climate make prediction of the future risk of increase in disease with climate change complicated. Diagnostics are imprecise and often complicated if the host is immunosuppressed. No vaccine exists and treatment is based on standard antifungal drugs.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Posadas A. Un nuevo caso de micosis fungoidea con posrospemias. An Cir Med Argentina. 1892;15:585–597.
- Ophuls W. Further observations on a pathogenic mould formerly described as a protozoon (Coccidioides immitis, Coccidioides pyogenes). J Exp Med. 1905;6(4–6):443–485. Epub 1905/ 02/01. PubMed PMID: 19866981; PMCID: 2124510.
- Wolbach SB. The life cycle of the organism of “Dermatitis coccidioides”. J Med Res. 1904;13(1):53–60. 5. Epub 1904/12/01. PubMed PMID: 19971658; PMCID: 2099147.
- Davis BL Jr, Smith R, Smith C. An epidemic of coccidioidal infection (coccidioidomycosis). J Am Med Assoc. 1942;118(14):1182–1186.
- Tamerius JD, Comrie AC, Coccidioidomycosis incidence in Arizona predicted by seasonal precipitation. PloS one. 2011;6(6):e21009. . Epub 2011/06/28. PubMed PMID: 21701590; PMCID: 3118810.
- Untereiner WA, Scott JA, Naveau FA, et al. The Ajellomycetaceae, a new family of vertebrate-associated Onygenales. Mycologia. 2004;96(4):812–821. PubMed PMID: 21148901.
- Lee CY, Thompson GR 3rd, Hastey CJ, et al. Coccidioides endospores and spherules draw strong chemotactic, adhesive, and phagocytic responses by individual human neutrophils. PLoS One. 2015;10(6):e0129522. PubMed PMID: 26070210; PMCID: PMC4466529.
- Petkus AF, Baum LL, Ellis RB, et al. Pure spherules of Coccidioides immitis in continuous culture. J Clin Microbiol. 1985;22(2):165–167. Epub 1985/08/01.PubMed PMID: 3897262; PMCID: 268352.
- Sun SH, Huppert M. A cytological study of morphogenesis in Coccidioides immitis. Sabouraudia. 1976;14(2):185–198. Epub 1976/07/01.PubMed PMID: 959944.
- Mandel MA, Barker BM, Kroken S, et al. Genomic and population analyses of the mating type loci in Coccidioides species reveal evidence for sexual reproduction and gene acquisition. Eukaryot Cell. 2007;6(7):1189–1199. Epub 2007/05/22. PubMed PMID: 17513566; PMCID: 1951113.
- Koufopanou V, Burt A, Szaro T, et al. Gene genealogies, cryptic species, and molecular evolution in the human pathogen Coccidioides immitis and relatives (Ascomycota, Onygenales). Mol Biol Evol. 2001;18(7):1246–1258. Epub 2001/ 06/23.PubMed PMID: 11420364.
- Fisher FS, Bultman MW, Johnson SM, et al. Coccidioides niches and habitat parameters in the southwestern United States: a matter of scale. Ann N Y Acad Sci. 2007;1111:47–72. . Epub 2007/03/09. PubMed PMID: 17344527.
- Mayorga RP, Espinoza H. Coccidioidomycosis in Mexico and Central America. Mycopathol Mycol Appl. 1970;41(1):13–23. Epub 1970/01/01.PubMed PMID: 4938834.
- Campins H. Coccidioidomycosis in South America. A review of its epidemiology and geographic distribution. Mycopathol Mycol Appl. 1970;41(1):25–34. Epub 1970/01/01.PubMed PMID: 5535369.
- Wanke B, Lazera M, Monteiro PC, et al. Investigation of an outbreak of endemic coccidioidomycosis in Brazil’s northeastern state of piaui with a review of the occurrence and distribution of Coccidioides immitis in three other Brazilian states. Mycopathologia. 1999;148(2):57–67. Epub 2001/ 02/28.PubMed PMID: 11220226.
- Fisher MC, Koenig GL, White TJ, et al. Molecular and phenotypic description of Coccidioides posadasii sp. nov., previously recognized as the non-California population of Coccidioides immitis. Mycologia. 2002;94(1):73–84. Epub 2002/ 01/01.PubMed PMID: 21156479.
- Engelthaler DM, Roe CC, Hepp CM, et al. Local population structure and patterns of western hemisphere dispersal for Coccidioides spp., the fungal cause of valley fever. MBio. 2016;7(2):e00550–16. . Epub 2016/04/28. PubMed PMID: 27118594; PMCID: PMC4850269.
- Teixeira MM, Barker BM. Use of population genetics to assess the ecology, evolution, and population structure of Coccidioides. Emerg Infect Dis. 2016;22(6):1022–1030. . Epub 2016/05/19. PubMed PMID: 27191589; PMCID: PMC4880095.
