ABSTRACT
Methicillin-resistant Staphylococcus aureus (MRSA) has been developing rapidly in recent years. It poses a severe peril to global health care, and the new strategies to against the MRSA is urgently needed. Sortase A (SrtA) regulates the anchoring of many surface proteins. Compounds repress Staphylococcus aureus (S. aureus) cysteine transpeptidase SrtA are considered adequate potent virulence inhibitors. Then, we describe the identification of an effective SrtA inhibitor, cyanidin chloride, a bioflavonoid compound isolated from various plants. It has a reversible inhibitory effect on SrtA activity at an IC50 of 21.91 μg/mL. As a SrtA inhibitor, cyanidin chloride antagonizes SrtA-related virulence phenotypes due to its breadth and specificity, including fibrinogen adhesion, A549 cell invasion, biofilm formation, and surface protein (SpA) anchoring. Subsequently, molecular docking and fluorescence quenching revealed that SrtA and cyanidin chloride had robust mutual affinity. Further mechanistic studies revealed that Arg-197, Gly-167, and Sep-116 were the key-binding sites mediating the interaction between SrtA and cyanidin chloride. Notably, a significant therapeutic effect of cyanidin chloride in vivo was also observed on the mouse pneumonia model induced by MRSA. In conclusion, our study indicates that cyanidin chloride potentially represents a new candidate SrtA inhibitor for S. aureus and potentially be developed as a new antivirulence agent.
Introduction
Staphylococcus aureus (S. aureus) is one of the commensal bacteria that colonizes approximately 20–30% of the population [Citation1,Citation2]. The bacteria in the anterior nostrils can spread from the original habitat to the skin, intestines, and deeper host tissues. Under pathogenic conditions, S. aureus may cause pneumonia, sepsis, endocarditis, bone and joint infection, and death in severe cases [Citation3]. Methicillin-resistant S. aureus (MRSA), which has higher pathogenicity, high complications, and a high mortality rate [Citation4], is considered a priority pathogen by the World Health Organization (WHO) [Citation5]. The broad and indiscriminate use of antibiotics has led to a severe problem of bacterial drug resistance, forcing researchers to develop new and effective strategies to fight infections with multidrug-resistant pathogens.
Aiming at bacterial virulence is the best strategy for combating multidrug-resistant pathogens. It creates less selective pressure on the bacterial resistance developing than conventional ways, and is not mediated by the necessary pathways, such as directly killing bacteria or inhibiting bacterial growth [Citation6,Citation7]. MRSA itself expresses many surface proteins and secretes different virulence factors, which provides mechanisms for bacteria to survive and escape from innate and adaptive immunity (adhesion, invasion, biofilm formation, etc.) [Citation8]. Therefore, developing a screen for specific virulence factor inhibitors will provide an effective therapeutic strategy for MRSA infection.
In S. aureus, sortase A (SrtA) is a polypeptide of 20 KD that catalyzes thioesterification and transpeptidation. SrtA anchors LPXTG-containing proteins to lipid II, thereby affecting bacterial adhesion [Citation9]. All S. aureus isolates encode at least 17–21 surface proteins with LPXTG classification signals [Citation10]. The lack of SrtA is associated with the inability to bind the surface proteins that anchor at their C-terminal ends [Citation11]. Therefore, surface adhesion is eliminated, resulting in a significant decrease in the pathogenicity of the pathogen. In vivo experiments also proved that the SrtA mutant significantly reduces pathogenicity in various infections, for instance, pneumonia, systemic infection, and sepsis [Citation12–15]. In summary, SrtA-mediated modification of surface proteins plays crucial roles in host adhesion, invasion, immune escape, blood clotting, biofilm formation, and transportation of nutrients across the microbial cell wall envelope. SrtA, membrane protein, is also easier to target, making it an ideal anti-virulence target.
Natural compounds have always been the first choice for new drug research and development because of their wide variety, novel and diverse structures, and rich biological activities [Citation16]. Natural compounds generally have the advantage of minor side effects and are a last resort for treating multidrug resistance [Citation17,Citation18]” In addition, studies have reported that natural products have been developed for therapeutic indications in a variety of diseases: anti-cancer, anti-infection, anti-diabetes, etc [Citation19,Citation20]. It is worth mentioning that natural products are more easily absorbed than synthetic agents. Subsequently, we found that cyanidin chloride, a bioflavonoid, effectively inhibited SrtA activity at low concentrations. It is a water-soluble natural pigment that widely exists in various plants and has been reported to have broad application prospects and multiple pharmacological functions. For example, cyanidin chloride has been used as a therapeutic drug for atherosclerosis, cancer, and cardiovascular diseases [Citation21,Citation22]. However, no report has described the anti-virulence effect of cyanidin chloride. Subsequently, we evaluated the anti-S. aureus SrtA activity and the related effect of cyanidin chloride on virulence in vitro. The therapeutic effect of cyanidin chloride on pneumonia in mice infected with S. aureus was also evaluated in vivo. Its significant effect suggests that it represents an ideal candidate compound for the effective treatment of MRSA infection by inhibiting virulence.
Materials and methods
Strains, growth conditions, and reagents
The S. aureus USA300 and S. aureus Newman strains used in this study were purchased from ATCC (Manassas, VA, USA). The S. aureus USA300 srtA deletion strain (ΔsrtA) and pET28a-SrtA plasmid were stocked in our laboratory. Escherichia coli (E. coli) and S. aureus were incubated in Luria-Bertani (LB) and tryptone soy broth (TSB) media at 37°C. DMSO was obtained from Biotechnology (Shanghai, China), and cyanidin chloride was purchased from Weikeqi Biology (Chengdu, China). The fluorescent substrate peptide Abz-LPATG-Dap (Dnp)-NH2 was synthesized by Lifetein, LLC (Beijing, China).
