ABSTRACT
MvaT and MvaU are global transcriptional regulators belonging to the H-NS family, and pyocyanin is an important virulence factor produced by Pseudomonas aeruginosa. mvaT mvaU double knockout mutant of P. aeruginosa PAO1 demonstrated pyocyanin abolishment in the previous study. Here, we further explored the mechanism. Two main directions were studied: pyocyanin biosynthesis pathway and QS system. The effect on the expression of the pyocyanin biosynthesis genes was evaluated by promoter strength determination and Real-Time PCR assay, and significant changes leading to low pyocyanin production were found. The effect on the QS system was studied by signal molecule quantification using LC-MS/MS and related gene expression measurements using Real-Time PCR. In mvaT mvaU double knockout, the production of 3-oxo-C12-HSL obviously increased, while those of C4-HSL and PQS obviously decreased, and the changes can be recovered by mvaT or mvaU complementation. The expressions of transcriptional activator genes binding with QS system signal molecules were all decreased, resulting in decreased formation of signal-transcriptional activator complexes. And the decreased expression of rhlR and pqsE also led to the lower expression of phzA1 and phzA2. Further exploration found that QS system downregulation may be related to QsrO, a QS system repressor, which was highly upregulated with mvaT mvaU double knockout. Hence, the synthesis of pyocyanin was suffocated and the biofilm formation ability was decreased. These results were also confirmed by transcriptome analysis, which demonstrated similar gene expression changes of the aforementioned genes together with decreased expression of other virulence factor genes regulated by QS system.
KEYWORDS:
Introduction
Pseudomonas aeruginosa (P. aeruginosa) is an aerobic Gram-negative bacterium that can cause both community-acquired and hospital-acquired infections, posing a particular threat to cystic fibrosis patients, traumatic burn victims, patients with implanted medical devices and immunocompromised individuals [Citation1–Citation3]. P. aeruginosa is formidable because of the intrinsic ability to develop antibiotic resistance, formation of impenetrable biofilms and releasing a large number of virulence factors [Citation4]. Pyocyanin (PYO) is a redox-active virulence factor produced by P. aeruginosa that can easily penetrate biological membranes. This secondary metabolite helps P. aeruginosa accept and transport electrons produced in respiration so the bacteria can survive under oxygen-poor conditions [Citation5]. Pyocyanin has been shown to induce oxidative stress, affect endothelial cell redox status, and cause loss of porosity in the liver sinusoidal endothelial cells [Citation6,Citation7]. Pyocyanin can increase intracellular levels of reactive oxygen species (ROS) and result in oxidative damage to components of the cell cycle and direct damage to DNA [Citation8,Citation9]. In addition, pyocyanin is associated with a decline in lung function and contributes to the dominance of P. aeruginosa in the CF lung [Citation10]. Significant levels of pyocyanin have been detected in sputum sol, ear secretions, wounds, and urine in chronic infections caused by P. aeruginosa [Citation11–Citation13]. Moreover, pyocyanin plays a major role in animal models of acute and chronic infection caused by P. aeruginosa [Citation14].
Pyocyanin is synthesized through a series of complex steps mediated by gene products encoded by two phzABCDEFG operons and the phzH, phzM, phzS genes. In the pyocyanin synthetic pathway, chorismic could be transformed into phenazine-1-carboxylic acid by the PhzA-G proteins firstly. Subsequently, phenazine-1-carboxylic acid could be converted to pyocyanin by PhzM and PhzS () [Citation15]. The synthesis is regulated by quorum sensing (QS), which involves in cell-density-dependent accumulation of signal molecules that enable bacteria to modulate the expression of virulence genes [Citation16–Citation18] () and can be repressed by qsrO [Citation19]. Several independent studies have revealed that other gene mutations could also influence the synthesis of pyocyanin, such as kinB [Citation20], gbuA [Citation21], gacA-gacS and vfr [Citation16]. MvaT and MvaU are global transcriptional regulators belonging to the H-NS family of P. aeruginosa, binding the same chromosomal regions, and coregulating the expression of about 350 target genes [Citation22]. Pyocyanin synthesis was induced in the mvaT mvaU single mutants but was completely abolished in the double knockout mutant [Citation23].
