ABSTRACT
Pseudomonas aeruginosa
is an opportunistic pathogen and a major cause of corneal infections worldwide. The bacterium secretes several toxins through its type III secretion system (T3SS) to subvert host immune responses. In addition, it is armed with intrinsic as well as acquired antibiotic resistance mechanisms that make treatment a significant challenge and new therapeutic interventions are needed. Type III secretion inhibitors have been studied as an alternative or in accompaniment to traditional antibiotics to inhibit virulence of bacteria. In this study, INP0341, a T3SS inhibitor, inhibited cytotoxicity by P. aeruginosa toward human corneal epithelial cells (HCEC) at 100 μM without affecting bacterial growth in the liquid media. An increased expression of antimicrobial peptides and reactive oxygen species generation was also observed in cells exposed to P. aeruginosa in the presence of INP0341. Furthermore, INP0341 efficiently attenuated corneal infection by P. aeruginosa in an experimental model of murine keratitis as evident from corneal opacity, clinical score and bacterial load. Thus, INP0341 appears to be a promising candidate to treat corneal infection caused by P. aeruginosa and can be further considered as an alternative therapeutic intervention.
Introduction
Pseudomonas aeruginosa is a gram-negative bacterium, ubiquitous in nature and a major opportunistic human pathogen. Corneal infections caused by P. aeruginosa are associated with both trauma and contact lens use and are a foremost cause of blindness worldwide [Citation1]. In the cornea, P. aeruginosa activates the Toll like receptors (TLRs) that results in prompt production of cytokines and chemokines, recruitment of immune cells to the cornea and development of corneal opacity [Citation2]. The corneal epithelium provides the first line of defense against invading bacteria [Citation3] and the host immune response to P. aeruginosa is regulated by TLR4-MD-2 and TLR5 leading to an elevated expression of proinflammatory cytokines and antimicrobial peptides (AMPs) [Citation2,Citation4–Citation6]. One of the fundamental virulence factors of P. aeruginosa is the type III secretion system (T3SS) which consists of a syringe-like apparatus that functions in a highly controlled manner to transport bacterial toxins and other proteins into the host cells [Citation7] and amend different functions of the host to survive [Citation8]. We and others have recently shown that wild-type PAO1 subverts the host immune responses including AMP expression [Citation6] and attenuates generation of reactive oxygen species (ROS) in neutrophils and epithelial cells by its T3SS [Citation6,Citation9].
P. aeruginosa infections are increasingly concerning with their rise in antibiotic resistance. In contrast to other gram-negative bacteria, P. aeruginosa is less vulnerable to various antibiotics due to low penetrance across their outer membrane and the presence of several multi-drug efflux pumps and intrinsic β-lactamases [Citation10,Citation11]. To make the situation worse, P. aeruginosa can form biofilms that have reduced susceptibility to antibiotics [Citation12]. Thus, it becomes important to identify and study novel therapeutic agents that are effective against P. aeruginosa.
T3SSs are highly conserved in several gram-negative pathogenic bacteria and play important roles by secreting toxins into the host cells and thus contributes to pathogenesis [Citation7]. Therefore, they are considered important targets for development of new anti-infective agents. T3SS inhibitors are expected to abolish bacterial virulence without directly killing the bacteria and thus reduce selective pressure that leads to the development of resistance. To date, several small compounds have been developed as T3SS inhibitors and salicylidene acylhydrazides are extensively studied among them. INP0010 [Citation13], INP0400 [Citation14] and INP0007 [Citation15] are examples of salicylidene acylhydrazides that effectively inhibit the T3SSs of several pathogens including Salmonella, Shigella and Yersinia. For this study we have selected INP0341 as it was earlier shown to be effective against P. aeruginosa [Citation16]. It is also known to attenuate the infectivity of C. trachomatis both in vitro and in vivo [Citation17,Citation18]. Uusitalo et al. have reported INP0341 to be effective in prevention of biofilms formed by Pseudomonas and to attenuate P. aeruginosa infection in a burn wound model in Balb/c mice[Citation16].
Herein we demonstrate that INP0341 prevents cytotoxicity induced by P. aeruginosa in human corneal epithelial cells and causes increased expression of antimicrobial peptides and reactive oxygen species generation in response to P. aeruginosa. In addition, topical application of INP0341 inhibits bacterial growth and facilitates bacterial clearance in a murine model of P. aeruginosa keratitis.
Materials and methods
INP0341
INP0341[Citation19] was synthesized as described previously and analytical data were in agreement with those previously reported. Stock solutions of INP0341 (25 mM) were prepared in dimethylsulfoxide (DMSO), stored under dark and dry conditions as described[Citation16]. An intermediate 5 mM solution was made in 50% aqueous DMSO, from which the working solutions were prepared further.