- Fisher MC, Koenig GL, White TJ, et al. Biogeographic range expansion into South America by Coccidioides immitis mirrors new world patterns of human migration. Proc Natl Acad Sci U S A. 2001;98(8):4558–4562. . Epub 2001/04/05. PubMed PMID: 11287648; PMCID: 31873.
- Smith CE, Beard RR, Whiting, EG, et al. Varieties of coccidioidal infection in relation to the epidemiology and control of the diseases. Am J Public Health Nations Health. 1946;36(12):1394–1402. Epub 1946/12/01.PubMed PMID: 20278046; PMCID: PMC1624510.
- Sharpton TJ, Stajich JE, Rounsley SD, et al. Comparative genomic analyses of the human fungal pathogens Coccidioides and their relatives. Genome Res. 2009;19(10):1722–1731. Epub 2009/09/01. PubMed PMID: 19717792; PMCID: PMC2765278.
- Kolivras KN, Comrie AC. Modeling valley fever (coccidioidomycosis) incidence on the basis of climate conditions. Int J Biometeorol. 2003;47(2):87–101. Epub 2003/ 03/21. PubMed PMID: 12647095.
- Emmons CW. Coccidioidomycosis in wild rodents. Public Health Rep. 1943;58(1):1–5. . PubMed PMID: WOS:000207351100001.
- Emmons CW, Ashburn LL. The isolation of Haplosporangium parvum n. sp. and Coccidioides immitis from wild rodents. Their relationship to coccidioidomycosis. Public Health Rep. 1942;57(46):1715–1727. PubMed PMID: WOS:000207358700001.
- Maddy KT, Coccozza J. The probable geographic distribution of Coccidioides immitis in Mexico. Bol Oficina Sanit Panam. 1964;57:44–54. Epub 1964/07/01.PubMed PMID: 14175564.
- Nguyen C, Barker BM, Hoover S, et al. Recent advances in our understanding of the environmental, epidemiological, immunological, and clinical dimensions of coccidioidomycosis. Clin Microbiol Rev. 2013;26(3):505–525. Epub 2013/07/05. PubMed PMID: 23824371; PMCID: PMC3719491.
- Barker BM, Tabor JA, Shubitz LF, et al. Detection and phylogenetic analysis of Coccidioides posadasii in Arizona soil samples. Fungal Ecol. 2012;5(2):163–176. PubMed PMID: WOS:000301633500007.
- Greene DR, Koenig G, Fisher MC, et al. Soil isolation and molecular identification of Coccidioides immitis. Mycologia. 2000;92(3):406–410.
- Comrie AC. Climate factors influencing coccidioidomycosis seasonality and outbreaks. Environ Health Perspect. 2005;113(6):688–692. Epub 2005/06/03.PubMed PMID: 15929890; PMCID: 1257592.
- Lacy GH, Swatek FE. Soil ecology of Coccidioides immitis at Amerindian middens in California. Appl Microbiol. 1974;27(2):379–388. Epub 1974/02/01.PubMed PMID: 4856715; PMCID: 380039.
- Elconin AF, Egeberg RO, Egeberg MC. Significance of soil salinity on the ecology of Coccidioides immitis. J Bacteriol. 1964;87:500–503. Epub 1964/03/01.PubMed PMID: 14127564; PMCID: 277045.
- Swatek FE, Omieczynski DT. Isolation and identificationof Coccidioides immitis from natural sources. Mycopathol Mycol Appl. 1970;41(1):155–166. Epub 1970/01/01.PubMed PMID: 5520711.
- Levine HB, Winn WA. Isolation of Coccidioides immitis from soil. Health Lab Sci. 1964;1:29–32. Epub 1964/01/01.PubMed PMID: 14116583.
- Emmons CW. Isolation of Coccidioides from soil and rodents. 433021. 1942;57(4):109–111. . PubMed PMID: WOS:000207359700001.
- Lauer A, Baal JD, Baal JC, et al. Detection of Coccidioides immitis in Kern County, California, by multiplex PCR. Mycologia. 2012;104(1):62–69. . Epub 2011/ 09/22. PubMed PMID: 21933931.
- Baptista-Rosas RC, Catalán-Dibene J, Romero-Olivares AL, et al. Molecular detection of Coccidioides spp. from environmental samples in Baja California: linking valley fever to soil and climate conditions. Fungal Ecol. 2012;5(2):177–190.
- Bowers JR, Parise KL, Kelley EJ, et al. Direct detection of Coccidioides from Arizona soils using CocciENV, a highly sensitive and specific real-time PCR assay. Med Mycol;2019;57(2):246–255. DOI:10.1093/mmy/myy007. Epub 2018/ 03/14. PubMed PMID: 29534236.
- Saubolle MA, Wojack BR, Wertheimer AM, et al. Multicenter clinical validation of a cartridge-based real-time PCR system for detection of Coccidioides spp. In lower respiratory specimens. J Clin Microbiol. 2018;56(2). DOI:10.1128/JCM.01277-17. Epub 2017/ 12/08. PubMed PMID: 29212702; PMCID: PMC5786707.
