ABSTRACT
Coronavirus disease 2019 (COVID-19) is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and has become a global pandemic since December 2019. Most of the patients are mild or asymptomatic and recovered well as those suffered from other respiratory viruses. SARS-CoV-2 infection is supposed to demonstrate more sequelae. Acute kidney injury (AKI) is common among COVID-19 patients and is associated with disease severity and outcomes. Only a few studies focused on a detailed analysis of kidney damage in asymptomatic or mildly symptomatic COVID-19 patients. Whether any minor viral infection is likely to exhibit similar minor effect on renal function as COVID-19 is still unclear, and the definite pathophysiology of viral invasion is not fully understood. Currently, the proposed mechanisms of AKI include direct effects of virus on kidney, dysregulated immune response, or as a result of multi-organs failure have been proposed. This study will discuss the difference between COVID-19 and other viruses, focusing on proposed mechanisms, biomarkers and whether it matters with clinical significance.
Introduction
Coronavirus disease 2019 (COVID-19) is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which escalated into a global pandemic since December 2019. It already resulted in more than 500 million cases and more than 6 million deaths globally. Initially, it mainly affects the lungs and is considered a unique respiratory virus-induced illness, but now multiple organ systems are recognised to be affected. According to current evidence, extrapulmonary involvement in COVID-19 patients has been reported, and the affected organs and systems include myocardium, gastrointestinal tract, vascular endothelium, neurological system, and kidneys [Citation1].
Acute kidney injury (AKI) is the most frequently encountered extrapulmonary manifestation among COVID-19 patients, and the reported rate are extremely variable (1%–80%), which is considered to be associated with disease severity and outcomes [Citation2,Citation3]. Previous systemic review showed that the pooled incidence of AKI in COVID-19 patients was 19.45%, which seems to be relatively higher than other respiratory coronavirus infections (5%–15%) [Citation1,Citation4]. Although the definite pathophysiology of AKI is still in the exploration stage, several studies discriminated the possible mechanisms. Further kidney damage might be due to direct effects of virus on kidney, dysregulated immune response, or as a result of multi-organs failure [Citation3]. Clinical signs suspicious of COVID-19 include cough, dyspnea, sore throat, fever, malaise, olfactory loss, and myalgia. In fact, no obvious difference from other respiratory virus infection, such as influenza virus and coronavirus, caused the SARS pandemic of 2003 [Citation5–7].
Influenza A is the most common infectious disease compared with COVID-19 because of similar transmission routes, clinical manifestation, and complications. AKI had also been reported in influenza A patients, but it usually occurred in those severe illness group [Citation8]. Asymptomatic COVID-19 patients or those with mild illness account for the majority, especially individuals infected by current Omicron variant. Only a few studies exist focused on detailed analysis of kidney damage on COVID-19 patients with mild illness. Whether any minor viral infection is likely to exhibit a similar minor effect on glomerular filtration rate (GFR) reduction like SARS-CoV-2 infection is still unclear. These proposed mechanisms and whether it matters with clinical significance are also unclear. This study aimed to discuss the unique feature of SARS-CoV-2 that differ considerably from other minor viral infection on kidney injury in mildly symptomatic and asymptomatic patients.
Point 1. Whether any minor viral infection is likely to demonstrate similar minor effect on kidney injury as SARS- CoV-2 in asymptomatic or mildly symptomatic patients?
First, the SARS-CoV-2 virus is a unique and novel virus, which belong to Betacoronavirus and is highly transmissible in humans. The positive-sense single-stranded RNA is contained within envelope and has approximately 80% genetic similarity with SARS-CoV and is less similar to the nucleotide sequence of Middle East respiratory syndrome coronavirus (about 50%) [Citation9]. The SARS-CoV-2 structure contains four structural proteins including spike (S), envelope (E), membrane (M), and nucleocapsid (N) proteins. The S protein of SARS-CoV-2 has intense binding affinity to angiotensin-converting enzyme 2 (ACE2) receptors on host cell surface and their interaction initiates the viral cell entry. Furthermore, cleavage of the S protein activates the infection process. Binding through cathepsin L or transmembrane serine protease 2 (TMPRSS2) can promote membrane fusion, then viral RNA is released into infected cell [Citation10–12]. To current consensus, ACE2 and TMPRSS2 are regarded as the crucial mediators for the entry step of SARS-CoV-2, so tissues enriched with these two proteins automatically become candidate for viral infection. That is why COVID-19 patients present more broadly extrapulmonary manifestations. Kidney is also supposed to be the target of SARS-CoV-2, and direct infection can lead to tubule-interstitial fibrosis without any systemic effect had been proved through stem cell-derived kidney organoid models by Jitske Jansen et al [Citation13].
