ABSTRACT
Post-transcriptional global carbon storage regulator A (CsrA) is a sequence-specific RNA-binding protein involved in the regulation of multiple bacterial processes. Hemolysin is an important virulence factor in the enterohemorrhagic Escherichia coli O157:H7 (EHEC). Here, we show that CsrA plays a dual role in the regulation of hemolysis in EHEC. CsrA significantly represses plasmid-borne enterohemolysin (EhxA)-mediated hemolysis and activates chromosome-borne hemolysin E (HlyE)-mediated hemolysis through different mechanisms. RNA structure prediction revealed a well-matched stem-loop structure with two potential CsrA binding sites located on the 5' untranslated region (UTR) of ehxB, which encodes a translocator required for EhxA secretion. CsrA inhibits EhxA secretion by directly binding to the RNA leader sequence of ehxB to repress its expression in two different ways: CsrA either binds to the Shine–Dalgarno sequence of ehxB to block ribosome access or to ehxB transcript to promote its mRNA decay. The predicted CsrA-binding site 1 of ehxB is essential for its regulation. There is a single potential CsrA-binding site at the 5'-end of the hlyE transcript, and its mutation completely abolishes CsrA-dependent activation. CsrA can also stabilize hlyE mRNA by directly binding to its 5' UTR. Overall, our results indicate that CsrA acts as a hemolysis modulator to regulate pathogenicity under certain conditions.
Introduction
Hemolysins are among the major virulence factors identified in various bacterial pathogens [Citation1]. Escherichia coli hemolysins can be divided into three types: E. coli α-hemolysin (HlyA), enterohemolysin (EhxA), and hemolysin E (HlyE) [Citation2]. Hemolysins EhxA and HlyE are present in the enterohemorrhagic E. coli (EHEC). EhxA shares high similarity with HlyA, which is a key virulence factor encoded on the chromosome and plasmid of pathogenic E. coli strains [Citation3,Citation4]. It belongs to the RTX family of proteins and is encoded on the virulence plasmids of typical EHEC strains, such as O157:H7 [Citation4]. EhxA is a potential virulence factor that significantly correlates with severe diseases, such as hemolytic uremic syndrome (HUS) and hemorrhagic colitis (HC) [Citation5]. However, EhxA normally exhibits weaker hemolytic activity than HlyA [Citation6].
The synthesis of EhxA is directed by the ehxCABD operon, which shares nearly 60% identity with the hlyCABD operon [Citation7]. Like hlyCABD, ehxA and ehxC encode a hemolytic toxin and an activator involved in the posttranslational modification process of EhxA, respectively [Citation4,Citation5]. The secretion structure is composed of three proteins: an ABC transporter, EhxB; a membrane fusion protein (MFP), EhxD; and an outer membrane protein (OMP), TolC. Unlike EhxB and EhxD, TolC is not a plasmid-coded protein, and it involved in many cellular processes [Citation8]. The inner membrane protein complex EhxB-EhxD and TolC form a nanomachine that exports EhxA through the bacterial cell wall [Citation5,Citation8]. Although both operons form similar type 1 secretion systems (T1SSs) that deliver effectors to the extracellular space, the biochemical properties of the hemolysins produced by each operon are different [Citation9]. EhxA can be found either free or in association with outer membrane vesicles released by EHEC strains. Most free-EhxA targets cell membranes and damages microvascular endothelial cells via pore formation [Citation10]. The outer membrane vesicle-associated EhxA targets mitochondria, causing apoptosis via the capase-9-dependent pathway [Citation5]. EhxA is also used as a phenotypic marker to identify typical EHEC strains [Citation11].
The ehxCABD operon of EHEC O157:H7 is located on the non-transmissible plasmid pO157 and is influenced by several regulators at the transcriptional level. Regulators such as the GrlA-GrlR regulatory system and Ler, which are encoded by the locus of enterocyte effacement (LEE) island can affect hemolysin expression [Citation12]. Deletion of grlR or overexpression of GrlA significantly increases the lytic activity of Ehx, thus indicating that GrlA is a positive regulator of Ehx expression, while GrlR is a negative regulator of Ehx expression at the transcriptional level [Citation13]. Ler is an expression activator of most LEE genes and can activate Ehx expression, either as a GrlA activator or in a GrlA-independent manner [Citation13]. This GrlA-independent EhxA expression may be facilitated via the regulation of ehxC by Ler, which may directly interact with the regulatory region of ehxC [Citation14]. LrhA, a non-LEE-encoded regulator, upregulates the expression of LEE genes and can also activate EhxA expression [Citation14]. Li et al. suggested that the expression of proteins by the ehxCABD operon could be regulated by the global transcriptional regulation factors H-NS, DsrA, and σ factor RpoS [Citation15]. The expression of proteins by the ehxCABD operon was silenced by H-NS, which could be antagonized by DsrA [Citation15]. A mutation in rpoS completely abolished ehxA transcription, indicating that RpoS is an essential regulator of Ehx expression [Citation16]. The regulation of Ehx expression therefore seems to be a complex, multifactorial process. However, examining the interactions between regulators and their influence on the ehxCABD operon can help to uncover the clinical importance of EhxA.
HlyE is a pore-forming toxin that has been identified in Enterobacteriaceae such as E. coli, Shigella flexneri, and Salmonella Typhi [Citation17]. It is unrelated to the E. coli hemolysins HlyA and EhxA [Citation18,Citation19]. Unlike HlyA and EhxA, which are synthesized as soluble protoxins that require proteolytic and posttranslational modification processes to become active toxins, HlyE does not require posttranslational processing [Citation17]. Previous studies have revealed that the genome of Salmonella Typhi, causing typhoid fever and that of S. flexneri, causing dysentery, encode proteins that are highly homologous to HlyE [Citation17]. HlyE plays an essential role in epithelial cell invasion and deep organ infection and can thus be regarded as a significant component of these pathogens’ armory of toxins [Citation20].
The global carbon storage regulator (Csr) system plays an important role in controlling many cellular processes, such as carbon metabolism, motility, stress response, quorum sensing, biofilm formation, iron storage, and pathogenicity [Citation21–29]. Csr is widely distributed among gram-negative bacteria, and it also occurs in the gram-positive bacterium Bacillus subtilis [Citation30]. Homologous regulatory factors of Csr are known to be repressors of secondary metabolites (Rsm) [Citation30]. The four major components of Csr in E. coli are as follows: CsrA is a homo-dimeric RNA-binding protein; CsrB and CsrC are two small RNAs acting as CsrA antagonists; and CsrD is a factor targeting CsrB and CsrC specifically for degradation by RNase E [Citation31–33]. CsrA, a key component of the Csr system, preferentially binds to mRNA in the 5' untranslated region (5' UTR) or the early coding region [Citation30]. The specific binding motif GGA is normally located in the single stranded loop of a hairpin structure [Citation34]. The CsrA binding to these sites can therefore affect translation, RNA stability, and RNA structure for Rho-dependent transcription termination [Citation35–39]. Several studies have revealed that CsrA is not only capable of directly targeting multiple RNA but also of controlling other regulators, confirming its crucial role in bacterial physiology and virulence [Citation24,Citation40].