Construction of the SrtA mutant plasmid
Using the pET28a-SrtA plasmid as a template, S116A, G167A, and R197A mutations in SrtA were generated according to the instructions of the Mut Express MultiS Fast Mutagenesis Kit V2 (Vazyme, Nanjing, China). SrtA-related mutation primer pairs are shown in Supplementary Table 1.
Expression and purification of SrtA and its mutant proteins
The recombinant pET28a-SrtA plasmid and its mutant plasmids were transferred into the E. coli expression host strain BL21 Star (DE3) chemically competent cell (Tsingke Biological Technology) using the heat shock method. Then, the bacteria were grown until OD600 reached 0.8, sufficient IPTG was added so that final concentration was 0.5 mM, and the bacteria were under 8 h culturing. Because we used the recombinant plasmid containing 6 × His tags, the induced protein was purified using Ni-NTA chromatography (Beyotime Biotechnology). The non-specifically bound proteins were eluted, and then the target protein was eluted with 300 mM imidazole.
Determination of SrtA transpeptidase activity
Fluorescence resonance energy transfer (FRET) has been used to identify SrtA transpeptidase activities. Using the method reported in a previous study [Citation23], a 100 μL reaction system containing the SrtA protein (10 μM) and different concentrations of cyanidin chloride was incubated under 37°C in the dark for 1 h. Subsequently, the fluorescent substrate peptide Abz-LPATG-Dap (Dnp)-NH2 (10 μM) was added. After incubating for 20 min at 37°C, fluorescence was detected by a fluorescence microplate reader (Thermo, USA). Then the inhibition rate was calculated. When inhibition rate was greater than 60%, the compound was considered as a potential inhibitor. Subsequently, a gradient concentration inhibition curve was performed and IC50 was calculated.
Reversible inhibition of SrtA
The reversible assay was conducted as previously described [Citation10]. Briefly, purified SrtA protein and cyanidin chloride were mixed into the reaction buffer (100 μL) at a concentration of 10 times the IC50 and incubated for 1 h at 37°C under darkness. The mock group was treated with an equal volume of DMSO. Subsequently, this reaction solution was diluted 100-fold, followed by the peptide substrate Abz-LPATG-Dap (Dnp)-NH2 was added and incubated at 37°C for 20 min. The fluorescence intensity was read using emission and excitation wavelengths of 420 and 309 nm with a microplate reader (Thermo, USA). Then the reversible inhibition rate was calculated. Reversible inhibition rate >60% is considered as non-covalent reversible binding, and <60% is considered as covalent irreversible binding.
Antibacterial activity
The minimum inhibitory concentration (MIC) of cyanidin chloride against S. aureus USA300 was obtained with the broth dilution method recommended by the NCCLS guide. Briefly, 100 μL of cation-adjusted Mueller–Hinton broth (CAMHB) medium and S. aureus USA300 (105 CFUs) were pipetted to 96-well plates. Then various concentrations of cyanidin chloride (2 to 512 μg/mL) were added using the double dilution method. S. aureus USA300 treated without cyanidin chloride was established as a negative control group, and CAMHB medium alone was established as a blank control. After incubating at 37°C for 16 h at a constant temperature, the absorbance was measured by a microplate reader.
Growth curve
The overnight cultures of S. aureus USA300 was diluted 1:100, transformed into fresh TSB medium, and continuously cultured and grown untill OD600 0.3. The cultures were divided into a solvent control group (WT + DMSO), drug group (WT + cyanidin chloride), and ΔsrtA group. The bacterial culture was collected at different time points to determine the absorbance at 600 nm.
Cell viability and LDH release assay
Inoculated series of diluted HepG2 cells (human hepatocellular carcinoma cells, 0, 1 × 103, 5 × 103, 1 × 104, 2 × 104) into 96-well plates and cultured in a cell incubator for 24 h. Then, multiple concentrations of cyanidin chloride were mixed and cells were incubated for 24 h. Next, 10 μL of CCK8 reagent (US EVERBRIGHT, Suzhou, China) were added. After incubating the plates in the cell incubator for 4 h, the absorptivity was recorded at 450 nm using a microplate reader. The LDH cytotoxicity assay kit was used to determine the cytotoxicity of cyaniding chloride. HepG2 cells were cultured at a density of 2 × 105 cells/well in 24-well plates and then incubated for 24 h. Subsequently, 400 μL of DMEM containing different concentrations of cyaniding chloride was added. After 6 h of cocultivation at 37°C, the supernatant of the culture was collected, and the LDH levels in the supernatant were measured using an LDH kit (Beyotime, Beijing, China) according to the instructions.
Fibronectin binding assay
Cyanidin chloride was added to the culture of S. aureus at OD600 0.3 and continuously grown until OD600 0.5. The bacteria were collected by centrifugation, and resuspended by PBS. Subsequently, resuspended bacteria were added to 96-well plates coated with 100 μL of coating solution (20 μg/mL bovine blood fibrinogen). After culturing at 37°C for 2 h, the unbound bacteria were gently eliminated by washing with PBS and fixed with 25% (v/v) formaldehyde for 30 min. After staining with crystal violet dye for 20 min, the absorbance was measured at 570 nm.
A549 cell invasion assay
A549 cells (3 × 105 cells per well) were inoculated into 24-well cell culture plates. After overnight culture in an atmosphere containing 5% CO2 at 37°C. Multiple concentrations of cyanidin chloride were mixed with S. aureus USA300 with growing to an OD600 1.0, respectively. Subsequently, the bacteria were collected by centrifugation and resuspended with the same volume of DMEM. Then, 1 mL of the resuspended bacterial solution was incubated in cell medium for 2 h at 37°C. After two washes with PBS, 1 mL of DMEM containing gentamicin (300 μg/mL) was added to the cell culture plates. After culture at 37°C for 1 h, the cells were washed with PBS. Then, Triton X-100 was added to the cell culture plate to lyse the cells, and an appropriate amount of lysate was coated on TSB medium until a single colony appeared and was counted.
