ABSTRACT
Eight members of a big family with laboratory-confirmed COVID-19 pneumonia were admitted to First Hospital of Jilin University, Changchun, China, from 28 January to 5 February 2020. The clinical records, laboratory results, and chest computed tomography (CT) scans were retrospectively reviewed. Throat swab samples were positive for severe acute respiratory syndrome coronavirus 2, confirmed by the Center for Disease Control and Prevention of Changchun. All eight patients had fever of different degrees; and 6, 3, and 2 had cough; diarrhea; and sore throat. With disease progression, the percentage of lymphocytes in older patients increased, CT images worsened, and the ratio of lymphocytes increased when images revealed inflammation absorption. Although the CT images showed ground-glass opacities in the youngest patient, his lymphocyte count did not decrease with mild clinical symptoms, and the images showed that inflammation was quickly absorbed. Only the oldest patient developed critical illness. The C reaction protein (CRP) levels of Patient 5 increased significantly, and the rate of decline was the slowest, while his condition was the most severe. The clinical manifestations of COVID-19 in this family cluster varied with contact, age, and underlying disease. Lymphocyte count and quality of chest CT images appeared inversely associated with disease severity. CRP changes may be an indicator of disease severity and prognosis.
KEYWORDS:
What is new?
Our study confirms the clustered-onset characteristics of COVID-19.
Clinical manifestations of family-clustered onset of COVID-19 are related to contact history, age, and underlying disease.
The degree of decline in lymphocytes correlates with worsening of CT images.
The lymphocyte count and quality of chest CT images may be inversely associated with severity of the disease.
An increase of CRP may correlate with disease severity, while the speed of CRP decrease appeared to correlate negatively with severity.
Introduction
Beginning late December 2019, a series of unexplained cases of pneumonia appeared in Wuhan, China, which increased dramatically in January 2020 [Citation1,Citation2]. The pathogen was a novel coronavirus [Citation3] that was termed severe acute respiratory syndrome coronavirus 2 (SARS-Cov-2) by the International Committee on Taxonomy of Viruses. The pneumonia associated with SARS-Cov-2 infection was named coronavirus disease 2019 (COVID-19) by the World Health Organization (WHO) on 11 February 2020.
COVID-19 is a self-limited disease with obvious human-to-human transmission [Citation4]. By 5 August 2020, there were 18,318,928 confirmed cases globally, with 695,043 confirmed deaths. WHO considers that COVID-19 is pandemic. According to the Sixth Chinese National Consensus Report on COVID-19, the disease progresses in four stages: mild, moderate, severe, and critical. At the critical stage, severe acute respiratory distress syndrome (ARDS) or fulminant myocarditis disease, increases the risk of death. Clustering onset is a main characteristic of COVID-19, although reports are limited. By 20 February 2020, there were 91 confirmed cases and 1 death reported in Jilin Province. Of these, 13 cases were diagnosed at First Hospital of Jilin University, with 8 cases within one family. Herein, we summarize the clinical characteristics of these eight cases, to provide some information toward prevention and control of family cluster-onset of COVID-19.
Methods
Study design and patients
The Medical Ethics Committee of First Hospital of Jilin University reviewed and approved this study (approval number 2020–048). Each patient provided written informed consent.
A retrospective review was conducted of the medical records of eight members of a big family. Each member had been admitted to First Hospital of Jilin University, Changchun, China with laboratory-confirmed COVID-19 pneumonia; admissions were from 28 January to 5 February 2020. Diagnosis of COVID-19 pneumonia was based on the New Coronavirus Pneumonia Prevention and Control Program (Fourth Edition) published by the National Health Commission of China. All eight patients tested positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) via quantitative RT-PCR (qRT-PCR), based on samples from throat swab samples confirmed by the Center for Disease Control and Prevention of Changchun city.
Data collection
The first patient presented with fever (maximum 39.0°C), cough without sputum, and obvious breathing difficulties and was admitted to the Department of Infectious Diseases, First Hospital of Jilin University on 28 January 2020, 9 days after his travel back from Wuhan, the epidemic center of COVID-19. Chest computed tomography (CT) revealed the presence of ground-glass opacities under the pleura in both lungs. The remaining seven patients, all of whom had a close familial relationship with the first patient, were admitted to the hospital 1 to 8 days later.