The present study investigated the dual role of CsrA in hemolysis regulation. CsrA can be considered as contributing to the EHEC pathogenicity by facilitating the coordination of virulence factors.
Materials and methods
Bacterial strains and culture conditions
Bacterial strains (Supplementary material, Table S1) were grown routinely in a Luria-Bertani (LB) medium at pH 7.4 and temperature 37°C with shaking (250 rpm). Antibiotics were added at the following final concentrations: tetracycline, 10 μg/mL; ampicillin, 100 μg/mL; chloramphenicol, 15 μg/mL; and kanamycin, 50 μg/mL. All chemical reagents were purchased from Sigma-Aldrich (St. Louis, MI, USA), unless stated otherwise.
Construction of plasmids and mutant strains
All primers used in this study (Supplementary material, Table S2) were synthesized by Sangon Biotech Co., Ltd (Shanghai, China). All mutants were constructed using the λ Red recombinase system [Citation41]. A truncated csrA strain exhibiting reduced RNA-binding affinity and partial activity was constructed using the method described by Wang et al. [Citation42]. The truncation of csrA was confirmed by PCR. For csrA complementation, csrA and its upstream 500-bp sequence were amplified using primers 184-CsrA-F and 184-CsrA-R. The amplified DNA fragment was then inserted into the plasmid cloning vector pACYC184 at restriction sites NcoI and ScaI to obtain the plasmid pCSRA. For EhxA overexpression, ehxA was PCR amplified using primers 80-ehxA-F and 80-ehxA-R. The obtained DNA fragment was inserted into the plasmid cloning vector pQE80-YX1 at restriction sites AfeI and FseI to construct plasmid pQE-ehxA.
EHEC ΔlacI-Z, a β-galactosidase-defective strain, was constructed following the method described by Jackson et al. [Citation26]. To construct lacZ reporter fusions, the plasmid cloning vector pACYC184 was employed. The lacZ gene, containing EcoRI and ScaI restriction sites, was amplified from E. coli O157:H7 EDL933 using the primers 184-lacZ-F and 184-lacZ-R. The amplified lacZ gene was ligated to pACYC184 and transformed into E. coli DH5α to obtain the recombinant p184-lacZ plasmid. The EcoRI site of lacZ in p184-lacZ was mutated using the primers lacZ-EcoRI-Mu-F and lacZ-EcoRI-Mu-R. lacZ was sequenced using primers 184-CHK-F and 184-CHK-R. The 600-bp region upstream of ehxC in E. coli O157:H7 EDL933 was amplified using primers Gb1-PehxC-F and Gb1-PehxC-R (PehxC). The 5' UTR of ehxB and its early coding region were amplified from 200 bp upstream the gene to the first 30 bp of the coding region using primers Gb2-UTRehxB-F and Gb2-UTRehxB-R (UTRehxB). The PehxC and UTRehxB were inserted into p184-lacZ to obtain plasmid p184-PehxC-UTRehxB::lacZ. For complementation of ehxB, the gene and its UTR were amplified using primers Gb2-UTRehxB-F and Gb2-ehxB-R; PehxC with the obtained fragment was inserted into pACYC184, carrying restriction sites EcoRI and ScaI, to obtain plasmid pEHXB. All plasmids mentioned above were constructed using ClonExpress II One Step Cloning Kit (Vazyme, Nanjing, China; #C112) according to the manufacturer’s recommendations.
UTRehxA (amplified by UTRehxA-F and UTRehxA-R) and UTRhlyE (amplified by UTRhlyE-F and UTRhlyE-R) were used to replace UTRehxB of p184-PehxC-UTRehxB::lacZ at SpeI and XhoI restriction sites to construct p184-PehxC-UTRehxA::lacZ and p184-PehxC-UTRhlyE::lacZ respectively. Plasmids p184-PehxC-UTRehxB::lacZ, p184-PehxC-UTRhlyE::lacZ, and p184-PehxC-UTRehxA::lacZ were used for the β-galactosidase assay. Site-directed mutagenesis was conducted at Genewiz Co., Ltd. (Suzhou, China). The GGA motifs in the DNA sequences of UTRehxB and UTRhlyE harboring potential CsrA binding were mutated to TTC. PehxC with each mutated UTR was inserted into p184-lacZ carrying EcoRI and NcoI restriction sites to obtain the various mutant plasmids using the ClonExpress II One-Step Cloning Kit (Vazyme; #C112).
CsrA binding sites prediction and in silico analysis
The genes containing multiple CsrA binding sites were predicted using the sequence-based algorithm reported by Kulkarni et al. [Citation43]. Two hundred twenty-five potential targets were obtained from the EHEC genome, including ehxB. We also analyzed genes containing a potential single CsrA binding site. The potential CsrA target sequences were predicted to form secondary structures using the tools available on the mfold and CentroidFold web servers [Citation44,Citation45].
Hemolytic activity assay
Hemolytic activity was detected using a washed blood agar plate (EHX plate) containing 3% washed sheep erythrocytes (Bersee, Beijing, China; #RCB001), 0.5 g/L sodium chloride, 10 mM CaCl2, 5 g/L yeast extract, and 10 g/L tryptone. Bacterial cells were cultured in LB medium overnight, collected by centrifugation, washed once with a fresh LB medium, and resuspended in the same medium to obtain optical density at 600 nm (OD600) = 0.8. Two microliters of the bacterial suspension were spotted on the EHX blood agar plate. The size of the hemolytic zone produced by each strain was recorded after incubation at 37°C for 24 h.
Secreted protein preparation and EhxA detection
EHEC and its derived mutants were cultured overnight, collected, and washed twice. The strain was inoculated in a fresh LB at 1:100 dilution for further growth at 37°C When the OD600 of the sample reached 0.4, isopropyl β-d-1-thiogalactopyranoside (IPTG, 0.5 mM) was added while shaking at 250 rpm for 0.5 h at 37°C to induce the expression of 6HisEhxA. The sample was centrifuged at 10,000 ×g for 15 min, and the resulting supernatant was passed through a 0.22 μm filter, precipitated with 10% trichloroacetic acid (TCA) overnight and centrifuged at 20,000 ×g for 30 min. The precipitate was washed twice with cold acetone and dried in a fume hood. The pellet was diluted in 500 μL phosphate-buffered saline (PBS, 20 mM, pH 7.4) and stored at −20°C for further analysis.