Biofilm formation
A variety of cyanogen chloride concentrations were added to S. aureus USA300 with an initial OD of 0.1, the bacterium was grown until OD 600 nm of 0.6 at 37°C. The bacterial culture (5 µL) was mixed to 200 µL BHI broth and incubated for 18 h to form biofilms. Then, the medium was carefully discarded and the cell plate was washed to eliminate residual bacteria. Biofilm formation was assessed by staining the samples with 0.1% crystal violet for 20 min. Unbound dyes were gently removed by rinsing with sterile PBS buffer and blown dry at room temperature. Then, 95% ethanol (100 µL) was added and the absorption was detected at 570 nm.
FITC-IgG binding to staphylococcal protein a (SpA)
The overnight cultures of S. aureus USA300 and ΔsrtA were diluted in TSB medium at a ratio of 1:1,000, and multiple concentrations of cyanidin chloride or DMSO were added, respectively. When the bacterial culture was cultured at 37°C at OD600 1.0, they were harvested by centrifuging and washed twice with PBS. Resuspension of the bacteria in 50 μL FITC-labeled rabbit IgG (1:200) were incubated in the dark for 2 h. The bacteria were resuspended in 4% formaldehyde after washing. The fluorescence intensity was measured using flow cytometry (Beckman Coulter, USA) to evaluate the amount of SpA.
RT-qPCR
S. aureus USA300 was grown to an OD600 of 0.3, and different concentrations of cyanidin chloride were then added until the OD600 reached 2.5. The bacteria were collected by centrifugation, and total RNA was extracted using TRIzol (Tiangen, Beijing, China). Subsequently, the RNA was quantified and reverse transcribed into cDNA using a PrimeScript RT Reagent Kit (Takara, Dalian, China) and stored at −80°C. The RT-qPCR primers are shown in Supplementary Table 1, and the 16S rRNA housekeeping gene served as an internal control to compare the transcript-level changes between samples.
Western blot
S. aureus USA300 treated with different concentrations of cyanidin chloride (0–64 μg/mL) were collected by centrifuging and resuspending in PBS. Total protein was extracted from S. aureus using the conventional method [Citation24]. Subsequently, an equal quantity of total bacterial protein (20 μg) was isolated and transferred. Then, the polyvinylidene fluoride membrane was blocked with 5% BSA for 2 h. the membrane was incubated with a rabbit anti-SrtA polyclonal antibody. After washing, it was incubated with a horseradish peroxidase (HRP)-labeled goat anti-rabbit antibody for 1 h. Subsequently, the bands were exposed and detected, and the target band was analyzed.
Fluorescence quenching assay
The binding constant (KA) of cyanidin chloride to SrtA and its mutants (S116A-srtA, G167A-srtA, and R197A-srtA) was measured using a fluorescence quenching assay, as previously described. Fluorescence emission spectra of SrtA were recorded in the absence or presence in various concentrations of cyanidin chloride. The excitation wavelength, and the emission peak was recorded. The excitation and emission slit widths were 5 and 10 nm, respectively. The fluorescence quenching data were plotted as the relative fluorescence intensity against different concentrations of cyanidin chloride, and the KA values were calculated using previously reported methods.
Molecular docking
SrtA structure was derived from the 3D X-ray crystal structure (PDB code: 1T2P) in the Protein Data Bank. The 3D structures of cyanidin chloride were constructed using the ChemBio3D Ultra 12.0 software package. Standard docking procedures for the SrtA protein and cyanidin chloride was performed with AutoDock-Tools 1a.5.6 software (La Jolla, CA, USA).
Murine model of pneumonia
The pneumonia infection model was established in female C57BL/6J mice selected at 6–8 weeks to study the therapeutic effect of cyanidin chloride on acute pneumonia caused by MRSA [Citation25,Citation26]. Mice were administered intranasal infection with 2 × 108 CFU of S. aureus USA300 and then held upright for 30 s to assure that each mouse aspirated bacteria into lungs. Mice were injected subcutaneously with cyanidin chloride (80 mg/kg) 1 h after the infection and then every 12 h thereafter. The survival rate of mice within 96 h was recorded at 12 h intervals. The bacterial load in lung tissue and the histopathological changes in the lung were analyzed to further estimate the therapeutic effect of cyanidin chloride on pneumonia in mice. Each group of mice were infected for two days by intranasal dripping of 30 μL (1 × 108 CFU) of S. aureus culture. Then, the mice were euthanized by cervical dislocation, and the lungs were collected, weighed, and homogenized. Then, the homogenate was appropriately diluted and coated on a BHI agar plate. After an incubation at 37°C overnight, the quantity of colonies was calculated. The left lungs were collected from mice in each group and fixed with 10% formalin after perfusion. Haematoxylin and eosin (H & E) staining was recorded, pathological changes in lung tissue were observed.
Statistical analysis
The data are presented as the means ± SD for each experimental group. The experimental data obtained in this study were analyzed using GraphPad Prism 8.0 software (GraphPad Software, La Jolla, CA, USA). Statistical significance was set to P < 0.05.
Results
Identification of cyanidin chloride as a SrtA inhibitor
FRET is the primary approach to screening for SrtA inhibitors. It is designed to carry fluorescent groups and quenching groups at both ends of the LPXTG motif [Citation27]. SrtA inhibitors are screened based on the principle that SrtA recognizes and cuts the LPXTG motif to transfer fluorescence [Citation28,Citation29] (). There were thirty-five compounds have been screened in this study, all of which belong to flavonoids, terpenoids, alkaloids, and quinones, and three were screened out for further study. Cyaniding chloride was selected for subsequent studies because of its high SrtA inhibitory activity at low doses during initial screening. The representative list of natural compounds in initial screening with their SrtA relative inhibition rate is displayed in Supplementary Table 2. In this experiment, we recorded that cyanidin chloride suppressed the activity of SrtA with an IC50 of 21.91 µg/mL in a dose-dependent manner (). Subsequently, the binding mode of cyanidin chloride and SrtA was further evaluated. When SrtA was incubated with cyanidin chloride (10-fold IC50), 83.53 ± 5.77% of SrtA activity was recovered compared with mock treated group (), indicating that cyanidin chloride functions as a reversible inhibitor that does not covalently modify the active site cysteine of SrtA [Citation30].