The symptoms, signs, and epidemiological data of these patients were collected. Blood routine tests and biochemical examinations were performed. Throat swabs were collected for detecting the viral nucleic acid. During hospitalization, blood routine and blood biochemical examinations were monitored. The nucleic acid tests of the throat swabs and chest CT examinations were performed in accordance with the guidelines for prevention and control of COVID-19 and biosafety management regulations.
Throat swab samples were collected and transferred to the Chinese Center for Disease Control and Prevention (CDC) of Changchun city for detecting the viral nucleic acid.
Results
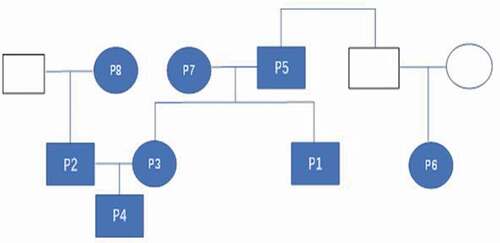
The family tree diagram of the eight patients is shown in . The disease onset of the first patient (Pt-1) occurred upon his return from Wuhan to Changchun on 19 January 2020. The onset of the other seven patients was due to close contact with Pt-1 ().
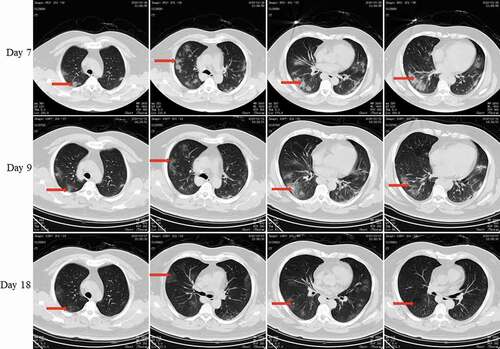
On 19 January, Pt-1 was together with his brother-in-law (Pt-2), younger sister (Pt-3), and nephew (Pt-4). Pt-1 developed fever on 24 January was admitted to the hospital on 28 January. The lung CT scan () revealed ground-glass opacities in both lungs. The throat swab collected on 29 January was positive for viral RNA, and Pt-1 was given a diagnosis of COVID-19. He was hospitalized for 13 days. The nucleic acid test was negative on the day 17 of the disease course.
Figure 3. Chest CT scans on days 7, 9, and 18 after onset for Pt-1. CT showed scattered bilateral multiple high-density effusions on day 7. The high-density effusions were absorbed on day 9 compared to day 7, and obvious absorption was observed on day 18. Red arrows indicate typical lesions.
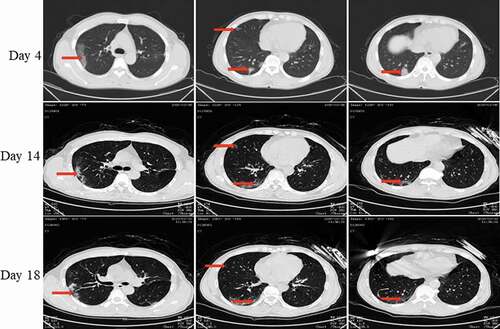
Pt-2 developed fever on 24 January and was admitted to the hospital on 28 January. The lung CT scan () revealed ground-glass opacities in both lungs. The throat swab on 30 January was viral RNA-positive, and the diagnosis was COVID-19. He was hospitalized for 8 days, and the nucleic acid converted to negative on the day 12 of the course of disease.
Figure 4. Chest CT scans on days 4, 14, and 18 after onset for Pt-2. CT showed scattered bilateral multiple high-density effusions, faintly exudative shadows along the lungs on day 4. Effusions were absorbed and partially fibrotic on day 14. Obvious absorption was observed on day 18. Red arrows indicate typical lesions.
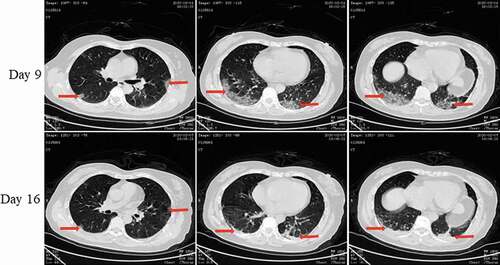
Pt-3 developed fever on 24 January and was admitted to the hospital on 31 January. She received a diagnosis of COVID-19 on 1 February when a throat swab tested positive for the viral RNA. The lung CT scan revealed ground-glass opacities in both lungs (). She was hospitalized for 8 days, and the nucleic acid converted to negative on day 14 of the course of disease.