Except for SARS-CoV-2, other sarbecoviruses like SARS-CoV do not exist in the step of S protein cleaved by furin-like proteases during viral maturation [Citation10]. As for further respiratory virus, influenza A viruses (IAVs) may affect host cell via glycan-dependent entry. IAVs initiate infection process by using the haemagglutinin (HA) RBD to connect to sialylated glycoconjugates on infected cell, and then, endocytosis is triggered. (). The participant of ACE2 and TMPRSS2 are not necessary for IAVs cell entry; nevertheless, TMPRSS2 still play a role in proteolytic activation of some H1N1 subtype IAVs. It demonstrates no involvement in viral maturation of incoming virions but cleavage nascent HA within the host cell [Citation14]. Prior studies found that IAVs infection may also increase ACE2 expression on airway epithelial cell, which might possibly explain the high rates of co-infection of COVID-19 and influenza [Citation15]. Despite these viruses exhibit close association, they still show obvious difference on pathophysiology and disease characteristics. Selected comparisons between SARS-CoV-2, SARS-CoV, and influenza virus are summarised in .
Figure 1. Difference of viral cell entry between SARS-CoV-2 and influenza a virus.
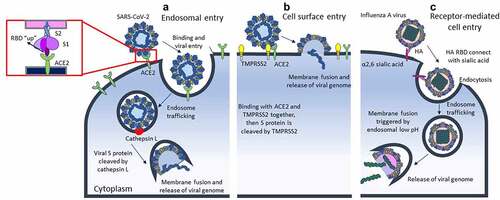
Table 1. Comparison between different respiratory viruses.
Second, not all the mechanisms of virus-induced kidney damage are the same. Divergent pathways of renal injury exist by viral infections, including direct invasion leading to cytopathic injury, inflammation-mediated tissue destruction and alterations in host haemodynamic responses [Citation23]. Nearly, all the virus infection may induce systemic immune response, but not all of the virus can invade the kidney directly. As mentioned above, SARS-CoV-2 tend to attack organs rich in ACE2 and TMPRSS2 because of their necessity for infection process of COVID-19. An organotropism is found beyond the respiratory tract, including the kidneys, liver, heart, and brain. According to both the protein expression profile and mRNA-sequencing data, we can find highly expression of ACE2 and TMPRSS2 across multiple cell types in the kidney. The most abundant site is epithelial cell on renal tubule [Citation24], and other nephron subunits include endothelial cells and mesangial cells. However, it is interesting to note that the main distribution of these two key proteins is distinct. ACE2 is enriched in proximal tubule and podocyte, whereas TMPRSS2 is mostly located in distal tubule and intercalated cells. Although double positive cells are rarely detected in kidney, evidences of direct invasion to above regions had been reported [Citation25]. In view of this, we suppose that SARS-CoV-2 enters proximal tubules assisted by ACE2 and cathepsin L. Another potential protein may cooperate with TMPRSS2 to facilitate direct invasion to distal tubule via non-ACE2 pathway. Additionally, some studies also propose the theory that transmembrane glycoprotein CD147 among with cyclophilin A serve as an alternative receptor for SARS-CoV-2 entry, which is present on tubular epithelia and podocyte [Citation26].
In spite of sialic acid α 2,6-galactose-linked receptors expression on renal glomeruli of pig [Citation27], its distribution in human still confines to ileal epithelium, ciliated and non-ciliated cells of the respiratory tract [Citation28]. No sufficient evidence showing kidney damage from influenza infection is attributable to direct viral invasion. In contrast to IAVs, SARS-CoV-2 exists diverse entry pathway to impact the kidney. Therefore, renal tissue is supposed to have higher susceptibility to SARS-CoV-2 infection and may be a reasonable explanation that AKI is prevalent among COVID-19 patients [Citation29,Citation30]. The degree of virus-induced inflammatory status or tissue invasion is heterogeneously different. Initially, viraemia occur first and then originate systemic inflammation or changed haemodynamic response subsequently induced kidney damage. Then, the virus can enter kidney cells directly or followed by penetration of the glomerular barrier into renal tubules. As stated above, we can understand that SARS‐CoV‐2 is quite unique and different from other viral infections structurally, which demonstrates the ability to induce kidney damage via assorted mechanisms.
