ABSTRACT
The control of Ostrinia furnacalis, a major pest of maize in Xinjiang, is challenging owing to the occurrence of resistant individuals. Entomopathogenic fungi (EPF) are natural insect regulators used as substitutes for synthetic chemical insecticides. The fungus Aspergillus nomius is highly pathogenic to O. furnacalis; however, its virulence characteristics have not been identified. This study aimed to analyse the lethal efficacy, mode of infection on the cuticle, and extracellular enzyme activity of A. nomius against O. furnacalis. We found that the mortality and mycosis of O. furnacalis were dose-dependent when exposed to A. nomius and varied at different life stages. The egg-hatching and adult emergence rates decreased with an increase in conidial suspension. The highest mortality (83.33%, 7 d post-infection [DPI]) and mycosis (74.33%, 7 DPI) and the lowest mortality response (8.52 × 103 conidia mL−1) and median lethal time (4.91 d) occurred in the 3rd instar larvae of O. furnacalis. Scanning electron microscopy indicated that numerous conidia germination and infection structure formation may have contributed to the high pathogenicity of A. nomius against O. furnacalis. There were significant correlations between O. furnacalis mortality and the activities of extracellular protease, lipase, and chitinase of A. nomius. This study revealed the infection process of the highly pathogenic A. nomius against O. furnacalis, providing a theoretical basis and reference for strain improvement and field application of EPF.
Introduction
The Asian corn borer, Ostrinia furnacalis (Guenée) (Lepidoptera: Crambidae), particularly during the larval stage, is an important pest of maize (Zea mays L.) in China [Citation1,Citation2]. It poses a serious threat to maize production and causes substantial economic losses owing to direct damage and transmission of several plant pathogens, such as Fusarium spp. and Ustilago maydis [Citation3,Citation4]. Currently, the primary strategy for managing this insect pest in Xinjiang, China, is applying novel diamide insecticides to control O. furnacalis populations; however, this strategy is limited by the occurrence of resistant individuals [Citation2]. Moreover, insecticides have undesirable side effects; therefore, the development of biological control methods is more advantageous for preventing and controlling agricultural pests than insecticides [Citation5].
Entomopathogenic fungi (EPF) are an environmentally friendly alternative to chemical insecticides and are important natural regulators of pest insect populations [Citation6]. Among the several groups of biocontrol agents used against insect pests, the most promising mitosporic fungi include Beauveria bassiana, Metarhizium anisopliae, Aspergillus nomius, Lecanicillium lecanii, and Isaria fumosorosea [Citation2]. Of these, the genus Beauveria has been commercially mass-produced and successfully used to control O. furnacalis and related insects in greenhouses in China [Citation7–9]. However, increasing evidence shows that the virulence of EPF is often associated with their high genetic diversity, environmental and host adaptations, and the extent of their additional non-target effects [Citation10–13]. Therefore, the application of local fungal strains, instead of exotic strains, to control insects is a promising strategy [Citation2,Citation14], suggesting the effective use of local fungal strains for the biological control of insect pests. However, EPF virulence differs depending on the instar stages of the insect host [Citation13,Citation15,Citation16].
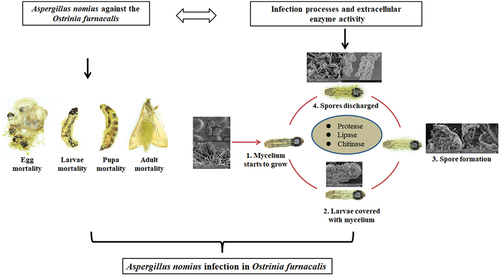
Unlike viruses and bacteria, EPF possesses the unique ability to infect their host directly through the integument [Citation17]; therefore, they are potent alternatives for insect management [Citation2]. The mode of action of EPF against host insects begins with the attachment of the conidia to the insect integument, followed by germination, formation of an infection structure, and subsequent penetration of the cuticle. The hemocoel is then colonized, and the insect is eventually killed. Sporulation occurs after the emergence of the fungus from a mycosed cadaver [Citation18]. Extracellular enzymes, including chitinase, protease, and lipase, which facilitate infection, are also produced by EPF when the insect’s integument is penetrated [Citation19]. Therefore, knowledge of the infection processes and extracellular enzyme activity of EPF is essential to determine its potential for biocontrol [Citation20–24]. Because EPF virulence can differ considerably according to the developmental stage of the pest [Citation25], it is essential to obtain knowledge on the effectiveness of EPF in all life stages of an insect to comprehensively evaluate its regulatory effect on insect pests [Citation26].