Figure 1. Biosynthesis and signaling system of pyocyanin [Citation15]. Chorismic acid is transformed into phenazine-1-carboxylic acid by the PhzA to G proteins. Then, phenazine-1-carboxylic acid is subsequently converted into different phenazines by the enzymes PhzH, PhzS, and PhzM, respectively. The product of the 5-methylphenazine-carboxylic acid betaine is further transformed into pyocyanin (PYO) by PhzS
![Figure 1. Biosynthesis and signaling system of pyocyanin [Citation15]. Chorismic acid is transformed into phenazine-1-carboxylic acid by the PhzA to G proteins. Then, phenazine-1-carboxylic acid is subsequently converted into different phenazines by the enzymes PhzH, PhzS, and PhzM, respectively. The product of the 5-methylphenazine-carboxylic acid betaine is further transformed into pyocyanin (PYO) by PhzS](/cms/asset/b8db6a41-6188-4136-8a7d-134a95f67523/kvir_a_1708052_f0001_oc.jpg)
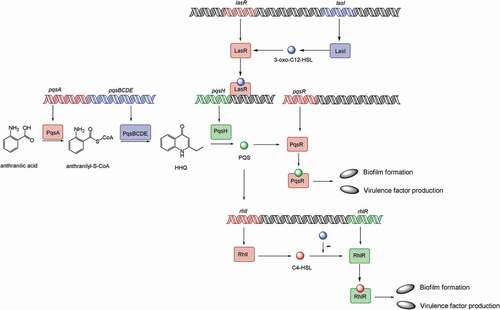
Figure 2. Model of the P. aeruginosa quorum-sensing hierarchy. When cells reach a threshold density, the las quorum sensing will be induced. LasI directs the synthesis of 3-oxo-C12-HSL, which then binds to and activates LasR. LasR regulates the production of PQS, which is conversed by PqsH from HHQ, catalyzed by PqsA-E. PQS either directly or indirectly induces rhlI, which leads to the production of C4-HSL that binds to and activates RhlR. Hence, PQS constitutes a regulatory link between the las and rhl quorum-sensing system. PQS binds to the transcriptional regulator PqsR to regulate biofilm formation and virulence factor production. The RhlR–C4-HSL complex can induce genes controlled by the rhl quorum-sensing system, such as biofilm formation and virulence factor production. 3-oxo-C12-HSL has an inhibitory effect on the association between RhlR and C4-HSL
In this study, we aim to demonstrate the mechanism of pyocyanin abolishment in P. aeruginosa PAO1 mvaT mvaU double knockout mutant. First, we compared the virulence of mvaT mvaU double mutant to the parental strain and the single knockout mutants by a mouse systemic infection model and biofilm formation assay. Then, the effects of mvaT mvaU double knockout on the pyocyanin synthesis genes and the quorum-sensing system were studied using promoter strength determination, Real-Time PCR and LC-MS/MS. The results were also confirmed by transcriptome analysis.
Materials and methods
Bacterial strains and culture conditions
Strains (from the CAMS Collection Center of Pathogen Microorganisms, CAMS-CCPM-A) and plasmids used in this study are listed in Table S1. P. aeruginosa PAO1 wild type strain and mvaT mvaU single or double knockout mutants were routinely cultured in Luria-Bertani (LB) broth or on LB agar plates.
Construction of mutant strains and plasmids
Primers used in this study are listed in Table S2. mvaT mvaU single or double knockout mutants were generated using an allelic exchange as previously described [Citation23]. The authenticity of the mutants was confirmed by PCR, RT-PCR and gene sequencing. For complementation of mvaT and mvaU genes, plasmids pUCP-T and pUCP-U carrying full lengths of mvaT or mvaU were constructed as before [Citation23] and confirmed by DNA sequence analysis.
Pyocyanin quantitation assay
Pyocyanin was extracted and quantified from P. aeruginosa PAO1 and the mvaT mvaU single or double knockout mutants as previously described [Citation24]. Pyocyanin was extracted with 3 mL chloroform from 4 mL cells cultures grown at 37°C for 24 h in glycerol-alanine medium and then reextracted into 2 mL of 0.2 M HCl. The A520 of the resulting solution was measured and the concentration of pyocyanin was determined using an extinction coefficient of 2460 M-1 cm-1. The experiments were performed in triplicate on different days.
β-galactosidase activity assays
DNA fragments containing the regulatory regions of phzA1-G1, phzA2-G2, phzH, phzM, or phzS were amplified by PCR from the genomic DNA of strain PAO1 with Prime STAR polymerase and the primers listed in Table S2. The PCR products were purified and ligated into pQF50, a broad-host-range lacZ transcriptional fusion vector. The nucleotide sequences of the resulting constructs were verified by nucleotide sequence determination. The β-galactosidase activity was measured with o-Nitrophenyl beta-D-galactopyranoside (ONPG) as the substrate according to procedures from the protocol of Bacterial Adenylate Cyclase Two-Hybrid System Kit, EUROMEDEX. Briefly, bacterial cells were grown in LB broth in the presence of 0.5 mM IPTG and 150 μg/mL of carbenicillin at 30°C till OD600 ≈ 0.3. After 30 μL of chloroform and 30 μL of 0.1% SDS solution were added to 2.5 mL of cell suspensions, the cultures were vigorously agitated in a shaker at 37°C for 40 min. 0.1 mL of the cells was added to 0.9 mL of PM2 buffer (70 mM Na2HPO4.12H2O, 30 mM NaH2PO4 H2O, 1 mM MgSO4, 0.2 mM MnSO4, pH 7.0, add 100 mM β-mercaptoethanol just before use) and were placed in a water bath at 28°C for 5 min. The enzymatic reaction was started by adding 0.25 mL of the ONPG substrate solution and stopped by 0.5 mL of the 1 M Na2CO3. The OD420 was recorded for calculating the enzymatic activities with the correction of bacterial cell absorbance by OD600. The experiments were performed in triplicate on different days.