Point 2. Whether evidence exists to support the pathogenesis of SARS- CoV-2 on the kidney in (asymptomatic or mildly symptomatic) COVID-19 patients?
First, renal tropism of SARS-CoV-2 and evidence of directly infected renal parenchyma had been reported. SARS-CoV-2 exhibits an extrapulmonary organotropism and tends to infect ACE2-enriched tissues, the kidney among the rest. SARS-CoV-2 binds to ACE2 as a receptor for cellular entry. The ACE2 RNA was expressed nearly 100-fold more in the kidney than in the lungs. ACE2 and TMPRSS, crucial roles for viral entry, were highly expressed in the brush border of tubular cells but less in podocytes [Citation31]. Thus, the absence of predominant glomerular involvement may be resulting from the ACE2 distribution difference.
The most common histopathologic features of autopsied COVID-19 kidney specimens include tubulointerstitial nephritis, acute tubular necrosis, and lymphocyte infiltration in varying degrees. Also, viral infection associated-syncytia were observed. Otherwise, both nucleocapsid protein antigen and viral RNA of SARS-CoV-2 have been detected in the kidney tissues by immunostaining and molecular biotechnology. Furthermore, recognition of coronavirus-like particle structure in podocytes and proximal renal tubules by electron microscopy and live virus recovered from kidney tissue have also been proved [Citation32]. Recently, Huang et al. disclosed the separation and identification of virus particles from urine sample of affected patients. These virions are considered to be kidney-originated and may pass through glomerulus, then enter the urinary stream directly [Citation33]. The viral load in urine sediments detected by quantitative real time polymerase chain reaction (qRT-PCR) also display a positive correlation with incidence of AKI and mortality [Citation34]. All the evidence supports that SARS-CoV-2 might exhibit viral tropism and direct persistence, which is a potential explanation of commonly occurring kidney injury in patients with COVID-19 [Citation24].
Second, the pathophysiology and mechanisms of kidney damage in COVID-19 patients have not been fully elucidated. Observation on the existance of coronavirus in the kidneys and urine supports the hypothesis that virus can directly invade the kidneys. However, some people claim that the presence of viral protein may not equal to direct viral damage, and these findings might not have absolute specificity [Citation32]. A retrospective cohort study showed that significant associations of SARS-CoV-2 viral load are found with in-hospital AKI, which may indicate the direct effect of SARS-CoV-2 virus on the kidney [Citation35]. In a Belgium study, Werion et al. present a proximal tubular dysfunction in patients with COVID-19 [Citation36]. A high prevalence of hypokalaemia was found among infected patients because of continuous renal potassium loss resulting from the presence of disordered rennin-angiotensin system activity [Citation37]. These data replenish current evidence regarding SARS-CoV-2 presence and potential infection in the kidney. Nevertheless, some studies suppose that relatively low viral load with uneven distribution detected in the renal tissues may not possess the ability to attack kidneys extensively [Citation38].
Similar to nearly all, the virus can induce systemic inflammatory response, strong presence of CD68+ macrophages and membrane attack complex depositions in the tubulointerstitium noted in the kidney of COVID-19 patients, which can further accelerate and amplify renal injury. As discussed previously, the virus may also invade renal parenchyma and cause further renal complications like acute tubular necrosis. Although podocytes are not predominantly affected site, collapsing focal segmental glomerulosclerosis due to direct viral effect has also been reported. The above kidney damage may also occur through dysregulation of the immune responses and microthrombi formation and the aggregation of fibrin deposition, which leads to acute ischaemia and AKI resulted from systemic inflammation [Citation32].
AKI may also occur in patients infected by other respiratory viruses, especially influenza. Prior studies showed approximately one third of hospitalised patients with pandemic influenza A (H1N1) virus infection presented with AKI [Citation39]. Like COVID-19, the definite pathogenic mechanisms that develop AKI remain unclear. Several hypotheses had been proposed, including severe infection-related rhabdomyolysis, acute immune complex-mediated glomerulonephritis, acute tubular necrosis due to renal hypoperfusion, or disseminated intravascular coagulation [Citation8]. Although one case series displays that IAV can be found in the cytoplasma of glomerular macrophages in four patients, no sufficient evidence of direct kidney invasion exists [Citation40].