Figure 1. Cyanidin chloride reversibly inhibited S. aureus SrtA activity. (a) SrtA inhibitors were screened from natural compounds based on FRET upon substrate cleavage, and cyanidin chloride was identified as an SrtA inhibitor. (b) Inhibitory effects of cyanidin chloride on the activity of S. aureus USA300 SrtA in vitro. (c) Cyanidin chloride could reversibly inhibit SrtA activity. (d) S. aureus USA300 growth curve with or without cyanidin chloride (64 μg/mL) and ΔsrtA group. (e) the percent cell viability of HepG2 cells measured by CCK8 assay after 24 h incubating with various concentrations of cyanidin chloride. (f) the LDH released from HepG2 cells treated with or without cyanidin chloride. * indicates P < 0.05, **indicates P < 0.01, and *** indicates P < 0.001 compared to the control group.
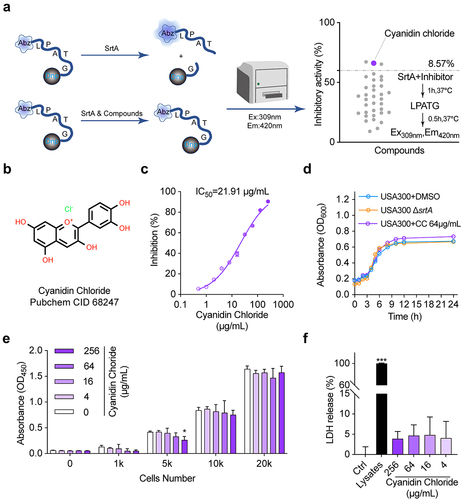
Cyanidin chloride does not affect the growth of S. aureus USA300 and HepG2 cells
Considering the efficient inhibition of SrtA activity by cyanidin chloride, we further evaluated the safety of its clinical application. The MIC of cyanidin chloride against S. aureus USA300 was 512 µg/mL. The administration of cyanidin chloride at 64 µg/mL, much greater than the IC50, had no inhibitory effect on S. aureus growth (). In addition, HepG2 cells were incubated with various concentrations of cyanidin chloride (4–256 μg/mL) for 24 h, and there was even no toxicity to HepG2 cells at 256 μg/mL. The CCK-8 assay confirmed that this molecule did not affect HepG2 cell viability (). Subsequently, the effect of cyanidin chloride on HepG2 cells was further assessed by LDH assay. Data shown that cyanidin chloride had no influence on LDH release under the concentrations of 4–256 μg/mL, indicating that cyanidin chloride did not have a cytotoxic effect at the concentration required to inhibit SrtA, and did not induce HepG2 cell death (). The safety of cyanidin chloride meets the characteristics of an ideal anti-virulence agent.
Effect of cyanidin chloride on the adhesion of S. aureus to fibrinogen
The absence of fibronectin-binding protein would reduce in the ability of bacteria adhesion to the host, which is mediated by SrtA and related to the pathogenicity of bacteria [Citation31,Citation32]. Therefore, the effect of cyanidin chloride on the ability of S. aureus to adhere to fibrinogen needs to be evaluated. As shown in , the adhesion of cyanuric chloride (64 µg/mL)-treated S. aureus to fibrinogen was significantly reduced to 16.93 ± 0.15% compared to the wild type (WT) group.
Figure 2. The influence of cyanidin chloride on SrtA-related phenotypes of S. aureus. (a) Effect of cyanidin chloride on the adhesion of S. aureus USA300 to fibrinogen. (b) the effect of cyanidin chloride on internalization of S. aureus into A549 cells. (c) Antagonistic effects of cyanidin chloride on S. aureus USA300 biofilm formation. (d) Flow cytometry analysis of S. aureus surface protein (SpA) stained with FITC-labeled rabbit IgG. (e) the transcript levels of spa in the presence of various concentrations of cyanidin chloride were determined by RT-qPCR. * indicates P < 0.05, **indicates P < 0.01, and *** indicates P < 0.001 compared to the untreated group.
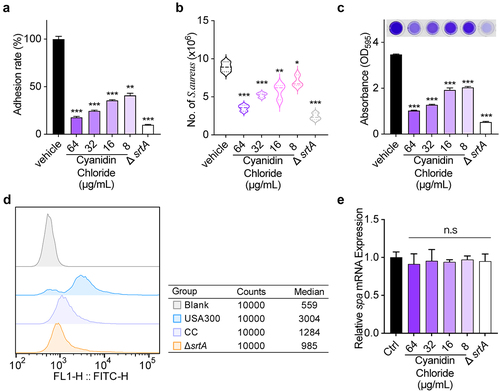
Effect of cyanidin chloride on S. aureus internalization
S. aureus SrtA is closely associated with the pathogenic mechanisms of host colonization and invasive disease [Citation33]. Therefore, we further evaluated the invasive effect of S. aureus treated with cyanidin chloride on A549 cells in vitro. As expected, Untreated S. aureus displayed an extreme invasive capacity against A549 cells, which was significantly reduced upon treating S. aureus with 64 μg/mL cyanidin chloride (P < 0.001, ). Therefore, cyanidin chloride effectively inhibited cell invasion by S. aureus through inhibition of SrtA.
