?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Streptococcus suis
serotype 2 (S. suis 2) is an important swine pathogen and also an emerging zoonotic agent. HtpsA has been reported as an immunogenic cell surface protein on the bacterium. In the present study, we constructed an isogenic mutant strain of htpsA, namely ΔhtpsA, to study its role in the development and virulence of S. suis 2. Our results showed that the mutant strain lost its typical encapsulated structure with decreased concentrations of sialic acid. Furthermore, the survival rate in whole blood, the anti-phagocytosis by RAW264.7 murine macrophage, and the adherence ability to HEp-2 cells were all significantly affected in the ΔhtpsA. In addition, the deletion of htpsA sharply attenuated the virulence of S. suis 2 in an infection model of mouse. RNA-seq analysis revealed that 126 genes were differentially expressed between the ΔhtpsA and the wild-type strains, including 28 upregulated and 98 downregulated genes. Among the downregulated genes, many were involved in carbohydrate metabolism and synthesis of virulence-associated factors. Taken together, htpsA was demonstrated to play a role in the morphological development and pathogenesis of the highly virulent S. suis 2 05ZYH33 strain.
Introduction
Streptococcus suis (S. suis) is an important pathogenic bacterium in swine worldwide and causes a variety of diseases, such as meningitis, endocarditis, septicemia, arthritis, pneumonia, and even acute death [Citation1]. This pathogen can also infect humans via close contact with infected swine or pork-derived products, causing meningitis, endocarditis, septicemia, permanent deafness, and streptococcal toxic shock-like syndrome (STSLS) [Citation2]. Based on the differentiation of capsule antigens, S. suis was divided into 35 serotypes, but several recent reports manifested that serotypes 20, 22, 26, 32, 33, and 34 did not belong to the S. suis taxon [Citation3–Citation5]. Among all serotypes, S. suis 2 is the most virulent and frequently isolated serotype from clinically diseased piglets [Citation6,Citation7]. During the last 20 years, sporadic or large outbreaks of human S. suis 2 infections have occurred occasionally worldwide [Citation8,Citation9]. In 1998 and 2005, two outbreaks of S. suis 2 in China caused severe streptococcal toxic shock-like syndrome in infected patients with mortalities as high as 62.7% to 81.3% [Citation10]. Over the past 20 years, a growing number of studies involving S. suis pathogenic mechanisms have identified over 70 bacterial virulence-associated factors using comparative genomics, transcriptomics, proteomics, suppression subtractive hybridization, and other methods [Citation11].
Many surface proteins were reported to contribute to bacterial adhesion to cells, blood invasion, immune evasion, and transmembrane nutrient delivery, such as enolase [Citation12,Citation13], glutamate dehydrogenase (GDH) [Citation14], elongation factor Tu (EF-Tu) [Citation15], sortases [Citation16], glyceraldehyde-3-phosphate dehydrogenase (GAPDH) [Citation17], factor H-combining protein (Fhb) [Citation18,Citation19], β-galactosidase (BgaC) [Citation20], laminin-binding protein (Lmb) [Citation21], and eukaryotic-like Ser/Thr protein kinase (STK) [Citation22]. Recently, a family of surface-exposed proteins containing multiple histidine triad (His-x-x-His-x-His) motifs was reported to be in the Streptococcus genus and has attracted widespread attention [Citation23]. The histidine triad proteins (Htps) were initially identified from Streptococcus pneumoniae and named pneumococcal histidine triad proteins (Phts) [Citation24–Citation26]. Subsequently, the homologs of Pht proteins were also identified in Streptococcus pyogenes (Slr and HtpA) [Citation27,Citation28], Streptococcus agalacticae (Blr and Sht) [Citation29,Citation30], and Streptococcus suis (HtpS) [Citation31]. Based on phylogenetic relationship and domain structure analyses, htps were classified into type I and type II subfamilies [Citation32]. Our previously study found three Htps in S. suis 2, HtpsA (HtpS, SSU05_0332), HtpsB (SSU05_1267), and HtpsC (SSU05_1577) [Citation31,Citation32]. Among them, HtpsA belongs to the HTP I type subfamilies, whereas HtpsB and HtpsC belong to the HTP II type subfamilies [Citation32]. The S. suis HtpsA is orthologous to the HtpA of S. pyogenes and PhtD of S. pneumoniae, which form an operon with an upstream laminin-binding protein (lmb)-encoding gene and is regulated by the AdcR protein [Citation24,Citation27,Citation33,Citation34]. Many studies have shown that S. pneumoniae PhtD is involved in a diverse range of important biological functions, including zinc-ion homeostasis, immune evasion, adherence of bacteria to host cells, and pathogenicity [Citation23].
To elucidate the biological functions of HtpsA and its potential role in the pathogenicity of S. suis 2, we constructed a gene knockout mutant, ΔhtpsA, of the S. suis 2 strain by homologous recombination. Comprehensive experimental studies showed obvious morphology change and attenuation of pathogenicity in the mutant strain. RNA sequencing (RNA-seq) analysis suggested that the inactivation of htpsA resulted in the downregulation of many genes involved in glucose metabolism and virulence-related factors.
Materials and methods
Bacterial strains, plasmids, and culture conditions
The bacterial strains and plasmids used in this study are listed in . The S. suis 2 virulent strain 05ZYH33 (wild-type, WT) was isolated from an infected patient during the 2005 outbreak in Sichuan, China [Citation10]. The 05ZYH33 strain and isogenic mutant strains were grown in Todd-Hewitt broth (THB; Difco Laboratories, Detroit, MI, USA) liquid medium or plated on THB agar plates at 37°C in a 5% CO2 atmosphere supplemented with 5% (v/v) sheep blood when needed. Spectinomycin (Spc, Sigma, St. Louis., MO, USA) was added to screen for the htpsA mutant strain, 100 µg/mL. The E. coli DH5α strain used in the construction of the recombinant gene knockout plasmid pUC::htpsA (consisting of a spectinomycin resistance (SpcR) cassette with flanking homology regions of the htpsA gene) was purchased from Transgen Co. (Beijing, China) and maintained in Luria-Bertani (LB) broth liquid medium or plated on LB agar at 37°C. The following antibiotics were added to the medium at the indicated concentrations: for the isogenic mutant strains, spectinomycin at 100 μg/mL; for E. coli, ampicillin or kanamycin [Amp, Sigma, St. Louis, MO, USA] at 100 μg/mL.
Table 1. Bacterial strains and plasmids used in this study.