The proposed mechanisms of COVID-19-induced kidney damage are demonstrated in . The COVID-19-induced kidney damages are involved through both direct and indirect mechanisms. The consensus report of the 25th Acute Disease Quality Initiative Workgroup concerning COVID-19-associated AKI stated that the pathophysiology and mechanisms of AKI in infected individuals still exist uncertainly and seem to be multifactorial. Also, they stated that whether kidney damage is caused by systemic inflammation and immune dysfunction remains controversial.
Figure 2. Mechanism of COVID-19-associated AKI.
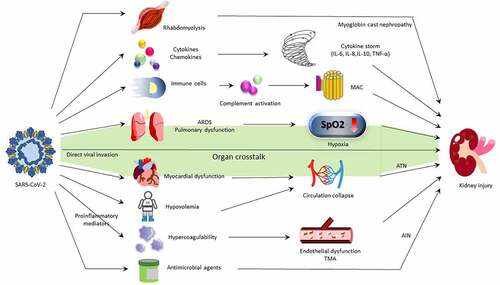
Third, further work is needed to clarify the precise aetiology of COVID-19-associated AKI. Although renal tropism is supported by majority of current researches and some studies even show evidences of direct viral invasion to the kidney, acute tubular injury is reported as the main histologic lesion [Citation38]. This finding implies the leading cause of kidney damage might be resorted to systemic dysfunction, such as acute respiratory distress syndrome, hypotension, nephrotoxin exposure or severe inflammatory response. Even though direct kidney infection of SARS-CoV-2 is indeed, it is considered to play a relatively minor role. For the current data, AKI caused by either a direct cytotoxicity of SARS-CoV-2 or by indirect immune-mediated pathogenesis is all possible. Further investigation about the definite pathophysiology and the effects of COVID-19 infection on kidney is our first imperative.
Point 3. Whether slight difference in GFR in asymptomatic COVID-19 patients indicated any clinical significance and value?
First, no evidence exists to support all viral infection in asymptomatic patient will induce similar minor effect on GFR as COVID-19. Whenever there is overt symptoms, viral infection may act on kidneys through the “direct effects” of invasive disease and/or “indirect effects” of inflammatory responses on kidneys. The virus-induced systemic inflammatory responses may exhibit effects on GFR; however, the degree is diversified. One retrospective cohort study investigated the renal presentations of COVID-19 victims with different disease severity. Proteinuria and microscopic haematuria tend to occur among severe individuals, and both of these manifestations are independent risk factors for mortality [Citation41]. No evidence was provided, and little is known about the effects on GFR among asymptomatic patients. Take an example of IAV infection. Despite the fact that development of IAV-related kidney injury has not been depicted, heterogeneous and dynamic prevalence of asymptomatic influenza virus infections was founded [Citation24,Citation42].
Only a small amount of studies focusing on the viral infections were involved in kidney allograft function [Citation43]. A variety of pathogenic viruses have been proven to cause renal complications in kidney transplant patients. Either acute or chronic graft dysfunction can be resulting from adenovirus and polyomavirus BK infection. Besides, adenovirus can also bring out haemorrhagic cystitis and tubulointerstitial nephritis. These viral infections may demonstrate effects on the long-term allograft survival [Citation43,Citation44]. As for the impact of SARS-CoV-2 infection in renal transplant recipient, most patients present cough, myalgia, chills, and fatigue, similar to general individuals. However, hospitalization is usually required and increased overall mortality has also been reported [Citation45]. Otherwise, renal function decline may also occur during initial infection, regardless of the clinical presentation or age [Citation46]. Hence, findings of COVID-19 in asymptomatic and mild patients are unique and informative.
Second, those asymptomatic patients are not absolutely caused by minor viral infection. Although some studies, mostly published in 2020, supported that a higher SARS-CoV-2 viral load might be associated with growing disease intensity and mortality [Citation47–49]. With mounting cases accumulated rapidly and further exploration of COVID-19, the significant correlation between initial viral load and disease progression seems to be overthrown [Citation50,Citation51]. Because of different host immune response, viral load is dynamic and vary over the course. Prior study has tried to constitute a model framework of variation of viral load for precise treatment policy, but additional investigation is needed [Citation52]. Besides, definite immune pathway when initial exposure to SARS-CoV-2 and the crucial determinant of symptomatic presentation remain unknown [Citation53]. Asymptomatic COVID-19 patients with higher viral load have also been broadly reported. This phenomenon represents that asymptomatic are not equal to undetectable or untransmittable, on the country, it might be the potential threat from the iceberg [Citation54–56]. Hence, SARS-CoV-2 viral loads may aid in the risk stratification of patients with COVID19 but not always demonstrate a positive correlation with severity of clinical manifestation. Future research should focus on SARS-CoV-2 viral load dynamics and its role in disease pathogenesis.