Effect of cyanidin chloride on biofilm formation
Biofilms are complex and organized bacterial assemblies with high levels of resistance to antibiotics and host defences [Citation34]. According to previous studies, the formation of S. aureus biofilms depends on a certain bacterial surface protein, and most of these surface proteins are modified by SrtA. Therefore, the role of cyanidin chloride on biofilm formation was further studied by performing crystal violet staining. The biofilm biomass of ΔsrtA was 19.76 ± 0.13% (), demonstrating the effect of SrtA in biofilm formation. In addition, cyanidin chloride significantly suppressed the biofilm formation of S. aureus. Upon treating with 64 μg/mL cyanidin chloride, biofilm formation in the WT group was significantly decreased to 29.31 ± 0.12% (P < 0.001).
Effect of cyanidin chloride on the anchoring of SpA
In S. aureus, SrtA anchors a wide range of surface proteins on the bacterial envelope, and thus SpA plays an essential role in host immunity and phagocytosis [Citation35,Citation36]. The main feature of SpA is specifically binding to mammalian IgG [Citation37]. Therefore, the fluorescence intensity of S. aureus bound to FITC-labeled IgG after treatment with different concentrations of cyanidin chloride was detected using flow cytometry to evaluate the content of SpA on the surface of the bacterial cell wall. ΔsrtA group exhibited a weak fluorescence intensity detected by flow cytometry, indicating that the bacteria nearly lost its ability to anchor SpA to the cell wall. In addition, the WT group showed a strong fluorescence intensity, and the median value was 3004. When S. aureus was dosed with 64 µg/mL cyanidin chloride, the fluorescence intensity was decreased, and the median value was 1284 (P < 0.001). Therefore, cyanidin chloride reduced the anchoring of SpA in the bacterial cell wall by inhibiting SrtA (). In addition, RT-qPCR was performed to further analyze the transcript levels of spa in the presence of various concentrations of cyanidin chloride. The results showed that there was no statistical difference in the transcript levels of spa among different concentrations (8–64 μg/mL) of cyanidin chloride-treated S. aureus (). These results revealed that cyanidin chloride interfered with the anchoring of SpA by inhibiting the activity of SrtA but not affecting spa transcription level.
Effect of cyanidin chloride on the expression of SrtA
The changes in SrtA expression in S. aureus USA300 treated with cyanidin chloride (0 to 64 µg/mL) was performed to further clarify whether cyanidin chloride inhibits SrtA activity by altering its expression. The addition of different concentrations of cyanidin chloride (0 to 64 µg/mL) did not affect natural form of SrtA expression (). This finding implied that cyanidin chloride effectively inhibited SrtA activity, but did not alter its expression.
Figure 3. The expression of SrtA in the presence of cyanidin chloride and between cyanidin chloride and StrA by fluorescence quenching assay. (a) Expression of SrtA in the S. aureus USA300 with different concentrations of cyanidin chloride by western blotting. (b) Binding affinity between cyanidin chloride and SrtA. The KA of cyanidin chloride and SrtA was calculated by plotting the Stern-Volmer SrtA quenching. (c) Molecular docking illustrated that the binding mode of cyanidin chloride in SrtA binding pocket. (d). WT-SrtA and SrtA mutants (S116A-SrtA, G167A-SrtA, R197A-SrtA) were incubated with 64 μg/mL cyanidin chloride, and the transepiptase activity of the recombinant SrtA was determined by FRET. *** P < 0.001 were calculated using one-way ANOVA.
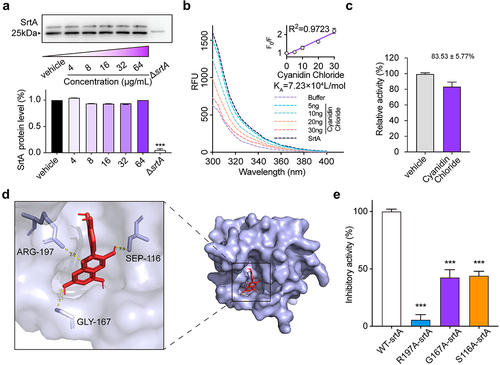
Determination of the binding of cyanidin chloride to SrtA
The binding affinity between cyanidin chloride and SrtA was subsequently assessed. As the concentration of cyanidin chloride increased, the fluorescence of SrtA was gradually quenched, and a linear dependence of F0/F on the quencher concentration was obtained (, inset). We further constructed a Stern-Volmer plot of SrtA quenching. The calculated binding constant KA of SrtA to cyanidin chloride was 7.23 × 104 L/mol, indicating a significant interaction between cyanidin chloride and SrtA.
Furthermore, the binding sites of cyanidin chloride and SrtA were simulated in vitro by molecular docking. Detailed analysis showed that the side chains of residues AGR-197 (bond lengths: 2.9 Å), SEP-116 (bond length: 2.3 Å) and GLY-167 (bond length: 3.1 Å) of SrtA formed three essential hydrogen bonds with the heteroatoms of cyanidin chloride. All these interactions contributed to the docking of cyanidin chloride with the SrtA binding site ().
Subsequently, we performed point mutagenesis of the major amino acid sites (AGR-197, SEP-116, GLY-167) and obtained purified SrtA mutant proteins. FRET assay was used to evaluate the transpeptidase activity of cyanidin chloride on SrtA mutant proteins (S116A-SrtA, G167A-SrtA), which showed a significantly reduced transpeptidase inhibitory capacity for SrtA mutant proteins () (P < 0.001). Furthermore, when the R197 site was mutated, nearly complete loss of its transpeptidase activity has been observed, consistent with previous reports. In conclusion, molecular docking and point mutation confirmed that AGR-197, SEP-116, GLY-167 were essential amino acid sites for SrtA binding to cyanogen chloride.
Cyanidin chloride protects mice from MRSA-induced pneumonia
Bacterial infectious pneumonia caused by MRSA is a common infectious disease in communities and hospitals. It has been a complex problem in clinical management due to its complexity, high complication rate, and high mortality rate [Citation31]. Therefore, cyanidin chloride was further evaluated for the treatment of MRSA-induced pneumonia in mice. The survival rate of mice in the infected group at 96 h was only 10%, while the survival rate of the ΔsrtA group was 100%. These results indicated a key role for SrtA in the acute pneumonia model of S. aureus infection. After treatment with 80 mg/kg cyanidin chloride, the survival rate of mice within 96 h increased from 10% to 50% ().