We have previously identified and isolated an EPF, A. nomius ACB1041, from a cadaver of O. furnacalis during a maise field investigation in Xinjiang from March-May 2019 and 2020 [Citation2]. Several studies have reported the potential of Aspergillus spp., viz. A. nomius, A. flavus, A. kanagawaensis, as a biocontrol agent to suppress pest populations, including those that threaten stored products and public health [Citation2,Citation17,Citation27–29], such as Dolichoderus thoracicus [Citation30], mosquitoes [Citation17], Diaphorina citris [Citation29], Spodoptera litura [Citation28,Citation31], Oligonychus coffeae [Citation32], Anopheles gambiae, and Culex quinquefasciatus [Citation33]. Although the insecticidal activity of some Aspergillus species may be attributed to the secretion of mycotoxins (e.g. aflatoxins, ochratoxins, fumonisins, zearalenone and various phenolic compounds viz. gallic acid, caffeic acid) [Citation34], the mycotoxins also showed its safety for mammals, as evidenced by negligible toxicity on rats [Citation28]. However, the mode of action of Aspergillus spp. against insects has not yet been reported. There is limited knowledge on the virulence of A. nomius in Xinjiang against the different instar stages of O. furnacalis. In addition, the mode of action and extracellular enzymes of A. nomius remain unknown, although its pathogenicity against insects, such as O. furnacalis, has been reported [Citation2,Citation19].
This study aimed to (i) evaluate the virulence of A. nomius against O. furnacalis at different life stages; (ii) use optical and scanning electron microscopy to observe the infection process on the O. furnacalis, which are highly susceptible to A. nomius; and (iii) study the potential of A. nomius to produce extracellular enzymes. Moreover, this study aimed to elucidate the mechanism involved in A. nomius infection and evaluate its efficacy in suppressing the O. furnacalis population. This highly pathogenic strain was eventually developed into an effective biopesticide for the eco-friendly management of O. furnacalis. Our findings provide a better understanding of the mode of action of A. nomius infection and reveal how A. nomius succeeds in infecting O. furnacalis.
Materials and methods
Fungus and culture conditions
For this study, A. nomius ACB1041 (registered as the benA and CaM sequence of A. nomius) was originally isolated from the cadavers of naturally infected O. furnacalis overwintering larvae [Citation2] and deposited in GenBank under Accession nos. OK625287 and OK625286. The fungal strain was also maintained at the China Center for Type Culture Collection (CCTCC), Wuhan, China, under the Accession number M 20,211,535 ACB1041.4. The strain was cultured on Sabouraud dextrose agar (SDA) and incubated at 26 °C for 15 d under a 16:8 h (light: dark) photoperiod. Conidia were harvested from 15-d-old SDA sporulated cultures [Citation2] and suspended in 10 mL 0.05% Tween 80 solution in universal bottles containing 6‒8 glass beads (3-mm diameter). To break the conidial clumps and ensure a homogeneous suspension, the suspension was vortexed for 5 min at approximately 700 rpm [Citation35].
Preparation of the conidial suspension
For the virulence bioassay, conidial suspensions were adjusted to eight strain concentrations ranging from 1.0 × 101 conidial mL−1 to 1.0 × 108 conidial mL−1 through dilution in a Neubauer chamber under a light microscope. Before the experiments, conidia viability tests were conducted according to the method of Akutse et al. [Citation27,Citation35].
Insect rearing
A laboratory colony of O. furnacalis was established using overwintering larvae collected from maize crops from Yining County in Yining City, Xinjiang Uygur Autonomous Region, China (81°52’ E, 43°98’ N) from January to March 2019.
The laboratory colony of O. furnacalis larvae was reared on a modified artificial diet [Citation36] until pupation under controlled conditions at 27 ± 1 °C, 70–80% relative humidity (RH), and under a 16:8 h (L:D) photoperiod [Citation2]. The pupae were placed in ventilated mating cages (25 × 25 × 40 cm with sulphate paper on the sides and top) until adult emergence. The newly emerged adults were paired, kept in a ventilated mating cage, and fed a 10% sugar solution [Citation2,Citation36]. Sulphate paper was removed 24 h after oviposition, and pupae were transferred to moistened plastic boxes until hatching, following the approach used by Guo et al. [Citation35,Citation37]. The newly hatched neonates to the 2nd instar larvae were reared in the same group and then placed in plastic containers (15 × 7 × 5 cm) lined with sterile moistened filter paper to absorb excess moisture. After moulting to the 3rd instar larvae stage, the larvae were individually fed in 12-well culture plates containing fresh modified artificial diet blocks. The larval instars were identified by their head width using a previously described method [Citation38].