Bacterial culture
PAO1[Citation20], the mutant strain PAO1ΔpscC[Citation21] and two clinical isolates of P. aeruginosa were used in this study. For identification of the clinical isolates, corneal ulcer materials were collected aseptically and investigated following the Institute protocol as described earlier[Citation22]. Briefly, ulcer materials were placed on glass slides for Gram staining and were inoculated in different specific media for bacterial cultures. The pure homogenous culture was then subjected to Vitek 2 compact (bioMerieux, France) analysis for identification of the bacterium along with Gram staining and series of biochemical tests. All strains of P. aeruginosa were grown as described earlier[Citation23]. In brief, bacteria were subcultured from overnight culture in Brain Heart Infusion broth (HiMedia Laboratories, West Chester, USA), washed twice in 1X phosphate buffered saline (PBS), centrifuged at 10,000 rpm for 10 min, and resuspended in 1X PBS. Dilutions of the sample were done with serum free media for the final inoculums.
Culture of HCEC
Immortalized human corneal epithelial cells (HCEC) 10.014 pRSV-T[Citation24] were maintained in keratinocyte serum free media containing bovine pituitary extract and recombinant human epidermal growth factors (Invitrogen, Carlsbad, USA) at 37°C and 5% CO2 and cultured as mentioned before. To study the AMP expression, HCEC were grown in 12-well plates (1 x 105 cells/well) and infected with PAO1 in the presence or absence of INP0341 for 4 h after which cells were processed further.
Toxicity of INP0341 against HCECs
Cytotoxicity of INP0341 toward HCEC was determined quantitatively by measuring the release of lactate dehydrogenase (LDH) into the culture media using CytoTox nonradioactive cytotoxicity assay kit (Promega, Madison, USA) following the manufacturer’s protocol. Briefly, cells were grown to confluency and 50 μM (1% DMSO), 100 μM (2% DMSO), 250 μM (5% DMSO) and 500 μM (10% DMSO) of INP0341was added in triplicate and incubated for 6 h. Cells incubated with Triton X-100 were used as a positive control. The culture supernatant was used for LDH estimation by a colorimetric assay, absorbance was recorded at 490 nm[Citation25].
Cell toxicity induced by P. aeruginosa
PAO1 or PAO1ΔpscC was grown overnight, and subcultured to an OD600 of 0.2 (108 colony forming units/ml, cfu/ml), centrifuged and resuspended in cell culture medium. HCECs were exposed to PAO1 in the presence or absence of INP0341 (100 μM, 2% final DMSO concentration), or PAO1ΔpscC at a multiplicity of infection (MOI) 10:1 (bacteria:cells) for 6 h, washed and imaged using phase contrast microscope. Cell death was measured by LDH assay using CytoTox nonradioactive cytotoxicity assay kit (Promega, Madison, USA) following the manufacturer’s protocol. Cells were fixed with 4% paraformaldehyde and stained with Alexa Fluor 488 phalloidin (ThermoFisher Scientific, Waltham, USA) for 15 min and were counterstained with DAPI (Vector Laboratories, Burlingame, USA) to visualize the nucleus. Images of the cells were captured on a fluorescent microscope (Olympus IX73, Zeiss, Germany) using the 100X objective.
Assay for reactive oxygen species
1 x 104 cells per well were cultured overnight in 96-well plates and infected with PAO1 in the presence or absence of INP0341 (100 μM, 2% final DMSO concentration) for 2 h at a MOI of 10. Cells were also infected with ΔpscC mutant PAO1 for the same period of time. After washing with 1X PBS, cells were incubated with 2ʹ7’-dichlorodihydrofluorescein diacetate dye containing media (H2CFDA; Invitrogen, Carlsbad, USA) for 15 min. The cells were further washed, media was added and the cells were observed under a fluorescent microscope (Olympus IX73, Zeiss, Germany) using a 10X objective. In separate experiments, cells were exposed to bacteria as mentioned above and the fluorescence intensity of the H2CFDA dye was measured quantitatively by SpectraMaxM3 (Softmax Pro 6.3).