Figure 5. Chest CT scans on days 9 and 16 after onset for Pt-3. CT showed scattered bilateral multiple high-density effusions, faintly exudative shadows along the lungs with partial fibrosis on day 9. Effusions were absorbed and most were fibrotic, and the lesions were incompletely absorbed on day 16. Red arrows indicate typical lesions.
Pt-4 developed fever and diarrhea on 30 January and was admitted to the hospital on 31 January. He received a diagnosis of COVID-19 on 1 February due to a positive test for viral RNA based on the throat swab; and the CT scan revealed ground-glass opacities in both lungs (). He was hospitalized for 10 days; the nucleic acid converted to negative on day 10 of the course of disease.
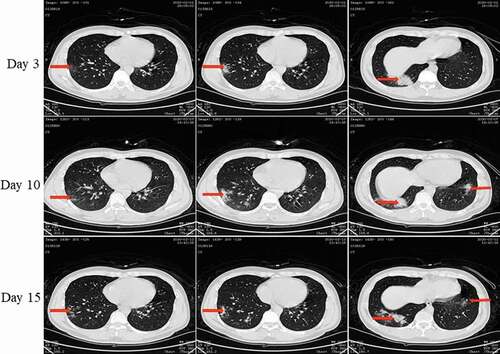
Figure 6. Chest CT scans on days 3, 10, and 15 after onset for Pt-4. CT showed scattered bilateral multiple high-density effusions with shadows along the lungs on the third day. Effusions were partially absorbed and fibrotic on day 10. Effusions were obviously absorbed and most were fibrotic on day 15. Red arrows indicate typical lesions.
Pt-1 lived with his father (Pt-5) and mother (Pt-7). Pt-5 developed diarrhea and fatigue on 22 January and was admitted to the hospital on 29 January. Pt-5 was given a diagnosis of COVID-19 on 30 January because of the positive nucleic acid in the throat swab, and ground glass opacities in both lungs revealed by CT scan (). He was hospitalized for 22 days, and the nucleic acid converted to negative on day 28 of the disease course. Pt-7 developed fever and cough on 30 January and was admitted to the hospital on 30 January. COVID-19 was determined on 1 February because the throat swab was viral RNA-positive. The lung CT scan revealed ground-glass opacities in both lungs (). He was hospitalized for 10 days, and the nucleic acid converted to negative on day 10 of the disease course.
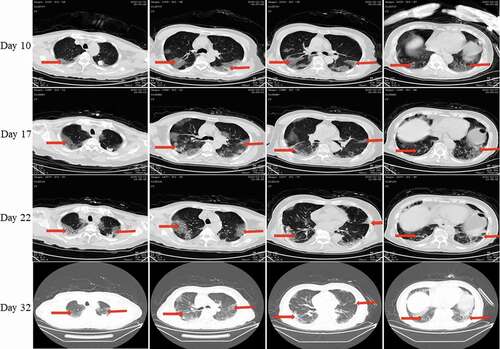
Figure 7. Chest CT scans on days 10, 17, 22, and 32 after onset for Pt-5. CT showed scattered bilateral multiple high-density effusions, faintly exudative shadows along the lungs on day 10. Effusions were gradually absorbed and partially fibrotic over time. Red arrows indicate typical lesions.
After being with Pt-1 on 23 January for half an hour at Pt-1’s home, his cousin (Pt-6) developed fever and cough on 2 February and was admitted to the hospital on 5 February. COVID-19 was determined on 6 February, because the throat swab was viral RNA-positive. The lung CT scan revealed ground-glass opacities in both lungs (). He was hospitalized for 14 days, and the nucleic acid converted to negative on day 16 of the disease course.
Figure 8. Chest CT scans on days 5, 14 and 19 after onset for Pt-6. CT showed scattered bilateral multiple high-density effusions, faintly exudative shadows along the lungs especially in the left lung on day 10. Effusions were gradually absorbed over time. Red arrows indicate typical lesions.