Construction of an htpsA knockout mutant
Homologous recombination was utilized to generate an htpsA mutant as described previously Citation22]. Primers used in this study are listed in Table S1. Using primers LA1/LA2 and RA1/RA2, the 5ʹ upstream region (976 bp) and 3ʹ fragment (847 bp) of htpsA gene were amplified by PCR from the genome of S. suis 2 05ZYH33, respectively. The SpcR gene was amplified from the pSET2 plasmid using primers Spc1/Spc2. The three fragments were double-digested by restriction enzymes and then ligated into pUC19 to form the knock-out plasmid pUC::htpsA. Then, the pUC::htpsA plasmids were used for the electronic transformation of the 05ZYH33 competent cells. The putative transformants were confirmed by combined PCR and then verified by RT-PCR and direct DNA sequencing using a series of specific primers (Table S1).
RNA-seq and quantitative real-time RT-PCR (qRT-PCR)
The 05ZYH33 and ∆htpsA strains were cultured to the middle of the logarithmic phase (OD600 = 0.6). Bacterial cells were harvested by centrifugation at 12, 000 × g for 2 minutes. Then, 3 mg/mL of fresh lysozyme (Sigma, St. Louis, MO, USA) was used to resuspend the collected cells and incubated for 5–10 minutes. Total RNA was extracted from the 05ZYH33 and ∆htpsA strains using an SV Total RNA Isolation System kit (Promega, Madison, Wisconsin, USA), according to the manufacturer’s instructions. The extracted RNA concentration and integrity were assessed by One Drop spectrophotometer (Pharmacia, Dübendorf, Switzerland) and electrophoresis on a 1.5% agarose gel, respectively. The RNA concentration of the 05ZYH33 and ∆htpsA strains were determined as 551.29 ng/µL and 539.77 ng/µL, respectively. The RNA samples were stored at −80°C until needed.
The RNA samples were sent to the GENEWIZ Company (Suzhou, China) for RNA-seq. The sequencing library was performed as reported previously [Citation36]. The amplified library was sequenced using an Illumina HiSeqTM 2500 according to the manufacturer’s protocol. Transcriptome data were analyzed as follows: i) the RPKM (reads per kilobase per million mapped reads) values were calculated for each gene using uniquely mapped reads; ii) differential expression genes were confirmed via the model |log2 (fold_change)| ≥1 and P-value ≤0.05. The categorization of biological processes was analyzed using the Gene Ontology (GO) project (http://www.geneontology.or).
To confirm the differentially expressed genes of RNA-seq, quantitative real-time RT-PCR was performed using primers listed in table S1 as follwing: first-strand cDNA was synthesized according to the PrimeScriptTM RT Master Mix (Perfect Real Time) Kit (TaKaRa, Dalian, China). The cDNA samples were used for real-time RT-PCR (Applied Biosystems QuantStudio 3 Real-time PCR System, ThermoFisher, Shanghai, China). The relative levels of target gene expression were normalized with the gapdh gene using the 2− ∆∆Ct method.
Detection of the genetic stability of the mutant strains and growth curves
The mutant ΔhtpsA was passaged more than 50 consecutive times in THB liquid containing spectinomycin (100 μg/mL) or no spectinomycin at 37°C. The genetic stability of ΔhtpsA was assessed by PCR using the primers Spc1/Spc2. The ΔhtpsA and 05ZYH33 strains were inoculated in Colombia sheep agar plates (bioMérieux, Shanghai, China) at 37°C for 48 hours. The single colonies were inoculated into fresh THB liquid medium. Cultures were then inoculated into fresh THB at 1:100 ratios for growth curve analysis. Subsequently, the absorbance of the cultures was monitored at 1 hour intervals using a spectrophotometer (Bio-Rad, Hercules, California, USA) at a wavelength of 600 nm, and sterile THB media was used as the blank control.
Morphological observation of the 05ZYH33 and mutant strain
The ΔhtpsA and 05ZYH33 strains were inoculated into THB liquid medium (containing 10% fetal bovine serum) and cultured to the middle of the logarithmic growth phase (OD600 = 0.6) at 37°C, and then washed twice with ddH2O. Each sample was fixed on glass slides by flaming. Gram staining was conducted according to the instructions provided in the Gram staining kit (Jian Cheng, Nanjing, China). The morphology of bacteria was observed under a light microscope (10 × 100 times).
Transmission electron microscope (TEM) observation was performed as previously described [Citation22]. Briefly, a single colony (05ZYH33, ΔhtpsC, ΔhtpsA, or Δcps2B) was picked from Colombia sheep agar plates and cultured in THB liquid medium adding 10% fetal bovine serum. The bacterial cells were harvested at OD600 = 0.8 and fixed in 2.5% glutaraldehyde (Sangon Biotech, Shanghai, China) for 2 hours, followed by washing with 0.01 M PBS. After that, bacterial cells were fixed in cacodylate buffer with 1% osmium tetroxide (Sigma, St. Louis, MO, USA) for 2 hours at 25°C in the dark. The cells were then dehydrated for 20 minutes with gradient acetone and then embedded in Epon-618 epoxy resin (BOC, New York, USA). Ultra-thin sections were post-stained with aqueous uranyl acetate and lead citrate and observed using a JEM-1010 TEM (JEOL, Ltd., Tokyo, Japan) at an accelerating voltage of 100 kV. The thickness of the bacterial capsule was determined by imageJ software by randomly selecting 30 cells.
The bacterial agglutination test
A single colony of both the 05ZYH33 and ΔhtpsA strains was picked from Colombia sheep agar plates and cultured in THB liquid medium at 37°C 12 hours. Equal amounts of each sample (5 μL) were evenly coated on two glass slides. One was dripped with 5 μL of specific S. suis 2 antiserum (Statens Serum Institut, Copenhagen, Denmark), and the other was a negative control. The results were observed under a microscope.
Determination of sialic acid content
Determination of sialic acid content was performed as described previously [Citation37], with slight modifications. Briefly, the single colonies (05ZYH33 and ΔhtpsA) were picked from Colombia sheep agar plates, inoculated into THB liquid medium (containing 10% fetal bovine serum), and cultured to OD600 = 0.3 at 37°C. The pellets were harvested by centrifugation at 8, 000 × g for 5 minutes. After washing with PBS, the resuspended pellet was centrifuged again at 8, 000 × g for 5 minutes and resuspended in 600 μL of PBS, of which 500 μL of the suspensions was disrupted by sonication. The supernatant was collected by centrifugation at 15, 000 × g for 15 minutes at 4°C. After adding 10 μL of sialidase (0.25 U), the supernatants were hydrolyzed for 1 hour at 37°C. The sialic acid concentration was detected using a Sialic Acid Assay Kit (Jian Cheng, Nanjing, China), according to the manufacturer’s instructions. The following formula was used for calculating the sialic acid concentration:
Note: SA represents sialic acid; OD represents optical density value at 560 nm wavelength; C represents concentration of standard mmol/L; V represents volume of standard; MW represents molecular weight of sialic acid, 309.3 g/mol.