- Nicas M. A point-source outbreak of coccidioidomycosis among a highway construction crew. J Occup Environ Hyg;2017. DOI:10.1080/15459624.2017.1383612. Epub 2017/ 10/21. doi PubMed PMID: 29053941.
- Colson AJ, Vredenburgh L, Guevara RE, et al. Large-scale land development, fugitive dust, and increased coccidioidomycosis incidence in the Antelope Valley of California, 1999–2014. Mycopathologia. 2017;182(5–6):439–458. Epub 2017/01/14. PubMed PMID: 28084574.
- Chow NA, Griffin DW, Barker BM, et al. Molecular detection of airborne Coccidioides in Tucson, Arizona. Med Mycol. 2016;54(6):584–592. Epub 2016/05/05. PubMed PMID: 27143633; PMCID: PMC4962330.
- Johnson L, Gaab EM, Sanchez J, et al. Valley fever: danger lurking in a dust cloud. Microbes Infect. 2014;16(8):591–600. PubMed PMID: 25038397; PMCID: PMC4250047.
- Das R, McNary J, Fitzsimmons K, et al. Occupational coccidioidomycosis in California: outbreak investigation, respirator recommendations, and surveillance findings. J Occup Environ Med. 2012;54(5):564–571. Epub 2012/ 04/17. PubMed PMID: 22504958.
- Schneider E, Hajjeh RA, Spiegel RA, et al. A coccidioidomycosis outbreak following the Northridge, Calif, earthquake. JAMA. 1997;277(11):904–908. Epub 1997/03/19. PubMed PMID: 9062329.
- Sievers ML, Fisher JR. Decreasing incidence of disseminated coccidioidomycosis among Piman and San Carlos Apache Indians. A probable environmental basis. Chest. 1982;82(4):455–460. Epub 1982/10/01.PubMed PMID: 7116964.
- Flynn NM, Hoeprich PD, Kawachi MM, et al. An unusual outbreak of windborne coccidioidomycosis. N Engl J Med. 1979;301(7):358–361. Epub 1979/08/16. PubMed PMID: 460324.
- Pappagianis D, Einstein H. Tempest from Tehachapi takes toll or Coccidioides conveyed aloft and afar. West J Med. 1978;129(6):527–530. Epub 1978/12/01.PubMed PMID: 735056; PMCID: 1238466.
- Smith CE, Beard RR, Rosenberger, HG, et al. Effect of season and dust control on coccidioidomycosis. J Am Med Assoc. 1946;132(14):833–838. Epub 1946/12/07. PubMed PMID: 20274881.
- Gorris ME, Cat LA, Zender CS, et al. Coccidioidomycosis dynamics in relation to climate in the Southwestern United States. GeoHealth. 2018;2(1):6–24.
- Alexander R, Nugent C, Nugent K. The dust bowl in the US: an analysis based on current environmental and clinical studies. Am J Med Sci. 2018;356(2):90–96. Epub 2018/ 09/17. PubMed PMID: 30219167.
- Tong DQ, Wang JXL, Gill TE, et al. Intensified dust storm activity and valley fever infection in the southwestern United States. Geophys Res Lett. 2017;44(9):4304–4312. Epub 2017/05/16. PubMed PMID: 30166741; PMCID: PMC6108409.
- Weil T, De Filippo C, Albanese D, et al. Legal immigrants: invasion of alien microbial communities during winter occurring desert dust storms. Microbiome. 2017;5(1):32. Epub 2017/03/12. PubMed PMID: 28283029; PMCID: PMC5345179.
- Baumgardner DJ. Soil-related bacterial and fungal infections. J Am Board Fam Med. 2012;25(5):734–744. Epub 2012/ 09/08. PubMed PMID: 22956709.
- Robert VA, Casadevall A. Vertebrate endothermy restricts most fungi as potential pathogens. J Infect Dis. 2009;200(10):1623–1626. Epub 2009/10/16. PubMed PMID: 19827944.
- Restrepo A, Baumgardner DJ, Bagagli E, et al. Clues to the presence of pathogenic fungi in certain environments. Med Mycol. 2000;38(Suppl 1):67–77. Epub 2001/02/24.PubMed PMID: 11204166.
- Vergara ML, Martinez R. Role of the armadillo Dasypus novemcinctus in the epidemiology of paracoccidioidomycosis. Mycopathologia. 1998;144(3):131–133. Epub 1999/ 10/26. PubMed PMID: 10531678.
- Baumgardner DJ, Paretsky DP. The in vitro isolation of Blastomyces dermatitidis from a woodpile in North Central Wisconsin, USA. Med Mycol. 1999;37(3):163–168. Epub 1999/07/28. PubMed PMID: 10421847.