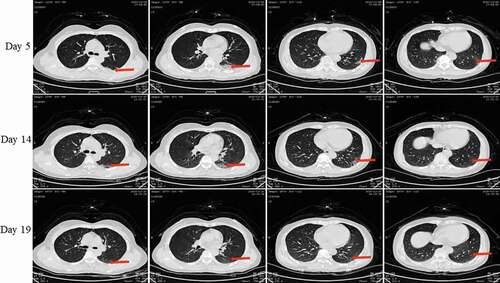
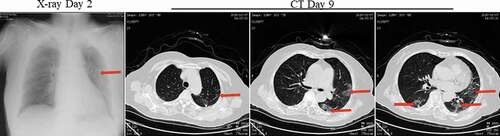
Figure 9. X-ray on day 2 and Chest CT scans on 9 after onset for Pt-7. X-ray showed high-density effusions in the middle lobe of the left lung on the second day. CT showed scattered bilateral multiple high-density effusions, faintly exudative shadows along the lungs especially in the left lung on day 9. Red arrows indicate typical lesions.
Pt-8 was the brother-in-law’s mother (Pt-8). After being in contact with Pt-2 for 1 week, she developed fever, cough, and chest tightness on 31 January. She was admitted to the hospital on 2 February and was revealed as viral RNA-positive on 3 February. The lung CT scan revealed ground-glass opacities in both lungs (). She was hospitalized for 21 days, and the nucleic acid converted o negative on day 22 of the disease course.
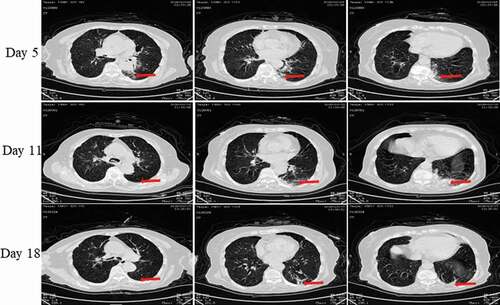
Figure 10. Chest CT scans on days 5, 11 and 18 after onset for Pt-8. CT showed scattered bilateral multiple high-density effusions, faintly exudative shadows along the lungs especially in the left lung on the fifth day. Effusions were gradually absorbed over time. Red arrows indicate typical lesions.
All eight patients showed fever. Six of them had cough, three had diarrhea, and two had sore throat. Routine blood examinations () showed that the total number of white blood cells in all eight patients was normal, while the percentage of lymphocytes in five patients was low. Pt-7 was aged 79 years and she had a significant decrease in lymphocytes. However, she did not develop severe oxygenation. Pt-4 was the youngest of these patients, and had no underlying disease. His lymphocytes remained normal throughout his illness.
Table 1. Blood routine tests for eight patients on days 1, 4, 7, and 14 after onseta.
The blood biochemical examinations of the eight patients showed CRP values that increased to different degrees (). The rise of CRP in Pt-5 was the most obvious, and the decline in the speed was the slowest, while Pt-5 had the only severe case among the eight patients. These results showed that the increase of CRP is positively correlated with severity, while the speed of the decrease of CRP is negatively correlated with severity. Other biochemical test results are shown in ; LDH, CK-MB, CTnI, MYO, AST, ALT in all patients changed to different degrees, but with no significant difference. Pt-5, Pt-6, and Pt-7 (ages 79, 79, and 85 y) showed obvious decrease of oxygen partial pressure. The above results show that old age is the main factor that causes severe lung lesions in COVID-19.
Table 2. Biochemical tests for the eight patients on days 1, 4, and follow-up day after onset*.
The chest CT scans of eight patients are shown in to 10. Pt-1 underwent chest CT scans on days 7, 9, and 18 after onset; these showed that pulmonary inflammation was gradually absorbed (). Thus, inflammatory lesions of the lungs appeared heaviest within 1 week of onset. This was consistent with the blood routine results, and that the lowest lymphocyte count was observed on day 7 after onset. The youngest patient, Pt-4, underwent chest CT scans on days 3, 10, and 15 after onset (). These also showed that pulmonary inflammation was the most serious on day 10, while lymphocytes were normal. The patient, Pt-5, underwent chest CT scans on days 10, 17, 22, and 32 after onset (). Pulmonary inflammation was still obviously exuding on day 17, with a small amount of pleural effusions. Partial pulmonary fibrosis appeared after 1 month. The blood lymphocytes count had significantly decreased during the course and returned to the normal range at 1 month. The lung CT scans of Pt-2, Pt-3, Pt-6, Pt-7, and Pt-8 revealed varying degrees of inflammatory ground-glass opacities and the inflammatory lesions were absorbed at different rates.