Figure 4. Cyanidin chloride alleviated the infection of the S. aureus-induced pneumonia in mice. (a) Effect of cyanidin chloride on survival of mice (n=10) with S. aureus USA300 pneumonia was recorded in 12 h intervals for 4 days. The statistical significance was determined with the log-rank test (*** P < 0.001). (b) the bacterial load of the S. aureus USA300 in the mice lung treated with or without cyanidin chloride. The limit of lung bacterial load detection was 1×103. the statistical significance was determined with the Mann–Whitney test (***P < 0.001). (c) H&E staining and histopathology analysis of the mice lung tissues with or without treatment of cyanidin chloride (80 mg/kg).
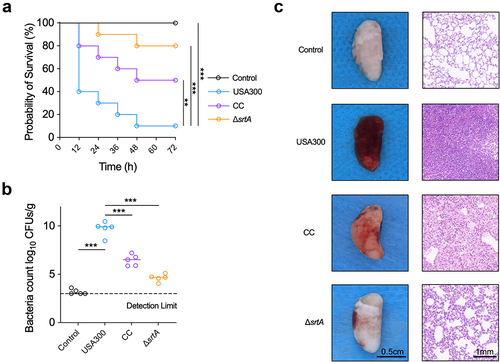
Subsequently, the number of bacteria in the lungs of each group of mice was further assessed. The lung bacterial load in the infected group was 9.91 ± 1.01 log CFU/g (). A significant reduction in bacterial load to 6.77 ± 0.24 log CFU/g after cyanidin chloride treatment reduced the invasion of S. aureus, thereby alleviating the lung damage. The appearance and pathological changes in the lungs of each group were further observed. The appearance of lungs from mice in the control group (uninfected) was light pink and soft, while the lungs infected with S. aureus USA300 showed obvious hyperaemia, were dark red and exhibited low elasticity. The degree of redness and swelling was significantly reduced in the lungs of mice in the cyanidin chloride treatment group (, left panel). In addition, the histopathological examination showed that the lung tissue of the WT group was significantly congested, a large number of inflammatory cells accumulated in the alveoli, inflammatory cell infiltration was significantly reduced after cyanidin chloride treatment, and the alveolar structure was relatively complete (, right panel).
In conclusion, cyanidin chloride reduced the virulence of S. aureus in vivo and provided significant protection against pneumonia caused by S. aureus.
Discussion
Researchers have long struggled to identify novel antibacterial targets. Intervention rather than direct sterilization has proven to be effective. The primary purpose of these approaches is to limit the pathogenesis by modifying or enhancing the host response and inhibiting the bacterial virulence mechanism [Citation38–40]. Virulence-specific therapy may also avoid unnecessary changes in the host microbiota. This approach avoids the limitation that the use of traditional antibiotics may damage beneficial bacteria and affect the balance. A range of virulence factors secreted by S. aureus are associated with a variety of diseases [Citation41]. SrtA mediates the secretion of these adhesions by S. aureus. Therefore, SrtA is considered an ideal target for designing new anti-infective agents, disturbing bacterial virulence, but it will not interfere with bacterial vitality like antibiotics [Citation42].
Here, we found that the natural product cyanidin chloride present in many plants significantly inhibits the activity of SrtA with an IC50 of 21.91 μg/mL. Cyanidin chloride has no antibacterial effect and does not affect the growth of S. aureus USA300 and HepG2 cells at the concentration required to inhibit SrtA. The safety of cyanidin chloride is consistent with the characteristics of an ideal anti-virulence agent, namely, to maintain the pathogen under low selective pressure and avoid the generation of resistant strains.
In addition, fibronectin-binding proteins are proteins expressed by S. aureus containing the amino acid sequence LPXTG. Fibronectin (Fn) is considered an intermediary between bacteria adhering to FnBPs and mammalian cell integrins, accelerating the process of bacterial invasion of the host [Citation43]. In our experiment, the adhesion of S. aureus to fibrinogen was inhibited by cyanidin chloride, thus reducing the invasion of host cells. This result was also confirmed in the A549 cell invasion experiment. In addition, bacteria in nature are predominantly observed as surface-attached, multicellular aggregates called biofilms, relating to bacterial infectious diseases in humans (60%−80%) [Citation44,Citation45]. The surface proteins involved in biofilm formation, such as ClfA, ClfB, FnBPA, and FnBPB, are modulated by SrtA. Therefore, we speculate that the inhibitory effect of cyanidin chloride on biofilm formation may be achieved by inhibiting adhesion factor aggregation mediated by SrtA [Citation46]. In addition, we also observed that cyanidin chloride reduced the anchoring of the S. aureus surface protein SpA. In summary, cyanidin chloride impairs bacterial adhesion and invasion, making it an ideal agent against S. aureus infection, especially in the early stage of the infection process. Subsequently, fluorescence quenching and molecular docking analyses further confirmed the direct interaction between cyanidin chloride and SrtA. The results of the molecular docking prompted us to study point mutations. Fluorescence quenching experiments showed that S116A-SrtA, G167A-SrtA, and R197A-SrtA were the direct interaction sites between SrtA and cyanidin chloride.
SrtA inhibitors can be classified as covalent or non-covalent, where covalent bonding is formed by the reaction of the inhibitor with the cysteine active site [Citation47,Citation48]. The nature compound cyanidin chloride screened in this study is a reversible inhibitor of SrtA with a reversible inhibition rate of 83.53 ± 5.77%, which is not covalently bound to the active site of SrtA. The non-covalent inhibitor has a wide development prospect and has become the first choice of enzymatic inhibitors, mainly based on its low toxicity, recyclable, recoverable, and other advantages. Based on this characteristic, the high efficiency and low toxicity of cyanidin chloride suggested that the agent can be further studied as a leading compound of SrtA inhibitor.