Adhesion assay and anti-phagocytosis assay
The bacterial strains were inoculated into THB liquid medium (containing 10% fetal bovine serum) and cultured to log phase at 37°C. Bacterial cells were collected by centrifugation at 5, 000 × rpm for 10 minutes, washed three times with PBS (pH = 7.4), and then opsonized to a density of 5 × 108 CFU/mL. 5(6)-carboxyfluorescein diacetate, succinimidyl ester (CFDA-SE) was added to the resuspension at a final concentration of 10 μM and then incubated at 37°C for 30 minutes. The pellets were collected again by centrifugation at 5, 000 × rpm for 10 minutes and then washed twice with PBS. Labeled bacterial pellets were put on ice until use.
The adherence assay was performed on HEp-2 cells (ATCC CCL23) according to previous report [Citation38]. The anti-phagocytosis ability was evaluated using Raw264.7 murine macrophages, as previously described [Citation22]. Briefly, 1 × 106 cells (HEp-2 or RAW264.7) were incubated with 1 × 108 CFU of CFSE-labeled bacterial pellets at a ratio of 100:1 at 37°C for 2 hours. Additionally, for anti-phagocytosis, the cells were treated with penicillin (5 μg/mL) and gentamycin (100 μg/mL) (Sigma, St. Louis, MO, USA) for 1 hour in the dark to eliminate extracellular bacteria. After incubation, the pellets were washed three times with PBS, and then 4% (wt/vol) paraformaldehyde was added and softly mixed to fix cells. Flow cytometry was performed with a FACSCalibur (BD, Franklin Lakes, New Jersey, USA). The adhesion rates and the phagocytosis rates were assessed based on the normalized mean fluorescence intensities (NMFI).
The survival of 05ZYH33 and ΔhtpsA strains in human whole blood
A whole-blood bactericidal activity was performed as previously reported [Citation39]. The ΔhtpsA and 05ZYH33 strains were grown to the middle of the logarithmic phase (OD600 = 0.6) in THB liquid medium at 37°C. The pellets were then collected by centrifugation at 8, 000 × g for 10 minutes and washed twice in sterile 0.01 M PBS. The bacterial was opsonized to a density of 5 × 108 CFU/mL. Then, 10 μL of the bacterial suspensions was added to 350 μL human anticoagulant whole blood and incubated in 5% CO2 at 37°C for 8 hours. Mixtures (50 μL) of each group sample were diluted, and the number of viable bacteria was determined by plating serial dilutions onto THB agar and incubating overnight at 37°C. The survival rate was calculated as CFU per mL. Experiments were performed in triplicate.
Experimental infection of mice
To compare the pathogenicity of the ΔhtpsA and 05ZYH33 strains, a bacterial challenge experiment was performed in a previously constructed mouse model [Citation22]. Four-week-old specific pathogen free (SPF) grade female BALB/c mice (SLAC, Nanjing, China) were randomly divided into three groups consisting of 10 mice and infected with 1 × 108 CFU of either ΔhtpsA or 05ZYH33 strains via intraperitoneal injection. Negative controls were treated with equal volumes of aseptic THB medium. Mice were monitored in terms of clinical symptoms for 2 weeks, and the dead mice were recorded. A Kaplan-Meier survival curve analysis was performed using the SPSS package to test the significant difference among different groups. All animal experiments were carried out according to the recommendations of the laboratory animal administration rules, State Scientific and Technological Commission. The protocol was approved by the Ethics Committee of Hua Dong Research Institute for Medicine and Biotechnics.
Statistical analysis
Statistics for the survival curves of mice were done with the Kaplan-Meier survival analysis. The statistical analyses of capsular thickness, sialic acid content, bacterial adherence capability, survival level in human whole blood, anti-phagocytotic ability, and qRT-PCR data were carried out by using independent-samples t test (two-tail). All experiments were performed at least three times, and data are displayed as the mean ± SD. A p-value <0.05 is considered significant. In the figures, * represents p < 0.05, ** represents p < 0.01, and NS means no significant difference.
Results
Construction of an htpsA mutant
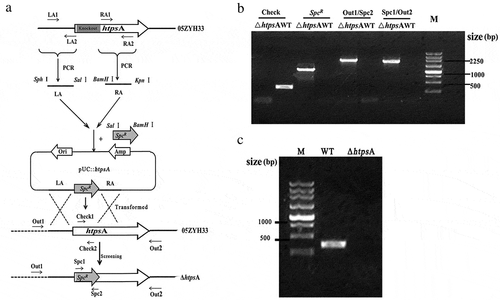
An isogenic mutant strain, ΔhtpsA, was constructed through homologous recombination as illustrated in . Using the primers of Check1/Check2, two htpsA negative strains were identified by PCR amplification after screening more than 100 SpcR transformants. Combined PCR analysis showed that the 5ʹ 970-bp region of htpsA gene was successfully replaced by the SpcR gene in the mutant strain (). RT-PCR also confirmed the lack of expression of htpsA from the mutant strain (). Finally, direct DNA sequencing showed the 5ʹ 970-bp region of htpsA gene was completely displaced by the SpcR gene in the mutant (data not show). Taken together, an htpsA mutant, named ΔhtpsA, was successfully constructed and used for subsequent experiments in this study.
Figure 1. Construction of an isogenic htpsA mutant of S. suis 05ZYH33. (a) Schematic diagram of the construction process of the ΔhtpsA strain. (b) Combined PCRs of the ΔhtpsA mutant. (c) Reverse-transcription PCR analysis of htpsA gene transcripts. The primer pairs and templates used in the PCR analysis are indicated above the lanes. WT and ΔhtpsA represent genome DNA of the wild-type strain 05ZYH33 and mutant strain, respectively.
Morphological alteration of the ΔhtpsA
To investigate whether inactivation of the htpsA gene affected the morphology of S. suis 2, several features were assessed and compared between the ΔhtpsA and 05ZYH33 strains, including growth rates, bacterial shape, capsule formation, and colony morphology. Under normal growth conditions without antibiotics, the growth density of ΔhtpsA was lower than the 05ZYH33 strain starting from the late logarithmic stage (7 hours), and the difference increased after entering the stationary stage (). There were no significant differences between the two groups regarding colony morphology, including size, color, shape, transparency, and stickiness when growing on the solid medium. Transmission electron microscopy (TEM) observation revealed that the capsular structure of ΔhtpsA was obviously thinner than that of the 05ZYH33 (). The capsular structure of ΔhtpsA is similar to a capsular deficiency strain by cps2B mutation. However, similar phenotype of capsular deficiency was not observed in the htpsC mutant strain (). We calculated the capsular thickness of the 05ZYH33, ΔhtpsA, ΔhtpsC and Δcps2B strain, and found that ΔhtpsA cells (6.11 ± 1.81 nm) were significantly thinner than that of 05ZYH33 (46.92 ± 6.30 nm) and ΔhtpsC (43.64 ± 5.55 nm), as shown in .