Even if patients did not present respiratory symptoms or recovered from COVID-19 clinically, viruses may still remain hidden in our body and attack extrapulmonary organs consequently, especially in groups with immunodeficiency. The concept of extrapulmonary reservoir indicates that there exists other transmission pathway rather than respiratory secretions, regardless of the detected viral load. Otherwise, SARS-CoV-2 is supposed to increase the risk of organ dysfunction [Citation57].
Third, is slight difference in GFR in asymptomatic COVID-19 patients meaningful? The exact dynamic changes of renal functions in those asymptomatic ones still need further explorations. According to previous studies, there is a positive correlation between COVID-19-associated renal complications and mortality rate. AKI is considered to be independent risk factors for both long-term renal outcome and all-cause in-hospital death in infected patients [Citation58].
Different to influenza, which may develop sequelae limited to respiratory system, such as susceptibility to secondary bacterial infection and pulmonary fibrotic changes [Citation59], Benjamin Bowe et al. demonstrated that COVID-19 may experience post-acute sequelae involving pulmonary and broad extrapulmonary organ system. Otherwise, the study exhibited that COVID-19 survivors demonstrate an increased incidence of AKI, renal function decline, end stage kidney disease and major adverse kidney events compared with those non-infected controls. Although a positive relation exists between eGFR loss and disease severity, increased risk of renal sequelae mentioned above is still evident among those individuals with mild symptoms or even asymptomatic ones. Based on this statistically significant finding, specialists strongly suggested to establish post-acute COVID-19 clinics because majority of COVID-19 victims are non-hospitalised, who may neglect further development of sequelae easily [Citation60]. The international HOPE COVID‑19 (Health Outcome Predictive Evaluation for COVID 19) Registry regards eGFR at admission as an independent factor of prognosis. When eGFR is below 150 mL/min/1.732 m2, including patients already have chronic kidney disease, a tendency towards conflicting relationship between renal function and all-cause mortality is found [Citation61]. Hence, we can tell that slight difference in GFR in asymptomatic COVID-19 patients is surely meaningful.
Finally, biomarkers are linked with changes of renal function in asymptomatic and mildly symptomatic COVID-19 patients. Some asymptomatic carriers also developed deterioration of renal function in various degrees during hospitalisation. Although it is difficult to accomplish in the real world, early detection through contact tracing may efficiently avoid presymptomatic transmission and reduces the occurrence of critical cases [Citation62]. Not all one is really assessing kidneys in asymptomatic people, so we just do not know what is going on. Some novel biomarkers like cystatin C, neutrophil gelatinase-associated lipocalin (NGAL), and kidney injury molecule 1 (KIM-1) are more specific for early detection of AKI. Recently, coronavirus detection in urine sediment suggests further kidney invasion and is associated with risk of AKI and poor prognosis. Considering this urinary viral detection is expensive and not easily accessible, it is also not practicable to utilise, not to mention that performing renal biopsy in such asymptomatic individuals. The majority of the studies demonstrated positive correlations between the use of CRP and neutrophil-to-lymphocyte ratio (NLR) in COVID-19 with disease severity or mortality. Elevation in neutrophil counts, CRP, and NLR had also been provided as useful biomarkers to link with the worsening of the renal function in asymptomatic patients. These biomarkers are non-invasive, convenient, and easy practice for clinical application [Citation47]. Long-term outcome of renal function progression in asymptomatic COVID-19 patient still need further evaluation.
Conclusion
SARS-CoV-2, different from other familiar respiratory viruses, had destroyed numerous families and forced the world to advance amidst turbulence. Through abundant researches focused on COVID-19, we understand that SARS-CoV-2 enter infected cell mainly mediated by ACE2 and TMPRSS2. Nevertheless, more potential pathways for renal invasion exist and the kidney is supposed to have strong susceptibility. Although there is still controversy, several studies had proved the theory of direct kidney infection and consequently tubulointerstitial injury. Besides renal tropism, systemic inflammatory response and organ crosstalk also participate in the development of AKI and eventually impact disease outcome. Even if patients with mild illness or those asymptomatic individuals may still exhibit high viral load. Condition of viral replication and influences on the human body are present as a dynamic variation. Most of the COVID-19 patients do not have life-threatening manifestations during early infection but a quantity of them do develop extrapulmonary sequelae at post-acute phase, which indicated the importance of regular health survey. Due to frequent mutation of this troublesome coronavirus, many issues remain unclear and need further exploration. We all hope improvement of technology and knowledge can terminate the disaster as soon as possible.
