ABSTRACT
Patients with Acinetobacter baumannii bacteremia treated with antipseudomonal cephalosporins showed higher 14-day mortality than patients treated with antipseudomonal carbapenems. We hypothesized that the bacterial membrane vesicles (BMVs) induced by antipseudomonal cephalosporins are more virulent than BMVs induced by antipseudomonal carbapenems.
To simulate the clinical condition with inadequate antimicrobial treatment, carbapenem-resistant A. baumannii was treated with ceftazidime (an antipseudomonal cephalosporin) or imipenem (an antipseudomonal carbapenem) at 1/2 the minimum inhibitory concentration. BMVs and BMV-carried lipopolysaccharide were measured by nanoparticle tracking analysis and western blotting, respectively. Cytokine expression in RAW264.7 macrophages or mice serum induced by the BMVs was determined by ELISA, fluorescent bead-based immunoassay or western blotting. The virulence of the BMVs was assessed in mice. Liquid chromatography tandem-mass spectrometry was used to determine the protein contents of the BMVs.
We found that ceftazidime induced a higher number of BMVs (CAZ-BMV), which carried more LPS, and induced higher expression levels of iNOS, IL-1β, and IL-6 in macrophages, higher expression of many cytokines in mice, more neutrophil infiltration in lung interstitium, and higher mortality in mice than imipenem-induced BMVs (IMP-BMV). When adjusted to same amount of LPS, CAZ-BMV still led to higher mortality than IMP-BMV. Proteomic analysis revealed different protein contents in CAZ-BMV and IMP-BMV. In conclusion, A. baumannii BMVs induced by ceftazidime are more virulent than BMVs induced by imipenem.
Introduction
In a previous study of 252 patients with monomicrobial Acinetobacter baumannii bacteremia [Citation1], we found that, despite comparable percentages of inappropriate antimicrobial therapy (56% vs. 51.4%) and Acute Physiology and Chronic Health Evaluation (APACHE II) scores (24.5 vs. 25), patients treated with antipseudomonal cephalosporins had a higher 14-day mortality rate than those treated with antipseudomonal carbapenems (42% vs. 25%, P < 0.05). This result suggested that factors other than the appropriateness of antimicrobial therapy and disease severity contribute to the mortality differences between the two groups.
The development of “sepsis-like” symptoms after antimicrobial therapy has long been reported. Such a paradoxical effect of penicillin treatment for syphilis was first described in 1895. These syphilis patients suffered from fever, chills, headache, rigor, myalgia, hypotension, tachycardia, hyperventilation, and vasodilation with flushing within a few hours of the first dose of penicillin [Citation2]. Similar symptoms have been reported in other diseases, such as borreliosis, leptospirosis, Q fever [Citation3], bartonellosis [Citation4], brucellosis [Citation5], typhoid fever [Citation6], trichinosis [Citation7], and cerebral trypanosomiasis [Citation8].
Treatment with different antimicrobials might cause different levels of these “sepsis-like” symptoms [Citation9], and several studies have shown that ceftazidime caused a higher incidence of symptoms related to cytokine release than imipenem in patients with Gram-negative bacilli infections [Citation10,Citation11]. This paradoxical phenomenon is thought to be caused by the rapid release of lipopolysaccharide (LPS) from the bacteria as a result of cell lysis [Citation12], as LPS is known to trigger the release of cytokines, such as interleukin (IL)-1, IL-6, and tumor necrosis factor alpha (TNF-α), from mononuclear leukocytes and other cells. However, another study revealed that cell lysis is not necessary for LPS release, especially when the antimicrobial concentration is below the minimum inhibitory concentration for the bacteria [Citation13].
Gram-negative bacteria secrete bacterial membrane vesicles (BMVs) for the transport of bacterial effectors to target cells [Citation14]. These vesicles may carry and transmit proteins, nucleic acids, and LPS [Citation14]. In a previous study, we demonstrated that treatment with sub-inhibitory concentrations of ceftazidime and imipenem could enhance the release of BMVs from carbapenem-resistant A. baumannii [Citation15]. In this study, we hypothesized that treatment of carbapenem-resistant A. baumannii with sub-inhibitory concentrations of ceftazidime and imipenem would enhance the release of BMVs with different virulence.
Materials and methods
Bacterial strains, chemicals, and cell lines
In this study, a carbapenem-resistant A. baumannii transformant was used; a recombinant plasmid harboring blaOXA-58, pOXA-58-2 [Citation16], was transformed into A. baumannii reference strain ATCC 17978 to generate ATCC17978 pOXA-58-2. The transformation and verification of the transformation were performed as previously described [Citation16]. All antimicrobials and adenosine triphosphate (ATP) were purchased from Sigma-Aldrich (St. Louis, MO). LPS (Ultra-pure LPS-EB) from Escherichia coli O111:B4 was purchased from InvivoGen (San Diego, CA) and mouse IL-4 was from R&D Systems (Minneapolis, MN). For fluorescent bead-based instrument Bio-Plex Pro™ Cytokine Assay, capture antibodies, and detection antibodies were obtained as a complete kit from Bio-Rad (Hercules, CA).