For EhxA detection, western blotting was performed according to the procedures described by Wan et al. [Citation46]. The membranes were probed with anti-His tag antibody (BBI, Shanghai, China; #D191001; 1:3000 dilution) overnight at 4°C and HRP-conjugated goat anti-mouse IgG (BBI; #D110087) as the secondary antibody (1:5000 dilution).
β-Galactosidase assay
To examine the expression effects of ehxB-lacZ and hlyE-lacZ translational fusions, β-galactosidase assays were performed using o-nitrophenyl-β-D-galactopyranoside (ONPG) [Citation47]. A single bacterial colony in 5 mL LB was cultured overnight with the corresponding antibiotics. The overnight culture was transferred to a fresh medium (1:100 dilution) and sub-cultured to the OD600 value that is optimal for investigation. The culture samples were prefilled with Z buffer and assayed using the method of Aviv and Gal-Mor [Citation47].
Expression and purification of the CsrA protein
CsrA expression was obtained as described by Sun et al. [Citation48]. To construct the expression vector pET-csrA, csrA was amplified and cloned into pET28a carrying NcoI and XhoI sites to enable the production of CsrA containing a His-tag at its N-terminus (6HisCsrA) (Supplementary material, Table S1). The plasmid was transformed into E. coli BL21 (DE3) (Tsingke, Beijing, China; #TSC-E01) to allow 6HisCsrA overexpression. The strain was cultured to OD600 = 0.5 at 37°C and 0.5 mM IPTG was then added while shaking at 250 rpm for 4 h at 37°C to induce 6HisCsrA expression. The same methods were applied to E. coli BL21 (DE3) cells harboring the pET28a vector as a control. The cells were collected by centrifugation at 5000 g, 4°C for 10 min, and frozen at −20°C until further use. CsrA purification was performed using the method described by Andrade et al. [Citation29]. Protein concentration was determined using a Pierce™ BCA Protein Assay kit (Thermo Fisher Scientific, Waltham, MA, USA; # 23,227). The purified sample was stored at −20 °C.
RNA electrophoretic mobility shift assay (RNA-EMSA)
RNA-EMSA was carried out using the method of Andrade et al. [Citation29] with slight modifications. 5'-end-FAM-labeled RNA oligonucleotides encoding ehxB and hlyE leader sequences, which contain potential GGA motifs, were designed based on their RNA secondary structure prediction. All RNA oligonucleotides including positive control R9–43 and negative control hns were synthesized by Genewiz Co. Ltd. (Suzhou, China) to test for interactions with 6HisCsrA (Supplementary materials, Table S3) [Citation34,Citation49]. The binding assays for the gradient 6HisCsrA protein and 1 μM FAM-labeled RNA were performed according to Andrade et al. [Citation29]. Signal bands were visualized using the Fusion FX fluorescence imaging instrument (VILBER, Paris, France).
Competition assays were used to calculate the apparent equilibrium binding constant (Kd) of the CsrA-RNA complex. The concentration of RNA probes was 1 μM. The concentrations of CsrA were 0, 0.9, 1.8, 3.6, 7.2, 14.4, 28.8, 43.2, 57.6, and 72 nM. The CsrA-RNA binding curve was determined by CsrA concentration and shift band intensity in RNA-EMSA. Kd was evaluated using ImageJ software [Citation50], following the manufacturer’s protocol.
mRNA stability assay
The assay for mRNA stability was performed according to Andrade et al. [Citation29] with slight modifications. For the detection of ehxB, EHEC wild-type and ΔcsrA mutant strains can be directly used in mRNA stability assays. For the detection of hlyE, both strains should be transformed into the pHLYE plasmid to increase the hlyE copy number for mRNA detection. Briefly, EHEC and its derived mutants were cultured overnight. The strain was inoculated in a fresh LB medium (1:100 dilution) for further growth at 37°C When OD600 reached 0.4, rifampicin (200 μg/mL) was added to inhibit transcription. Bacterial cultures were collected at 0, 2, 4, 6, 8, and 15 min after rifampicin treatment. The cells were harvested, and TRIzol (Invitrogen, Waltham, MA, USA; #15596026) was added for further treatment [Citation29]. RNA extraction was performed using the RiboPureTM Bacteria kit (Invitrogen; #AM1925), and RNA was quantified using Nanodrop One (Thermo Fisher Scientific; #ND-ONE-W) according to the manufacturer’s instructions. The purified RNA was used to obtain cDNA by reverse-transcription PCR (Takara Bio Inc., Kusatsu, Shiga, Japan; #RR014A). The amount of mRNA was detected using agarose gel electrophoresis [Citation29]. To further detect the ratio of the transcript degradation in the wild type and ΔcsrA strains, quantitative real-time PCR (qPCR) was employed according to the method described by Wan et al. [Citation46]. The reactions were performed using SYBR Premix Ex Taq II (Takara Bio Inc.; #RR820A) in the QuantStudioTM 3 Real-Time PCR System (Applied Biosystems, Waltham, Ma, USA; #A28567).
Statistical analysis
GraphPad Prism software (version 6.0; GraphPad Software, Inc., La Jolla, CA, USA) was used for statistical analysis. The results are expressed as the mean of the replicate measurements ± standard deviation (SD). A P-value of less than 0.05 indicated statistical significance in all tests performed.
Results
CsrA plays a central role in the repression of plasmid-coded hemolysis in EHEC
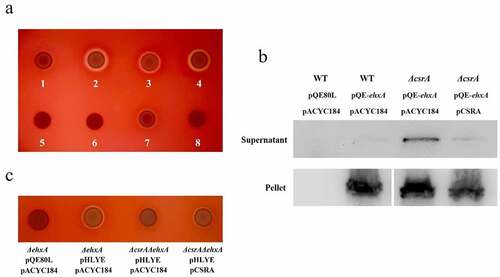
To study the involvement of CsrA on the hemolytic activity of EHEC, a truncated csrA mutant with kanamycin resistance was constructed to reduce its RNA binding affinity and retain partial activity [Citation42]. We first used a standard sheep blood agar plate to investigate the hemolytic activity of various EHEC strains (Supplementary material, Fig. S1). Similar to the previous reports [Citation2], wild-type EHEC did not form an observable hemolytic circle in the standard blood agar plate. However, the ΔcsrA mutant strain showed a clear hemolytic circle, indicating that CsrA may downregulate hemolysis in EHEC (Supplementary material, Fig. S1a-S1d). We used an EHX blood agar plate to investigate CsrA-regulated hemolysis in EHEC. As evidenced in , the wild-type EHEC formed a weak hemolytic circle on the EHX blood plate after 18 h incubation at 37°C. The ΔcsrA EHEC formed a large, clear hemolytic circle but it was reduced after complementation by a low-copy number plasmid with csrA, thus indicating that CsrA represses EHEC hemolysis. A similar, large hemolytic circle was formed by ΔcsrA/ΔhlyE while ΔcsrA/ΔehxA showed no evident hemolytic activity. This observation suggested that, in laboratory conditions, EHEC plasmid-borne hemolysin EhxA has a major hemolytic role, unlike HlyE. EhxB is a translocator of the EhxA secretion system [Citation5]. The ΔehxB strain does not form a hemolytic circle by itself, but its hemolytic activity can be rescued by a plasmid copy of ehxB complementation, suggesting that like HlyB [Citation4], EhxB is required for EhxA secretion and hemolytic activity in EHEC ().