In vivo infection evaluation
All mice (ICR, female, 18–20 g) were obtained from Beijing Vital River Laboratory Animal Technology Co. Ltd. Mice were infected intraperitoneally with 0.5 mL bacterial suspensions of P. aeruginosa PAO1 or mvaT mvaU single/double knockouts in 5% mucin. The experiments were performed in triplicate on different days. The animal husbandry and experiments were performed according to national standards of laboratory animals in China (GB/T 35892–2018) [Citation25]. A log-rank test was applied to compare the survival distributions of animals infected by different strains.
Biofilm formation assay
Biofilm quantification assays were performed in microtiter plates using crystal violet staining according to published protocols [Citation26]. Overnight cultures were diluted to 1 ~ 2 × 106 CFU/mL in Brain Heart Infusion (BHI) broth, 200 μL was allocated to each well of a flat 96-well microtiter plate (Corning, 3599) and cultured at 37°C for 24 h. Planktonic cells were removed and wells were washed with physiological saline. 200 μL crystal violet solution (0.1%, v/v) was added to each well and incubated at 37°C for 15 min. Then, crystal violet solution was removed and wells were washed with double-distilled water 3 times. 200 μL of glacial acid (30%, v/v) was added to each well and absorbance was measured at 595 nm using Perkin Elmer 2300 EnSpire Multilabel Plate Reader.
Relative growth rate assay
The exponential growth rates of the mutant strains were measured in CAMH broth at 37°C by taking optical density at 600 nm (OD600) every 4 min in a Bioscreen C reader (Oy Growth Curves Ab Ltd, FP-1100-C). Four independent cultures per strain were grown overnight until saturation. The cultures were 1000-fold diluted and aliquoted into a Bioscreen C plate in duplicate (0.3 mL/well). The growth rates were estimated from the OD600 interval between 0.01 and 0.1, where the growth was observed to be exponential. Relative growth rates of the strains were calculated by comparing the growth rates with that of PAO1.
Gene expression determined by real-time PCR
P. aeruginosa PAO1 and mvaT mvaU mutants were grown in LB broth at 37°C with shaking (220 rpm) until OD600 ≈ 0.4 (in log phase) or OD600 ≈ 1.0 (in early stationary phase); then, 1.5 mL of cells was harvested by centrifugation at 4°C. The RNA extraction of bacteria was performed using RNAprep Pure Cell/Bacteria Kit (TIANGEN), and mRNA was reversed to cDNA using FastQuant RT Kit (TIANGEN). The Real-Time PCR reaction mixture (20 μL) contained 10 μL 2 × Power SYBR Green PCR Master Mix (Applied biosystems by Life technologies), 2 μL forward and reverse primer mix (10 μM each, sequences are listed in Table S2), μL template cDNA and 7 μL nuclease-free water. The cycling conditions were 50°C for 2 s, 95°C for 10 min followed by 40 cycles at 95 oC for 15 s and 60°C for 1 min using 7500 Fast Real-Time PCR System (Applied Biosystems TM). The experiments were performed in triplicate on different days.
Table 1. Quantitation of pyocyanin in P. aeruginosa PAO1 and the knockout mutants
Quantitation of quorum-sensing signaling molecules using LC-MS/MS
The quantitation of AHLs (3-oxo-C12-HSL, C4-HSL) and PQS was performed as previously described [Citation27–Citation30]. Briefly, strains were grown at 37°C in LB broth until OD600 ≈ 1.0. For extraction of AHLs, samples were centrifuged at 10,000 g in a Thermo Fresco21 tabletop centrifuge for 20 min at 4°C. 0.6 mL of liquid supernatant was extracted with acid ethyl acetate. The organic phase was dried using a vacuum freeze dryer (CHRIST, ALPHA2-4 LD pius), resolubilized by methanol and filtered with millex (0.22 μM, MERCK). For extraction of PQS, 0.5 mL of cultures were mixed with isovolumic methanol, and samples were centrifuged at 10,000 g in a Thermo Fresco21 tabletop centrifuge for 20 min at 4°C. The supernatant was filtered with millex (0.22 μM, MERCK) and used for LC-MS/MS analysis. The following standards were used: N-(3-oxododecanoyl)-L-homoserine lactone (3-oxo-C12-HSL, Sigma Aldrich), N-[(3S)-Tetrahydro-2-oxo-3-furanyl] butanamide (C4-HSL, Cayman Chemical), 2-heptyl-3-hydroxy-4(1H)-quinolone (PQS, Sigma Aldrich). Three samples were performed for each group.