The murine macrophage cell line RAW264.7 (ATCC) was maintained in Dulbecco’s modified Eagle’s medium (Corning, NY) supplemented with 10% fetal bovine serum (Corning) and 1% penicillin-streptomycin (Sigma-Aldrich). Cells were cultured at 37°C with 5% CO2 and were seeded in 96-well plates at a concentration of 2 × 106 cells/well and incubated for 4 h before stimulation. All experiments were performed at least in triplicate, and representative experiments are shown.
Preparation of BMVs and supernatant from bacterial cultures
BMVs were isolated from ATCC17978 pOXA-58-2 cultured with or without antimicrobials using a previously published protocol with some modifications [Citation17]. Briefly, 200 ml of Luria-Bertani (LB) broth (BD Diagnostic System, Sparks, MD) was inoculated with 2 ml of an overnight culture of ATCC17978 pOXA-58-2 and incubated for 2 h at 37°C with shaking. Antimicrobials were added at 1/2 the minimum inhibitory concentration, and the cultures were incubated for another 4 h at 37°C with shaking. Then, the cultures were centrifuged (10,000 rpm, 15 min), and the supernatant was collected and filtered (0.22 μm filter; Jet Biofil, Canada) to remove the bacteria. BMVs were isolated from the supernatant by high speed centrifugation (200,000 × g, 2 h, at 4°C), washed with phosphate-buffered saline (PBS), and collected by a second ultracentrifugation (200,000 × g, 2 h). The BMV pellet was then resuspended in 800 μl of PBS and stored at −80°C until use. Supernatant (~200 ml) samples from the ultracentrifugation were stored at −80°C for further tests.
Cytokine analysis
BMVs or bacterial culture supernatants were diluted to the same volume, and 10 µl aliquots were used to treat a monolayer of murine RAW264.7 macrophages for 4 h. Then, the culture medium was collected, and the expression of cytokines were determined either by using the DuoSet ELISA development system [Citation18] for IL-1β (R&D Systems), the Opt EIA cytokine ELISA set for TNF-α (BD Sciences, San Diego, CA) or the fluorescent bead-based instrument Bio-Plex Pro™ Cytokine Assay for IL-1β, TNF-α, IL-6, IL-10, IL-12 and IFN-ϒ (Bio-Rad) [Citation19] . All the BMV samples and supernatant samples used to inoculate with RAW264.7 were in the same volume and thus derived from the same initial bacterial counts. RAW264.7 macrophages treated with LPS plus ATP were used as a positive control, and IL-4 and PBS were used as negative controls.
To determine the effect of LPS in the BMVs on the expression of IL-1β, the BMVs were pretreated with polymyxin B (25 μg/ml), a cationic antimicrobial peptide that can neutralize LPS [Citation20], before being used to treat the macrophages.
Nanoparticle tracking of BMVs
The size and concentration of the prepared BMVs was determined via nanoparticle tracking analysis (NTA) using a NanoSight LM10-HS instrument (NanoSight Ltd.) according to the manufacturer’s protocol [Citation21].
Western blot analysis
To determine the amount of LPS in the BMVs and supernatant, the contents of the BMV preparation and supernatant were separated by polyacrylamide gel electrophoresis and immobilized on nitrocellulose membranes (Millipore, Molsheim, France). The membranes were incubated with primary antibodies, followed by horseradish peroxidase-conjugated goat anti-rabbit (Sigma-Aldrich) secondary antibodies in blocking buffer at room temperature for 4 h. Primary polyclonal antibodies against LPS were generated in rabbits [Citation22] and were a gift from Dr. Shih-Hsiung Wu. The ECL Western blot kit (PerkinElmer) was used for chemiluminescent detection. All the BMV samples and supernatant samples used to determine the LPS amount here were in the same volume and thus derived from the same initial bacterial counts.
The production of iNOS in RAW264.7 cells exposed to the prepared BMVs was also determined by western blot analysis. The information about iNOS detection could be found in the supplementary information.
In vivo study of the mice mortality caused by A. baumannii BMVs
The animal study was approved by the Ethical Committee for Animal Experiments of the National Health Research Institutes. BMVs were intraperitoneally administered to 8-week-old male mice as described previously, with some modifications [Citation23]. The survival of the mice was monitored every 12 h for 5 days. In the first study, mice were intraperitoneally administered 25, 40, or 65 μg of imipenem-induced BMVs (IMP-BMV) [Citation24], which is equal to 30, 48.3, or 77.5 μl of the 800 μl stock solution. Equal volumes (30, 48.3, and 77.5 μl) of ceftazidime-induced BMVs (CAZ-BMV) and BMVs collected from A. baumannii cultured in LB without any antimicrobial (LB-BMV) were also administered. Thus, the tested mice were separated into nine groups: CAZ-BMV- 30μl (five mice), CAZ-BMV- 48.3μl (five mice), CAZ-BMV- 77.5 μl (five mice), IMP-BMV- 30μl (five mice), IMP-BMV- 48.3μl (five mice), IMP-BMV- 77.5 μl (five mice), LB-BMV- 30μl (five mice), LB-BMV- 48.3μl (five mice), and LB-BMV- 77.5 μl (five mice). All the BMV samples used to administer the mice here were in the same volume and thus derived from the same initial bacterial counts. In the second study, to evaluate the role of the BMV contents other than LPS, quantitative chromogenic Limulus amebocyte lysate test was performed to make each BMVs samples derived owing to different antibiotics treatment contained equal amounts of LPS (270 EU). The inoculum was adjusted to 100 μl by adding PBS before administration.