Figure 2. Phenotypic analysis of the 05ZYH33 and ΔhtpsA mutant strains. (a) Cell density was measured spectrophotometrically at a wavelength of 600 nm. (b) Antiserum aggregation reaction of the ΔhtpsA and 05ZYH33. (c) Observation of capsular morphology of the ΔhtpsA, ΔhtpsC, Δcps2B and the wild-type strains by transmission electron microscopy. (d) Determination and analysis of capsule thickness of the ΔhtpsA, ΔhtpsC, Δcps2B and wild-type strains. ** represents significant differences (P < 0.01), NS represents no significant differences.
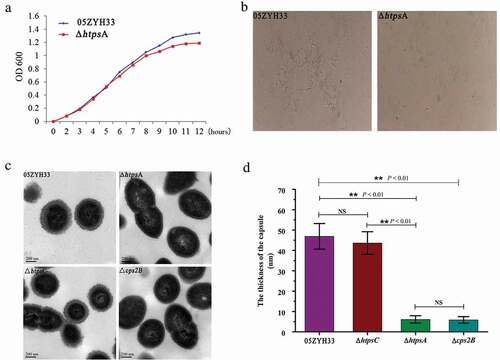
We further performed an agglutination test using a specific antibody against the capsule of S. suis 2 in order to confirm the deficiency in capsular development of the ΔhtpsA. The ΔhtpsA aggregated weakly 3 minutes after adding the specific antibody, while apparent cell aggregation for the 05ZYH33 strain was observed at 1 minute after the treatment (). These results suggested that the insensitivity of the ΔhtpsA to specific antiserum may be related to the loss of capsular structure.
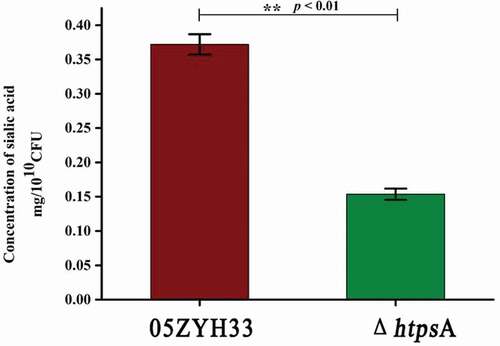
Reduced sialic acid production in the ΔhtpsA
Sialic acid is an important component of the capsule of S. suis 2. Inactivation of the sialic acid synthesis pathway resulted in the loss of the capsular structure. In order to examine whether the observed capsule-less structure of the ΔhtpsA strain was associated with the lack of sialic acid, we detected the sialic acid content of the ΔhtpsA strain. Our results showed that the content of sialic acid in the capsule of the ΔhtpsA cells was only half of the sialic acid content in 05ZYH33 cells ().
Attenuated pathogenicity of the ΔhtpsA
To evaluate the adhesive capacity of the ΔhtpsA strain, CFSE-labeled ΔhtpsA and 05ZYH33 cells were co-cultured with HEp-2 cells. Our results showed that the NMFI value of HEp-2 cells incubated with the ΔhtpsA declined nearly 40% as compared to the 05ZYH33 strain (P < 0.01, ). This result indicated that the inactivation of htpsA significantly impaired the adherence of S. suis 2.
Figure 4. Effect of HtpsA deficiency on virulence and pathogenicity of bacteria. (a) Comparison of bacterial adherence capability of the ΔhtpsA mutant with the wild-type 05ZYH33 strain. The normalized mean fluorescence intensities (NMFI) of HEp-2 cells after incubation with the bacteria are shown as columns with standard errors. (b) Survival of the 05ZYH33 and ΔhtpsA mutant in human whole blood. Mixtures were incubated at 37°C for 8 hours, and then the dilutions were coated on agar plates. The number of single colonies that grew after incubating overnight was counted. (c) Evaluation of the anti-phagocytotic ability of S. suis strains in macrophage RAW264.7 cells (* indicates P < 0.05; ** indicates P < 0.01, Student’s t-test).
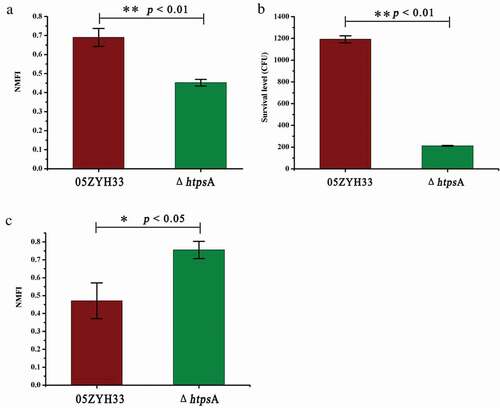
Subsequently, a whole-blood killing test showed that the survival rate of the ΔhtpsA strain dropped sharply from 1188 CFU to 213 CFU as compared with the 05ZYH33 strain (P < 0.05, ). We further compared the anti-phagocytosis ability of the ΔhtpsA and 05ZYH33 strains using murine RAW264.7 macrophages. As shown in , the NMFI value of the mutant strain was 0.75 ± 0.047, which was approximately a 1.27-fold increase as compared with the 05ZYH33 strain (0.47 ± 0.099). This indicated that the sensitivity of ΔhtpsA to phagocytic cells increased as compared to the 05ZYH33 strain, which resulted in a significantly weakened anti-phagocytosis ability in the ΔhtpsA (P < 0.05). All these results suggested that the deletion of htpsA attenuated the pathogenicity of S. suis 2 via different aspects.
Weakened virulence of the htpsA mutant in the mouse infection model
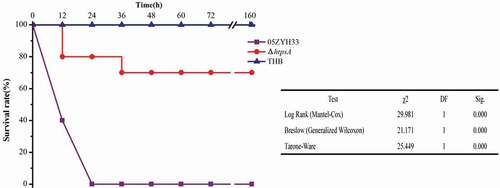
A mouse infection assay was used to clarify the contribution of the htpsA gene to bacterial virulence. The mice were randomly divided into three groups, namely ΔhtpsA, 05ZYH33, and THB groups as negative control. After 12 hours of infection, the survival rate of the ΔhtpsA group was 80%, while the 05ZYH33 group was only 40%. Moreover, after infection 24 hours, the survival rate was still 80% in the ΔhtpsA group, while all the mice in the 05ZYH33-infected group died. The survival rate was 70% in the ΔhtpsA group at 36 hours after infection (). No symptoms were found in the surviving mice through the end of the 7-day experiment. In the THB group, all mice were in good condition. Statistical analysis revealed that the rate of mortality was significantly reduced in the ΔhtpsA group (P < 0.01, Kaplan-Meier survival analysis). The results of animal experiments demonstrated that knockout of the htpsA gene impaired the full virulence of 05ZYH33.