Murine models of corneal infection
C57BL/6 mice (6–8 week old) were purchased from Cyagen Biosciences and experiments were performed at Vivo Bio Tech Ltd, Hyderabad, a Contract Research Organization. All animal experiments were approved by the Institutional Animal Ethics Committee of the test facility (VB/IAEC/09/2018/281/Mouse/C57BL/6). The animals were housed in specific pathogen free conditions in microisolator cages and were treated in accordance with the guidelines provided in the ARVO Statement for the Use of Animals in Ophthalmic and Vision research. Mice were anesthetized by intraperitoneal injection of ketamine (8.7 mg/ml) and xylazine (0.5 mg/ml) at a dose of 0.01 ml/g body weight and the corneal epithelium was abraded with three parallel 1 mm scratches using a 26 gauge needle and separated in two random groups. A 2.5 μL aliquot containing approximately 1 × 105 PAO1 was added topically to one eye, and 1X PBS was added to the fellow eye of one group, and mice remained in this position for 5 min. To the second group, 5 μL of 500 μM of INP0341 in 10% DMSO was added immediately after addition of PAO1 to one eye, and only INP0341 (in 10% DMSO) was added to the other eye. 5 μL of DMSO was also added to the infected group untreated with INP0341. The second dose of INP0341 was added topically 6 h post infection to the second group. Mice were euthanized and examined under a stereomicroscope for corneal opacification, ulceration, or perforation 24 h post infection. Clinical scores for the opacity were determined in a blinded fashion according to the scale earlier reported[Citation26]. To measure cfu, whole eyes were homogenized in sterile 1X PBS using a tissue homogenizer (Genetix Biotech, Hyderabad, India) and serial dilutions were plated on LB agar plates, and cfu was counted manually.
Histology and immunohistochemistry
Eyes from control and infected mice were enucleated and placed in 10% formalin and corneal sections were prepared. Hematoxylin and eosin (H&E) staining was performed following deparaffinization of 5 μm corneal sections as described earlier[Citation2].
RNA isolation, cDNA synthesis and quantitative PCR analysis
Quantitative real-time PCR was used to determine mRNA expression of different AMP genes from murine corneas and HCEC. The primers used are shown in . Relative quantities of mRNA expression of respective genes were normalized using the 2−ΔΔct method using GAPDH as the housekeeping gene.
Table 1. Oligonucleotide sequences.
Statistical analysis
Bar graphs represent mean and error bars represent standard error of mean (SEM). Statistical analysis was performed using either a one-way ANOVA or an unpaired t test (Prism; GraphPad Software). p values less than 0.05 were considered significant.
Results
INP0341 prevents cytotoxic effects of P. aeruginosa on HCECs
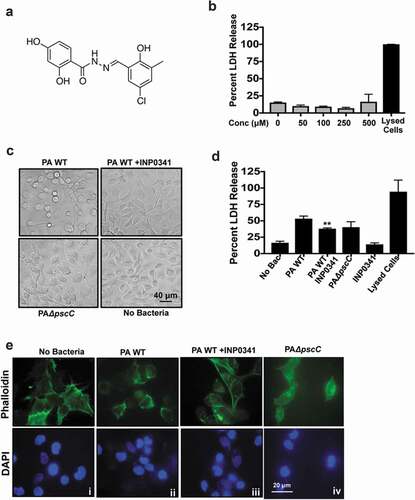
INP0341 ()) was earlier shown to reduce cytotoxicity by P. aeruginosa in HeLa cells[Citation16]. Building on these results we first investigated if INP0341 exerted any cytotoxic effect on HCEC or bacteria. HCECs were incubated with increasing concentrations of INP0341 for 6 h, and cell viability was determined by measuring the release of cytosolic enzyme lactate dehydrogenase (LDH) into the culture media[Citation27]. Cells lysed using detergent were used as a positive control. No significant cytotoxicity was observed in INP0341 treated cells even at higher concentrations ()). Toxicity toward bacteria was also determined by monitoring the growth at 6 and 24 h in the presence of increased concentrations of INP0341 and no significant difference in growth was observed in the presence of higher concentrations of the inhibitor (Fig. S1). Since INP0341 was not toxic to HCECs, we then examined the effect of INP0341 on the cytotoxicity of P. aeruginosa toward HCECs. PAO1 is known to cause cell cytotoxicity in airway epithelial cells[Citation28], and murine macrophages[Citation23] . HCECs were exposed to PAO1 in the presence or absence of INP0341 (100 μM) or PAO1ΔpscC, and cytotoxicity was determined after 6 h by observing the cell phenotype and LDH assay. Cells exposed to PAO1 changed morphologically and about seventy percent of the cells became rounded up. This was significantly reduced in the presence of the inhibitor, and cells were of a similar phenotype to those of uninfected cells ()). In comparison to the cells completely lysed by triton-X 100, PAO1 infection caused fifty percent of LDH maximum release, which was significantly lowered in the presence of INP0341 ()). HCEC treated with INP0341 only showed less than 15% LDH maximum release. Several bacteria including P. aeruginosa cause T3SS mediated disruption of the actin cytoskeleton of the host cells[Citation29]. To observe if INP0341 inhibits actin cytoskeleton rearrangements in cells induced by P. aeruginosa, HCECs were infected with P. aeruginosa in the presence or absence of INP0341 and stained with phalloidin-Alexa Fluor 488. We observed redistribution of the actin cytoskeleton along with cell rounding in cells infected with P. aeruginosa () ii). This effect was visibly inhibited in the presence of INP0341 () iii).