Cytokine expression of serum and histology of lungs in mice inoculated with A. baumannii BMVs
Mice were intraperitoneally administered 30μl of BMVs derived from different antimicrobials treatment, respectively. Blood samples were obtained at 8 h from the ceftazidime (5 mice), imipenem (5 mice), and LB (5 mice) groups. Each sample was centrifuged, and the supernatants were assessed using the Bio-Plex Pro Mouse Cytokine 23-plex panel. The cytokines analyzed include Eotaxin, G-CSF, GM-CSF, IFN-γ, IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-9, IL-10, IL-12 (p40), IL-12 (p70), IL-13, IL-17A, KC, MCP-1 (MCAF), MIP-1α, MIP-1β, RANTES and TNF-α. In brief, 50 ul antibody-coupled beads per well was added to the flat bottom plate and wash two times. Then 50ul serum sample was incubated with antibody-coupled beads for 30 minutes at room temperature. After washing three times to remove unbound materials, the beads were incubated with 25 ul biotinylated detection antibodies for 30 minutes at room temperature. After washing away the unbound biotinylated antibodies for three times washes, the beads were incubated with 50 ul streptavidin-PE for 10 minutes at room temperature. Following removal of excess streptavidin-PE for three times washes, the beads were resuspended in 125 ul assay buffer. Beads were read on the Bio-Plex suspension array system, and the data were analyzed using Bio-Plex Manager software version 6.0.
After the BMVs-treated mice were killed, the lungs were fixed in 10% formalin for pathological analysis. After formalin fixation (72 h), the lungs were multiply transected in preparation for paraffin embedding. The paraffin blocks were cut at 4 μm and stained with hematoxylin and eosin. A pathologist, blinded to treatment arms, subjectively evaluated the pathology in 10 random fields of tissue sections viewed at 400 ╳ total magnification.
Liquid chromatography tandem-mass spectrometry (LC-MS/MS)
The protein concentrations in the BMV samples were determined using a bicinchoninic acid protein assay kit (Thermo Scientific, Rockford, IL). Each sample (contained 20 µg of proteins) was first reduced with 5 mM tris-(2-carboxyethyl)-phosphine (Sigma-Aldrich) at 60°C for 30 min, followed by cysteine-blocking with 10 mM methyl methanethiosulfonate (Sigma-Aldrich) at 25°C for 30 min, and digested with 1 µg of trypsin (Promega, Madison, WI) in solution containing 150 mM triethylammonium bicarbonate at 37°C for 16 hours. The peptides were then labeled with isobaric tags for relative and absolute quantitation (iTRAQ) reagent (AB Sciex, Foster City, CA) according to the manufacturer’s protocol. After incubation at room temperature for 1 h, the peptide mixtures were pooled, dried by vacuum centrifugation, and stored at −80°C until use.
The dried peptide mixtures were reconstituted and desalted with a C18-microcolumn. The resulting peptides (5 µg) were reconstituted in 30 µl of high performance liquid chromatography (HPLC) buffer A (30% acetonitrile/0.1% formic acid) and loaded onto a homemade column (Luna strong cation exchange, 5 µm, 0.5 × 50 mm) at flow rate of 3 µl/min for 30 min. The peptides were then separated into 7 fractions by eluting with 0–100% HPLC buffer B (0.5 M ammonium chloride/30% acetonitrile/0.1% formic acid) using an on-line 2D-HPLC (Dionex Ultimate 3000; Thermo Fisher, San Jose, CA). Each strong cation exchange fraction was diluted in-line prior to separation on a reverse-phase column (Zorbax 300SB-C18, 0.3 × 5 mm; Agilent Technologies, Wilmington, DE). The desalted peptides were then separated on a homemade column (HydroRP 2.5 µm, 75 μm I.D. × 24 cm with a 15 μm tip) using a multi-step gradient of HPLC buffer C (99.9% acetonitrile/0.1% formic acid) for 60 min, with a flow rate of 0.23 μl/min. The LC apparatus was coupled with a 2D linear ion trap mass spectrometer (LTQ-Orbitrap ELITE; Thermo Fisher) operated using Xcalibur 2.2 software (Thermo Fisher). The full-scan MS was performed in the Orbitrap over a range of 400 to 2,000 Da.
The data analysis was carried out using Proteome Discoverer software (version 1.4; Thermo Fisher Scientific), including the reporter ions quantifier node for tandem mass tags quantification. The MS/MS spectra were searched against the UniProt sequence database using the Mascot search engine (version 2.5; Matrix Science London, UK).
Statistical analysis
For continuous variables, Student’s t-test or the Mann-Whitney rank sum test was used to assess differences. Time to mortality was analyzed using Kaplan-Meier survival analysis and the log-rank test. A P value less than 0.05 was considered statistically significant. All analyzes were performed using Statistical Package for the Social Sciences software version 20 (SPSS).