Figure 5. Survival curves of mice infected with the ΔhtpsA mutant or wild-type strain 05ZYH33 strains. Four-week-old BALB/c mice were challenged intraperitoneally with 1 × 108 CFU bacteria, and the survival time was monitored. * represents a significant difference of P < 0.05, and ** represents a significant difference of P < 0.01.
Altered transcription profiles of the htpsA mutant
To explore the mechanisms underlying the function of htpsA, the transcriptome profile of both the ΔhtpsA and 05ZYH33 strain was determined by RNA-seq. Compared with the 05ZYH33 strain, there were 98 genes downregulated and 28 genes upregulated in the ΔhtpsA, among which five were located on the 89 K pathogenic island (Table S2). The differentially expressed genes were classified into different functional categories (), including physiological metabolism (45.2%), enzymes associated with transport systems (21.4%), genetic information processing (10.3%), and function-unknown genes (21.4%). Notably, 51% (50 of 98 genes) of the downregulated genes encoded proteins involving in saccharometabolism and sugar transporters, such as GalK, GalT, GlgC, and MalM (). This may be one of the most important reasons for the reduction in the bacterial capsular structure in the ΔhtpsA strain. The expression of several virulence-related factors was also observed, including suilysin and genes encoding hyaluronidase (Table S2).
Table 2. Functional classification of differentially expressed genes between the ΔhtpsA mutant and 05ZYH33 strain.
Table 3. Classification of downregulated genes related to saccharometabolism.
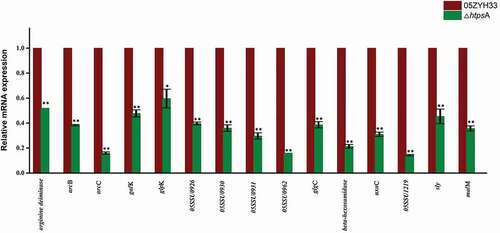
Fifteen genes from different functional catagories were selected for further qRT-PCR analysis to confirm their differential expression after htpsA inactivation. As shown in , the results obtained from the qRT-PCR analysis are highly consistent with those in the RNA-seq data. Specifically, the expression of several proved or potential virulence factor-related genes including sly, arcB, arcC and arginine deiminase was downregulated 2.22, 2.61, 6.28 and 1.93 folds compared to the 05ZYH33 strain, respectively. The expression level of four genes (05SSU0926, 05SSU0930, 05SSU0931, 05SSU0962) from 89 K virulence island was also decreased by two to six folds. The expression of genes associated with glycometabolism including malM, uxaC, galK, beta-hexosamidase and 05SSU1219 (PTS system, mannose specific) were downregulated 2.8, 3.21, 2.11, 4.68 and 7.04 folds, respectively. Taken together, our data revealed that inactivation of htpsA influenced expression of multiple genes involved in metabolism processes, capsule synthesis and virulence of the bacterium. We also evaluated the expression of the other two htp family members htpsB and htpsC by qRT-PCR. No significant differential expression was observed for the two genes as revealed by the RNA-seq data (Figure S1).
Figure 6. qRT-PCR validation of expression profiles of 15 differentially expressed genes identified by RNA-seq. The house-keeping gene gapdh was used as internal control, and error bars represent SEM of three replicates. * represents a significant difference of P < 0.05, and ** represents a significant difference of P < 0.01. Significant difference as determined by Student’s t-test.
Discussion
Streptococci express many surface proteins to promote host infection [Citation40]. htps are a group of genes widely distributed within the Streptococcus genus and play important roles in bacterial infection [Citation23]. According to the phylogenetic relationship and gene structure, htps can be divided into two subfamilies: HTP I and HTP II [Citation32]. Research regarding four HTP I subfamily members, phtA, phtB, phtD, and phtE, in S. pneumoniae found that the virulence of the bacteria was weakened or even lost when deleting two or more members at the same time, but there was no effect on bacterial virulence when only one gene was inactivated [Citation34]. This report suggested that the pht family proteins are very important for the pathogenesis of S. pneumonia, although functional redundancy existed between family members. Furthermore, deletion of phtD orthologous genes resulted in decreased virulence of S. agalactiae and S. pyogenes, indicating that the family proteins are closely related to the pathogenesis [Citation27,Citation30]. Recently, our study showed that a type II Htp protein, HtpsC, of S. suis 2 could bind to laminin and fibronectin of the human ECM complex. Inactivation of htpsC significantly affected adherence and attenuated the virulence of S. suis 2 in mice [Citation39]. In this study, we found that the deletion of the type I Htp member, htpsA, in S. suis 2, impaired the full virulence and the capsular structure of the bacterium. The results from our present and previous studies suggested that type I and type II proteins play important, but not redundant, roles in the virulence of S. suis 2.
The deficiency in capsular development is one of the reasons for the weakened virulence in the ΔhtpsA strain
Capsular polysaccharide (CPS) is an important virulence factor for several pathogens. It is involved in many infection processes in S. suis, such as bacterial adhesion, invasion, survival, and blocking neutrophil and monocyte/macrophage-mediated phagocytosis and killing [Citation41]. CPS biosynthesis involves 25 open reading frames in S. suis 2, including orf2Z, orf2Y, orf2X, orf2L, orf2M, orf2N, orf2U, orf2V, Cps2A-Cps2K, and Cps2O-Cps2T [Citation42]. An unencapsulated S. suis 2 mutant generated by inactivating the cps2 gene exhibits a 15–60% decrease in the adherence to HEp-2 cells [Citation43]. And inactivation of cps2B caused a significant loss of the capsule and decreased the pathogenicity of S. suis 2 in a mouse infection model [Citation44]. The deletion of four genes (cps2E, cps2 G, cps2 J, and cps2 L) in S. suis 2 SC19 caused a significant decrease in the capsular sialic acid synthesis and virulence [Citation45]. In this study, we observed that the ΔhtpsA had a thinner capsule than 05ZYH33, which likely caused an increase in the sensitivity of this strain to phagocytosis by macrophages. The reduced content of sialic acid, one of the main components of the capsular polysaccharide, in the ΔhtpsA also supports its deficiency in capsular development. Furthermore, our RNA-seq analysis of the ΔhtpsA strain revealed that among the 98 downregulated genes, 51% are involved in the metabolism of glucose, galactose, mannose, PTS, and ABC-type sugar transport systems (). A possible hypothesis is that the absence of htpsA caused a dysfunction in glucose metabolism and glucose transport system in the ΔhtpsA, which resulted in the hindrance of bacterial capsular polysaccharide synthesis and loss of the typical capsular structure. Notably, capsular deficiency was not detected for the htpsC mutant strain, suggesting functional divergence of the two htp family members in S. suis 2 morphology development. Together, these resulted in the decreased anti-phagocytosis ability and attenuated virulence of the ΔhtpsA in a murine infection model.