Dose-response bioassay of A. nomius ACB1041 against different stages of life of O. furnacalis
Against different instar stages of O. furnacalis larvae
Before the dose-response bioassays, the different life stages of O. furnacalis (egg, larvae from the 1st to the 5th instar larvae, pupae, and adults) were identified within the colony described above. A dose-response bioassay of all O. furnacalis life stages was conducted. Similar-sized pieces (approximately 5 × 5 × 6 mm) of fresh but modified artificial food were prepared, and one piece was placed in each well of a 12-well cell culture plate. The O. furnacalis were transferred using a camel hairbrush onto the modified artificial diet piece in each well of the 12-well cell culture plates and allowed to settle. Individual larvae were placed in each separate well. Three cell culture plates represented a replicate (n = 36), and three were used for each conidial suspension concentration (n = 108 insects per treatment). The modified cut food and larvae within the cell culture plates were then sprayed with 3 mL spore suspension using a Potter spray tower PDE0012 (Burkard, Burkard Scientific, UK). Before spraying, a sterilised paper towel was placed under the food to absorb any excess fungal suspension. Treatments were applied from the lowest to the highest concentration to prevent cross-contamination. The spray tower was washed twice with 75% alcohol after each application and once with 0.05% (v/v) Tween 80 between the application of different concentrations. The control group was sprayed with 0.05% Tween 80. All experiments were repeated three times.
Treatment and control groups were placed separately in a chamber under controlled conditions (27 ± 1 °C, 70% ± 5% RH, 12 h light: dark photoperiod) [Citation2]. Mortality, mycosis, and larval development were monitored daily for 15 d until all individuals had died or a new O. furnacalis instar had emerged [Citation39]. Mycosis was confirmed based on the method used in our previous research [Citation2]. The mortality rate was corrected using the number of larvae that exhibited natural death/paralysis in the 0.5% Tween 80 control according to the Schneider – Orelli formula [Citation40].
Against O. furnacalis pupae
O. furnacalis pupae were placed in Petri dishes (90-mm diameter) (lined with sterilised moistened filter paper to absorb excess spore suspension) and then treated with 3 mL of spore suspension using a Burkard Potter. The inoculated pupae (30) were treated as one replicate, and three replicates were used for each conidial suspension concentration (n = 90 insects per treatment). The controls were treated with 0.05% Tween 80. After treatment, pupae were transferred into Petri dishes at 26 ± 1 °C and 70%–80% RH, and each treatment was replicated thrice. The emergence rate and mortality were monitored daily for 15 d until all individuals had died or adults had emerged, and mycosis was confirmed by the presence of hyphae and conidia on the surface of the cadaver using the method reported in our previous research [Citation2].
Against O. furnacalis adult
A total of 15 pairs of 2-d-old newly emerged adults were placed in a 200-mL beaker with a 10-mesh nylon sieve (2 mm) and then treated with 3 mL of spore suspension using a Burkard Potter. The controls were treated with 0.05% Tween 80 [Citation30]. The inoculated adults were then placed separately in mating cages (25 × 25 × 40 cm), and the moths were fed a 5% honey solution. The mating cages were maintained in a rearing room at 26 ± 1 °C and 70–80% RH. Mortality was monitored daily for 15 d until all individuals had died, and mycosis was confirmed based on the method used in our previous research [Citation2].
Against O. furnacalis eggs and neonates
Fresh sulphate paper containing similar-sized O. furnacalis eggs (24 h) was cut into pieces measuring approximately 0.5 × 0.5 cm, and each piece was placed in a Petri dish (90-mm diameter) lined with sterile moistened filter paper. The paper and eggs were treated with 3 mL of spore suspension using a Burkard Potter, and the controls were treated with 0.05% Tween 80. After treatment, the paper and eggs were air dried for 30 min on a clean bench (25 ± 2 °C) and then transferred into Petri dishes and incubated at 26 ± 1 °C and 70–80% RH. Each treatment was replicated three times. The hatched larvae and egg mortality numbers were recorded daily for 15 d until all individuals died or neonate larvae emerged.
The neonate larvae that emerged from the O. furnacalis eggs on each piece of treated sulphate paper were counted, fed with fresh modified artificial diet blocks (approximately 5 × 5 × 6 mm) in clear plastic containers (15 × 7 × 5 cm) lined with sterile moistened filter paper, incubated at 26 ± 1 °C, and monitored daily. Mortality of the neonate larvae due to fungal infection was recorded daily for 15 d post-emergence. Mycosis was also evaluated for dead neonate larvae, as described above, and mycosis was confirmed using the method reported in our previous research [Citation2]. All treatments were replicated three times.