Figure 1. EHEC hemolysis regulation by CsrA.
Considering the high similarity of the intensively studied E. coli hemolysin cluster hlyCABD with ehxCABD, we evaluated the effect of CsrA on the hemolytic activity of uropathogenic E. coli (UPEC). We found that the hemolytic activity of UPEC ΔcsrA was similar to that of the wild-type, indicating that unlike that observed for ehxCABD from EHEC, CsrA had no obvious effect on hlyCABD regulation (Supplementary material, Fig. S1e and S1f). A similar phenotype of a classical repeats-in-toxin (RTX) leukotoxin (β-hemolysis, coded by ltxCABD), which is secreted by Aggregatibacter actinomycetemcomitans, a bacterial pathogen suspected to be involved in localized aggressive periodontitis and endocarditis, was also investigated [Citation51] (Supplementary material, Fig. S1g and S1h). Our results indicated that CsrA might specifically regulate ehxA-derived hemolysis in EHEC.
CsrA represses ehxA-derived hemolysis by interrupting EhxA secretion
EhxA is secreted by a classical T1SS in EHEC [Citation5]. Hence, we hypothesized CsrA may be involved in the regulation of EhxA secretion. We constructed the plasmid pQE-ehxA to overexpress 6HisEhxA and detected 6HisEhxA secretion in the supernatant. The large amount of 6HisEhxA detected in the pellets of different bacterial strains indicated 6HisEhxA can be successfully overexpressed in EHEC (). However, the signal of the target protein was very weak in the EHEC wild-type supernatant, suggesting low 6HisEhxA secretion in the extracellular space (). The protein level of 6HisEhxA in the ΔcsrA strain supernatant was significantly higher than that in the wild-type and it was reduced by complementation of csrA in EHEC (). The significant amount of intracellular 6HisEhxA in the wild type and ΔcsrA strains further suggested CsrA represses 6HisEhxA secretion. Overall, these results demonstrate CsrA represses ehxA-derived hemolysis by interrupting EhxA secretion.
Deletion of csrA impairs hlyE-derived hemolysis
EHEC ΔcsrA/ΔehxA showed no hemolytic activity on EHX blood agar plates, suggesting the plasmid pO157, which comprised the ehxCABD gene cluster, could play a major role in hemolysis (). To verify hlyE regulation by CsrA, we constructed a pHLYE plasmid containing the hlyE gene and its promoter to increase both transcript copy number and protein levels. A clear hemolytic zone was observed in ΔehxA-harboring pHLYE on EHX blood agar plates (). The hemolytic activity was significantly reduced in the ΔehxA/ΔcsrA strain, but it could be recovered by complementing the plasmid with csrA (). These results suggest CsrA positively regulates hlyE-derived hemolysis.
In silico prediction of potential CsrA binding sites in hemolysin-coding genes
CsrA normally binds to the 5' UTR or early coding region at sites containing a GGA motif that is often located in the single-stranded hairpin structure [Citation30]. We found multiple GGA motifs in the 5' UTR of ehxB in the EHEC ehxCABD operon (). Binding site 1 (BS1) and binding site 2 (BS2) are located on the well-matched hairpin structure, which overlapped the ehxB Shine–Dalgarno (SD) sequence (). For the UPEC hlyCABD operon, two GGA motifs were found in the leader sequence of hlyB, but neither of them formed a well-matched stem-loop structure (), suggesting that this is not a potential CsrA-binding site. The transcription starting site of the E. coli general hemolysin coding gene hlyE has been described in a previous study [Citation52]. We found only one GGA motif located on the loop of the hairpin structure at the 5' UTR of hlyE mRNA (), which is a potential CsrA-binding site and may be stabilized by CsrA (). Based on our sequence analysis and phenotype investigation, we inferred that CsrA might regulate both ehxB and hlyE in EHEC, with completely different implications in EHEC hemolysis.
Figure 2. Overview of ehxCABD (EHEC), hlyCABD (UPEC), and hlyE (EHEC) operons.

CsrA inhibits ehxB but activates hlyE expression in vivo
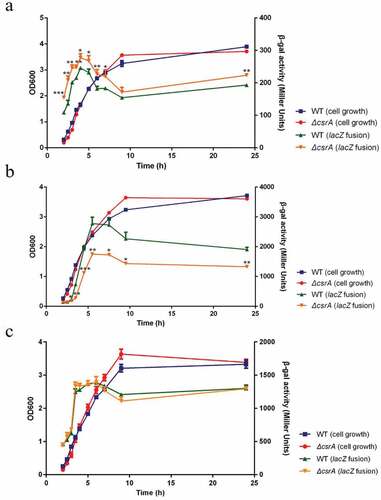
To determine the effects of CsrA on the expression of ehxB and hlyE in vivo, we used ehxB-lacZ and hlyE-lacZ translational fusions. We constructed low copy number plasmids that integrated PehxC-UTRehxB::lacZ, PehxC-UTRhlyE::lacZ, and PehxC-UTRehxA::lacZ. Based on our prediction in silico, the ehxA gene should not be affected by CsrA and therefore PehxC-UTRehxA::lacZ was set as a negative control. The plasmids were electroporated into wild-type and ΔcsrA strains. The ΔcsrA strain grew slightly slower than the isogenic wild-type strain for 4 h but overtook it in the late exponential phase in LB medium (). Expression of all fusion proteins was increased in the early exponential phase but decreased in the middle and late exponential phases (). The ehxB-lacZ fusion expression in the ΔcsrA mutant showed a slightly higher activity compared to that in the wild-type strain throughout the exponential phase of the growth curve (). In this phase, the expression of hlyE-lacZ fusion was significantly higher in the wild-type than in the ΔcsrA strain (). No obvious difference in the expression of ehxA-lacZ fusion was found between the wild-type and ΔcsrA strains, indicating that CsrA does not directly affect ehxA expression (). These results demonstrate that CsrA represses ehxB expression but activates hlyE expression at the post-transcriptional level.
Figure 3. CsrA-Dependent regulation of ehxB-lacZ, hlyE-lacZ, and ehxA-lacZ translational fusion in vivo.