Author contributions
Ya-Chieh Chang and Ding-Jie Lee have participated in designed study and manuscript writing; Pauling Chu, Kuo‐Cheng Lu and Chia-Chao Wu organized the study. Besides, all authors have reviewed and approved final version of the manuscript.
Acknowledgement
The authors gratefully acknowledge the cooperation of every clinician fighting against the COVID-19 pandemic.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Zheng KI, Feng G, and Liu W-Y, et al. Extrapulmonary complications of COVID-19: a multisystem disease?. J Med Virol. 2021 Jan;93(1):323–335.
- Cheng Y, Luo R, Wang K, et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020 May;97(5):829–838.
- Moledina DG, Simonov M, Yamamoto Y, et al. The association of COVID-19 with acute kidney injury independent of severity of illness: a multicenter cohort study. Am J Kidney Dis. 2021 Apr;77(4):490–499.e1.
- Raina R, Mahajan ZA, Vasistha P, et al. Incidence and outcomes of acute kidney injury in COVID-19: a systematic review. Blood Purif. 2022;51(3):199–212.
- Monto AS, Gravenstein S, Elliott M, et al. Clinical signs and symptoms predicting influenza infection. Arch Intern Med. 2000 Nov 27;160(21):3243–3247.
- Shu-Cheong Hui D, Wong P-C, Wang C. SARS: Clinical features and diagnosis. Respirology. 2003 Nov;8(Suppl(Suppl1)):S20–4.
- Rebholz H, Braun RJ, and Ladage D, et al. Loss of olfactory function-early indicator for covid-19, other viral infections and neurodegenerative disorders. Front Neurol. 2020 Oct 26;11:569333.
- Watanabe T. Renal complications of seasonal and pandemic influenza a virus infections. Eur J Pediatr. 2013 Jan;172(1):15–22.
- Hu B, Guo H, Zhou P, et al. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol. 2021 Mar;19(3):141–154.
- Jackson CB, Farzan M, Chen B, et al. Mechanisms of SARS-CoV-2 entry into cells. Nat Rev Mol Cell Biol. 2022 Jan;23(1):3–20.
- Rahbar Saadat Y, Mahdi Hosseiniyan Khatibi S, Zununi Vahed S, et al. Host serine proteases: a potential targeted therapy for COVID-19 and influenza. Front Mol Biosci. 2021 Aug 30;8:725528.
- Berdowska I, Matusiewicz M. Cathepsin L, transmembrane peptidase/serine subfamily member 2/4, and other host proteases in COVID-19 pathogenesis - with impact on gastrointestinal tract. World J Gastroenterol. 2021 Oct 21;27(39):6590–6600.
- Jansen J, Reimer KC, Nagai JS, et al. SARS-CoV-2 infects the human kidney and drives fibrosis in kidney organoids. Cell Stem Cell. 2022 Feb 3;29(2):217–231.e8.
- Dou D, Revol R, Östbye H, et al. Influenza a virus cell entry, replication, virion assembly and movement. Front Immunol. 2018 Jul 20;9:1581.
- Schweitzer KS, Crue T, Nall JM, et al. Influenza virus infection increases ACE2 expression and shedding in human small airway epithelial cells. Eur Respir J. 2021 Jul 1;58(1):2003988.
- Belouzard S, Chu VC, Gary R GR. Activation of the SARS coronavirus spike protein via sequential proteolytic cleavage at two distinct sites. Proc Natl Acad Sci U S a. 2009 Apr 7;106((14)):5871–5876.
- Abdelrahman Z, Li M, Wang X. Comparative review of SARS-CoV-2, SARS-CoV, MERS-CoV, and influenza a respiratory viruses. Front Immunol. 2020 Sep 11;11:552909.
- Gu J, Gong E, Zhang B, et al. Multiple organ infection and the pathogenesis of SARS. J Exp Med. 2005 Aug 1;202(3):415–424.