MRSA has widely spread in hospitals worldwide, and it is one of the primary pathogens causing pneumonia. Therefore, we further evaluated the therapeutic effect of cyanidin chloride on MRSA-induced pneumonia in mice. The degree of lung tissue injury in MRSA-infected mice was significantly alleviated after cyanidin chloride treatment. Importantly, treatment reduced the number of colonies colonizing the lung tissue of infected mice, thus reducing the invasion of pathogenic bacteria into the lung tissue and protecting mice from MRSA-induced pneumonia.
In conclusion, cyanidin chloride possesses significant anti-SrtA activity in vivo and in vitro, and it has the potential to be developed as an anti-virulence agent with a minimal effect on normal microbiota and antibiotic resistance. In addition, SrtA exists in almost all gram-positive species with low GC% content [Citation49], suggesting that SrtA inhibitors can be used to fight a variety of gram-positive pathogen infections.
Data availability statements
The data that support the findings of this study are available from the corresponding author.
Ethics statement
The Experimental Animal Ethics Committee of Changchun University of Chinese Medicine were approved all animal experiments and surgical procedures conducted according to guidelines.
Supplemental Material
Download MS Word (20.9 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/21505594.2022.2112831.
Additional information
Funding
References
- Wertheim HF, Melles DC, Vos MC, et al. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect Dis. 2005;5(12):751–762. DOI:10.1016/S1473-3099(05)70295-4
- Becker K, Schaumburg F, Fegeler C, et al. Staphylococcus aureus from the German general population is highly diverse. Int J Med Microbiol. 2017;307(1):21–27. DOI:10.1016/j.ijmm.2016.11.007
- van Alen S, Ballhausen B, Peters G, et al. In the centre of an epidemic: Fifteen years of LA-MRSA CC398 at the University Hospital Münster. Vet Microbiol. 2017;200:19–24.
- Cosgrove SE, Qi Y, Kaye KS, et al. The impact of methicillin resistance in Staphylococcus aureus bacteremia on patient outcomes: mortality, length of stay, and hospital charges. Infect Control Hosp Epidemiol. 2005;26(2):166–174. DOI:10.1086/502522
- Islam MA, Parveen S, Rahman M, et al. Occurrence and characterization of methicillin resistant Staphylococcus aureus in processed raw foods and ready-to-eat foods in an urban setting of a developing country. Front Microbiol. 2019;10:503.
- Cai X, Zheng W, Li Z. High-Throughput screening strategies for the development of anti-virulence inhibitors against Staphylococcus aureus. Curr Med Chem. 2019;26(13):2297–2312.
- Lee ZW, Kim BS, Jang KK, et al. Small-Molecule inhibitor of HlyU attenuates virulence of Vibrio species. Sci Rep. 2019;9(1):4346. DOI:10.1038/s41598-019-39554-y
- Speziale P, Pietrocola G. Monoclonal antibodies targeting surface-exposed and secreted proteins from Staphylococci. Vaccines (Basel). 2021;9(5). DOI:10.3390/vaccines9050459
- Cascioferro S, Raffa D, Maggio B, et al. Sortase a inhibitors: recent advances and future perspectives. J Med Chem. 2015;58(23):9108–9123. DOI:10.1021/acs.jmedchem.5b00779
- Zhang J, Liu H, Zhu K, et al. Antiinfective therapy with a small molecule inhibitor of Staphylococcus aureus sortase. Proc Natl Acad Sci U S A. 2014;111(37):13517–13522. DOI:10.1073/pnas.1408601111
- Maresso AW, Schneewind O. Sortase as a target of anti-infective therapy. Pharmacol Rev. 2008;60(1):128–141.
- Mazmanian SK, Liu G, Jensen ER, et al. Staphylococcus aureus sortase mutants defective in the display of surface proteins and in the pathogenesis of animal infections. Proc Natl Acad Sci U S A. 2000;97(10):5510–5515. DOI:10.1073/pnas.080520697
- Bubeck Wardenburg J, Patel RJ, Schneewind O. Surface proteins and exotoxins are required for the pathogenesis of Staphylococcus aureus pneumonia. Infect Immun. 2007;75(2):1040–1044.
- Lee JC, Betley MJ, Hopkins CA, et al. Virulence studies, in mice, of transposon-induced mutants of Staphylococcus aureus differing in capsule size. J Infect Dis. 1987;156(5):741–750. DOI:10.1093/infdis/156.5.741
- Albus A, Arbeit RD, Lee JC. Virulence of Staphylococcus aureus mutants altered in type 5 capsule production. Infect Immun. 1991;59(3):1008–1014.
- Deng LJ, Qi M, Li N, et al. Natural products and their derivatives: Promising modulators of tumor immunotherapy. J Leukoc Biol. 2020;108(2):493–508. DOI:10.1002/JLB.3MR0320-444R
- Bode HB, Müller R The impact of bacterial genomics on natural product research. Angewandte Chemie. 2005;44(42):6828–6846.
- Rasool M, Malik A, Qureshi MS, et al. Recent updates in the treatment of neurodegenerative disorders using natural compounds. 2014;2014:979730. DOI:10.1155/2014/979730
- Zhang J, Wu C, Gao L, et al. Astragaloside IV derived from Astragalus membranaceus: A research review on the pharmacological effects. Adv Pharmacol. 2020;87:89–112.
- Vafadar A, Shabaninejad Z, Movahedpour A, et al. Quercetin and cancer: New insights into its therapeutic effects on ovarian cancer cells. Cell Biosci. 2020;10(1):1–17. DOI:10.1186/s13578-020-00397-0
- Fimognari C, Berti F, Nüsse M, et al. Induction of apoptosis in two human leukemia cell lines as well as differentiation in human promyelocytic cells by cyanidin-3-O-β-glucopyranoside. Biochem Pharmacol. 2004;67(11):2047–2056. DOI:10.1016/j.bcp.2004.02.021
- Jayaprakasam B, Vareed SK, Olson LK, et al. Insulin secretion by bioactive anthocyanins and anthocyanidins present in fruits. J Agric Food Chem. 2005;53(1):28–31.