- Baumgardner DJ, Buggy BP, Mattson BJ, et al. Epidemiology of blastomycosis in a region of high endemicity in North Central Wisconsin. Clin Infect Dis. 1992;15(4):629–635. Epub 1992/ 10/01.PubMed PMID: 1420675.
- Chaturvedi VP, Randhawa HS, Chaturvedi S, et al. In vitro interactions between Blastomyces dermatitidis and other zoopathogenic fungi. Can J Microbiol. 1988;34(7):897–900. Epub 1988/07/01.PubMed PMID: 3058276.
- DiSalvo AF. The Ecology of Blastomyces dermatitidis. In: Al-Doory Y, DiSalvo AF, editors. Blastomycosis. Boston, MA: Springer; 1992. p. 43–73.
- Lewis ER, Bowers JR, Barker BM. Dust devil: The life and times of the fungus that causes valley fever. PLoS Pathog. 2015;11(5):e1004762. Epub 2015/ 05/15. PubMed PMID: 25973899; PMCID: PMC4431877.
- Tamreihao K, Mukherjee S, Khunjamayum R, et al. Feather degradation by keratinolytic bacteria and biofertilizing potential for sustainable agricultural production. J Basic Microbiol;2018. DOI:10.1002/jobm.201800434. Epub 2018/ 10/26. PubMed PMID: 30353928.
- Maddy KT, Crecelius HG. Establishment of Coccidiodies immitis in negative soil following burial of infected animal tissues. In: Ajello L, editor. The second symposium on Coccidioidomycosis. Phoenix (AZ): The University of Arizona Press; 1965. p. 309–312.
- Vargas-Gastelum L, Romero-Olivares AL, Escalante AE, et al. Impact of seasonal changes on fungal diversity of a semi-arid ecosystem revealed by 454 pyrosequencing. FEMS Microbiol Ecol. 2015;91(5). DOI:10.1093/femsec/fiv044. PubMed PMID: 25877341.
- Del Rocio Reyes-Montes M, Perez-Huitron MA, Ocana-Monroy JL, et al. The habitat of Coccidioides spp. and the role of animals as reservoirs and disseminators in nature. BMC Infect Dis. 2016;16(1):550. PubMed PMID: 27724885.
- Eulalio KD, de Macedo RL, Cavalcanti MA, et al. Coccidioides immitis isolated from armadillos (Dasypus novemcinctus) in the state of Piaui, northeast Brazil. Mycopathologia. 2001;149(2):57–61. Epub 2001/ 03/29.PubMed PMID: 11270394.
- Cordeiro RA, E Silva KR, Brilhante RS, et al. Coccidioides posadasii infection in bats, Brazil. Emerg Infect Dis. 2012;18(4):668–670. Epub 2012/04/04. PubMed PMID: 22469192; PMCID: 3309697.
- Brown J, Benedict K, Park BJ, et al. Coccidioidomycosis: epidemiology. Clin Epidemiol. 2013;5:185–197. Epub 2013/ 07/12. PubMed PMID: 23843703; PMCID: 3702223.
- Cramer RA, Hohl TM. Some fungi in the air. Interview by Sophia Hafner. Microbes Infect. 2013;15(4):255–258. Epub 2013/02/05. PubMed PMID: 23376622; PMCID: PMC5563158.
- Willger SD, Grahl N, Cramer RA Jr. Aspergillus fumigatus metabolism: clues to mechanisms of in vivo fungal growth and virulence. Med Mycol. 2009;47(Suppl 1):S72–9. Epub 2009/ 03/03. PubMed PMID: 19253141; PMCID: PMC2905159.
- Latge JP. Aspergillus fumigatus and aspergillosis. Clin Microbiol Rev. 1999;12(2):310–350. Epub 1999/04/09.PubMed PMID: 10194462; PMCID: PMC88920.
- Valdes ID, van Den Berg J, Haagsman A, et al. Comparative genotyping and phenotyping of Aspergillus fumigatus isolates from humans, dogs and the environment. BMC Microbiol. 2018;18(1):118. Epub 2018/09/19. PubMed PMID: 30223790; PMCID: PMC6142626.
- Park BJ, Sigel K, Vaz V, et al. An epidemic of coccidioidomycosis in Arizona associated with climatic changes, 1998–2001. J Infect Dis. 2005;191(11):1981–1987. Epub 2005/05/05. PubMed PMID: 15871133.
- Hugenholtz P. Climate and Coccidioidomycosis. In: Proceedings of the Symposium on Coccidioidomycosis. Phoenix (AZ): U.S. Public Health Service Publication 575; 1957. p. 136–143.
- Maddy KT. The geographic distribution of Coccidioides immitis and possible ecologic implications. Arizona Med. 1958;15(3):178–188. Epub 1958/03/01. PubMed PMID: 13510095.
- Maddy KT. Ecological factors of the geographic distribution of Coccidioides immitis. J Am Vet Med Assoc. 1957;130(11):475–476. Epub 1957/06/01. PubMed PMID: 13438738.