The downregulated expression of virulence-related factors may also contribute to the weakened virulence of the ΔhtpsA strain
Virulence factors play important roles in different stages of pathogen infection and pathogenicity [Citation40]. Among numerous virulence factors, suilysin (SLY), a vital virulence factor, has been verified to participate in the bacterial infection process through activating phagocytes and inducing the release of proinflammatory cytokines [Citation46]. In this study, the SLY gene was downregulated 2.22-fold in the htpsA mutant strain as compared with the 05ZYH33 strain. We also observed the decreased expression of arginine deiminase (arcA, SSU05_0624), ornithine carbamoyltransferase (arcB, SSU05_0626), and carbamate kinase (arcC, SSU05_0627) in the htpsA mutant strain. The ArcA is a member of arginine deiminase system (ADS), which is a secondary metabolic system that exists in many different bacterial pathogens and is often associated with virulence [Citation47]. In S. pyogenes, the ADS is involved in adhesion and invasion of epithelial cells [Citation48]. In S. suis, ADS is responsible for survival under acidic conditions, where it catalyzes the conversion of arginine to ornithine, ammonia, and carbon dioxide [Citation49]. Additionally, the genes encoding hyaluronidase (SSU05_1212 ~ SSU05_1215), which catalyzes the degradation of hyaluronic acid (HA), were downregulated 3.25 to 3.62-fold in the ΔhtpsA strain. In Streptococcus and Staphylococcus, hyaluronidases are virulence factors that contribute to the destruction of the polysaccharide in the basement layer to facilitate dissemination through the tissues of the host organism [Citation50]. Hyaluronidase activity enables GBS to subvert uterine immune responses, leading to increased rates of ascending infection and preterm birth [Citation51]. Hyaluronate lyase may be also a potential virulence factor in S. suis, which requires hyaluronic acid as a carbon source [Citation52].
Sugar Phosphotransferase System (PTS) mediates the uptake and phosphorylation of carbohydrates and controls the carbon and nitrogen metabolism in response to the availability of sugars [Citation53]. In Listeria monocytogenes, two pairs of soluble PTS components (EIIACel1/EIIBCel1 and EIIACel2/EIIBCel2) and the permease EIICCel1 were responsible for cellobiose uptake and repression of PrfA, which is a virulence gene activator [Citation54]. As a virulence gene, the ptsP mutant caused a decrease in the colonization ability and pathogenicity of Legionella pneumophila [Citation55]. These reports together suggested that the PTS components are closely related to virulence. There are 27 genes encoding PTS components in S. suis 2 05ZYH33 [Citation56]. We noted the knockout of htpsA resulted in a significantly downregulated expression of 11 genes. These genes may play an important role in the transport and metabolism of bacterial carbohydrates and the regulation of virulence. Additionally, the deletion of htpsA in this study caused a significant downregulation of 15 ABC-type transporters – most of these genes were annotated as relating to sugar metabolism. Furthermore, the ABC transporter plays an important role in the pathogenesis of several pathogenic bacteria [Citation57–Citation59]. Taken together, it could be inferred that the downregulation of the virulence-related factors mentioned above may also contribute to the attenuated infection and pathogenicity of S. suis 2.
The disruption of zinc homeostasis may be the third reason for the weakened virulence of the ΔhtpsA strain
The histidine triad protein is not only related to adhesion and virulence but also to the absorption of zinc ions. The crystal structure analysis of the PhtA protein fragment and the high-resolution NMR structure of PhtD form S. pneumoniae have indicated that the histidine triad domain is a Zn2+ binding domain [Citation60,Citation61]. Other studies from S. pyogenes HtpA and S. pneumonia PhtD confirmed that Htp proteins did have zinc ion-binding activity [Citation27,Citation62]. Ogunniyi et al. revealed that Pneumococcal Pht proteins were regulated by the Zn2+-dependent repressor AdcR [Citation34]. These studies suggested that this family of proteins play an important role in maintaining the zinc ion balance in bacteria during bacterial infection. S. suis 2 HtpsA exhibits high amino acid similarity (57% and 46%) to HtpA of S. pyogenes and PhtD of S. pneumoniae [Citation31]. S. suis AdcR protein is able to bind to the promoter region of the hptsA gene [Citation63], suggesting that it plays a role in zinc homeostasis in S. suis 2. Zinc ions are not only a necessary nutritional requirement for the growth of all cells and the activity of a wide variety of enzymes but also play an important role in the process of sugar metabolism. A recent study demonstrated that disordered zinc balance impairs glucose metabolism through the inhibition of the glycolytic enzymes phosphofructokinase and glyceraldehyde-3-phosphate dehydrogenase, resulting in decreased capsule biosynthesis via the inhibition of phosphoglucomutase [Citation64]. This supports the observed transcriptional alteration of glycometabolism and capsular biosynthesis-related genes in the ΔhtpsA.
The involvement of htpsA in multiple biological processes largely explained the phenotype alteration of the ΔhtpsA strain. As observed in this study, the survival rate of ΔhtpsA in whole blood sharply decreased comparing to the wild-type strain. This is in accordance with the results of our previous study, which showed that incubating S. suis 2 with anti-HtpsA antiserum could reduce its survival rate in whole blood [Citation31]. The previous study also showed that immunization of mice with recombinant HtpsA confers significant protection against wild-type S. suis 2 infection [Citation31], which mirrors the active role of HtpsA during bacterial infection. Besides the obvious morphological alteration, the ΔhtpsA also showed slight growth reduction. Although many of the observed morphological change, including capsule deficiency, low sialic acids content, decreased adherence ability and macrophage resistance would not be affected a lot by the subtle delay in growth, we could not rule out that the growth reduction may indirectly contribute to the pathogenicity attenuation of the bacteria.
In conclusion, this work demonstrated that inactivation of htpsA disturbed a diverse of cellular activities in S. suis 2, including glycometabolism, nutrient transport (PTS, ABC transporter), zinc homeostasis, and virulence factor (e.g., ADS, suilysin, and hyaluronidase) expression. Among these downregulated genes, the altered expression of saccharometabolism and sugar transporters-related genes was the most obvious. These resulted in the thinning of the bacterial capsule and attenuation of pathogenicity of the ΔhtpsA strain. In summary, our findings provide evidence that htpsA contributes to the virulence of S. suis 2 in a murine infection model through a complicated mechanism.
Supplemental Material
Download MS Word (32.9 KB)Supplemental Material
Download MS Word (20.9 KB)Acknowledgments
We thank Dr. Daisuke Takamatsu at the National Institute of Animal Health of Japan for the generous gifts of the E. coli-S. suis shuttle vectors pSET2. We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript. The raw sequencing data were submitted to NCBI’s Gene Expression Omnibus (accession number PRJNA644160).
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplemental data
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Dutkiewicz J, Sroka J, Zajac V, et al. Streptococcus suis: a re-emerging pathogen associated with occupational exposure to pigs or pork products. Part I - Epidemiology. Ann Agric Environ Med. 2017;24:683–695.