In accordance with the Sixth Chinese National Consensus Report on COVID-19, all patients were given arbidol (200 mg, tid, po.) as an antiviral treatment for 7 days. Because Pt-5 and Pt-8 were hospitalized for a long time, and considering their advanced age (and especially the significantly elevated CRP for Pt-5), the two patients were given piperacillin-tazobactam (4.5 g, q8h, ivd.) as an antimicrobial treatment. Because of the low oxygen partial pressure, slow inflammation effusion absorption, and positive nucleic acid, on the third day after hospitalization Pt-5 was given methylprednisolone (40 mg, q12h, ivd.) for 5 days, which was reduced to 40 mg once daily for 3 days. All the patients were discharged after clinical symptoms were absent and two consecutive nucleic acid tests indicated negative conversion (test interval >24 h). All patients were followed for 1 month after discharge.
Discussion
The present study summarized the clinical characteristics of eight cases of a family-clustered onset of COVID-19 in Changchun, Jilin Province. Changchun is more than 2000 km from Wuhan, Hubei Province, the epicenter of the original outbreak. COVID-19 has obvious characteristics of human-to-human transmission [Citation5,Citation6]. Among the eight patients in the present population, only one had returned from Wuhan. Other family members developed symptoms after direct or indirect contact with the first patient. During the entire course, the other seven patients had no history of traveling and had not been to a seafood market, which is thought to be the source of the virus that causes COVID-19. Therefore, they were probably infected by droplets and contact transmission.
Human coronaviruses (HCoVs) have long been considered inconsequential pathogens, causing the “common cold” in otherwise healthy people. However, in the twenty-first century, two highly pathogenic HCoVs emerged from animal reservoirs to cause global epidemics with alarming morbidity and mortality: severe acute respiratory syndrome coronavirus (SARS-CoV), and Middle East respiratory syndrome coronavirus (MERS-CoV) [Citation7,Citation8] SARS-CoV-2, the causal virus of COVID-19, is the third highly pathogenic HCoV to emerge and cause serious illness and death; it was first recognized in Wuhan, China in December 2019 [Citation3]. COVID-19 originating in Wuhan appears clinically milder than SARS or MERS in severity and fatality rate, but is more highly contagious [Citation9], which increases the difficulty of prevention and control.
In the present study, the clinical manifestations of COVID-19 included fever, sore throat, cough, diarrhea, and fatigue, without other obvious symptoms in the upper respiratory tract. All the patients presented with low fever and dry cough, and most had no sputum. All eight patients had fever (100%), six had dry cough (75%), three had mild diarrhea (26.7%), and two had sore throat (25%). The oldest patient, Pt-5, had a slower absorption of pulmonary inflammation and a longer course because of the underlying disease (chronic bronchitis), which was consistent with previous studies [Citation10–12].
ARDS may occur in some patients with severe COVID-19 [Citation13], in which case mechanical ventilation and extracorporeal membrane oxygenation treatments are needed. Fortunately, although the eight patients in this study all had pulmonary inflammation, this was mild and the patients did not experience significant breathing difficulties.
Blood routine tests in patients with COVID-19 show a normal or decreased white blood cell and/or lymphocyte count, and sometimes a decreased platelet count. A substantial decrease in lymphocytes may indicate that coronavirus consumes many immune cells and inhibits the body’s cellular immune function; decreased T lymphocytes may be an important factor leading to exacerbation [Citation14]. A low absolute lymphocyte count may be used as a reference index in the clinical diagnosis of COVID-19.
Among these patients, the rise of CRP was most significant, and decline slowest, in Pt-5, who had the only severe case. Therefore, we speculate that an increase of CRP may positively correlate with severity, while the speed of decrease of CRP may negatively correlate with severity.