Optical microscopy (OM) and scanning electron microscopy (SEM)
OM observations
A Nikon Eclipse 80i OM (Nikon, Nikon Instruments Inc., Tokyo, Japan) was used to capture photographs of the immersed different life stages of O. furnacalis and observe the morphological characteristics of O. furnacalis following inoculation with the A. nomius ACB1041 strain from days 1‒15 post-inoculation. In each studied time interval and different life stages, five O. furnacalis were selected randomly, representing five replicates used to observe the morphological characteristics of O. furnacalis by the A. nomius ACB1041 infection process.
SEM observations
To document the epidermal infection process of ACB1041 strains, specimens were collected at 4, 8, 12, 24, 48, 72, 96, 120, and 144 h post-inoculation and fixed with 2.5% glutaraldehyde solution (Bodi Chemical Co., Ltd., Tianjin, China) at 4 °C for 12 h. The specimens were then dehydrated for 10 min each in a series of ascending order prepared the serial dilutions of ethanol (30, 50, 70, 80, and 100%), were dipped in isoamyl acetate for 30 min, and dried using a critical-point drying apparatus using carbon dioxide (Eiko XD-1). Finally, the samples were sputter-coated with gold and observed under a VEGA3 tungsten SEM (TESCAN, Brno, Czech).
Estimation of extracellular enzyme activity of ACB1041 strain
Liquid culture for enzyme production of ACB1041 strain
To investigate the extracellular enzyme activity of the ACB1041 strain during the epidermal infection process of O. furnacalis, a liquid medium was prepared containing 0.02% KH2PO4, 0.01% CaCl2, 0.01% MgSO4, 0.02% Na2HPO4, 0.01% ZnCl2, 0.01% yeast extract (Merck & Co., Rahway, NJ, USA), and 5% (weight) of larval cuticle; this was then sterilised in an autoclave (LDZX-75KB) at 121 °C for 30 min. Before preparing the liquid medium, the cuticles of O. furnacalis were obtained according to Velavan et al. [Citation24,Citation29]. The liquid medium was then inoculated in a flask with an ACB1041 isolate concentration of 3 mL of 108 conidia/mL, repeated three times. The flask was maintained on a 125 rpm cryogenic shaker (MAXQ5000) at 25 ± 1 °C. Subsequently, 1 mL was harvested from the culture flask daily for 15 d, placed in a 1.5-mL EP tube, and centrifuged (Eppendorf-5804 R) at 4 °C and 12,000 rpm for 15 min to obtain the supernatant for the enzyme assay.
Extracellular enzyme activities
Lipase, chitinase, and protease activities were determined based on the manufacturer’s instructions (Grace Biotechnology Co., Ltd., Suzhou, China). The lipase enzyme reaction mixture comprised 10 μL of fresh enzyme sample supernatant, 40 μL of Reagent I, and 150 μL of Reagent II placed in a 1.5-mL EP tube (with six replicates). After homogenization, a 200 μL mixture was added to 96-well cell culture plates (200 μL per well cell) (Grace Biotechnology Co., Ltd., Suzhou, China). Absorbance was recorded at 405 nm (A1) and then after 10 min at 405 nm (A2), where ΔA = A2 − A1.
The chitinase enzyme reaction mixture of the test group within each 1.5-mL EP tube comprised 80 μL of fresh enzyme sample supernatant, 80 μL of Reagent I, and 100 μL of Reagent II. After homogenization, the mixture was incubated for 90 min before centrifugation at 4,000 rpm and 4 °C for 5 min. Subsequently, 150 μL of mixture supernatant was obtained, and 10 μL of Reagent III and 15 μL of Reagent Ⅳ were added to the supernatant in a new 1.5-mL EP tube. This was then homogenized, incubated for 60 min at 37 °C, and 50 μL of Reagent Ⅴ was added to the 1.5-mL EP tube. The mixture was homogenized and centrifuged at 4,000 rpm and 4 °C for 5 min to obtain 150 μL of supernatant. The 150 μL supernatant of the mixture was added to a new 1.5-mL EP tube with 200 μL of Reagent Ⅵ, homogenized, and then incubated for 10 min at 95–100 °C. Finally, 200 μL of the mixture was added to 96-well cell culture plates at 200 μL per well cell.
The composition of the chitinase enzyme reaction mixture of the control group was similar to that of the test group, except that 80 μL of fresh enzyme sample supernatant was first incubated for 1 min at 100 °C before adding Reagents I and II to the 1.5-mL EP tube. The method used was then identical to that of the test group and followed the manufacturer’s instructions. The absorbance of both groups was recorded as A control group and A test group at 420 nm, and ΔA= Acontrol group - Atest group.