- Dutkiewicz J, Zajac V, Sroka J, et al. Streptococcus suis: a re-emerging pathogen associated with occupational exposure to pigs or pork products. Part II - Pathogenesis. Ann Agric Environ Med. 2018;25:186–203.
- Hill JE, Gottschalk M, Brousseau R, et al. Biochemical analysis, cpn60 and 16S rDNA sequence data indicate that Streptococcus suis serotypes 32 and 34, isolated from pigs, are Streptococcus orisratti. Vet Microbiol. 2005;107:63–69.
- Nomoto R, Maruyama F, Ishida S, et al. Reappraisal of the taxonomy of Streptococcus suis serotypes 20, 22 and 26: streptococcus parasuis sp. nov. Int J Syst Evol Microbiol. 2015;65:438–443.
- Tien le HT, Nishibori T, Nishitani Y, et al. Reappraisal of the taxonomy of Streptococcus suis serotypes 20, 22, 26, and 33 based on DNA-DNA homology and sodA and recN phylogenies. Vet Microbiol. 2013;162:842–849.
- Gottschalk M, Xu J, Calzas C, et al. Streptococcus suis: a new emerging or an old neglected zoonotic pathogen? Future Microbiol. 2010;5:371–391.
- Goyette-Desjardins G, Auger J-P, Xu J, et al. Streptococcus suis, an important pig pathogen and emerging zoonotic agent-an update on the worldwide distribution based on serotyping and sequence typing. Emerg. Emerg Microbes Infect. 2014;3:e45.
- Huong VTL, Turner HC, Kinh NV, et al. Burden of disease and economic impact of human Streptococcus suis infection in Viet Nam. Trans R Soc Trop Med Hyg. 2019;113:341–350.
- Tan C, Zhang A, Chen H, et al. Recent proceedings on prevalence and pathogenesis of Streptococcus suis. Curr Issues Mol Biol. 2019;32:473–520.
- Tang J, Wang C, Feng Y, et al. Streptococcal toxic shock syndrome caused by Streptococcus suis serotype 2. PLoS Med. 2006;3:e151.
- Xia X, Qin W, Zhu H, et al. How Streptococcus suis serotype 2 attempts to avoid attack by host immune defenses. J Microbiol Immunol Infect. 2019;52:516–525.
- Feng Y, Pan X, Sun W, et al. Streptococcus suis enolase functions as a protective antigen displayed on the bacterial cell surface. J Infect Dis. 2009;200:1583–1592.
- Sun Y, Li N, Zhang J, et al. Enolase of Streptococcus suis serotype 2 enhances blood-brain barrier permeability by inducing IL-8 Release. Inflammation. 2016;39:718–726.
- Xia XJ, Wang L, Shen ZQ, et al. Development of an Indirect Dot-PPA-ELISA using glutamate dehydrogenase as a diagnostic antigen for the rapid and specific detection of Streptococcus suis and its application to clinical specimens. Antonie Van Leeuwenhoek. 2017;110:585–592.
- Whitney JC, Quentin D, Sawai S, et al. An interbacterial NAD(P)(+) glycohydrolase toxin requires elongation factor Tu for delivery to target cells. Cell. 2015;163(3):607–619.
- Wang C, Li M, Feng Y, et al. The involvement of sortase A in high virulence of STSS-causing Streptococcus suis serotype 2. Arch Microbiol. 2009;191:23–33.
- Zhu WF, Zhang Q, Li JT, et al. Glyceraldehyde-3-phosphate dehydrogenase acts as an adhesin in Erysipelothrix rhusiopathiae adhesion to porcine endothelial cells and as a receptor in recruitment of host fibronectin and plasminogen. Vet Res. 2017;48:16.
- Kong DC, Chen Z, Wang JP, et al. Interaction of factor H-binding protein of Streptococcus suis with globotriaosylceramide promotes the development of meningitis. Virulence. 2017;8:1290–1302.
- Li XQ, Liu P, Gan SZ, et al. Mechanisms of host-pathogen protein complex formation and bacterial immune evasion of streptococcus suis protein Fhb. J Biol Chem. 2016;291:17122–17132.
- Hu D, Zhang FY, Zhang HM, et al. The beta-galactosidase (BgaC) of the zoonotic pathogen Streptococcus suis is a surface protein without the involvement of bacterial virulence. Sci Rep. 2014;4:4140.
- Zhang YM, Shao ZQ, Wang J, et al. Prevalent distribution and conservation of Streptococcus suis Lmb protein and its protective capacity against the Chinese highly virulent strain infection. Microbiol Res. 2014;169:395–401.
- Ni H, Fan WW, Li CL, et al. Streptococcus suis DivIVA Protein Is a Substrate of Ser/Thr Kinase STK and involved in cell division regulation. Front Cell Infect Microbiol. 2018;8:85.
- Plumptre CD, Ogunniyi AD, Paton JC. Polyhistidine triad proteins of pathogenic streptococci. Trends Microbiol. 2012;20:485–493.
- Adamou JE, Heinrichs JH, Erwin AL, et al. Identification and characterization of a novel family of pneumococcal proteins that are protective against sepsis. Infect Immun. 2001;69:949–958.
- Wizemann TM, Heinrichs JH, Adamou JE, et al. Use of a whole genome approach to identify vaccine molecules affording protection against Streptococcus pneumoniae infection. Infect Immun. 2001;69:1593–1598.
- Zhang Y, Masi AW, Barniak V, et al. Recombinant PhpA protein, a unique histidine motif-containing protein from Streptococcus pneumoniae, protects mice against intranasal pneumococcal challenge. Infect Immun. 2001;69:3827–3836.
- Kunitomo E, Terao Y, Okamoto S, et al. Molecular and biological characterization of histidine triad protein in group A streptococci. Microbes Infect. 2008;10:414–423.
- Reid SD, Montgomery AG, Voyich JM, et al. Characterization of an extracellular virulence factor made by group A Streptococcus with homology to the Listeria monocytogenes internalin family of proteins. Infect Immun. 2003;71:7043–7052.
- Maruvada R, Prasadarao NV, Rubens CE. Acquisition of factor H by a novel surface protein on group B Streptococcus promotes complement degradation. Faseb J. 2009;23:3967–3977.
- Waldemarsson J, Areschoug T, Lindahl G, et al. The streptococcal Blr and Slr proteins define a family of surface proteins with leucine-rich repeats: camouflaging by other surface structures. J Bacteriol. 2006;188:378–388.
- Shao Z, Pan X, Li X, et al. HtpS, a novel immunogenic cell surface-exposed protein of Streptococcus suis, confers protection in mice. FEMS Microbiol Lett. 2011;314:174–182.
- Shao ZQ, Zhang YM, Pan XZ, et al. Insight into the evolution of the histidine triad protein (HTP) family in Streptococcus. PLoS One. 2013;8:e60116.