Results
BMVs isolated following ceftazidime treatment (CAZ-BMV) induced higher IL-1β, IL-6, and iNOS expression in macrophages and higher mortality in mice than BMVs isolated following imipenem treatment (IMP-BMV)
Cytokine analysis revealed that CAZ-BMV induced higher expression of IL-1β and IL-6, but similar level of TNF-α, IL-12 and IFN-γ in macrophage compared to from IMP-BMV (). The expression of IL-10 in macrophage triggered by CAZ-BMV, IMP-BMV, and BMV from untreated A. baumannii (LB-BMV) were all too low to be detected. The addition of polymyxin B abolished the expression of IL-1β and TNF-α ()), indicating that these two cytokines were induced by LPS contained in the BMVs, and CAZ-BMV consisted higher level of LPS than IMP-BMV. In addition, CAZ-BMV also induced higher level of iNOS expression (Supplementary Figure S1) in macrophages than IMP-BMV. In contrast, supernatant from ceftazidime- and imipenem-treated samples induced similar levels of IL-1β and TNF-α expression ()).
Figure 1. Cytokine expression in macrophages after treatment with bacterial membrane vesicles (BMVs) from antimicrobial-treated Acinetobacter baumannii. BMVs induced by ceftazidime (CAZ) treatment led to higher expression of IL-1β (a) and IL-6 (c) in murine RAW264.7 macrophages than BMVs induced by imipenem (IMP) treatment or BMVs from untreated A. baumannii (LB). BMVs from ceftazidime and imipenem-treated cultures induced similar levels of TNF-α (b), IL-12 (d), and IFN-ϒ (e) expression, which were similar to that induced by untreated supernatant (LB). RAW264.7 macrophages treated with LPS plus ATP were used as a positive control, and phosphate-buffered saline (PBS) were used as negative controls. * P < 0.05 between CAZ-BMV and IMP-BMV
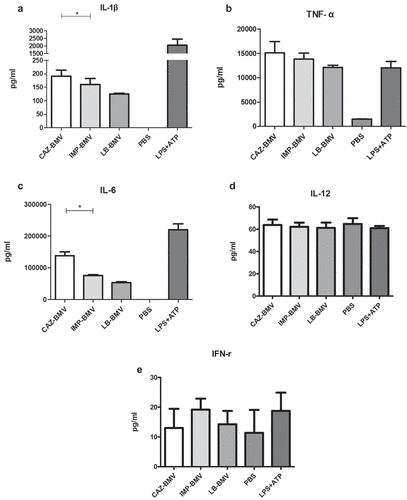
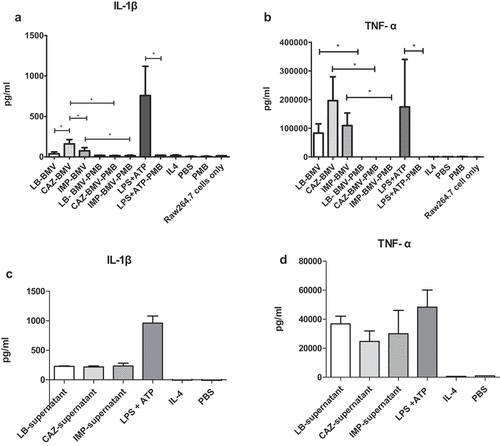
Figure 2. IL-1β and TNF-α expression in macrophages after treatment with bacterial membrane vesicles (BMVs) and culture supernatants from antimicrobial-treated Acinetobacter baumannii. BMVs pretreated with polymyxin B (PMB) did not induce IL-1β (a) or TNF-α (b) expression. Supernatants from ceftazidime and imipenem-treated cultures induced similar levels of IL-1β (c) and TNF-α (d) expression, which were similar to that induced by untreated supernatant (LB). RAW264.7 macrophages treated with LPS plus ATP were used as a positive control, and IL-4 and phosphate-buffered saline (PBS) were used as negative controls. LB-BMV-PMB, CAZ-BMV-PMB, and IMP-BMV-PMB are BMVs pretreated with polymyxin B (25 μg/ml). Shown are the mean and SD of results from triplicate studies. * P < 0.05 between two compared groups
CAZ-BMV induced higher mortality in mice, higher cytokine expression, and more neutrophil infiltration in lung interstitium than IMP-BMV
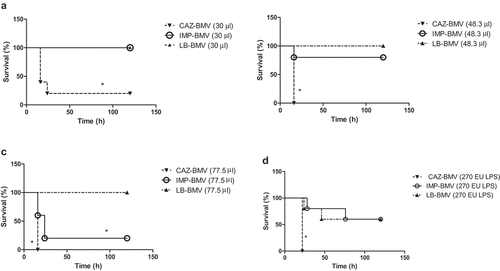
The in vivo study showed that the administration of CAZ-BMV caused significantly higher mortality in mice ()). All mice inoculated with 30, 48.3, or 77.5 μl of LB-BMV survived. Mice inoculated with 30 μl and 48.3 μl of CAZ-BMV showed significantly higher mortality rates than mice inoculated with the same volume of IMP-BMV [(80% vs. 0) and (100% vs. 20%), respectively]. Mice treated with 77.5 μl of either CAZ-BMV (100%) or IMP-BMV (80%) showed significantly higher mortality rates than mice treated with LB-BMV.