Figure 1. INP0341 impedes cytotoxic effects of P. aeruginosa on HCEC. Chemical structure of INP0341 (a). HCEC were exposed to different concentrations of INP0341 for 6 h to test its effect on cell viability using the lactate dehydrogenase (LDH) assay. Cells lysed with detergent were used as a positive control and cytotoxicity was measured as a percentage of total LDH (b). HCEC were infected with PAO1 in the presence or absence of INP0341 (100 μM), or PAO1ΔpscC for 6 h and cell morphology was imaged under a bright field microscope (c) and cell viability was determined by LDH assay (d). Cells were stained with phalloidin- Alexa Fluor 488 and imaged under a fluorescent microscope using a 100x objective and oil-immersion (e). The experiments were repeated at least three times.
Inhibition of T3SS by INP0341 enhanced AMP expression by HCEC in response to P. aeruginosa
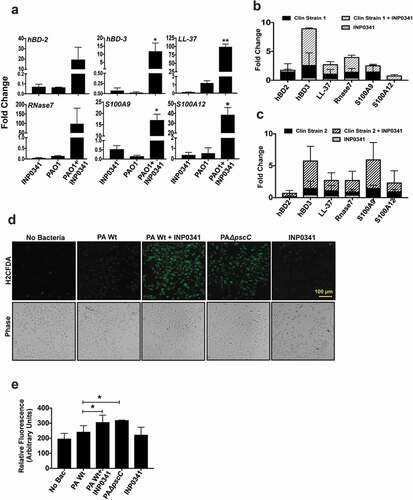
In our earlier study we investigated the expression of AMPs from corneal scrapings of patients with P. aeruginosa corneal infections and saw differential expression of several AMPs, including human β defensins (hBD) 2, and 3, S100A9, S100A12 and LL-37. We also found that PAO1 subverts the expression of these AMPs in HCECs in vitro[Citation6]. However, we observed that the expression of the AMPs increased significantly when cells were exposed to PAO1ΔpscC, a T3SS mutant that fails to transfer exotoxins to the host cells. We therefore continued to investigate if pharmacological inhibition of the T3SS has a similar effect on AMP expression. Two clinical isolates of P. aeruginosa along with PAO1 were included for this experiment. Both the clinical isolates were positive for exoS, T and Y genes but not exoU, similar to PAO1 (data not shown), and thus harbor the T3SS. HCECs were infected either with PAO1 or the clinical isolates at MOI 10 in presence or absence of 100 μM INP0341 and the expression of AMPs was determined. We found increased expression of hBD-2, hBD-3, LL-37, RNase7, S100A9 and S100A12 in HCEC in response to PAO1 in the presence of INP0341 compared to PAO1 alone ()). Increased AMP expression was also observed in cells exposed to clinical isolates of P. aeruginosa in the presence of INP0341 compared to bacteria alone in agreement with our hypothesis (,)).
Figure 2. Increased expression of AMPs and generation of ROS in HCEC exposed to P. aeruginosa in presence of INP0341. HCEC were infected with PAO1 (a) or clinical isolates (b and c) in the presence or absence of INP0341 for 4 h, and AMP gene expression was determined by quantitative PCR using the 2−ΔΔCt method. GAPDH was used as a housekeeping gene. For detection of ROS, HCEC were exposed to PAO1 in the presence or absence of INP0341 (100 μM) or PAO1ΔpscC for 2 h and incubated with H2CFDA for 15 min and then observed and imaged using a fluorescent microscope (d). The generation of ROS was also quantitated using a fluorescent plate reader (e). The experiments were done in duplicate and repeated three times. (*indicates p < 0.05).
Effect of INP0341 on PAO1 induced ROS generation by HCEC
ROS generation in response to infections is not only to kill the invading pathogen but also to mediate the host immune responses. Like several other bacteria, P. aeruginosa has the ability to subvert ROS generation mediated by its T3SS in neutrophils[Citation9] and epithelial cells[Citation6], thus reducing the phagocytic killing by cells. Therefore we investigated if INP0341 could inhibit this subversion of ROS generation in host cells. HCECs were exposed to PAO1 in the presence or absence of INP0341 for 2 h and ROS generation was determined using fluorescent probe H2CDFA. Similar to our earlier observations[Citation6], we saw a minimum level of ROS in cells infected with PAO1 () ii). However, a significant increase in ROS generation was observed in HCECs infected with PAO1 in the presence of INP0341 () iii) compared to cells infected without INP0341 () ii). Increased ROS generation was also observed in PAO1ΔpscC infected cells () iv) compared to PAO1 infected cells as reported earlier[Citation6]. The ROS generation was also quantitatively measured by fluorimeter, and significantly increased levels of ROS were detected in cells infected with PAO1 in the presence of INP0341 ()) compared to infected cells alone.