The protease enzyme reaction mixture of the test group comprised 500 μL of fresh enzyme sample supernatant, 500 μL of Reagent I, and 500 μL of Reagent III in a 1.5-mL EP tube. After homogenization and incubation for 10 min, the mixture was centrifuged at 1,500 rpm and 4 °C for 10 min to obtain 250 μL of the supernatant of the mixture. This supernatant was added to a new 1.5-mL EP tube with 375 μL of Reagent Ⅳ and 250 μL Reagent Ⅴ, and the mixture was incubated for 20 min at 40 °C. Subsequently, 200 μL of the supernatant mixture was added to 96-well cell culture plates at 200 μL per well cell. Again, the procedure for the control group was similar to that of the test group and followed the manufacturer’s instructions; however, the remaining Reagent I was first incubated for 10 min at 40 °C before being added along with Reagents I and III to the 1.5-mL control EP tube. Finally, absorbance was recorded as A test group and A control group at 680 nm, and ΔA= Atest group - Acontrol group. All bioassays described above were repeated six times.
Statistical analysis
The effect of the spore concentration of A. nomius ACB1041 on different life stages of O. furnacalis was compared using the “chi-squared test” functions in the “contingency table analyses” program of Graph Pad Prism 8.0. A survival analysis was conducted using the “survival curve” functions in the “survival analysis” program of Graph Pad Prism 8.0. The median lethal concentration (LC50) and median lethal time (LT50) were determined by conducting a probit analysis using IBM SPSS Statistics software (version 20.0.). The data of LC50 and LT50 values were subjected to analysis of variance (ANOVA), and differences among treatments were compared using the Tukey’s test (P > 0.05) to determine significance using IBM SPSS Statistics software (version 20.0.). The relationships between mortality and concentration and extracellular enzyme activities were analysed using the “linear regression” and “Fit spline/LOWESS” functions, respectively, in the “XY analyses” program of Graph Pad Prism 8.0. The results were considered statistically significant when p < 0.05. Data were analysed using IBM SPSS Statistics software (version 20.0.), and figures were constructed using Graph Pad Prism 8.0 (Graph Pad Prism).
Results
Virulence bioassay of A. nomius against different life stages of O. furnacalis
O. furnacalis eggs were successfully infected with A. nomius ACB1041 at all spore concentrations; a significant effect was observed between the concentration and ovicidal response of the eggs (χ2 = 162.8, df = 7, p = 0.0150). The A. nomius concentration invoked a strong and increasing ovicidal response (15.56%, hatching rate) in 108 conidia/mL, followed by 106 conidia/mL (32.22%) and 104 conidia/mL (57.78%); in contrast, 102 conidia/mL provoked a weak response (65.56%) (). Infection with A. nomius was confirmed when the fungus sporulated, and the eggs did not hatch ().
Figure 1. Effect of O. furnacalis eggs on the hatching rate and the survival, mortality, and mycosis of the 1st instar larvae of O. furnacalis (n = 90) following spray infection with different A. nomius concentrations. (a) hatching rate of O. furnacalis eggs at 7 d post-infection (DPI) with A. nomius; (b and c) healthy eggs and mycosis of eggs; (d) survival probability of the 1st instar larvae of O. furnacalis; (e) mortality and mycosis of the 1st instar larvae of O. furnacalis infected withA. nomius; (f) A. nomiusmycosis of the 1st instar larvae of O. furnacalis; (g and h) log-probit regression line of concentration-mortality and mycosis of the 1st instar larvae of O. furnacalisto A. nomius.