- Maddy KT. Observations on Coccidioides immitis found growing naturally in soil. Arizona Med. 1965;22:281–288. Epub 1965/04/01.PubMed PMID: 14262164.
- Benedict K, Thompson GR 3rd, Deresinski S, et al. Mycotic infections acquired outside areas of known endemicity, United States. Emerg Infect Dis. 2015;21(11):1935–1941. Epub 2015/10/21. PubMed PMID: 26485441; PMCID: PMC4622235.
- Garfin G, Franco G, Blanco H, et al., editors. Southwest: the third national climate assessment. Washington, DC: U.S. Global Change Research Program; 2014.
- Cayan DR, Tyree M, Kunkel KE, et al. Future climate: projected average. Washington, DC: Southwest Climate Alliance; 2013.
- Friedman L, Smith CE. Vaccination of mice against Coccidoides immitis. Am Rev Tuberc. 1956;74(2 Part 1):245–248. Epub 1956/08/01.PubMed PMID: 13340152.
- Vogel RA, Fetter BF, Conant NF, et al. Preliminary studies on artificial active immunization of guinea pigs against respiratory challenge with Coccidioides immitis. Am Rev Tuberculosis. 1954;70(3):498–503. Epub 1954/09/01.PubMed PMID: 13189065.
- Vogel RA, Conant NF. Coccidioides immitis spherule antigen in a complement fixation test for experimental coccidioidomycosis. Proc Soc Exp Biol Med. 1952;79(3):544–547. Epub 1952/03/01.PubMed PMID: 14920489.
- Converse JL, Deauville GA, Snyder EM, et al. Control of tissue reactions in monkeys vaccinated with viable Coccidioides immitis by prevaccination with killed coccidioides immitis. J Bacteriol. 1965;90(3):783–788. Epub 1965/09/01.PubMed PMID: 16562081; PMCID: 315725.
- Castleberry MW, Converse JL, Sinski JT, et al. Coccidioidomycosis: studies of canine vaccination and therapy. J Infect Dis. 1965;115:41–48. Epub 1965/ 02/01.PubMed PMID: 14260175.
- Levine HB, Kong YC, Smith C. Immunization of mice to Coccidioides immitis: dose, regimen and spherulation stage of killed spherule vaccines. J Immunol. 1965;94:132–142. Epub 1965/ 01/01.PubMed PMID: 14253511.
- Levine HB, Cobb JM, Smith CE. Immunity to coccidioidomycosis induced in mice by purified spherule, arthrospore, and mycelial vaccines. Trans N Y Acad Sci. 1960;22:436–449. Epub 1960/04/01.PubMed PMID: 14416255.
- Pappagianis D. Evaluation of the protective efficacy of the killed Coccidioides immitis spherule vaccine in humans. The valley fever vaccine study group. Am Rev Respir Dis. 1993;148(3):656–660. Epub 1993/09/01. PubMed PMID: 8368636.
- Shubitz L, Peng T, Perrill R, et al. Protection of mice against Coccidioides immitis intranasal infection by vaccination with recombinant antigen 2/PRA. Infect Immun. 2002;70(6):3287–3289. Epub 2002/05/16.PubMed PMID: 12011027; PMCID: 127985.
- Pan S, Cole GT. Molecular and biochemical characterization of a Coccidioides immitis-specific antigen. Infect Immun. 1995;63(10):3994–4002. Epub 1995/10/01.PubMed PMID: 7558310; PMCID: 173561.
- Peng T, Orsborn KI, Orbach MJ, et al. Proline-rich vaccine candidate antigen of Coccidioides immitis: conservation among isolates and differential expression with spherule maturation. J Infect Dis. 1999;179(2):518–521. Epub 1999/ 01/07. PubMed PMID: 9878042.
- Shubitz LF, Yu JJ, Hung CY, et al. Improved protection of mice against lethal respiratory infection with Coccidioides posadasii using two recombinant antigens expressed as a single protein. Vaccine. 2006;24(31–32):5904–5911. Epub 2006/06/09. PubMed PMID: 16759762.
- Xue J, Chen X, Selby D, et al. A genetically engineered live attenuated vaccine of Coccidioides posadasii protects BALB/c mice against coccidioidomycosis. Infect Immun. 2009;77(8):3196–3208. Epub 2009/06/03. PubMed PMID: 19487479; PMCID: 2715678.
- Shubitz LF, Powell DA, Trinh HT, et al. Viable spores of Coccidioides posadasii delta-cps1 are required for vaccination and provide long lasting immunity. Vaccine. 2018;36(23):3375–3380. . Epub 2018/05/05. PubMed PMID: 29724507.
- Narra HP, Shubitz LF, Mandel MA, et al. A Coccidioides posadasii CPS1 deletion mutant is avirulent and protects mice from lethal infection. Infect Immun. 2016;84(10):3007–3016. PubMed PMID: 27481239.