- Rodrigues Guimarães Alves V, Helena Perosa A, de Souza Luna LK, et al. Influenza A(H1N1)pdm09 infection and viral load analysis in patients with different clinical presentations. Mem Inst Oswaldo Cruz. 2020 ;115:e200009.
- Peiris JSM, Chu CM, Cheng VCC, et al. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003 May 24;361(9371):1767–1772.
- Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med. 2021 Apr;27(4):601–615.
- Chan KS, Zheng JP, Mok YW, et al. SARS: prognosis, outcome and sequelae. Respirology. 2003 Nov;8(Suppl(Suppl 1)):S36–40.
- Kotton CN, Fishman JA. Viral infection in the renal transplant recipient. J Am Soc Nephrol. 2005 Jun;16(6):1758–1774.
- Puelles VG, Lütgehetmann M, Lindenmeyer MT, et al. Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med. 2020 Aug 6;383(6):590–592.
- Chen Z, Hu J, Liu L, et al. SARS-CoV-2 causes acute kidney injury by directly infecting renal tubules. Front Cell Dev Biol. 2021 May;31(9):664868.
- Su H, Wan C, Wang Z-D, et al. Expression of CD147 and cyclophilin a in kidneys of patients with COVID-19. Clin J Am Soc Nephrol. 2021 Apr 7;16(4):618–619.
- Nelli RK, Kuchipudi SV, White GA, et al. Gavin a white et al.comparative distribution of human and avian type sialic acid influenza receptors in the pig. BMC Vet Res. 2010 Jan 27;6:4.
- Kuchipudi SV, Nelli RK, Gontu A, et al. Sialic acid receptors: the key to solving the enigma of zoonotic virus spillover. Viruses. 2021 Feb 8;13(2):262.
- Dong M, Zhang J, Ma X, et al. ACE2, TMPRSS2 distribution and extrapulmonary organ injury in patients with COVID-19. Biomed Pharmacother. 2020 Nov;131:110678.
- Salamanna F, Maglio M, Paola Landini M, et al. Body localization of ACE-2: on the trail of the keyhole of SARS-CoV-2. Front Med (Lausanne). 2020 Dec;3(7):594495.
- Ertuğlu LA, Kanbay A, Afşar B, et al. COVID-19 and acute kidney injury. Tuberk Toraks. 2020 Dec;68(4):407–418. DOI:10.5578/tt.70010.
- Benedetti C, Waldman M, Zaza G, et al. COVID-19 and the kidneys: an update. Front Med (Lausanne). 2020 Jul 21;7:423.
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020 Feb 15;395(10223):497–506.
- Caceres PS, Savickas G, Murray SL, et al. High SARS-CoV-2 viral load in urine sediment correlates with acute kidney injury and poor COVID-19 outcome. J Am Soc Nephrol. 2021 Oct;32(10):2517–2528.
- Paranjpe I, Chaudhary K, Johnson KW, et al. Association of SARS-CoV-2 viral load at admission with in-hospital acute kidney injury: a retrospective cohort study. PLoS One. 2021 Feb 24;16(2):e0247366.
- Braun F, Huber TB, Puelles VG. Proximal tubular dysfunction in patients with COVID-19: What have we learnt so far? Kidney Int. 2020 Nov;98(5):1092–1094.
- Chen D, Li X, Song Q, et al. Assessment of hypokalemia and clinical characteristics in patients with coronavirus disease 2019 in Wenzhou, China. JAMA Network Open. 2020 Jun 1;3(6):e2011122.
- Wang M, Xiong H, Chen H, et al. Renal injury by SARS-CoV-2 infection: a systematic review. Kidney Dis (Basel). 2021 Mar;7(2):100–110.
- Demirjian SG, Raina R, Bhimraj A, et al. Influenza a infection and acute kidney injury: incidence, risk factors, and complications. Am J Nephrol 2011. 2009;34(1):1–8.
- Carmona F, Carlotti APCP, Ramalho LNZ, et al. Evidence of renal infection in fatal cases of 2009 pandemic influenza a (H1N1). Am J Clin Pathol. 2011 Sep;136(3):416–423.
- Hong D, Long L, Wang AY, et al. Kidney manifestations of mild, moderate and severe coronavirus disease 2019: a retrospective cohort study. Clin Kidney J. 2020 May 9;13(3):340–346.
- Furuya-Kanamori L, Cox M, Milinovich GJ, et al. Heterogeneous and dynamic prevalence of asymptomatic influenza virus infections. Emerg Infect Dis. 2016 Jun;22(6):1052–1056.