- Aranda J, Garrido ME, Fittipaldi N, et al. Protective capacities of cell surface-associated proteins of Streptococcus suis mutants deficient in divalent cation-uptake regulators. Microbiology-Sgm. 2009;155(5):1580–1587.
- Ogunniyi AD, Grabowicz M, Mahdi LK, et al. Pneumococcal histidine triad proteins are regulated by the Zn2+-dependent repressor AdcR and inhibit complement deposition through the recruitment of complement factor H. Faseb J. 2009;23:731–738.
- Takamatsu D, Osaki M, Sekizaki T. Construction and characterization of Streptococcus suis-Escherichia coli shuttle cloning vectors. Plasmid. 2001;45(2):101–113.
- Chen HY, Shen H, Jia B, et al. Differential gene expression in ovaries of Qira black sheep and Hetian sheep using RNA-Seq technique. PLoS One. 2015;10(3):e0120170.
- Charland N, Kobisch M, MartineauDoize B, et al. Role of capsular sialic acid in virulence and resistance to phagocytosis of Streptococcus suis capsular type 2. FEMS Immunol Med Microbiol. 1996;14:195–203.
- Wang Y, Zhang W, Wu Z, et al. Functional analysis of luxS in Streptococcus suis reveals a key role in bio-film formation and virulence. Vet Microbiol. 2011;152(1–2):151–160.
- Li M, Shao ZQ, Guo Y, et al. The type II histidine triad protein HtpsC is a novel adhesion with the involvement of Streptococcus suis virulence. Virulence. 2015;6:631–641.
- Fittipaldi N, Segura M, Grenier D, et al. Virulence factors involved in the pathogenesis of the infection caused by the swine pathogen and zoonotic agent Streptococcus suis. Future Microbiol. 2012;7:259–279.
- Houde M, Gottschalk M, Gagnon F, et al. Streptococcus suis Capsular Polysaccharide Inhibits Phagocytosis through Destabilization of Lipid Microdomains and Prevents Lactosylceramide-Dependent Recognition. Infect Immun. 2012;80:506–517.
- Smith HE, de Vries R, Van’t Slot R, et al. The cps locus of Streptococcus suis serotype 2: genetic determinant for the synthesis of sialic acid. Microb Pathog. 2000;29:127–134.
- Benga L, Goethe R, Rohde M, et al. Non-encapsulated strains reveal novel insights in invasion and survival of Streptococcus suis in epithelial cells. Cell Microbiol. 2004;6(9):867–881.
- Hu D, Wang CJ, Hu FQ, et al. Development of a unencapsulated mutant of Streptococcus suis serotype2 Chinese highly virulent strain 05ZYH33. Acta Academiae Medicinae Militaris Tertiae. 2009;31:93–97.
- Zhang YY, Ding DD, Liu ML, et al. Effect of the glycosyltransferases on the capsular polysaccharide synthesis of Streptococcus suis serotype 2. Microbiol Res. 2016;185:45–54.
- Segura M, Vanier G, Al-Numani D, et al. Proinflammatory cytokine and chemokine modulation by Streptococcus suis in a whole-blood culture system. FEMS Immunol Med Microbiol. 2006;47:92–106.
- Xu B, Yang XY, Zhang P, et al. The arginine deiminase system facilitates environmental adaptability of Streptococcus equi ssp zooepidemicus through pH adjustment. Res Microbiol. 2016;167:403–412.
- Marouni MJ, Ziomek E, Sela S. Influence of group A streptococcal acid glycoprotein on expression of major virulence factors and internalization by epithelial cells. Microb Pathog. 2003;35:63–72.
- Gruening P, Fulde M, Valentin-Weigand P, et al. Structure, regulation, and putative function of the arginine deiminase system of Streptococcus suis. J Bacteriol. 2006;188:361–369.
- Starr CR, Engleberg NC. Role of hyaluronidase in subcutaneous spread and growth of group A streptococcus. Infect Immun. 2006;74:40–48.
- Vornhagen J, Quach P, Boldenow E, et al. Bacterial Hyaluronidase Promotes Ascending GBS Infection and Preterm Birth. MBio. 2016;7:e00781-16.
- Allen AG, Lindsay H, Seilly D, et al. Identification and characterisation of hyaluronate lyase from Streptococcus suis. Microb Pathog. 2004;36:327–335.
- Jeckelmann JM, Erni B. Carbohydrate transport by group translocation: the bacterial phosphoenolpyruvate: sugar phosphotransferase system. Subcell Biochem. 2019;92:223–274.
- Cao TN, Joyet P, Ake FMD, et al. Studies of the listeria monocytogenes cellobiose transport components and their impact on virulence gene repression. J Mol Microbiol Biotechnol. 2019;1–17. DOI:https://doi.org/10.1159/000500090
- Higa F, Edelstein PH. Potential virulence role of the Legionella pneumophila ptsP ortholog. Infect Immun. 2001;69:4782–4789.
- Chen C, Tang J, Dong W, et al. A glimpse of streptococcal toxic shock syndrome from comparative genomics of S. suis 2 Chinese isolates. PLoS One. 2007;2:e315.
- Garmory HS, Titball RW. ATP-binding cassette transporters are targets for the development of antibacterial vaccines and therapies. Infect Immun. 2004;72:6757–6763.
- Hollenstein K, Frei DC, Locher KP. Structure of an ABC transporter in complex with its binding protein. Nature. 2007;446:213–216.
- Zwiers LH, Stergiopoulos I, Van Nistelrooy JG, et al. ABC transporters and azole susceptibility in laboratory strains of the wheat pathogen Mycosphaerella graminicola. Antimicrob Agents Chemother. 2002;46:3900–3906.
- Bersch B, Bougault C, Roux L, et al. New insights into histidine triad proteins: solution structure of a Streptococcus pneumoniae PhtD domain and zinc transfer to AdcAII. PLoS One. 2013;8(11):e81168.
- Riboldi-Tunnicliffe A, Isaacs NW, Mitchell TJ. 1.2 Angstroms crystal structure of the S. pneumoniae PhtA histidine triad domain a novel zinc binding fold. FEBS Lett. 2005;579:5353–5360.
- Loisel E, Chimalapati S, Bougault C, et al. Biochemical characterization of the histidine triad protein PhtD as a cell surface zinc-binding protein of pneumococcus. Biochemistry. 2011;50:3551–3558.
- Aranda J, Garrido ME, Fittipaldi N, et al. Protective capacities of cell surface-associated proteins of Streptococcus suis mutants deficient in divalent cation-uptake regulators. Microbiology. 2009b;155(5):1580–1587.
- Ong C, Walker M, McEwan A. Zinc disrupts central carbon metabolism and capsule biosynthesis in Streptococcus pyogenes. Sci Rep. 2015;5:10799.