- Cooksey GS, Nguyen A, Knutson K, et al. Notes from the Field: increase in coccidioidomycosis - California, 2016. MMWR Morb Mortal Wkly Rep. 2017;66(31):833–834. . Epub 2017/ 08/11. PubMed PMID: 28796756.
- Hector RF, Rutherford GW, Tsang CA, et al. The public health impact of coccidioidomycosis in Arizona and California. Int J Environ Res Public Health. 2011;8(4):1150–1173. Epub 2011/06/23. PubMed PMID: 21695034; PMCID: 3118883.
- Palmer CE, Edwards PQ, Allfather WE. Characteristics of skin reactions to coccidioidin and histoplasmin, with evidence of an unidentified source of sensitization. Am J Hyg. 1957;66(2):196–213. PubMed PMID: 13458183.
- Edwards PQ, Palmer CE. Prevalence of sensitivity to coccidioidin, with special reference to specific and nonspecific reactions to coccidioidin and to histoplasmin. Dis Chest. 1957;31(1):35–60. PubMed PMID: 13384171.
- Levine HB, Gonzalez-Ochoa A, Ten Eyck DR. Dermal sensitivity to Coccidioides immitis. A comparison of responses elicited in man by spherulin and coccidioidin. Am Rev Respir Dis. 1973;107(3):379–386. Epub 1973/03/01.PubMed PMID: 4690486.
- Ampel NM. What’s behind the increasing rates of coccidioidomycosis in Arizona and California? Curr Infect Dis Rep. 2010;12(3):211–216. Epub 2011/ 02/11. PubMed PMID: 21308532.
- Coopersmith EJ, Bell JE, Benedict K, et al. Relating coccidioidomycosis (valley fever) incidence to soil moisture conditions. Geohealth. 2017;1:51–63. Epub 2017/ 11/11. PubMed PMID: 29124249; PMCID: PMC5672948.
- Talamantes J, Behseta S, Zender CS. Fluctuations in climate and incidence of coccidioidomycosis in Kern County, California: a review. Ann N Y Acad Sci. 2007;1111:73–82. Epub 2007/ 03/10. PubMed PMID: 17347336.
- Comrie AC, Glueck MF. Assessment of climate-coccidioidomycosis model: model sensitivity for assessing climatologic effects on the risk of acquiring coccidioidomycosis. Ann N Y Acad Sci. 2007;1111:83–95. Epub 2007/ 03/09. PubMed PMID: 17344540.
- Odio CD, Marciano BE, Galgiani JN, et al. Risk factors for disseminated coccidioidomycosis, United States. Emerg Infect Dis. 2017;23(2). DOI:10.3201/eid2302.160505. PubMed PMID: 28098554.
- Lee LA, Yuan J, Vugia D, et al. Increased coccidioidomycosis among inmates at a California prison: initial investigation in 2005 to 2006. J Correct Health Care. 2017;23(3):347–352. Epub 2017/ 06/29. PubMed PMID: 28656821.
- Wheeler C, Lucas KD, Mohle-Boetani JC. Rates and risk factors for Coccidioidomycosis among prison inmates, California, USA, 2011. Emerg Infect Dis. 2015;21(1):70–75. PubMed PMID: 25533149; PMCID: 4285255.
- Huang JY, Bristow B, Shafir S, et al. Coccidioidomycosis-associated deaths, United States, 1990–2008. Emerg Infect Dis. 2012;18(11):1723–1728. Epub 2012/10/25. PubMed PMID: 23092645; PMCID: 3559166.
- Ruddy BE, Mayer AP, Ko MG, et al. Coccidioidomycosis in African Americans. Mayo Clin Proc Mayo Clin. 2011;86(1):63–69. Epub 2011/01/05. PubMed PMID: 21193657; PMCID: 3012635.
- Tabor JA, O’Rourke MK. A risk factor study of coccidioidomycosis by controlling differential misclassifications of exposure and susceptibility using a landscape ecology approach. Sci Total Environ. 2010;408(10):2199–2207. . Epub 2010/03/02. PubMed PMID: 20188397.
- Ampel NM. Coccidioidomycosis in persons infected with HIV-1. Ann N Y Acad Sci. 2007;1111:336–342. Epub 2007/ 03/17. PubMed PMID: 17363429.
- Santelli AC, Blair JE, Roust LR. Coccidioidomycosis in patients with diabetes mellitus. Am J Med. 2006;119(11):964–969. Epub 2006/10/31. PubMed PMID: 17071165.
- Blair JE, Douglas DD. Coccidioidomycosis in liver transplant recipients relocating to an endemic area. Dig Dis Sci. 2004;49(11–12):1981–1985. Epub 2005/ 01/05.PubMed PMID: 15628738.
- Bergstrom L, Yocum DE, Ampel NM, et al. Increased risk of coccidioidomycosis in patients treated with tumor necrosis factor alpha antagonists. Arthritis Rheumatism. 2004;50(6):1959–1966. Epub 2004/06/10. PubMed PMID: 15188373.