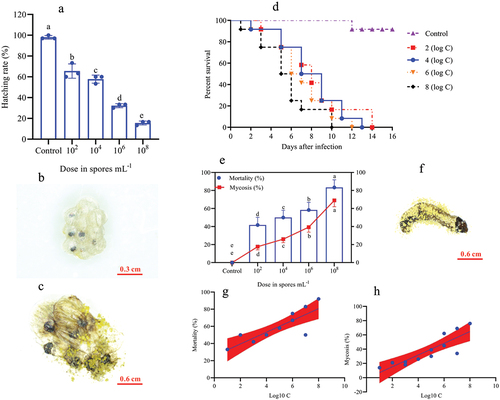
In the larval stage, the mortality and mycosis frequency increase at different spore concentrations was dose-dependent. The survival probability test indicated that the survival rates of O. furnacalis at the 1st (, Log-rank test: χ2 = 31.13, df = 7, p = 0.0001), 2nd (, Log-rank test: χ2 = 30.59, df = 7, p = 0.0001), 3rd (, Log-rank test: χ2 = 32.04, df = 7, p = 0.0001), 4th (, Log-rank test: χ2 = 17.64, df = 7, p = 0.0015), and 5th (, Log-rank test: χ2 = 16.81, df = 7, p = 0.0021) instar larvae were significantly lower when exposed to higher A. nomius concentrations. Furthermore, O. furnacalis mortality occurred earlier when exposed to higher concentrations () and increased with an increase in spore concentration (). All 1st, 2nd, and 3rd O. furnacalis instar larvae had died by 11 d post-infection (DPI) (Figs. ), whereas the survival rates of 4th and 5th instar larvae were 33.33% () and 36.36% () at 1.0 × 108 conidia mL−1, respectively. Similarly, mycosis also increased with increased spore concentration (). Furthermore, the mortality and mycosis of 1st (, F = 24.37, p = 0.0006, R2 = 0.7091; , F = 28.60, p = 0.0003, R2 = 0.7409), 2nd (, F = 11.31, p = 0.0072, R2 = 0.5308; , F = 22.66, p = 0.0008, R2 = 0.6930), 3rd (, F = 28.57, p = 0.0003, R2 = 0.7407; , F = 37.01, p = 0.0001, R2 = 0.7873), 4th (, F = 7.555, p = 0.0205, R2 = 0.4304; , F = 14.24, p = 0.0036, R2 = 0.5875), and 5th (, F = 27.26, p = 0.0004, R2 = 0.7316 and , F = 24.44, p = 0.0003, R2 = 0.7399) instar larvae were positively correlated with the spore concentrations of A. nomius.
Figure 2. Survival, mortality, and mycosis of the 2nd and 3rd instar larvae of O. furnacalis (n = 90) following spray infection with different concentrations of A. nomius. (a and e) survival probability of the (a) 2nd and (e) 3rd instar larvae of O. furnacalis; (b and g) mortality and mycosis of the (b) 2nd and (g) 3rd instar larvae of O. furnacalis infected with A. nomius; (c and h) A. nomius mycosis on the (c) 2nd and (h) 3rd instar larvae of O. furnacalis; (d, e, i, and j) log-probit regression line of (d and i) concentration-mortality and (e and j) mycosis response of the 2nd and 3rd instar larvae of O. furnacalis to A. nomius.
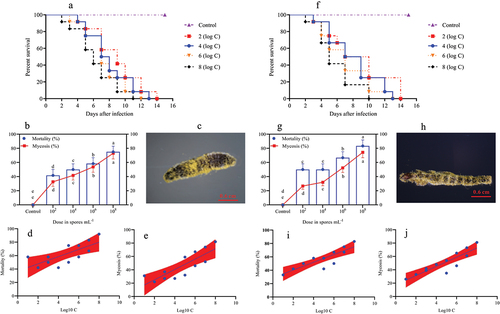
Figure 3. Survival, mortality, and mycosis of the 4th and 5th instar larvae of O. furnacalis (n = 90) following spray infection with different A. nomius concentrations. (a and f) survival probability of the (a) 4th and (f) 5th instar larvae of O. furnacalis; (b and g) mortality and mycosis of the (b) 4th and (g) 5th instar larvae of O. furnacalis infected with A. nomius; (c and h) A. nomius mycosis of the (c) 4th and (h) 5th instar larvae of O. furnacalis; (d, e, i, and j) log-probit regression line of (d and i) concentration-mortality and (e and j) mycosis response of 4th and 5th instar larvae of O. furnacalis to A. nomius.
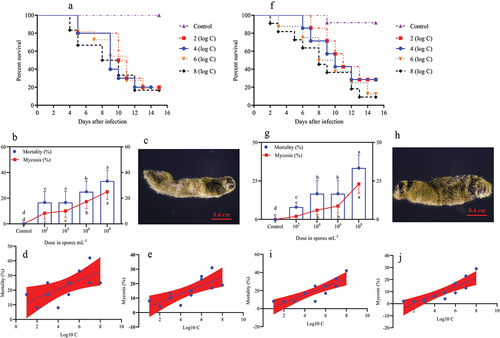
Figure 4. Survival, mortality, and mycosis of pupae and adults of O. furnacalis (n = 90) following spray infection with different A. nomius concentrations. (a and f) survival probability of O. furnacalis (a) pupae and (f) adults; (b and g) mortality and mycosis of O. furnacalis (b) pupae and (g) adults infected with A. nomius; (c and h) mycosis of A. nomius on O. furnacalis (c) pupae and (h) adults; (d, e, i, and j) log-probit regression line of (d and i) concentration-mortality and (e and j) mycosis response of O. furnacalispupae and adult to A. nomius.