Figure 3. Bacterial membrane vesicles (BMVs) induced by ceftazidime (CAZ) caused higher mortality in mice than BMVs induced by imipenem (IMP) treatment. In the study, mice were inoculated with 30 μl (a), 48.3 μl (b), or 77.5 μl (c) of BMVs derived from treatment of A. baumannii with CAZ, IMP, or no antimicrobial (LB). In the second study, the mice were inoculated with BMVs that carried the same amount of LPS (270 EU) (d). * P < 0.05 vs. BMVs derived from A. baumannii grown in Luria-Bertani broth without antibiotics (LB-BMV)
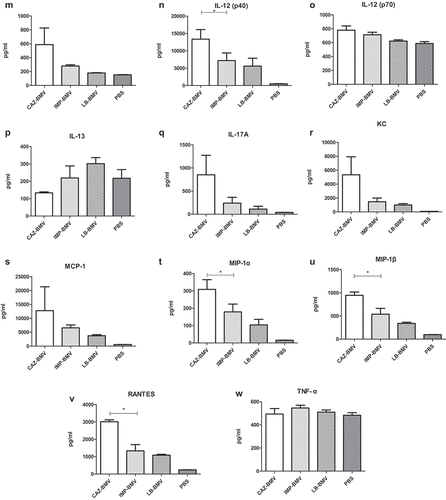
CAZ-BMV induced higher expression of many cytokines in mice, including exotoxin, IFN-γ, IL-1α, IL-1β, IL-6, IL-12 (p40), MIP-1α, MIP-1β and RANTES than IMP-BMV or LB-BMV (). Neutrophils infiltration increased within the interstitium of lungs in the CAZ-BMV treatment group ()), whereas no significant neutrophil infiltration in the IMP-BMV ()), LB-BMV ()), and control ()) group.
Figure 4. (a) Cytokine expression in mice serum after inoculated with bacterial membrane vesicles (BMVs) from antimicrobial-treated Acinetobacter baumannii. BMVs induced by ceftazidime (CAZ) treatment led to higher expression of many cytokines in mice than BMVs induced by imipenem (IMP) treatment or BMVs from untreated A. baumannii (LB). Phosphate-buffered saline (PBS) were used as negative controls. * P < 0.05 between CAZ-BMV and IMP-BMV. (b) Cytokine expression in mice serum after inoculated with bacterial membrane vesicles (BMVs) from antimicrobial-treated Acinetobacter baumannii. BMVs induced by ceftazidime (CAZ) treatment led to higher expression of many cytokines in mice than BMVs induced by imipenem (IMP) treatment or BMVs from untreated A. baumannii (LB). Phosphate-buffered saline (PBS) were used as negative controls. * P < 0.05 between CAZ-BMV and IMP-BMV
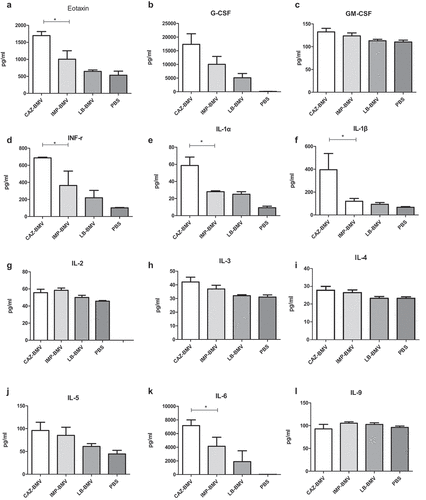
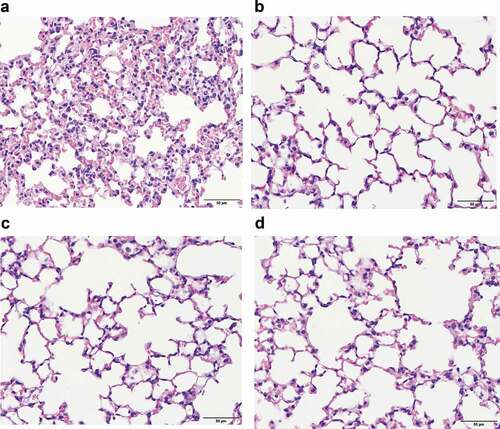
Figure 5. Lung pathology study showed that more neutrophil infiltration was found in lung interstitium of mice treated with ceftazidime-induced bacterial membrane vesicles (CAZ-BMV) than those treated with imipenem-induced bacterial membrane vesicles (IMP-BMV). Increased neutrophil infiltration occurred in the CAZ-BMV group (a), whereas no significant neutrophil recruitment in the IMP-BMV (b), LB-BMV (c), and control (d) group. Mice intraperitoneally administered 30 μl of phosphate-buffered saline (PBS) were used as control group
Ceftazidime induced greater numbers of BMVs than imipenem did
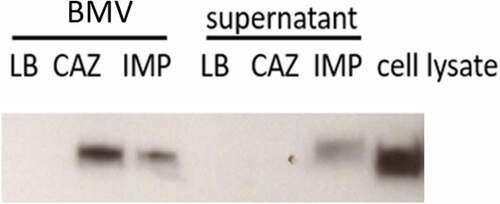
Sub-inhibitory concentrations of ceftazidime induced the production of higher numbers of BMVs than imipenem (). The size of BMVs derived from ceftazidime, imipenem, and no antibiotics treatment was 126.2 ± 5.9 nm, 136.7 ± 16.2 nm, and 114.6 ± 15.0nm, respectively. As expected, the CAZ-BMV carried more LPS than the IMP-BMV (). By using Limulus Amebocyte Lysate test, the LPS concentration of LB-BMV, CAZ-BMV, and IMP-BMV samples were 5.3 x 103, 9.5 x 103, 5.35 × 103 EU/ml, and 4.08 x 10−7, 2.34 x 10−7, 3.34 × 10−7 EU/per BMV, respectively. In contrast, supernatant from the ceftazidime-treated samples did not contain more LPS than supernatant from the imipenem-treated samples ().