INP0341 attenuates P. aeruginosa infection in a murine model of keratitis
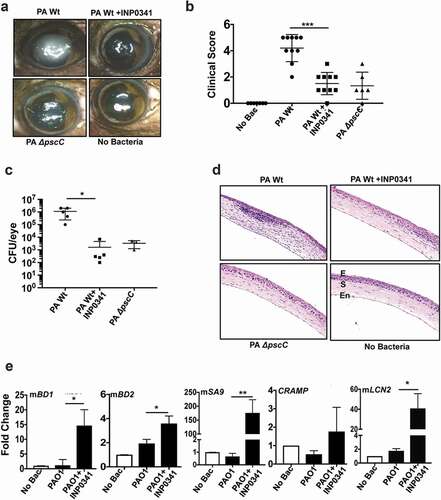
To determine the effect of INP0341 in P. aeruginosa keratitis in vivo, we used our established murine model of keratitis[Citation2] in which corneas of C57BL/6 mice were scratched and infected with PAO1. INP0341 (500 μM in 10% DMSO) was applied topically at 0, and 6 h post infection. Mice were also infected with PAO1ΔpscC as a separate group. Animals were euthanized after 24 h and corneas were imaged for opacification, and cfu in whole eyes were quantified after enucleation. Increased corneal opacification was detected in mice infected with wild-type PAO1, but significantly less opacity was observed in PAO1 infected mice treated with INP0341, or in mice infected with PAO1ΔpscC ()). Significant differences were noted in the clinical scores of the corneal opacity between the groups ()). Consistent with these data, cfu per eye obtained from INP0341 treated corneas were significantly lower by almost three log compare to untreated corneas that were infected with PAO1 (p < 0.0219) ()). There was significantly reduced opacity and cfu in corneas infected with PAO1ΔpscC compared to PAO1 infected corneas as was reported earlier[Citation30]. Hematoxylin and eosin stained sections of mice corneas showed reduced cellular infiltration in the corneal stroma of PAO1 infected mice treated with INP0341 compared to untreated infected mice ()). There was also less infiltration in PAO1ΔpscC infected mice compared to PAO1 infected ones. Since expression of AMPs in HCECs was augmented in the presence of INP0341 in response to PAO1, we further studied the expression of AMPs in the presence of the inhibitor during corneal infections in vivo. Corneas were infected with PAO1 in the presence or absence of INP0341 as mentioned earlier, and AMP expression was determined 24 h post infection by QPCR. Similar to results seen with human corneal epithelial cells in vitro, there was reduced expression of mBD-1, mBD-2, mS100A9, lipocalin (mLCN2) and CRAMP in PAO1 infected corneas, however AMP expression increased significantly in infected corneas treated with INP0341 ()).
Figure 3. INP0341 attenuates P. aeruginosa infection and facilitates bacterial clearance in a murine model of keratitis. C57BL/6 mice were infected with P. aeruginosa and topically treated with INP0341 at 0, and 6 h post infection. Mice were euthanized 24 h post infection and representative images of corneal opacification (a) and their clinical score (b) were recorded. Cfu was measured from whole eye homogenates 24 h post infection (n = 5 mice). Data points represent individual infected corneas (c). Corneal sections were stained with hematoxylin and eosin to visualize cellular infiltration. E, epithelium; S, stroma; En, endothelium (d). Corneas (n = 3) were excised 24 h post infection and homogenized for RNA isolation and expression of AMPs was determined by quantitative PCR using 2−ΔΔCtmethod. GAPDH was used as a housekeeping gene. (*indicates p < 0.05; ** indicates p < 0.005).
In a subsequent experiment, infected corneas were treated either at 0 and 6 h (group I) or at 3 and 6 h (group II) post infection with INP0341 (500 μM in 10% DMSO). Mice were euthanized and corneas were imaged 24 h post infection and reduced opacity was observed in both the group I and group II mice compared to the infected group (Fig. S2A). Significant reduction in clinical scores of the opacity was observed in mice of group I compared to the infected group (p < 0.0014). Although clinical scores of mice of group II were less than the infected group, the difference was not significant (Fig. S2B). Additionally, reduced cfu were observed in mice of group I but not of group II compared to the infected group (Fig. S2C). Consistent with these data, the hematoxylin and eosin staining of corneas of group I mice show reduced cellular infiltrations in the corneal stroma compared to PAO1 infected mice (Fig. S2D). The infiltrations observed in the corneal sections of group II mice were more than that of group I but lower than PAO1 infected group. Taken together, these findings indicate that INP0341 is effective in controlling infection in an experimental model of Pseudomonas keratitis.