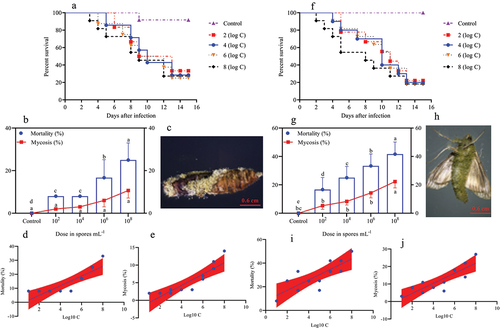
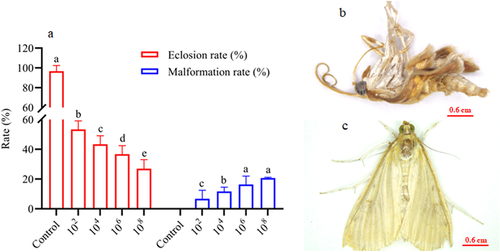
In the pupae and adult stages, a similar trend of dose-dependent spore concentration was observed between A. nomius and mortality and mycosis (). The mortality of pupae (; Log-rank test: χ2 = 10.96, df = 7, p = 0.0270) and adult O. furnacalis (; Log-rank test: χ2 = 17.57, df = 7, p = 0.0002) occurred earlier when exposed to higher concentrations, and a positive relation was observed between spore concentrations and mortality () and mycosis () of pupae and adults. Notably, several adults that emerged from the pupae infected with A. nomius were malformed (), and the malformation rate increased with an increase in the spore concentration ().
Figure 5. Effect of infection with A. nomius on the rate of pupa eclosion and pupa development of O. furnacalis.(a) pupa eclosion rate of O. furnacalis and malformed adults ofO. furnacalis emerged in a group of pupae infected with different A. nomius concentrations; (b) malformed O. furnacalis adults; (c) healthy O. furnacalis adults.
Aspergillus nomius infection was observed in all O. furnacalis life stages, and the maximum mortality and mycosis of early-stage larvae (including 1st, 2nd, and 3rd instar larvae) exceeded 70% at 7 DPI. However, the late-stage larvae (including 4th and 5th instar larvae), pupae, and adults showed lower mortality and mycosis of less than 50% at 7 DPI, indicating that the early-stage larvae are more susceptible to A. nomius. Therefore, we selected early-stage O. furnacalis larvae for subsequent experiments on the pathogenicity of A. nomius toward O. furnacalis and determining the LC50 and LT50 values.
LC50 and LT50 values of A. nomius
Significant differences in the LC50 and LT50 values of A. nomius across the different O. furnacalis life stages were identified based on dose and time mortality response studies (). The LC50 values of A. nomius against O. furnacalis larvae from the 1st to 5th instar larvae, pupae, and adults were 1.42 × 105, 2.17 × 105, 8.52 × 103, 2.30 × 108, 2.19 × 108, 8.20 × 107, and 2.00 × 105 conidia/mL, respectively. The LT50 values of A. nomius at 1.0 × 108 spores/mL and 1.0 × 106 spores/mL of the corresponding developmental stages were 3.90, 4.42, 4.91, 7.23, 7.16, 8.88, 6.35 d, and 5.72, 5.47, 6.05, 9.29, 11.16, 11.91, 8.83 d, respectively.
Table 1. LC50 values (conidia/mL) for A. nomius against different life-cycle stages of O. furnacalis..
Table 2. LT50 values (days) of A. nomius against different life-cycle stages of O. furnacalis..
Ultramicroscopic changes during A. nomius infection in O. furnacalis
The O. furnacalis larvae mortality and mycosis rates and LC50 and LT50 ranking results showed that the 3rd instar larvae of O. furnacalis were highly susceptible to infections with A. nomius. Therefore, the 3rd instar larvae were selected for SEM analysis to determine how A. nomius successfully infected O. furnacalis.
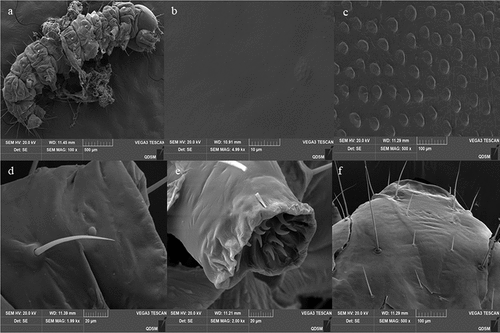
Surface topography analysis classified the O. furnacalis larval cuticle as having both a gentle and strumae surface topography (). The strumae surface topography was covered with densely regular protuberances, including those in the central segments and on sites beside the setae and proleg (). The gentle surface topography was flat and smooth with no protuberances (), and the areas included the head capsule (), setal alveolus (), and proleg ().