Transcriptome analysis
The total RNA extraction of PAO1 wild type and mvaT mvaU single/double knockouts were performed using RNAprep Pure Cell/Bacteria Kit (TIANGEN) as described in Real-Time PCR assay with cells harvested at OD600 ≈ 0.4, and transcriptome analysis was performed by Novogene (Beijing, China). Briefly, the concentration of purified RNA was measured and the integrity was assessed first. A total amount of 3 μg RNA per sample was then used for transcriptome analysis. Sequencing libraries were generated using NEBNext® Ultra™ Directional RNA Library Prep Kit for Illumina® (NEB, USA) and index codes were added to attribute sequences to each sample. After cluster generation, the library preparations were sequenced on an Illumina Hiseq platform and paired-end reads were generated. Then, differential expression was analyzed using the DESeq R package (1.18.0), which provides statistical routines for determining differential expression in digital gene expression data by a model based on the negative binomial distribution. P-values were adjusted using the Benjamini and Hochberg’s approach for controlling the false discovery rate, and genes with an adjusted P-value of <0.05 were assigned as differentially expressed. Three biological repeats were used for each group.
Results
Pyocyanin production influenced by mvaT and/or mvaU mutations
To conduct this study, we constructed a new set of mvaT and mvaU single and double knockout mutants in the wild type strains of PAO1 as described in Materials and Methods. Consistent with the results reported previously [Citation23,Citation31], pyocyanin synthesis was enhanced in mvaT and mvaU single knockout mutants while it was totally abolished in the double knockout mutant. Furthermore, the observed deficiency of pyocyanin synthesis in the double knockout mutant could be partly complemented by plasmids carrying mvaT or mvaU genes ().
The influence of mvaT and/or mvaU knockouts on phenotypes of P. aeruginosa PAO1
Firstly, we investigated the survival percentages of mice infected by different P. aeruginosa. The mvaT and mvaU single mutants showed hypervirulent features. Mice infected by mvaT and mvaU single mutants were all died within 20 h. In contrast, the survival rate of mice infected with mvaT mvaU double knockout mutation was improved from 10% to 30% compared to PAO1 wild type ()), suggesting decreased virulence of the double mutant.
Figure 3. Effect of mvaT mvaU knockouts on the phenotype of P. aeruginosa PAO1. A: Percent survival of mice infected by PAO1 wild type and mvaT mvaU knockout mutants (n = 10), ***P< 0.001 via log-rank test. PAO1: 6.5 × 103 CFU/mice; PAO1ΔT: 4 × 103 CFU/mice; PAO1ΔU: 6.5 × 103 CFU/mice; PAO1ΔTΔU:1.5 × 104 CFU/mice. B: Biofilm formation of PAO1 wild type and mvaT mvaU knockout mutants, calculated with one-way ANOVA and Bonferroni’s multiple comparisons (n = 6), *P< 0.05, ***P< 0.001, ****P< 0.0001. C: Relative growth of the mvaT mvaU knockout mutants in comparison to PAO1, calculated with one-way ANOVA and Bonferroni’s multiple comparisons (n = 4), ***P< 0.001
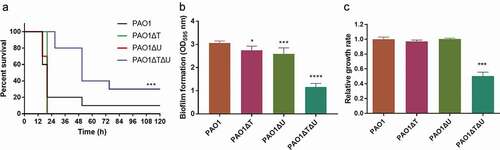
Secondly, the biofilm-forming ability was compared. As shown in ), knockout of mvaT and/or mvaU led to decreased biofilm-forming ability, with that of the mvaT mvaU double knockout mutant decreased to 38% of the wild type strain. In addition, the relative growth rates of the mutants were compared to that of the wild type strain, and the double knockout strain demonstrated a growth rate of about two times slower than that of the wildtype strain, while the single knockouts had no obvious changes ()).
Effects of mvaT and/or mvaU knockouts on the expression of pyocyanin biosynthesis-related genes
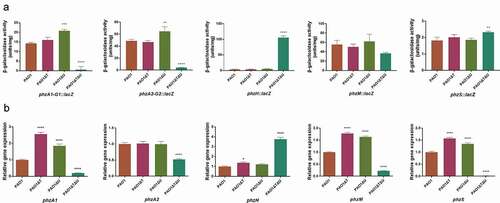
Firstly, the promoter activities of the related genes or operons were compared by measurements of β-galactosidase activities using lacZ transcriptional fusion plasmids carrying the promoter regions of the pyocyanin biosynthesis-related genes. As shown in ), the mvaT single knockout did not affect the promoter activities of the genes in general, while the promoter activities of phzA1-G1 and phzA2-G2 operons were increased 1.21–1.46 times in the mvaU single knockout in comparison to the wild type. In the mvaT mvaU double knockout mutant, the promoter activities were decreased to 4%-9% for phzA1-G1 and phzA2-G2, and 66% for phzM, while those of phzH and phzS were increased (29.15 times for phzH, 1.27 times for phzS). Regardless, these changes eventually led to a drastically reduced activity of pyocyanin biosynthesis. Secondly, the transcript levels of pyocyanin synthesis related genes were evaluated by Real-Time PCR. The results ()) in the log phase showed that the transcript levels of the pyocyanin synthesis-related genes or operons (except for phzA2) were generally increased (1.22–2.56 times) in mvaT or mvaU single knockout mutants in comparison to PAO1. However, in the double knockout mutant, most genes showed decreased expression by RT-PCR (20% for phzA1, 52% for phzA2, 23% for phzM, 1% for phzS) except for phzH (3.76 times increased), resulting in a lowered level of pyocyanin production. In early stationary phase, the transcript levels of these genes showed similar trends as in the log phase (Fig S4).