Discussion
In this study, we investigated the application of INP0341, a T3SS inhibitor, for the treatment of P. aeruginosa keratitis in a murine model. P. aeruginosa employs T3SS to translocate toxins into eukaryotic cells resulting in infection. It also has a unique ability to develop antibiotic resistance[Citation31]. The increase in antibiotic resistance has generated interests in targeting T3SS to prevent or treat infection by mechanisms distinct from those of conventional antibiotics. Furthermore, since T3SSs is typically found in pathogenic bacteria, targeting this system will not affect the vast commensal population aside[Citation32], thus reducing the likelihood of the emergence of resistant bacteria in this population. It has been reported that hydroxyquinolines effectively inhibited T3SS in Y. pseudotuberculosis, C. trachomatis[Citation33], and P. aeruginosa mediated lung injury[Citation34]. Phenoxyactemaide inhibitors also selectively inhibit T3SS by presumably targeting the needle protein PscF[Citation35]. Recently, Berube et al. reported that several phenoxyacetamide inhibitors could inhibit abscess formation in a murine model of P. aeruginosa infection[Citation36]. Aurodox, a polyketide compound, that inhibits T3SS, was also found to be effective against C. rodentium induced colonic hyperplasia in a mouse model[Citation37].
The salicylidene acylhydrazides block the T3SS in several pathogens including Y. pseudotuberculosis [Citation38,Citation39], C. trachomatis[Citation40], S. enterica, and P. aeruginosa. This class of compounds has been shown to inhibit the S. flexeneri invasion of HeLa to induce apoptosis in macrophages in vitro[Citation14], and to inhibit S. enterica protein secretion and invasion of Madin-Darby Canine Kidney cells[Citation13]. Of the several salicylidene acylhydrazides, INP0341 has been extensively studied and was found to be effective against several pathogens, including P. aeruginosa and C. trachomatis. The salicylidene acylhydrazides have the capacity to chelate iron and INP0341 was shown to exhibit in vitro activity against N. gonorrhoeae[Citation17] and HIV[Citation41] through an iron restriction mechanism. A recent study revealed that the topical application of INP0341 significantly increased the survival of mice with burn wounds infected with P. aeruginosa[Citation16]. We have extended our studies to determine the effect of INP0341 on P. aeruginosa in corneal epithelial cells. Here we showed that INP0341 protected corneal epithelial cells from P. aeruginosa infection. It has previously been shown that PAO1 inhibited host immune responses both in vivo and in vitro. Increased bacterial load was observed in the corneas of C57BL/6 mice infected with PAO1 compared with those infected with a T3SS deficient strain[Citation30]. PAO1 was also found to impede the expression of AMPs, reduce the generation of ROS, and inhibit T3SS mediated p38 and ERK signaling in HCEC [Citation6]. However, in the present study, a significant increase in the expression of AMPs was observed when HCECs were infected with PAO1 in the presence of INP0341. Treatment with INP0341 was also found to be effective against clinical isolates of PAO1 expressing ExoS. Furthermore, in the presence of INP0341, increased ROS generation was observed in PAO1 infected HCEC. Finally, we also demonstrated that INP0341 exerted a therapeutic effect following topical application to mouse corneas infected with PAO1. Significantly reduced bacterial load and increased expression of AMPs were observed in murine corneas infected with PAO1 in the presence of INP0341 applied at the time of infection. Although administered prophylactically and in higher doses than those required in vitro, our data suggested that INP0341 might be used to treat Pseudomonas infections as it improved bacterial clearance in infected corneas. However, further research is required to develop an optimal formulation and to determine the pharmacokinetic profile of INP0341 for ocular administration of the compound that can be useful to treat these blinding corneal infections. In conclusion, this study determines the potential of INP0341 to treat corneal infections caused by Pseudomonas and suggests that virulence inhibitors can be utilized for therapeutic intervention to combat antibiotic resistance.
Supplemental Material
Download Zip (1.1 MB)Acknowledgments
We are thankful to Ophthalmic Pathology Laboratory, LVPEI for the help with tissue sections. Authors acknowledge Dr. Rajesh Karunanithi for animal studies and Apurwa Samarth for technical help. We sincerely thank Prof. Sheila MacNeil for English revision of the paper. The support of Hyderabad Eye Research Foundation is also acknowledged.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Stapleton F, Carnt N. Contact lens-related microbial keratitis: how have epidemiology and genetics helped us with pathogenesis and prophylaxis. Eye (Lond). 2012;26:185–193.