CsrA directly interacts with the 5' UTR of ehxB and hlyE
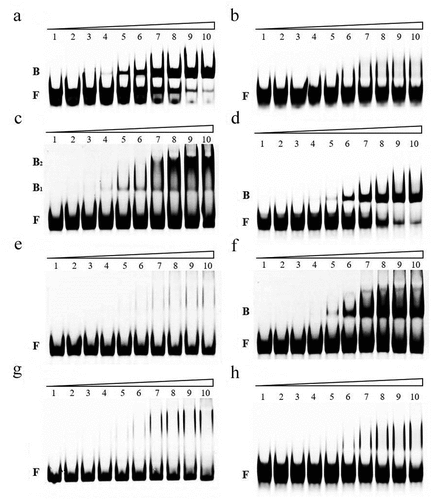
To investigate whether EHEC CsrA directly binds to the GGA motifs that were previously predicted in the leader sequence of ehxB and hlyE transcripts, RNA-EMSA was performed. R9–43 and the leader sequence of hns were used as positive and negative controls, respectively [Citation34,Citation49]. Our results demonstrate that EHEC 6HisCsrA promotes a band shift of the RNA probe R9–43, but not of the RNA probe hns, suggesting that the recombinant 6HisCsrA retains binding affinity (). The gel mobility shift analysis of the interaction between 6HisCsrA and FAM-labeled ehxB and hlyE leader probes was performed according to the binding reactions reported by Yakhnin et al. [Citation53] The fraction of bound ehxB and hlyE increased proportionally to the concentration of 6HisCsrA (). The complex was observed at 3.6 nM 6HisCsrA for ehxB and 7.2 nM 6HisCsrA for hlyE, and most of the starting RNA was shifted at 72 nM 6HisCsrA. The apparent Kd values of ehxB and hlyE were 21.39 nM and 27.25 nM, respectively.
Figure 4. RNA mobility shift assays using various concentrations of purified 6hisCsrA on EHEC.
CsrA binding sites in the ehxB and hlyE transcripts
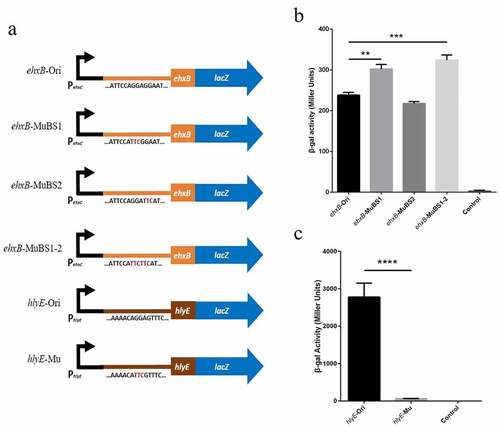
The RNA-EMSA results showed that CsrA has high affinity for ehxB and hlyE transcripts in vitro (). Mutation of the GGA motifs in potential binding sites to TTC was performed in accordance with our in silico predictions to investigate which site was crucial for CsrA interaction (). Mutations in BS1 and BS2 (ehxB-MuBS1–2) abolished the binding affinity for ehxB. The binding activity of BS1 with a single mutation (ehxB-MuBS1) was similar to the activity of ehxB-MuBS1–2, but mutation of BS2 (ehxB-MuBS2) slightly reduced the activity compared to that of the wild-type probe (ehxB-Ori) (). These results demonstrate that the BS1 of ehxB transcript is essential for CsrA binding affinity. Only one GGA motif was located in the hlyE 5' UTR (). The hlyE-Ori probe exhibited a significant band shift (). However, no obvious band shift was observed for the CsrA and hlyE-Mu interaction, indicating that the motif located in the 5' UTR of hlyE was crucial for CsrA binding ().
The same motif substitution (GGA to TTC) was used to construct the PehxC-UTRehxB::lacZ and PehxC-UTRhlyE::lacZ translational fusions, and expression was detected using the ONPG method (). The ehxB expression of the double motif substitution strain was nearly 1.4-fold higher than that of the wild-type strain and that of the BS1 motif substitution strain was nearly 1.3-fold higher than that of the wild-type strain (). However, the expression of the wild-type leader sequence of hlyE was nearly 43-fold higher than that of the motif substitution strain (). These results are consistent with that of RNA-EMSA and confirm the presence of CsrA binding sites in ehxB and hlyE transcripts.
Figure 5. Effect of site-directed mutation in potential binding sites in the expression of lacZ translational fusion in vivo.
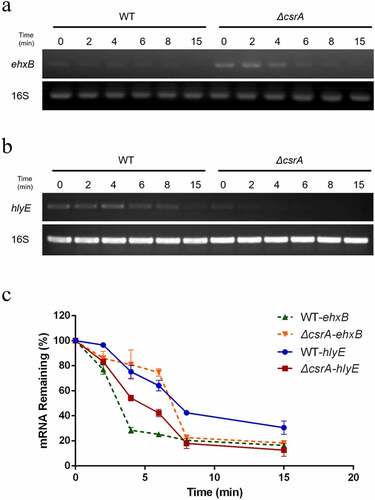
Effects of CsrA on ehxB and hlyE stability
As demonstrated above, CsrA repressed ehxA-derived hemolysis and promotes hlyE-derived hemolysis on EHX blood agar plates (). The translational fusion and RNA-EMSA results demonstrated that CsrA directly binds to the 5' UTR of ehxB and leader region of the hlyE transcripts to affect their expression (). As translation efficiency can be influenced by mRNA stability [Citation29], we examined the relative abundance of ehxB and hlyE transcripts using qPCR. Significantly more ehxB transcripts were found in ΔcsrA than in the wild-type, and their degradation rate in ΔcsrA was lower than that in the wild-type (). Conversely, significantly less hlyE transcripts were found in ΔcsrA than in the wild-type, and their degradation rate in ΔcsrA was higher than that in the wild-type (). We employed qPCR to analyze hlyE mRNA levels in the wild type and ΔcsrA strains. The ehxB transcript half-life in the wild-type was approximately 3.1 min, but in the ΔcsrA strain, it was approximately 7 min (). However, the hlyE transcript half-life in the wild-type was approximately 7.5 min, but in the ΔcsrA strain, it was approximately 4.7 min (). These results suggest that CsrA regulates ehxB and hlyE expression by affecting their mRNA stability.
Figure 6. Stability of ehxB and hlyE mRNA as affected by CsrA in EHEC.
Discussion
EhxA and HlyE are two hemolysins identified in EHEC [Citation2,Citation7]. According to our data, EhxA is the major contributor of EHEC hemolysis in vitro. Mutation of csrA led to the increased hemolytic activity of ΔcsrA strain on EHX blood agar plates compared to that of the wild type (). Other similar classical RTX toxins from bacterial pathogens, such as α-hemolysin HlyA from UPEC and leukotoxin LtxA (β-hemolysis) from A. actinomycetemcomitans, do not show different hemolytic activity between their wild type and ΔcsrA strains [Citation51,Citation54]. This indicates that CsrA may regulate ehxA-derived hemolysis specifically in EHEC.