Figure 6. Treatment with 1/2 the minimal inhibitory concentration of ceftazidime (CAZ) induced the release of a higher number of bacterial membrane vesicles (BMVs) than treatment with imipenem (IMP). The number of BMVs (a), surviving colonies (b), and secreted BMVs per surviving colony (c) of A. baumannii after CAZ or IMP treatment, or without antimicrobial treatment (LB). Shown are the mean and SD of results from triplicate studies. *P < 0.05 vs. the sample derived from A. baumannii grown in Luria-Bertani broth without antimicrobials (LB)
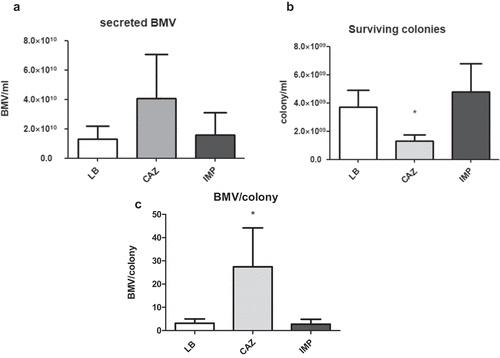
Figure 7. Treatment with 1/2 the minimal inhibitory concentration of ceftazidime (CAZ) increased the amount of lipopolysaccharide (LPS) carried by bacterial membrane vesicles (BMVs) but not the amount in supernatants, compared to treatment with imipenem (IMP). LPS was detected with an anti-LPS antibody by western blot analysis. Cell lysate of ATCC 17978 was used as a positive control
The in vivo study demonstrated that contents other than LPS present in the CAZ-BMV might also responsible for the higher mortality
Because IL-1β and TNF-α are mainly induced by LPS, we used an animal study to determine the virulence of contents other than LPS present in the CAZ-BMV and IMP-BMV. For this experiment, quantitative chromogenic Limulus Amebocyte Lysate test was performed to determine the LPS amount of our BMVs samples. Different amount of BMVs samples were used (LB-BMV: 50.9μl, CAZ-BMV: 28.4μl, IMP-BMV: 50.4μl) to administer mice so they contained equal amounts of LPS (270 EU). In the experiment, all mice inoculated with CAZ-BMV died, while 60% of the mice inoculated with IMP-BMV or LB-BMV survived (log-rank test, P = 0.018; )). This result indicated that other contents in the CAZ-BMV might also responsible for the higher mortality compared to those in the IMP-BMV.
The proteomics analysis showed that different protein contents were present in the BMVs induced by ceftazidime and imipenem
Based on the above results, we hypothesized that the protein contents of the BMVs induced by different antimicrobial treatments might vary. Proteomic comparison of the BMVs induced by different antimicrobial treatments are shown in and . We compared the proteomic results between these 3 different antimicrobials treatment groups in the same diluted sample volume, so the tested BMVs samples were derived from the same initial bacterial counts.
Table 1. The top 15 proteins with the greatest increase in the bacterial membrane vesicles (BMVs) from Acinetobacter baumannii induced by antimicrobial treatment when compared to BMVs collected from an untreated LB culture
Table 2. The proteins with the greatest decrease in the bacterial membrane vesicle (BMVs) from Acinetobacter baumannii induced by antimicrobial treatment when compared to BMVs collected from an untreated LB culture
The 15 proteins showing the greatest variance and fold difference between the antimicrobial-treated and untreated BMVs are listed (). Using the protein content in the LB-BMV preparation as a baseline, the 15 proteins increased with the greatest variance following induction by ceftazidime treatment included proteins involved in iron chelate transport (6 proteins), channels or transporters other than iron transport (4 proteins), external encapsulating structure (2 proteins) and others. In IMP-BMV, proteins increased included those involved in iron chelate transport (1 protein), channels or transporters other than iron transport (3 proteins), and external encapsulating structure (3 proteins). Many proteins were decreased in IMP-BMV compared to LB-BMV (), including those involved in peptide biosynthetic process (5 proteins) and iron chelate (1 protein), while fewer proteins were decreased in amount in CAZ-BMV compared to LB-BMV.
Discussion
Our previous study revealed that patients treated with antipseudomonal cephalosporins had a higher 14-day mortality rate than patients treated with antipseudomonal carbapenems. In this study, we found that BMVs induced by sub-inhibitory concentrations of ceftazidime led to higher expression of many cytokines in RAW264.7 macrophages and mice serum, caused higher mortality, more neutrophil infiltration in lung interstitium in mice than BMVs induced by imipenem. This might be due to the higher number of BMVs induced by ceftazidime, which also contained higher amounts of LPS and other contents that may be more pathogenic. In this study, we demonstrated that supernatant from a ceftazidime-treated culture of A. baumannii did not contain more LPS and did not induce higher levels of IL-1β and TNF-α expression than supernatant from an imipenem-treated culture, indicating that BMV release, but not cell lysis, was a more important contributor to the difference in pathogenicity. A proteomic study showed that the protein contents in the ceftazidime- and imipenem-induced BMVs were different.