- Rosenstein NE, Emery KW, Werner SB, et al. Risk factors for severe pulmonary and disseminated coccidioidomycosis: Kern County, California, 1995–1996. Clin Infect Dis. 2001;32(5):708–715. Epub 2001/ 03/07. PubMed PMID: 11229838.
- Freedman M, Jackson BR, McCotter O, et al. Coccidioidomycosis outbreaks, United States and Worldwide, 1940–2015. Emerg Infect Dis. 2018;24(3):417–423. Epub 2018/02/21. PubMed PMID: 29460741; PMCID: PMC5823332.
- Laws RL, Cooksey GS, Jain S, et al. Coccidioidomycosis outbreak among workers constructing a solar power farm - Monterey County, California, 2016–2017. MMWR Morb Mortal Wkly Rep. 2018;67(33):931–934. Epub 2018/08/24. PubMed PMID: 30138303; PMCID: PMC6107319 potential conflicts of interest. No potential conflicts of interest were disclosed.
- Wilken JA, Marquez P, Terashita D, et al. Centers for Disease Control and Prevention. Coccidioidomycosis among cast and crew members at an outdoor television filming event–California, 2012. MMWR Morb Mortal Wkly Rep. 2014;63(15):321–324. PubMed PMID: 24739339.
- Cummings KC, McDowell A, Wheeler C, et al. Point-source outbreak of coccidioidomycosis in construction workers. Epidemiol Infect. 2010;138(4):507–511. Epub 2009/ 10/23. PubMed PMID: 19845993.
- Petersen LR, Marshall SL, Barton-Dickson C, et al. Coccidioidomycosis among workers at an archeological site, northeastern Utah. Emerg Infect Dis. 2004;10(4):637–642. Epub 2004/06/18. PubMed PMID: 15200853; PMCID: 3323065.
- Gehlbach SH, Hamilton JD, Conant NF. Coccidioidomycosis. An occupational disease in cotton mill workers. Arch Internal Med. 1973;131(2):254–255. Epub 1973/02/01.PubMed PMID: 4682985.
- Levan NE, Huntington RW Jr. Primary cutaneous coccidioidomycosis in agricultural workers. Arch Dermatol. 1965;92:215–220. Epub 1965/09/01.PubMed PMID: 14329224.
- Benedict K, Purfield AE, Mohle-Boetani J, et al. Awareness and environmental exposures related to coccidioidomycosis among inmates at two California prisons, 2013. J Correct Health Care. 2016;22(2):157–163. Epub 2016/ 03/18. PubMed PMID: 26984139.
- de Perio MA, Niemeier RT, Burr GA. Coccidioides exposure and coccidioidomycosis among prison employees, California, United States. Emerg Infect Dis. 2015;21(6):1031–1033. PubMed PMID: 25989420.
- Burwell LA, Park BJ, Wannemuehler KA, et al. Outcomes among inmates treated for coccidioidomycosis at a correctional institution during a community outbreak, Kern county, California, 2004. Clin Infect Dis. 2009;49(11):e113–9. . Epub 2009/ 11/06. PubMed PMID: 19886797.
- Pappagianis D. Coccidioidomycosis in California state correctional institutions. Ann N Y Acad Sci. 2007;1111:103–111. . Epub 2007/ 03/03. PubMed PMID: 17332089.
- Shubitz LF, Dial SM. Coccidioidomycosis: a diagnostic challenge. Clin Tech Small Anim Pract. 2005;20(4):220–226. Epub 2005/ 12/02. PubMed PMID: 16317911.
- Shubitz LE, Butkiewicz CD, Dial SM, et al. Incidence of Coccidioides infection among dogs residing in a region in which the organism is endemic. J Am Vet Med Assoc. 2005;226(11):1846–1850. Epub 2005/06/09.PubMed PMID: 15938056.
- Navalkar KA, Johnston SA, Woodbury N, et al. Application of immunosignatures for diagnosis of valley fever. Clin Vaccine Immunol. 2014;21(8):1169–1177. Epub 2014/06/27. PubMed PMID: 24964807; PMCID: PMC4135907.
- Smith CE. Diagnosis of pulmonary coccidioidal infections. Calif Med. 1951;75(6):385–394. Epub 1951/12/01.PubMed PMID: 14886741; PMCID: PMC1521097.
- Smith CE, Saito MT, Beard RR, et al. Serological tests in the diagnosis and prognosis of coccidioidomycosis. Am J Hyg. 1950;52(1):1–21. Epub 1950/07/01.PubMed PMID: 15432437.
- Wack EE, Ampel NM, Sunenshine RH, et al. The return of delayed-type hypersensitivity skin testing for coccidioidomycosis. Clin Infect Dis. 2015;61(5):787–791. . PubMed PMID: 25979308.