To clarify how CsrA regulates ehxA-derived hemolysis, we overexpressed EhxA in various EHEC strains and examined its secretion. We observed that the level of secreted EhxA in the ΔcsrA strain was significantly increased compared to that in the wild-type but it was reduced by complementation with csrA. However, there was no obvious change in the levels of intracellular EhxA (). These results suggest that CsrA represses EhxA secretion.
The gene cluster ehxCABD encodes a classic bacterial T1SS and shares high similarity with hlyCABD in UPEC [Citation5,Citation7]. Based on genome screening and RNA secondary structure prediction, a well-matched stem-loop structure with two potential CsrA binding motifs was observed on the 5′ UTR of ehxB, which encodes the required translocator for T1SS (). However, no potential CsrA binding sites were found in the EHEC 5′ UTR of ehxA and UPEC hlyCABD gene cluster (). This led us to hypothesize that CsrA specifically regulates ehxB, not ehxA. Further β-galactosidase assays for detecting the activity of ehxB-lacZ and ehxA-lacZ translation fusion confirmed our prediction ().
RNA-EMSA revealed CsrA’s high affinity to the non-coding region of ehxB transcript. The BS1–2 double mutation, as well as the BS1 single mutation, completely abolished CsrA binding affinity, while the BS2 single mutation slightly reduced the binding activity (). These results indicate BS1 is crucial for the CsrA-ehxB interaction. Our hypothesis is further supported by the results of the β-galactosidase assay performed for detecting ehxB-lacZ translation fusion at the protein level, as CsrA specifically bound to the 5′ UTR of ehxB and repressed its translation (). The region containing the ehxB ribosome binding site may fold into a well-matched hairpin structure, and the CsrA binding site is located in the loop, blocking ribosome access and preventing translation (). In addition, the RNA stability assay revealed CsrA promotes ehxB transcript degradation, indicating that CsrA may repress ehxB translation in two different manners. This proposed mechanism is similar to the regulation of glgC by CsrA [Citation36].
Figure 7. CsrA dual regulation of EHEC hemolysis.
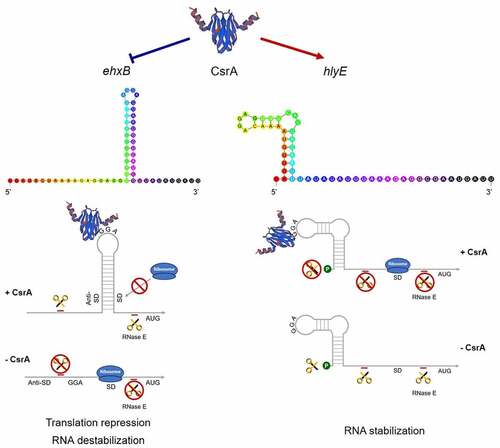
HlyE is a pore-forming toxin unrelated to the RTX family. The gene is usually silent because it is strictly controlled by the global regulator H-NS at the transcriptional level [Citation15]. We first demonstrated that CsrA directly regulates hlyE expression at the post-transcriptional level. The β-galactosidase assay for detecting hlyE-lacZ translational fusion confirmed the wild-type strain has significantly higher hlyE-derived hemolysis than the ΔcsrA strain (). There is only one GGA motif on the 5′ UTR of the hlyE transcript, which is located on the loop of the hairpin structure. Mutation of this motif led to significantly decreased hlyE expression ()). Our results further showed that hlyE transcripts were less abundant in the ΔcsrA mutant than in the wild type and that hlyE transcript degradation was faster in the ΔcsrA mutant than that in the wild type (). This indicates that CsrA upregulates hlyE expression by stabilizing hlyE mRNA. This is similar to the upregulation mechanism of the type III secretion system (T3SS) master regulator coding gene, hrpG, in Xanthomonas citri, and flagellum biosynthesis and chemotaxis master regulator coding gene, flhDC, in E. coli [Citation29,Citation38]. The latter mechanism is well-characterized in terms of flhDC regulation by CsrA in E. coli, with CsrA binding to the mRNA leader region and protecting it against 5′-end-dependent RNase E cleavage [Citation38]. However, a recent report revealed that CsrA-upregulated ymdA expression not only stabilizes ymdA mRNA but also promotes 30S ribosome subunit binding [Citation55]. In addition to RNA stabilization, CsrA could also bind to the leader region of ymdA mRNA, destabilizing the SD-blocking stem-loop structure and leading to the promotion of 30S ribosome subunit binding and translational activation [Citation55]. In the present study, a single binding was found for the transcript of hlyE; it is located at the 5′-end of the leader sequence, far from the SD-sequence, and no other potential-binding motif was observed. Thus, hlyE regulation by CsrA seems to be mediated by RNA stabilization only (). These results suggest a novel CsrA-mediated activation of hlyE-derived hemolysis in bacteria and elucidate the mechanism of hlyE regulation at the post-transcriptional level.
Our results also suggest that CsrA plays a dual role in the regulation of hemolytic activity. The global regulator CsrA normally acts as an organizer to switch metabolic pathways during the bacterial life cycle. Fine-tuning of CsrA activity allows bacterial pathogen to overcome many challenges in the host and promote pathogenicity. In Pseudomonas aeruginosa, the switch between acute and chronic infections is controlled by RsmA, a CsrA homolog [Citation30]. It positively regulates acute virulence factors such as type IV pili and T3SS, and negatively regulates chronic virulence factors, such as biofilm formation and the type VI secretion system (T6SS) [Citation30,Citation56]. CsrA also regulates the switch from replicative to transmissive virulence phases of infection in Legionella pneumophila [Citation30]. We hypothesize that CsrA regulates a switch between two hemolytic activities in EHEC. It negatively regulates ehxA-derived hemolysis and positively regulates hlyE-derived hemolysis. Results of the present study show that CsrA represses ehxA-derived hemolysis by interrupting its secretion but it activates hlyE-derived hemolysis by stabilizing its mRNA ( ). CsrA specifically binds to the ehxB 5′ UTR to repress EhxB expression and, in turn, blocks T1SS function. The suggested mechanism of ehxB repression is that CsrA prevents ehxB translation initiation by blocking ribosome access and promoting mRNA decay. The mechanism of hlyE activation is that CsrA specifically binds to the 5′-end of the transcript and reduces mRNA degradation by protecting it from hydrolysis by RNase (). The two-component system (TCS) BarA/UvrY is a primary regulator of the Csr regulatory system of E. coli and is activated by short-chain fatty acids (SCFAs) or Krebs cycle intermediates [Citation30,Citation57]. In the intestinal tract, abundant SCFAs may activate the TCS BarA/UvrY during EHEC colonization and infection [Citation30]. The activated TCS positively regulates expression of sRNA CsrB/C, leading to a decrease of free-CsrA in bacteria [Citation30]. Lacking of free-CsrA upregulates ehxB expression, and in turn promotes ehxA-derived hemolysis. Meanwhile, the hlyE-derived hemolysis is downregulated due to its mRNA destabilization. The high level of secreted EhxA may promote cell damage via pore formation and cause apoptosis via the capase-9-dependent pathway [Citation5,Citation10]. Conversely, if the sRNA CsrB/C expression is repressed or hydrolyzed by RNase, more CsrA will be released. This abundance of free-CsrA may inhibit ehxA-derived hemolysis by repressing ehxB expression. On the contrary, hlyE-derived hemolysis will be upregulated because CsrA prevents its mRNA decay. This effect may promote EHEC to invade epithelial cells [Citation20]. The circuit may assist EHEC to regulate the switch of ehxA- and hlyE-derived hemolysis according to different signals in the host’s intestinal tract or environment and perform distinct functions. Many factors function through or have connection with the BarA/UvrY, indicating the regulation circuits are more complex [Citation30].