Figure 4. Effect of mvaT mvaU knockout mutations on gene expression of pyocyanin biosynthesis system. A: β-galactosidase activity of phzA-G1/A2-G2/H/M/S: lacZ fusion covering the regulatory region. B: Relative gene expression of pyocyanin biosynthesis genes detected by Real-Time PCR. Data were calculated with one-way ANOVA and Bonferroni’s multiple comparisons, in comparison to P. aeruginosa PAO1, *P< 0.05, **P< 0.01, ***P< 0.001, ****P< 0.0001
Effects of mvaT and/or mvaU knockouts on the quorum-sensing system
It was well established that pyocyanin synthesis is regulated by the QS system [Citation16–Citation18], a global level of gene regulation that involves intercellular communication by means of cell-density dependent signal molecules. Hence, we conducted experiments to measure the expression levels of genes coding signal molecule catalyzing enzymes (pqsE, lasI, pqsH, and rhlI), the level of AHLs and PQS compounds, and the expression levels of genes coding for transcriptional activators binding with signal molecules (lasR, pqsR, and rhlR). We also measured the expression level of qsrO, a regulator in the QS system which can down-regulate all QS system regulatory and target genes [Citation19].
The Real-Time PCR results showed that in the mvaT single knockout mutant the levels of lasI, lasR, pqsR and rhlR expression were increased to 1.05–3.02 times, while those of pqsH and rhlI were decreased (83% for pqsH, 81% for rhlI). In the mvaU single knockout mutant, generally no obvious changes were seen, except for lasI and pqsH. In the mvaT mvaU double knockout, all these genes exhibited lower levels of expression (11%-74%) except lasI ().
Figure 5. Effect of mvaT mvaU knockout mutations on the expression of genes involved in QS system determined by Real-Time PCR. A: Relative expressions of genes coding QS signal molecule synthetase. B: Relative expressions of genes coding transcriptional activator proteins binding with signal molecules. Data were calculated with one-way ANOVA and Bonferroni’s multiple comparisons, *P< 0.05, **P< 0.01, ***P< 0.001, ****P< 0.0001
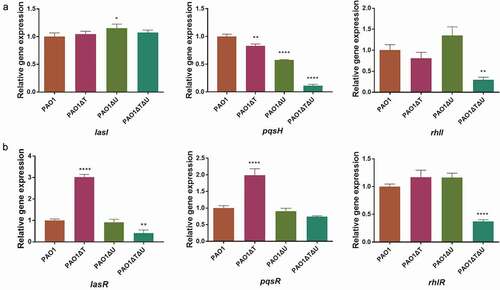
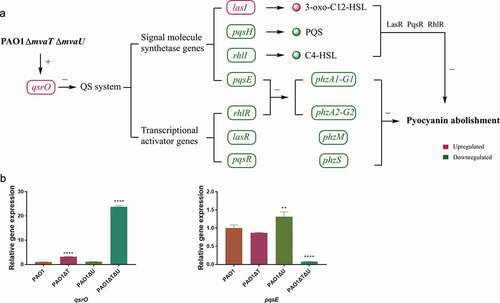
The level of qsrO expression increased 23.8 times in the mvaT mvaU double knockout, 3.18 times in the mvaT single knockout mutant, and showed no obvious change in the mvaU knockout mutant in comparison to those in the wild type strain PAO1 ()). The expression level of pqsE was decreased to 7.8% in the mvaT mvaU double knockout mutant and 87% in the mvaT single knockout mutant but increased to 1.31 times in the mvaU single knockout mutant ()).
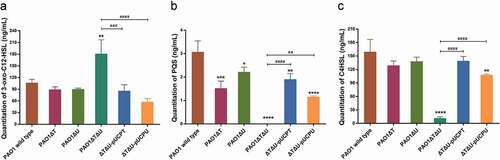
In addition, we used LC-MS/MS to quantitate the production levels of QS signal molecules AHLs (3-oxo-C12-HSL and C4-HSL) and PQS. For the production of 3-oxo-C12-HSL, no obvious change was observed in single knockouts. However, a significant increase (1.70 times) was observed in the mvaT mvaU double knockout mutant. When the deleted genes were complemented by plasmids carrying the corresponding genes, the level of 3-oxo-C12-HSL was decreased to wild type level ()). The levels of PQS and C4-HSL were significantly decreased (0.5% for PQS, 7% for C4-HSL) by mvaT mvaU double knockouts, and were recovered to 38%-62% for PQS and 68%-87% for C4-HSL with the introduction of plasmids carrying mvaT or mvaU. (,)).