- Zhang B, Wang X, Wang L, et al. Molecular mechanism of the flavonoid natural product Dryocrassin ABBA against Staphylococcus aureus Sortase A. Molecules. 2016;21(11):1428. DOI:10.3390/molecules21111428
- Arvidson S, Tegmark K. Regulation of virulence determinants in Staphylococcus aureus. Int J Med Microbiol. 2001;291(2):159–170.
- Labandeira-Rey M, Couzon F, Boisset S, et al. Staphylococcus aureus Panton-Valentine leukocidin causes necrotizing pneumonia. Science. 2007;315(5815):1130–1133. DOI:10.1126/science.1137165
- Brown EL, Dumitrescu O, Thomas D, et al. The Panton–Valentine leukocidin vaccine protects mice against lung and skin infections caused by Staphylococcus aureus USA300. Clin Microbiol Infect. 2009;15(2):156–164. DOI:10.1111/j.1469-0691.2008.02648.x
- Hussain M, Kohler C, Becker K. Role of SrtA in Pathogenicity of Staphylococcus lugdunensis. Microorganisms. 2020;8(12):1975.
- Maresso AW, Wu R, Kern JW, et al. Activation of inhibitors by sortase triggers irreversible modification of the active site. J Biol Chem. 2007;282(32):23129–23139. DOI:10.1074/jbc.M701857200
- Suree N, Liew CK, Villareal VA, et al. The structure of the Staphylococcus aureus sortase-substrate complex reveals how the universally conserved LPXTG sorting signal is recognized. J Biol Chem. 2009;284(36):24465–24477. DOI:10.1074/jbc.M109.022624
- Coan KE, Maltby DA, Burlingame AL, et al. Promiscuous aggregate-based inhibitors promote enzyme unfolding. J Med Chem. 2009;52(7):2067–2075. DOI:10.1021/jm801605r
- Rosenkranz KW, Rothenanger E, Brodard I, et al. Nasal carriage of methicillin-resistant Staphylococcus aureus (MRSA) among Swiss veterinary health care providers: Detection of livestock- and healthcare-associated clones. J Schweiz Arch Tierheilkd. 2014;156(7):317–325.
- McCourt J, O’Halloran DP, McCarthy H, et al. Fibronectin-Binding proteins are required for biofilm formation by community-associated methicillin-resistant Staphylococcus aureus strain LAC. FEMS Microbiol Lett. 2014;353(2):157–164. DOI:10.1111/1574-6968.12424
- Schneewind O, Missiakas D, Sandkvist M Sortases, surface proteins, and their roles in Staphylococcus aureus disease and vaccine development. Microbiol Spectr. 2019;7(1). DOI:10.1128/microbiolspec.PSIB-0004-2018
- Ofek I Hasty D L , Doyle R J . Bacterial adhesion to animal cells and tissues[M]. ASM press, 2003.
- Mazmanian SK, Ton-That H, Su K, et al. An iron-regulated sortase anchors a class of surface protein during Staphylococcus aureus pathogenesis. Proc Natl Acad Sci U S A. 2002;99(4):2293–2298. DOI:10.1073/pnas.032523999
- Falugi F, et al. Role of protein a in the evasion of host adaptive immune responses by Staphylococcus aureus. Mbio. 2013;4(5): e00575-13. doi:10.1128/mBio.00575-13.
- Forsgren A, Svedjelund A, Wigzell H. Lymphocyte stimulation by protein a of Staphylococcus aureus. Eur J Immunol. 1976;6(3):207–213.
- Spellberg B, Bartlett JG, Gilbert DN. The future of antibiotics and resistance. N Engl J Med. 2013;368(4):299–302.
- Nathan C Fresh approaches to anti-infective therapies. Sci Transl Med. 2012;4(140):140sr2.
- Park B, Liu GY. Targeting the host-pathogen interface for treatment of Staphylococcus aureus infection. Semin Immunopathol. 2012;34(2):299–315.
- Reddy PN, Srirama K, Dirisala VR. An update on clinical burden, diagnostic tools, and therapeutic options of Staphylococcus aureus. Infect Dis (Auckl). 2017;10:1179916117703999.
- Barlocco D, Meneghetti F. Special issue: frontiers in antimicrobial drug discovery and design. Molecules. 2017;22(7):1127.
- Roche FM, Massey R, Peacock SJ, et al. Characterization of novel LPXTG-containing proteins of Staphylococcus aureus identified from genome sequences. Microbiology (Reading). 2003;149(3):643–654. DOI:10.1099/mic.0.25996-0
- Joo HS, Otto M. Molecular basis of in vivo biofilm formation by bacterial pathogens. Chem Biol. 2012;19(12):1503–1513.
- Römling U, Balsalobre C. Biofilm infections, their resilience to therapy and innovative treatment strategies. J Intern Med. 2012;272(6):541–561.
- Graf AC, Leonard A, Schäuble M, et al. Virulence factors produced by Staphylococcus aureus biofilms have a moonlighting function contributing to biofilm integrity. Mol Cell Proteomics. 2019;18(6):1036–1053. DOI:10.1074/mcp.RA118.001120
- Wang L, Wang G, Qu H, et al. Taxifolin, an inhibitor of Sortase A, interferes with the adhesion of methicillin-resistant Staphylococcal aureus. Front Microbiol. 2021;12:1876.
- Wang L, Jing S, Qu H, et al. Orientin mediates protection against MRSA-induced pneumonia by inhibiting Sortase a. Virulence. 2021;12(1):2149–2161. DOI:10.1080/21505594.2021.1962138
- Paterson GK, Mitchell TJ. The biology of Gram-positive sortase enzymes. Trends Microbiol. 2004;12(2):89–95.