In the present study, the regulatory mechanism of EHEC hemolysis at the post-transcriptional level is reported for the first time. According to our results, the global regulator CsrA represses ehxA-derived hemolysis and activates hlyE-derived hemolysis, therefore regulating the switch between these two hemolytic activities. This reverse regulation effect of CsrA suggests that EhxA and HlyE may have other functions in bacterial metabolism and pathogenicity. Therefore, future studies should focus on comprehensively understanding the functions of EHEC hemolysins.
Supplemental Material
Download Zip (66.9 KB)Acknowledgment
We thank Prof. Rahul V. Kulkarni at the University of Massachusetts, Boston, for providing the computer code (CSRA_TARGET) for CsrA-binding site prediction.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/21505594.2022.2073023.
Additional information
Funding
References
- Welch RA, Dellinger EP, Minshew B, et al. Haemolysin contributes to virulence of extra-intestinal E. coli infections. Nature. 1981;294:665–667.
- Murase K, Ooka T, Iguchi A, et al. Haemolysin E- and enterohaemolysin-derived haemolytic activity of O55/O157 strains and other Escherichia coli lineages. Microbiol SGM. 2012;158:746–758.
- Burgos Y, Beutin L. Common origin of plasmid encoded alpha-hemolysin genes in Escherichia coli. BMC Microbiol. 2010;10:193.
- Welch RA. The Escherichia coli hemolysin. EcoSal Plus. 2005;1. DOI:10.1128/ecosalplus.8.7.2
- Bielaszewska M, Rüter C, Kunsmann L, et al. Enterohemorrhagic Escherichia coli hemolysin employs outer membrane vesicles to target mitochondria and cause endothelial and epithelial apoptosis. PLoS Pathog. 2013;9:e1003797.
- Beutin L, Montenegro M, Zimmermann S, et al. Characterization of hemolytic strains of Escherichia coli belonging to classical enteropathogenic O-serogroups. Zentralbl Bakteriol Mikrobiol Hyg a. 1986;261:266–279.
- Schmidt H, Beutin L, Karch H. Molecular analysis of the plasmid-encoded hemolysin of Escherichia coli O157:H7 strain EDL 933. Infect Immun. 1995;63:1055–1061.
- Koronakis V, Eswaran J, Hughes C. Structure and function of TolC: the bacterial exit duct for proteins and drugs. Annu Rev Biochem. 2004;73:467–489.
- Bauer ME, Welch RA. Characterization of an RTX toxin from enterohemorrhagic Escherichia coli O157:H7. Infect Immun. 1996;64:167–175.
- Schmidt H, Maier E, Karch H, et al. Pore-Forming properties of the plasmid-encoded hemolysin of enterohemorrhagic Escherichia coli O157:H7. Eur J Biochem. 1996;241:594–601.
- Beutin L, Montenegro MA, Orskov I, et al. Close association of verotoxin (Shiga-like toxin) production with enterohemolysin production in strains of Escherichia coli. J Clin Microbiol. 1989;27:2559–2564.
- Deng W, Puente JL, Gruenheid S, et al. Dissecting virulence: Systematic and functional analyses of a pathogenicity island. Proc Natl Acad Sci U S A. 2004;101:3597–3602.
- Saitoh T, Iyoda S, Yamamoto S, et al. Transcription of the ehx enterohemolysin gene is positively regulated by GrlA, a global regulator encoded within the locus of enterocyte effacement in enterohemorrhagic Escherichia coli. J Bacteriol. 2008;190:4822–4830.
- Iyoda S, Honda N, Saitoh T, et al. Coordinate control of the locus of enterocyte effacement and enterohemolysin genes by multiple common virulence regulators in enterohemorrhagic Escherichia coli. Infect Immun. 2011;79:4628–4637.
- Li H, Granat A, Stewart V, et al. RpoS, H-NS, and DsrA influence EHEC hemolysin operon (ehxCABD) transcription in Escherichia coli O157:H7 strain EDL933. FEMS Microbiol Lett. 2008;285:257–262.
- Majdalani N, Cunning C, Sledjeski D, et al. DsrA RNA regulates translation of RpoS message by an anti-antisense mechanism, independent of its action as an antisilencer of transcription. Proc Natl Acad Sci U S a. 1998;95:12462–12467.
- Wallace AJ, Stillman TJ, Atkins A, et al. E. coli hemolysin E (HlyE, ClyA, SheA): X-ray crystal structure of the toxin and observation of membrane pores by electron microscopy. Cell. 2000;100:265–276.
- Mueller M, Grauschopf U, Maier T, et al. The structure of a cytolytic α-helical toxin pore reveals its assembly mechanism. Nature. 2009;459:726–730.
- Eifler N, Vetsch M, Gregorini M, et al. Cytotoxin ClyA from Escherichia coli assembles to a 13-meric pore independent of its redox-state. Embo J. 2006;25:2652–2661.
- Fuentes JA, Villagra N, Castillo-Ruiz M, et al. The Salmonella Typhi hlyE gene plays a role in invasion of cultured epithelial cells and its functional transfer to S. Typhimurium promotes deep organ infection in mice. Res Microbiol. 2008;159:279–287.
- Sabnis NA, Yang H, Romeo T. Pleiotropic regulation of central carbohydrate metabolism in Escherichia coli via the gene csrA. J Biol Chem. 1995;270:29096–29104.
- Morin M, Ropers D, Letisse F, et al. The post-transcriptional regulatory system CSR controls the balance of metabolic pools in upper glycolysis of Escherichia coli. Mol Microbiol. 2016;100:686–700.
- Wei BL, Brun-Zinkernagel AM, Simecka JW, et al. Positive regulation of motility and flhDC expression by the RNA-binding protein CsrA of Escherichia coli. Mol Microbiol. 2001;40:245–256.