- Pappagianis D, Zimmer BL. Serology of coccidioidomycosis. Clin Microbiol Rev. 1990;3(3):247–268. Epub 1990/07/01.PubMed PMID: 2200605; PMCID: 358158.
- Huppert M, Peterson ET, Sun SH, et al. Evaluation of a latex particle agglutination test for coccidioidomycosis. Am J Clin Pathol. 1968;49(1):96–102. Epub 1968/01/01.PubMed PMID: 5635281.
- Martins TB, Jaskowski TD, Mouritsen CL, et al. Comparison of commercially available enzyme immunoassay with traditional serological tests for detection of antibodies to Coccidioides immitis. J Clin Microbiol. 1995;33(4):940–943. Epub 1995/04/01.PubMed PMID: 7790465; PMCID: 228072.
- Blair JE, Coakley B, Santelli AC, et al. Serologic testing for symptomatic coccidioidomycosis in immunocompetent and immunosuppressed hosts. Mycopathologia. 2006;162(5):317–324. Epub 2006/11/24. PubMed PMID: 17123029; PMCID: 2780641.
- de Aguiar Cordeiro R, Patoilo KR, Praciano SB, et al. Antigens of Coccidioides posadasii as an important tool for the immunodiagnosis of coccidioidomycosis. Mycopathologia. 2013;175(1–2):25–32. Epub 2012/ 12/18. PubMed PMID: 23242703.
- Zangeneh TT, Malo J, Luraschi-Monjagatta C, et al. Positive (1–3) B-d-glucan and cross reactivity of fungal assays in coccidioidomycosis. Med Mycol. 2015;53(2):171–173. PubMed PMID: 25541557.
- Millar BC, Jiru X, Walker MJ, et al. False identification of Coccidioides immitis: Do molecular methods always get it right? J Clin Microbiol. 2003;41(12):5778–5780. Epub 2003/ 12/10. PubMed PMID: 14662981; PMCID: 308958.
- Bowers JR, Parise KL, Kelley E, et al. Direct detection of Coccidioides from Arizona soils using cocciENV, a highly sensitive and specific real-time PCR assay. Med Mycol. 2019;57(2):246–255.
- Neafsey DE, Barker BM, Sharpton TJ, et al. Population genomic sequencing of Coccidioides fungi reveals recent hybridization and transposon control. Genome Res. 2010;20(7):938–946. . Epub 2010/06/03. PubMed PMID: 20516208; PMCID: 2892095.
- Galgiani JN, Ampel NM, Blair JE, et al. 2016 infectious diseases society of America (IDSA) clinical practice guideline for the treatment of coccidioidomycosis. Clin Infect Dis. 2016;63(6):e112–46. PubMed PMID: 27470238.
- Stewart ER, Eldridge ML, McHardy I, et al. Liposomal amphotericin B as monotherapy in relapsed Coccidioidal meningitis. Mycopathologia. 2018;183(3):619–622. . Epub 2018/01/18. PubMed PMID: 29340909.
- Oura M, Sternberg TH, Wright ET. A new antifungal antibiotic, amphotericin B. Antibiot Annu. 1955;3:566–573. PubMed PMID: 13355328.
- Ostrosky-Zeichner L, Marr KA, Rex JH, et al. Amphotericin B: time for a new “gold standard”. Clin Infect Dis. 2003;37(3):415–425. Epub 2003/ 07/29. PubMed PMID: 12884167.
- Catanzaro A, Fierer J, Friedman PJ. Fluconazole in the treatment of persistent coccidioidomycosis. Chest. 1990;97(3):666–669. Epub 1990/03/01.PubMed PMID: 2306969.
- Classen DC, Burke JP, Smith CB. Treatment of coccidioidal meningitis with fluconazole. J Infect Dis. 1988;158(4):903–904. Epub 1988/10/01.PubMed PMID: 2844925.
- Hartmann CA, Aye WT, Blair JE. Treatment considerations in pulmonary coccidioidomycosis. Expert Rev Respir Med. 2016;10(10):1079–1091. . PubMed PMID: 27635942.
- Kriesel JD, Sutton DA, Schulman S, et al. Persistent pulmonary infection with an azole-resistant coccidioides species. Med Mycol. 2008;46(6):607–610. . Epub 2008/ 07/09 PubMed PMID: 18608910.
- Ramani R, Chaturvedi V. Antifungal susceptibility profiles of coccidioides immitis and coccidioides posadasii from endemic and non-endemic areas. Mycopathologia. 2007;163(6):315–319. . Epub 2007/05/08. PubMed PMID: 17484074.
- Thompson GR 3rd, Barker BM, Wiederhold NP. Large-scale evaluation of in vitro amphotericin B, triazole, and echinocandin activity against Coccidioides species from U.S. Institutions. Antimicrob Agents Chemother. 2017;61(4). DOI:10.1128/AAC.02634-16. Epub 2017/01/18. PubMed PMID: 28096163; PMCID: PMC5365710.