Figure 6. Effect of mvaT mvaU knockout mutations on the production of QS system signal molecules AHLs (3-oxo-C12-HSL and C4-HSL) and PQS determined by LC-MS/MS. A: Quantitation of 3-oxo-C12-HSL. B: Quantitation of PQS. C: Quantitation of C4-HSL. Data were calculated with one-way ANOVA and Bonferroni’s multiple comparisons, *P< 0.05, **P< 0.01, ***P< 0.001, ****P< 0.0001
Further transcriptome analysis confirmed that in comparison to wild type PAO1 or single knockouts, all genes except lasI in the QS system had decreased levels of expression in the mvaT mvaU double knockout mutant. This double knockout mutant also decreased the expression of genes for virulence factors and biofilm formation related genes that were regulated by the QS system, such as rhamnolipid and elastase related genes ().
Table 2. List of selected genes whose transcriptions were affected by mvaT mvaU mutations
Discussion
Pyocyanin production was previously reported to be completely abolished in PAO1 mvaT mvaU double mutant [Citation23]. In this study, we aim to reveal the related regulatory mechanism that causes this phenotype. Compared to PAO1 wild type, mvaT mvaU double knockout mutant demonstrated lower pathogenicity, lower biofilm formation ability and decreased growth rate. The virulence changes induced by mvaT mvaU double knockout were in accordance with the abolishment of pyocyanin production. However, no apparent change in cell morphology can be observed in the mvaT mvaU single or double knockout mutants by scanning electron microscopy (SEM) or transmission electron microscopy (TEM) in comparison to the wild type (Fig S3).
Pyocyanin is a secondary metabolite produced by P. aeruginosa in the stationary phase, which is synthesized through a series of enzymatic reactions by PhzA-G, PhzM, and PhzS proteins [Citation15]. In mvaT mvaU double knockout, the expression of genes for pyocyanin biosynthesis was reduced, which resulted in the abolishment of pyocyanin production. It was noted that the promotor activity of phzS was increased while the transcript level of phzS was decreased in mvaT mvaU double knockout. The discrepancy of phzS-lacZ promoter activities and phzS transcript measurements by RT-PCR strongly suggested that phzS expression is mainly controlled by the distal phzA1 promoter(s) (downregulated) apart from the proximal phzS-only promoter (upregulated) of minor contribution in the growth conditions we tested in this study.
It is well known that pyocyanin synthesis is regulated by the QS system [Citation16–Citation18], and knockout of both mvaT and mvaU also had an obvious influence on the QS system. In the mvaT mvaU double knockout mutant, the production of 3-oxo-C12-HSL was significantly increased, while the transcriptional level of lasR was obviously decreased. As a result, the level of LasR/3-oxo-C12-HSL complex, which has been reported to regulate PQS production [Citation32], was decreased. Indeed, in the mvaT mvaU double mutant, the transcriptional levels of genes related to PQS syntheses such as pqsA, pqsBCDE, and pqsH [Citation33,Citation34] were all significantly decreased. Consequently, there was almost no PQS produced in the mvaT mvaU double knockout mutant. At the same time, the expression of pqsR for a transcriptional activator that binds with PQS to function was slightly decreased. Hence, it is very likely that the PQS/PqsR complex level was decreased to a very low residual level. The PQS system was reported to act as a link between the las and rhl QS system, and it is able to enhance the transcription of rhlI in P. aeruginosa [Citation17]. In mvaT mvaU double knockout, transcriptional expression of rhlI and rhlR, and the production of C4-HSL were all significantly decreased, which led to decreased C4-HSL/RhlR complex level. What is more, 3-oxo-C12-HSL was reported to have an inhibitory effect on the rhl QS system [Citation17], the free 3-oxo-C12-HSL in the double knockout mutant may block the association between RhlR and C4-HSL, and further lower the level of C4-HSL/RhlR complex. The decreased levels of PQS/PqsR complex and C4-HSL/RhlR complex resulted in the decreased levels of biofilm formation and expression of a variety of virulence factors, such as pyocyanin, elastase, and rhamnolipids. In addition, RhlR and PqsE are both required to induce phzA1 and phzA2 [Citation35]. The obviously decreased transcriptional levels of rhlR and pqsE further led to the decreased expression of phzA1 and phzA2 in mvaT mvaU double knockout mutant.