- Sun Y, Karmakar M, Roy S, et al. TLR4 and TLR5 on corneal macrophages regulate Pseudomonas aeruginosa keratitis by signaling through MyD88-dependent and -independent pathways. J Immunol. 2010;185:4272–4283.
- Evans DJ, Fleiszig SM. Why does the healthy cornea resist Pseudomonas aeruginosa infection? Am J Ophthalmol. 2013;155(961–70):e2.
- Roy S, Sun Y, Pearlman E. Interferon-gamma-induced MD-2 protein expression and lipopolysaccharide (LPS) responsiveness in corneal epithelial cells is mediated by Janus tyrosine kinase-2 activation and direct binding of STAT1 protein to the MD-2 promoter. J Biol Chem. 2011;286:23753–23762.
- Karthikeyan RS, Priya JL, Leal SM Jr., et al. Host response and bacterial virulence factor expression in Pseudomonas aeruginosa and streptococcus pneumoniae corneal ulcers. PLoS One. 2013;8:e64867.
- Sharma P, Guha S, Garg P, et al. Differential expression of antimicrobial peptides in corneal infection and regulation of antimicrobial peptides and reactive oxygen species by type III secretion system of Pseudomonas aeruginosa. Pathog Dis. 2018;76. DOI:https://doi.org/10.1093/femspd/fty001
- Hauser AR. The type III secretion system of Pseudomonas aeruginosa: infection by injection. Nature Rev Microbiol. 2009;7:654–665.
- Sundin C, Hallberg B, Forsberg A. ADP-ribosylation by exoenzyme T of Pseudomonas aeruginosa induces an irreversible effect on the host cell cytoskeleton in vivo. FEMS Microbiol Lett. 2004;234:87–91.
- Vareechon C, Zmina SE, Karmakar M, et al. Pseudomonas aeruginosa effector ExoS inhibits ROS production in human neutrophils. Cell Host Microbe. 2017;21:611–8 e5.
- Lambert PA. Mechanisms of antibiotic resistance in Pseudomonas aeruginosa. J R Soc Med. 2002;95(Suppl 41):22–26.
- Rocchetta HL, Burrows LL, Lam JS. Genetics of O-antigen biosynthesis in Pseudomonas aeruginosa. Microbiol Mol Biol Rev. 1999;63:523–553.
- Hoiby N, Bjarnsholt T, Moser C, et al. ESCMID guideline for the diagnosis and treatment of biofilm infections 2014. Clin Microbiol Infect. 2015;21(Suppl 1):S1–25. .
- Negrea A, Bjur E, Ygberg SE, et al. Salicylidene acylhydrazides that affect type III protein secretion in Salmonella enterica serovar typhimurium. Antimicrob Agents Chemother. 2007;51:2867–2876.
- Veenendaal AK, Sundin C, Blocker AJ. Small-molecule type III secretion system inhibitors block assembly of the Shigella type III secreton. J Bacteriol. 2009;191:563–570.
- Kauppi AM, Nordfelth R, Uvell H, et al. Targeting bacterial virulence: inhibitors of type III secretion in Yersinia. Chem Biol. 2003;10:241–249.
- Uusitalo P, Hagglund U, Rhoos E, et al. The salicylidene acylhydrazide INP0341 attenuates Pseudomonas aeruginosa virulence in vitro and in vivo. J Antibiot (Tokyo). 2017;70:937–943.
- Chu H, Slepenkin A, Elofsson M, et al. Candidate vaginal microbicides with activity against Chlamydia trachomatis and Neisseriagonorrhoeae. Int J Antimicrob Agents. 2010;36:145–150.
- Pedersen C, Slepenkin A, Andersson SB, et al. Formulation of the microbicide INP0341 for in vivo protection against a vaginal challenge by Chlamydia trachomatis. PLoS One. 2014;9:e110918.
- Slepenkin A, Enquist PA, Hagglund U, et al. Reversal of the antichlamydial activity of putative type III secretion inhibitors by iron. Infect Immun. 2007;75:3478–3489.
- Bleves S, Soscia C, Nogueira-Orlandi P, et al. Quorum sensing negatively controls type III secretion regulon expression in Pseudomonas aeruginosa PAO1. J Bacteriol. 2005;187:3898–3902.
- Cisz M, Lee PC, Rietsch A. ExoS controls the cell contact-mediated switch to effector secretion in Pseudomonas aeruginosa. J Bacteriol. 2008;190:2726–2738.