- Masutani K. Viral infections directly involved in kidney allograft function. Nephrology (Carlton). 2018 Jul;23(Suppl 2):31–37.
- Benotmane I, Gautier-Vargas G, Wendling M-J, et al. In-Depth virological assessment of kidney transplant recipients with COVID-19. AAm J Transplant. 2020 Nov;20(11):3162–3172.
- Mohan S, King KL, Ali Husain S, et al. COVID-19-associated mortality among kidney transplant recipients and candidates in the United States. Clin J Am Soc Nephrol. 2021 Nov;16(11):1695–1703.
- Chang Y-C, Tsai P-H, Chou Y-C, et al. Biomarkers linked with dynamic changes of renal function in asymptomatic and mildly symptomatic COVID-19 Patients. J Pers Med. 2021 May 19;11(5):432.
- Fajnzylber J, Regan J, Coxen K, et al. SARS-CoV-2 viral load is associated with increased disease severity and mortality. Nat Commun. 2020 Oct 30;11(1):5493.
- Pujadas E, Chaudhry F, McBride R, et al. SARS-CoV-2 viral load predicts COVID-19 mortality. Lancet Respir Med. 2020 Sep;8(9):e70.
- Bryan A, Fink SL, Gattuso MA, et al. SARS-CoV-2 viral load on admission is associated with 30-day mortality. Open Forum Infect Dis. 2020 Nov 3;7(12):ofaa535.
- Cocconcelli E, Castelli G, Onelia F, et al. Disease severity and prognosis of SARS-CoV-2 infection in hospitalized patients is not associated with viral load in nasopharyngeal swab. Front Med (Lausanne). 2021 Sep 10;8:714221.
- Trunfio M, Calcagno A, Bonora S, et al. Lowering SARS-CoV-2 viral load might affect transmission but not disease severity in secondary cases. Lancet Infect Dis. 2021 Jul;21(7):914–915.
- Challenger JD, Foo CY, Wu Y, et al. Modelling upper respiratory viral load dynamics of SARS-CoV-2. BMC Med. 2022 Jan 13;20(1):25.
- Boyton RJ, Altmann DM. The immunology of asymptomatic SARS-CoV-2 infection: What are the key questions? Nat Rev Immunol. 2021 Dec;21(12):762–768.
- Long Q-X, Tang X-J, Shi Q-L, et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med. 2020 Aug;26(8):1200–1204.
- Hasanoglu I, Korukluoglu G, Asilturk D, et al. Higher viral loads in asymptomatic COVID-19 patients might be the invisible part of the iceberg. Infection. 2021 Feb;49(1):117–126. DOI:10.1007/s15010-020-01548-8.
- Catherine McEllistrem M, Clancy CJ, Buehrle DJ, et al. SARS-CoV-2 is associated with high viral loads in asymptomatic and recently symptomatic healthcare workers. PLoS One. 2021 Mar 18;16(3):e0248347.
- Kalkeri R, Goebel S, Dutt Sharma G. SARS-CoV-2 shedding from asymptomatic patients: contribution of potential extrapulmonary tissue reservoirs. Am J Trop Med Hyg. 2020 Jul;103(1):18–21.
- Nadim MK, Forni LG, Mehta RL, et al. COVID-19-associated acute kidney injury: consensus report of the 25th acute disease quality initiative (ADQI) workgroup. Nat Rev Nephrol. 2020 Dec;16(12):747–764.
- Cipolla EM, Huckestein BR, Alcorn JF. Influenza sequelae: from immune modulation to persistent alveolitis. Clin Sci (Lond). 2020 Jul 17;134(13):1697–1714.
- Bowe B, Xie Y, Xu E, et al. Kidney outcomes in long COVID. J Am Soc Nephrol. 2021 Nov;32(11):2851–2862.
- Uribarri A, Núñez-Gil IJ, Aparisi A, et al. Impact of renal function on admission in COVID-19 patients: an analysis of the international HOPE COVID-19 (health outcome predictive evaluation for COVID 19) registry. J Nephrol. 2020 Aug;33(4):737–745.
- Yu C, Zhou M, Liu Y, et al. Characteristics of asymptomatic COVID-19 infection and progression: a multicenter, retrospective study. Virulence. 2020 Dec;11(1):1006–1014. DOI:10.1080/21505594.2020.1802194.