One major issue to justify the hypothesis as described above was that MvaT and MvaU must possess a potential function as transcriptional activators. However, current knowledge of MvaT and MvaU all indicated that these two proteins are transcriptional repressors [Citation22,Citation31,Citation36]. In fact, most genes under the control of MvaT and MvaU were suppressed in the wild type PAO1, and the expression of which was induced in the mvaT mvaU double knockout mutant (GSE135506). Few exceptions that display decreased expression include genes in the QS system and pyocyanin synthesis. Therefore, we hypothesized that the downregulation of the QS system by double knockout of mvaT mvaU is mediated by another transcriptional repressor that is subjected to direct control by MvaT and MvaU. Further exploration found that QsrO, a repressor of the QS system [Citation19], was significantly upregulated in the mvaT mvaU double knockout mutant. Our current hypothesis was that genes repressed in the mvaT mvaU double knockout mutant are direct targets of QsrO repressor, a member of the MvaT MvaU regulon. mvaT mvaU double knockout results in an increased level of QsrO, which can suppress the expression of the QS system and subsequently genes in the pyocyanin biosynthetic pathway ()). Future study will be needed to establish the proposed link of MvaT and MvaU to the QS system by QsrO.
Figure 7. Interactions of mvaT and mvaU to control biosynthesis and signaling systems of pyocyanin. A: When mvaT and mvaU were knockout, expression of qsrO was significantly increased, which led to the decreased expression of genes coding signal molecule synthetases (pqsH, rhlI, and pqsE) and transcriptional activators (rhlR, lasR, and pqsR). The lower expression levels of phzA1-G1 and phzA2-G2 caused by decreased expression of rhlR and pqsE, together with lower expression levels of phzM, phzS and the decreased formation of signal-transcriptional activator complexes resulted in pyocyanin abolishment. B: Relative gene expressions of qsrO and pqsE detected by Real-Time PCR. Data were calculated with one-way ANOVA and Bonferroni’s multiple comparisons, in comparison to P. aeruginosa PAO1, **P< 0.01, ****P< 0.0001
In conclusion, this study demonstrated the mechanism of mvaT mvaU double knockout on the abolishment of pyocyanin, providing evidence for using mvaT and mvaU as targets to decrease the pathogenicity or virulence of P. aeruginosa.
Supplemental Material
Download PDF (585.4 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Mathee K, Narasimhan G, Valdes C, et al. Dynamics of Pseudomonas aeruginosa genome evolution. Proc Natl Acad Sci U S A. 2008;105:3100–3105.
- Silby MW, Winstanley C, Godfrey SA, et al. Pseudomonas genomes: diverse and adaptable. FEMS Microbiol Rev. 2011;35:652–680.
- Gellatly SL, Hancock RE. Pseudomonas aeruginosa: new insights into pathogenesis and host defenses. Pathog Dis. 2013;67:159–173.
- Lau GW, Hassett DJ, Ran H, et al. The role of pyocyanin in Pseudomonas aeruginosa infection. Trends Mol Med. 2004;10:599–606.
- Rada B, Leto TL. Pyocyanin effects on respiratory epithelium: relevance in Pseudomonas aeruginosa airway infections. Trends Microbiol. 2013;21:73–81.
- Muller M. Pyocyanin induces oxidative stress in human endothelial cells and modulates the glutathione redox cycle. Free Radic Biol Med. 2002;33:1527–1533.
- Cheluvappa R, Jamieson HA, Hilmer SN, et al. The effect of Pseudomonas aeruginosa virulence factor, pyocyanin, on the liver sinusoidal endothelial cell. J Gastroenterol Hepatol. 2007;22:1350–1351.
- Gardner PR. Superoxide production by the mycobacterial and pseudomonad quinoid pigments phthiocol and pyocyanine in human lung cells. Arch Biochem Biophys. 1996;333:267–274.
- Ran H, Hassett DJ, Lau GW. Human targets of Pseudomonas aeruginosa pyocyanin. Proc Natl Acad Sci U S A. 2003;100:14315–14320.
- Carlsson M, Shukla S, Petersson AC, et al. Pseudomonas aeruginosa in cystic fibrosis: pyocyanin negative strains are associated with BPI-ANCA and progressive lung disease. J Cyst Fibros. 2011;10:265–271.
- Reimer A, Edvaller B, Johansson B. Concentrations of the Pseudomonas aeruginosa toxin pyocyanin in human ear secretions. Acta Otolaryngol Suppl. 2000;543:86–88.
- Wilson R, Sykes DA, Watson D, et al. Measurement of Pseudomonas aeruginosa phenazine pigments in sputum and assessment of their contribution to sputum sol toxicity for respiratory epithelium. Infect Immun. 1988;56:2515–2517.
- Cruickshank CN, Lowbury EJ. The effect of pyocyanin on human skin cells and leucocytes. Br J Exp Pathol. 1953;34:583–587.
- Fila G, Kawiak A, Grinholc MS. Blue light treatment of Pseudomonas aeruginosa: strong bactericidal activity, synergism with antibiotics and inactivation of virulence factors. Virulence. 2017;8:938–958.
- Mavrodi DV, Bonsall RF, Delaney SM, et al. Functional analysis of genes for biosynthesis of pyocyanin and phenazine-1-carboxamide from Pseudomonas aeruginosa PAO1. J Bacteriol. 2001;183:6454–6465.