- Edwards AN, Patterson-Fortin LM, Vakulskas CA, et al. Circuitry linking the Csr and stringent response global regulatory systems. Mol Microbiol. 2011;80:1561–1580.
- Yakhnin H, Aichele R, Ades SE, et al. Circuitry linking the global Csr and σE-dependent cell envelope stress response systems. J Bacteriol. 2017;199:e00484.
- Jackson DW, Suzuki K, Oakford L, et al. Biofilm formation and dispersal under the influence of the global regulator CsrA of Escherichia coli. J Bacteriol. 2002;184:290–301.
- Pourciau C, Pannuri A, Potts A, et al. Regulation of iron storage by CsrA supports exponential growth of Escherichia coli. Mbio. 2019;10:e01034.
- Yakhnin H, Baker CS, Berezin I, et al. CsrA represses translation of sdiA, which encodes the N-acylhomoserine-L-lactone receptor of Escherichia coli, by binding exclusively within the coding region of sdiA mRNA. J Bacteriol. 2011;193:6162–6170.
- Andrade MO, Farah CS, Wang N. The post-transcriptional regulator rsmA/csrA activates T3SS by stabilizing the 5′ UTR of hrpG, the master regulator of hrp/hrc genes, in Xanthomonas. PLoS Pathog. 2014;10:e1003945.
- Vakulskas CA, Potts AH, Babitzke P, et al. Regulation of bacterial virulence by Csr (Rsm) systems. Microbiol Mol Biol Rev. 2015;79:193–224.
- Liu MY, Gui G, Wei B, et al. The RNA molecule CsrB binds to the global regulatory protein CsrA and antagonizes its activity in Escherichia coli. J Biol Chem. 1997;272:17502–17510.
- Suzuki K, Babitzke P, Kushner SR, et al. Identification of a novel regulatory protein (CsrD) that targets the global regulatory RNAs CsrB and CsrC for degradation by RNase E. Genes Dev. 2006;20:2605–2617.
- Weilbacher T, Suzuki K, Dubey AK, et al. A novel sRNA component of the carbon storage regulatory system of Escherichia coli. Mol Microbiol. 2003;48:657–670.
- Dubey AK, Baker CS, Romeo T, et al. RNA sequence and secondary structure participate in high-affinity CsrA-RNA interaction. RNA. 2005;11:1579–1587.
- Baker CS, Eöry LA, Yakhnin H, et al. CsrA inhibits translation initiation of Escherichia coli hfq by binding to a single site overlapping the Shine-Dalgarno sequence. J Bacteriol. 2007;189:5472–5481.
- Baker CS, Morozov I, Suzuki K, et al. CsrA regulates glycogen biosynthesis by preventing translation of glgC in Escherichia coli. Mol Microbiol. 2002;44:1599–1610.
- Patterson-Fortin LM, Vakulskas CA, Yakhnin H, et al. Dual posttranscriptional regulation via a cofactor-responsive mRNA leader. J Mol Biol. 2013;425:3662–3677.
- Yakhnin AV, Baker CS, Vakulskas CA, et al. CsrA activates flhDC expression by protecting flhDC mRNA from RNase E-mediated cleavage. Mol Microbiol. 2013;87:851–866.
- Figueroa-Bossi N, Schwartz A, Guillemardet B, et al. RNA remodeling by bacterial global regulator CsrA promotes Rho-dependent transcription termination. Genes Dev. 2014;28:1239–1251.
- Holmqvist E, Wright PR, Li L, et al. Global RNA recognition patterns of post-transcriptional regulators Hfq and CsrA revealed by UV crosslinking in vivo. Embo J. 2016;35:991–1011.
- Datsenko KA, Wanner BL. One-Step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A. 2000;97:6640–6645.
- Wang S, Yang F, Yang B. Global effect of CsrA on gene expression in enterohemorrhagic Escherichia coli O157:H7. Res Microbiol. 2017;168:700–709.
- Kulkarni PR, Jia T, Kuehne SA, et al. A sequence-based approach for prediction of CsrA/rsma targets in bacteria with experimental validation in Pseudomonas aeruginosa. Nucleic Acids Res. 2014;42:6811–6825.
- Zuker M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 2003;31:3406–3415.
- Sato K, Hamada M, Asai K, et al. CENTROIDFOLD: a web server for RNA secondary structure prediction. Nucleic Acids Res. 2009;37:W277–80.
- Wan B, Zhang Q, Ni J, et al. Type VI secretion system contributes to Enterohemorrhagic Escherichia coli virulence by secreting catalase against host reactive oxygen species (ROS). PLoS Pathog. 2017;13:e1006246.
- Aviv G, Gal-Mor O. lacZ reporter system as a tool to study virulence gene regulation in bacterial pathogens. Methods Mol Biol. 2018;1734:39–45.
- Sun Z, Qin R, Li D, et al. A novel bacterial type II L-asparaginase and evaluation of its enzymatic acrylamide reduction in French fries. Int J Biol Macromol. 2016;92:232–239.
- Berndt V, Beckstette M, Volk M, et al. Metabolome and transcriptome-wide effects of the carbon storage regulator a in enteropathogenic Escherichia coli [Sci. rep.:138]. Sci Rep. 2019;9:138.
- Hartig SM Basic image analysis and manipulation in Image. J Curr Protoc Mol Biol 2013; Chapter:Unit14.15. DOI:10.1002/0471142727.mb1415s102
- Balashova NV, Crosby JA, Al Ghofaily LA, et al. Leukotoxin confers beta-hemolytic activity to Actinobacillus actinomycetemcomitans. Infect Immun. 2006;74:2015–2021.
- Shimada T, Yamazaki Y, Tanaka K, et al. The whole set of constitutive promoters recognized by RNA polymerase RpoD holoenzyme of Escherichia coli. PLoS One. 2014;9:e90447.
- Yakhnin AV, Yakhnin H, Babitzke P. Gel mobility shift assays to detect protein-RNA interactions. Methods Mol Biol. 2012;905:201–211.
- Linhartova I, Osicka R, Bumba L, et al. Repeats-In-Toxin (RTX) toxins: a review. In: Gopalakrishnakone P; B Stiles; A Alape-Girón; J Dubreuil and M Mandal, editors. Microbial toxins. Toxinology. Dordrecht: Springer; 2018. 10.1007/978-94-007-6449-1_13
- Renda A, Poly S, Lai YJ, et al. CsrA-Mediated translational activation of ymdA expression in Escherichia coli. Mbio. 2020;11:e00849.
- Goodman AL, Kulasekara B, Rietsch A, et al. A signaling network reciprocally regulates genes associated with acute infection and chronic persistence in Pseudomonas aeruginosa. Dev Cell. 2004;7:745–754.
- Timmermans J, Melderen LV. Post-Transcriptional global regulation by CsrA in bacteria. Cell Mol Life Sci. 2010;67:2897–2908.