In this study, sub-inhibitory concentration of antibiotics was used due to the following reason. Most of the A. baumannii clinical isolates are multidrug resistant nowadays, including resistant to ceftazidime and carbapenem. Therefore, most of the time the serum concentration of ceftazidime and carbapenem used in clinical setting is under the concentration that can kill the isolates. We used sub-inhibitory concentration to simulate this clinical condition. We avoided higher concentration of antibiotics that might cause the lysis of the bacterial isolates, which could confound the role of the BMVs.
BMVs are naturally secreted products from Gram-negative bacteria. The release of BMVs is enhanced under stress [Citation25, Citation26]. However, how ceftazidime and imipenem enhance BMV formation is still unknown. The bacterial membrane is bound to peptidoglycan via several lipoproteins [Citation27], inhibition of peptidoglycan synthesis by these β-lactams may cause increased vesiculation and BMV formation [Citation28]. Why ceftazidime induced more BMVs than imipenem is also unknown. Many rod-shaped bacteria use two different peptidoglycan biogenesis systems during growth [Citation29]. The actin-like MreB protein is part of the Rod system, which catalyzes the insertion of new peptidoglycan along the cell body to promote cell elongation [Citation29], and the tubulin-like FtsZ protein organizes the divisome to synthesize peptidoglycan for the new daughter cell poles [Citation30]. Each of these machineries requires an essential class B penicillin binding protein (PBP) for activity: PBP2 for the Rod system and PBP3 for the divisome [Citation31]. Antipseudomonal cephalosporins, such as ceftazidime, preferentially bind to PBP3, leading to filament formation, while carbapenems bind to PBP2, leading to the conversion of rod-shaped cells into spherical cells [Citation32]. The effects of these two β-lactams on peptidoglycan biogenesis and the accompanying morphology changes may impact the number and contents of the secreted BMVs.
In the animal study, we found that the administration of CAZ-BMV caused higher mortality than IMP-BMV. When we adjusted the amount of BMVs such that they contained equal amount of LPS, the mortality of mice administered CAZ-BMV was still higher. These results suggested that in addition to LPS, which is a well-known virulence factor, other contents in the CAZ-BMV may also play a role in virulence.
In the proteomic study, we demonstrated that the bacterial membrane proteins, receptors, and transporters in the BMVs between the two types could vary in quantity by more than 100-fold. In both CAZ-BMV and IMP-BMV, the proteins involved in the processes of “channels or transporters other than iron transport” and “external encapsulating structure” were increased, while proteins involved in “peptide biosynthetic process” were decreased. However, some virulence factors were increased in CAZ-BMV but not in IMP-BMV, and some virulence factors were decreased in IMP-BMV but not in CAZ-BMV. For example, in CAZ-BMV, six proteins involved in iron acquisition were increased, while only one protein involved in iron acquisition was increased in IMP-BMV. Moreover, the TonB-dependent siderophore receptor family protein (A0A009HBC2_ACIBA), the only protein involved in iron acquisition that was increased in IMP-BMV was increased only 4-fold in IMP-BMV but was increased 105- fold in CAZ-BMV. Bacterioferritin was decreased in IMP-BMV, but not in CAZ-BMV. The TonB-dependent receptor family proteins and bacterioferritin are known virulence factors of A. baumannii [Citation33,Citation34]. The differences in the amounts of these virulence factors in the BMVs maybe the reason the CAZ-BMV are more virulent. Further studies are needed to clarify the role of these BMV-related proteins on the virulence of CAZ-BMV.
In conclusion, treatment of A. baumannii with a sub-inhibitory concentration of ceftazidime enhanced the release of BMVs that showed higher virulence than the BMVs induced by treatment with imipenem. This finding needs to be validated in a clinical setting to determine whether treatment with sub-inhibitory concentrations of different β-lactams indeed lead to different clinical outcomes in patients with A. baumannii bacteremia.
Supplemental Material
Download MS Word (127.5 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Lee YT, Kuo SC, Yang SP, et al. Impact of appropriate antimicrobial therapy on mortality associated with Acinetobacter baumannii bacteremia: relation to severity of infection. Clinl Infect Dis. 2012;55:209–215.
- Herxheimer K. Ueber eine bei Syphilitischen vorkommende Quecksilberreaktion. DMW-Deutsche Medizinische Wochenschrift. 1902;28:895–897.
- Loscalzo J, Fauci AS, Braunwald E, et al. Harrison’s principles of internal medicine. Dubuque: McGraw-Hill Medical; 2008. p. 1048–1067.
- Rolain JM, Brouqui P, Koehler JE, et al. Recommendations for treatment of human infections caused by Bartonella species. Antimicrob Agents Chemother. 2004;48:1921–1933.
- Madkour MM. Brucellosis. In: Warrell DA, Cox TM, Firth JD, editors. Oxford textbook of medicine. Oxford: Oxford University Press; 2003. p. 1542–1545.
- Hopkin DB. Frapper fort ou frapper doucement: a gram-negative dilemma. Lancet. 1978;312:1193–1194.
- DI G. Nematode infections of lesser importance. In: Warrell DA, Cox TM, Firth JD, editors. Oxford textbook of medicine. Oxford: Oxford University Press; 2003. p. 809.
- Mackie F. The Jarisch-Herxheimer reaction in trypanosomiasis with a note on the morular cells of mott. Trans R Soc Trop Med Hyg. 1935;28:377 IN3379–378IN4384.