- Fuqua C, Parsek MR, Greenberg EP. Regulation of gene expression by cell-to-cell communication: acyl-homoserine lactone quorum sensing. Annu Rev Genet. 2001;35:439–468.
- McKnight SL, Iglewski BH, Pesci EC. The Pseudomonas quinolone signal regulates rhl quorum sensing in Pseudomonas aeruginosa. J Bacteriol. 2000;182:2702–2708.
- Diggle SP, Winzer K, Chhabra SR, et al. The Pseudomonas aeruginosa quinolone signal molecule overcomes the cell density-dependency of the quorum sensing hierarchy, regulates rhl-dependent genes at the onset of stationary phase and can be produced in the absence of LasR. Mol Microbiol. 2003;50:29–43.
- Kohler T, Ouertatani-Sakouhi H, Cosson P, et al. QsrO a novel regulator of quorum-sensing and virulence in Pseudomonas aeruginosa. PLoS One. 2014;9:e87814.
- Chand NS, Hung DT. The two-component sensor kinase KinB acts as a non-canonical switch between acute and chronic infection. Virulence. 2011;2:553–558.
- Jagmann N, Bleicher V, Busche T, et al. The guanidinobutyrase GbuA is essential for the alkylquinolone-regulated pyocyanin production during parasitic growth of Pseudomonas aeruginosa in co-culture with Aeromonas hydrophila. Environ Microbiol. 2016;18:3550–3564.
- Castang S, McManus HR, Turner KH, et al. H-NS family members function coordinately in an opportunistic pathogen. Proc Natl Acad Sci U S A. 2008;105:18947–18952.
- Li C, Wally H, Miller SJ, et al. The multifaceted proteins MvaT and MvaU, members of the H-NS family, control arginine metabolism, pyocyanin synthesis, and prophage activation in Pseudomonas aeruginosa PAO1. J Bacteriol. 2009;191:6211–6218.
- Essar DW, Eberly L, Hadero A, et al. Identification and characterization of genes for a second anthranilate synthase in Pseudomonas aeruginosa: interchangeability of the two anthranilate synthases and evolutionary implications. J Bacteriol. 1990;172:884–900.
- Standardization Administration of the People’s Republic of China. 2018. Laboratory animal—guideline for ethical review of animal welfare. Beijing, China. (GB/T 35892-2018).
- Merritt JH, Kadouri DE, O’Toole GA. Growing and analyzing static biofilms. Curr Protoc Microbiol. 2005;Chapter 1:Unit 1B.1.
- Ortori CA, Dubern JF, Chhabra SR, et al. Simultaneous quantitative profiling of N-acyl-L-homoserine lactone and 2-alkyl-4(1H)-quinolone families of quorum-sensing signaling molecules using LC-MS/MS. Anal Bioanal Chem. 2011;399:839–850.
- Purohit AA, Johansen JA, Hansen H, et al. Presence of acyl-homoserine lactones in 57 members of the Vibrionaceae family. J Appl Microbiol. 2013;115:835–847.
- Chen-Chen M, Bai-lin L, Jie O, et al. Detection of N-Acyl-homoserine lactones class signal molecules of quorum sensing secreted by bacteria using high performance liquid chromatography-mass spectrometry/mass spectrometry. Chin J Anal Chem. 2010;38:1428–1432.
- Lepine F, Deziel E, Milot S, et al. A stable isotope dilution assay for the quantification of the Pseudomonas quinolone signal in Pseudomonas aeruginosa cultures. Biochim Biophys Acta. 2003;1622:36–41.
- Diggle SP, Winzer K, Lazdunski A, et al. Advancing the quorum in Pseudomonas aeruginosa: mvaT and the regulation of N-acylhomoserine lactone production and virulence gene expression. J Bacteriol. 2002;184:2576–2586.
- Pesci EC, Milbank JB, Pearson JP, et al. Quinolone signaling in the cell-to-cell communication system of Pseudomonas aeruginosa. Proc Natl Acad Sci U S A. 1999;96:11229–11234.
- Dulcey CE, Dekimpe V, Fauvelle DA, et al. The end of an old hypothesis: the pseudomonas signaling molecules 4-hydroxy-2-alkylquinolines derive from fatty acids, not 3-ketofatty acids. Chem Biol. 2013;20:1481–1491.
- Dubern JF, Diggle SP. Quorum sensing by 2-alkyl-4-quinolones in Pseudomonas aeruginosa and other bacterial species. Mol Biosyst. 2008;4:882–888.
- Higgins S, Heeb S, Rampioni G, et al. Differential regulation of the phenazine biosynthetic operons by quorum sensing in pseudomonas aeruginosa PAO1-N. Front Cell Infect Microbiol. 2018;8:252.
- Rescalli E, Saini S, Bartocci C, et al. Novel physiological modulation of the Pu promoter of TOL plasmid: negative regulatory role of the TurA protein of Pseudomonas putida in the response to subop-timal growth temperatures. J Biol Chem. 2004;279:7777–7784.