For these eight patients, the leukocyte counts were normal. The lymphocyte count was low in five patients, especially in the older patients Pt-5, Pt-7, and Pt-8. With disease progression in the oldest patient, Pt-5, the changes in lymphocyte count correlated with lung CT manifestations. Therefore, the lymphocyte count and lung CT may be important predictors of severity and outcome.
The chest CT image of COVID-19 differs from that of pneumonia caused by other pathogens. It mainly appears with scattered sub-pleural and lateral field ground-glass opacities in bilateral lungs. During acute progression of the disease, the density of ground-glass opacities is not high, showing a homogeneous and faint exudation [Citation15,Citation16].
Pt-5 and Pt-8 are older patients, respectively aged 79 and 85 years. These patients were each hospitalized for over 20 days, because their nucleic acids for the virus remained positive. Our data indicated that older age and underlying diseases may be risk factors for the progression of COVID-19 into severe pneumonia, and recovery needs more time than for younger and healthier patients. They should be monitored closely, and isolated. Chest CT images and lymphocyte count may indicate the severity and prognosis of the disease.
Conclusion
This study confirms the clustered-onset characteristics of COVID-19. The elderly with underlying diseases are at high risk for the development of severe or critical illness. CRP changes may reflect the severity status and prognosis of the disease. The degree of decline in lymphocyte count, which seems to correlate with the worsening of CT images, may be an important predictor for the severity of the disease.
Author contributions
1) Conceived and designed the experiments: Wanguo Bao, Shucheng Hua;
2) Performed the experiments: Na Du;
3) Analyzed and interpreted the data: Yanfang Jiang;
4) Contributed reagents, materials, analysis tools or data: Lihe Che, Xiaohua Li, Lixin Lou;
5) Wrote the paper*: Na Du.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Government CfHPotHKSAR. CHP closely monitors cluster of pneumonia cases on Mainland. [updated 2019 Dec 31; cited 2020 Jan 21]. Available from: https://www.info.gov.hk/gia/general/201912/31/P2019123100667.htm
- Juan D Wuhan wet market closes amid pneumonia outbreak. China Daily. [cited 2020 Jan 21]. Available from: https://www.chinadaily.com.cn/a/202001/01/WS5e0c6a49a310cf3e35581e30.html
- Zhang YZ, Novel 2019 coronavirus genome. Virological. [cited 2020 Jan 21]. Available from: http://virological.org/t/novel-2019-coronavirus-genome/319
- Chan JF, Yuan S, Kok KH, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523.
- Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513.
- Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan. of Novel Coronavirus-Infected Pneumonia: China; 2020.
- Yin Y, Wunderink RG. MERS, SARS and other coronaviruses as causes of pneumonia. Respirology. 2018;23:130–137.
- Zaki AM, van Boheemen S, Bestebroer TM, et al. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367:1814–1820.
- Riou J, Althaus CL. Pattern of early human-to-human transmission of Wuhan 2019 novel coronavirus (2019-nCoV), December 2019 to January 2020. Euro Surveill. 2020;25. DOI:https://doi.org/10.2807/1560-7917.ES.2020.25.4.2000058
- Shi H, Han X, Jiang N, et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China:a descriptive study. Lancet Infect Dis. 2020;20:425–434.
- Zhao W, Zhong Z, Xie X, et al. Relation between chest CT findings and clinical conditions of coronavirus disease (COVID-19) pneumonia: a multicenter study. AJR Am J Roentgenol. 2020;214:1072–1077.
- Xu X, Yu C, Qu J, et al. Imaging and clinical features of patients with 2019 novel coronavirus SARS-CoV-2. Eur J Nucl Med Mol Imaging. 2020;47:1275–1280.
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506.
- Liu WJ, Zhao M, Liu K, et al. T-cell immunity of SARS-CoV: implications for vaccine development against MERS-CoV. Antiviral Res. 2017;137:82–92.
- Desai SR, Wells AU, Rubens MB, et al. Acute respiratory distress syndrome: CT abnormalities at long-term follow-up. Radiology. 1999;210:29–35.
- Desai SR. Acute respiratory distress syndrome: imaging of the injured lung. Clin Radiol. 2002;57:8–17.