- Ikeda Y, Fukuoka Y, Motomura K, et al. Paradoxical activity of beta-lactam antibiotics against Proteus vulgaris in experimental infection in mice. Antimicrob Agents Chemother. 1990;34:94–97.
- Simon DM, Koenig G, Trenholme GM. Differences in release of tumor necrosis factor from THP-l cells stimulated by filtrates of antibiotic-killed Escherichia coli. J Infect Dis. 1991;164:800–802.
- Dofferhoff A, Esselink M, de Vries-Hospers H, et al. The release of endotoxin from antibiotic-treated Escherichia coli and the production of tumour necrosis factor by human monocytes. J Antimicrob Chemother. 1993;31:373–384.
- Kirikae T, Nakano M, Morrison DC. Antibiotic-induced endotoxin release from bacteria and its clinical significance. Microbiol Immunol. 1997;41:285–294.
- Jackson JJ, Kropp H. ß-Lactam antibiotic-induced release of free endotoxin: in vitro comparison of penicillin-binding protein (PBP) 2-specific imipenem and PBP 3-specific ceftazidime. J Infect Dis. 1992;165:1033–1041.
- Kulp A, Kuehn MJ. Biological functions and biogenesis of secreted bacterial outer membrane vesicles. Annu Rev Microbiol. 2010;64:163–184.
- Liao YT, Kuo SC, Chiang MH, et al. Acinetobacter baumannii extracellular OXA-58 is primarily and selectively released via outer membrane vesicles after sec-dependent periplasmic translocation. Antimicrob Agents Chemother. 2015;59:7346–7354.
- Liao YT, Kuo SC, Lee YT, et al. Sheltering effect and indirect pathogenesis of carbapenem-resistant Acinetobacter baumannii in polymicrobial infection. Antimicrob Agents Chemother. 2014;58:3983–3990.
- Schaar V, Nordström T, Mörgelin M, et al. Moraxella catarrhalis outer membrane vesicles carry beta-lactamase and promote survival of Streptococcus pneumoniae and Haemophilus influenzae by inactivating amoxicillin. Antimicrob Agents Chemother. 2011;55:3845–3853.
- Weiss L, Barak V, Zeira M, et al. Cytokine production in linomide-treated NOD mice and the potential role of a Th1/Th2 shift on autoimmune and anti-inflammatory processes. Cytokine. 2002;19:85–93.
- De Jager W, Te VH, Prakken BJ, et al. Simultaneous detection of 15 human cytokines in a single sample of stimulated peripheral blood mononuclear cells. Clin Diagn Lab Immunol. 2003;10:133–139.
- Tsuzuki H, Tani T, Ueyama H, et al. Lipopolysaccharide: neutralization by polymyxin B shuts down the signaling pathway of nuclear factor κB in peripheral blood mononuclear cells, even during activation. J Surg Res. 2001;100(1):127–134.
- Dragovic RA, Gardiner C, Brooks AS, et al. Sizing and phenotyping of cellular vesicles using nanoparticle tracking analysis. Nanomedicine. 2011;7:780–788.
- Yang FL, Lou TC, Kuo SC, et al. A medically relevant capsular polysaccharide in Acinetobacter baumannii is a potential vaccine candidate. Vaccine. 2017;35:1440–1447.
- Shah B, Sullivan CJ, Lonergan NE, et al. Circulating bacterial membrane vesicles cause sepsis in rats. Shock. 2012;37:621–628.
- Park KS, Choi KH, Kim YS, et al. Outer membrane vesicles derived from Escherichia coli induce systemic inflammatory response syndrome. PLoS One. 2010;5:e11334.
- McBroom AJ, Kuehn MJ. Release of outer membrane vesicles by Gram‐negative bacteria is a novel envelope stress response. Mol Microbiol. 2007;63:545–558.
- MacDonald IA, Kuehn MJ. Stress-induced outer membrane vesicle production by Pseudomonas aeruginosa. J Bacteriol. 2013;195:2971–2981.
- Deatherage BL, Lara JC, Bergsbaken T, et al. Biogenesis of bacterial membrane vesicles. Mol Microbiol. 2009;72:1395–1407.
- Koning RI, de Breij A, Oostergetel GT, et al. Cryo-electron tomography analysis of membrane vesicles from Acinetobacter baumannii ATCC19606T. Res Microbiol. 2013;164:397–405.
- Typas A, Banzhaf M, Gross CA, et al. From the regulation of peptidoglycan synthesis to bacterial growth and morphology. Nature Rev Microbiol. 2012;10:123.
- De Boer PA. Advances in understanding E. coli cell fission. Curr Opin Microbiol. 2010;13:730–737.
- Cho H, Uehara T, Bernhardt TG. Beta-lactam antibiotics induce a lethal malfunctioning of the bacterial cell wall synthesis machinery. Cell. 2014;159:1300–1311.
- Moyá B, Zamorano L, Juan C, et al. Affinity of the new cephalosporin CXA-101 to penicillin-binding proteins of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2010;54:3933–3937.
- Zimbler DL, Arivett BA, Beckett AC, et al. Functional features of TonB energy transduction systems of Acinetobacter baumannii. Infect Immun. 2013;81(9):3382–3394.
- Jin JS, Kwon SO, Moon DC, et al. Acinetobacter baumannii secretes cytotoxic outer membrane protein A via outer membrane vesicles. PLoS One. 2011;6:e17027.