- Sharma P, Sharma N, Mishra P, et al. Differential expression of antimicrobial peptides in streptococcus pneumoniae keratitis and STAT3-dependent expression of LL-37 by streptococcus pneumoniae in human corneal epithelial cells. Pathogens. 2019;8:31–48.
- Roy S, Bonfield T, Tartakoff AM. Non-apoptotic toxicity of Pseudomonas aeruginosa toward murine cells. PLoS One. 2013;8:e54245.
- Araki-Sasaki K, Ohashi Y, Sasabe T, et al. An SV40-immortalized human corneal epithelial cell line and its characterization. Invest Ophthalmol Vis Sci. 1995;36:614–621.
- Ponsoda X, Jover R, Castell JV, et al. Measurement of intracellular LDH activity in 96-well cultures: A rapid and automated assay for cytotoxicity studies. J Tissue Culture Methods. 1991;13:21–24.
- Pan Z, Chen Y, Zhang W, et al. Rat corneal allograft survival prolonged by the superantigen staphylococcal enterotoxin B. Invest Ophthalmol Vis Sci. 2003;44:3346–3351.
- Allen M, Millett P, Dawes E, et al. Lactate dehydrogenase activity as a rapid and sensitive test for the quantification of cell numbers in vitro. Clin Mater. 1994;16:189–194.
- O’Grady EP, Mulcahy H, O’Callaghan J, et al. Pseudomonas aeruginosa infection of airway epithelial cells modulates expression of Kruppel-like factors 2 and 6 via RsmA-mediated regulation of type III exoenzymes S and Y. Infect Immun. 2006;74:5893–5902.
- Cowell BA, Evans DJ, Fleiszig SM. Actin cytoskeleton disruption by ExoY and its effects on Pseudomonas aeruginosa invasion. FEMS Microbiol Lett. 2005;250:71–76.
- Sun Y, Karmakar M, Taylor PR, et al. ExoS and ExoT ADP ribosyltransferase activities mediate Pseudomonas aeruginosa keratitis by promoting neutrophil apoptosis and bacterial survival. J Immunol. 2012;188:1884–1895.
- Breidenstein EB, de la Fuente-nunez C, Hancock RE. Pseudomonas aeruginosa: all roads lead to resistance. Trends Microbiol. 2011;19:419–426.
- Gauthier A, Robertson ML, Lowden M, et al. Transcriptional inhibitor of virulence factors in enteropathogenic Escherichia coli. Antimicrob Agents Chemother. 2005;49:4101–4109.
- Enquist PA, Gylfe A, Hagglund U, et al. Derivatives of 8-hydroxyquinoline–antibacterial agents that target intra- and extracellular Gram-negative pathogens. Bioorg Med Chem Lett. 2012;22:3550–3553.
- Anantharajah A, Faure E, Buyck JM, et al. Inhibition of the injectisome and flagellar type III secretion systems by INP1855 Impairs Pseudomonas aeruginosa pathogenicity and inflammasome activation. J Infect Dis. 2016;214:1105–1116.
- Bowlin NO, Williams JD, Knoten CA, et al. Mutations in the Pseudomonas aeruginosa needle protein gene pscF confer resistance to phenoxyacetamide inhibitors of the type III secretion system. Antimicrob Agents Chemother. 2014;58:2211–2220.
- Berube BJ, Murphy KR, Torhan MC, et al. Impact of type III secretion effectors and of phenoxyacetamide inhibitors of type III secretion on abscess formation in a mouse model of Pseudomonas aeruginosa infection. Antimicrob Agents Chemother. 2017;61. DOI:https://doi.org/10.1128/AAC.01202-17.
- Kimura K, Iwatsuki M, Nagai T, et al. A small-molecule inhibitor of the bacterial type III secretion system protects against in vivo infection with Citrobacter rodentium. J Antibiot (Tokyo). 2011;64:197–203.
- Bailey L, Gylfe A, Sundin C, et al. Small molecule inhibitors of type III secretion in Yersinia block the Chlamydia pneumoniae infection cycle. FEBS Lett. 2007;581:587–595.
- Nordfelth R, Kauppi AM, Norberg HA, et al. Small-molecule inhibitors specifically targeting type III secretion. Infect Immun. 2005;73:3104–3114.
- Slepenkin A, Chu H, Elofsson M, et al. Protection of mice from a Chlamydia trachomatis vaginal infection using a Salicylidene acylhydrazide, a potential microbicide. J Infect Dis. 2011;204:1313–1320.
- Forthal DN, Phan TB, Slepenkin AV, et al. In vitro anti-HIV-1 activity of salicylidene acylhydrazide compounds. Int J Antimicrob Agents. 2012;40:354–360.