Figure 6. Cuticle topography of the 3rd instar larvaeofO. furnacalis. (a) full view of a larva showing strumae and gentle surface topography; (b) gentle surface topography; (c) strumae surface topography; (d and e) sites beside the setae and proleg; (f) head capsule.
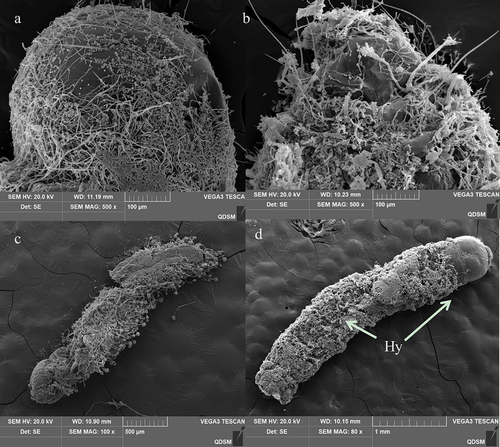
The infection process began with the attachment of A. nomius conidia to the larval cuticle of the 3rd instar larvae O. furnacalis (). SEM analysis of infected insects showed that the conidia of A. nomius were firmly attached to the surface topography of both types of larval cuticle on the 3rd instar larvae of O. furnacalis, including surfaces containing protrusions (), thoracic foot (), head capsule () and setal alveolus ().
Figure 7. Conidial attachment and germination of A. nomius on the cuticle of the 3rd instar larvae of O. furnacalis observed under a scanning electron microscope. (a) conidium (Co) attached to the setal alveolus and the germination of a few conidia with formed germ tube (gt) within 8 h and appressorium (ap) at 24 h post-inoculation; seta = se. (b) appressorium (ap) and penetration pegs (pp) formed at 36 h post-inoculation. (c and d) numerous conidia germinated to form a germ tube (gt), conidial germination rates increased, and mycelium formed on the host surface within 36 h; penetration pegs = pp, hyphae = hy.
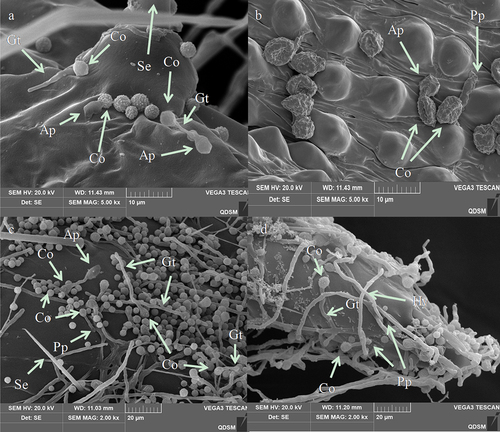
Morphogenetic events of A. nomius were easily observed near the setal alveolus (), surfaces containing protrusions (), sites beside the setae (), and thoracic foot cuticle of the larvae (). Differentiation of conidia into a germ tube, which is a sign of A. nomius conidia germination, occurred within 8 h following inoculation (). Two types of specialized infection structures, including appressoria and penetration pegs, were produced during germination (). The germ tubes from the conidia () and the penetration pegs and appressoria from the germ tubes (arrowhead in ) were observed near the setae and on surfaces containing protrusions and the thoracic foot. Appressoria and penetration pegs were formed at the end of the germ tubes, most of which were covered by a thin amorphous mucilage layer that firmly adhered the appressoria and penetration pegs to the O. furnacalis integument and then penetrated through the cuticle of the 3rd instar larvae of O. furnacalis (). Within 36 h, conidial germination rates increased. Most germ tubes and mycelium had formed on the surfaces of the 3rd instar larvae of O. furnacalis ().
Figure 8. Penetration and conidia development and secondary conidiogenous structure of A. nomiuson the cuticle surface of the 3rd instar larvae ofO. furnacalis. (a) several hyphae penetrating the body wall from the setal alveolus outward from the body at 60 h; hyphae = hy. (b) hyphae of A. nomiusdeveloped, branched, and formed a dense mycelial mass on the cuticle 72 h after inoculation; hyphae = hy. (c and d) secondary conidiogenous structure formation of A. nomius84 h after inoculation; phialides = Ph, conidiophores = cp, vesicle = ve.

Hyphae extrusion from sites near the protrusions on the larval cuticle was observed at 60 h following inoculation (). Extensive mycelial networking and a secondary conidiogenous A. nomius structure were noted on the cadaver 84 h after inoculation (). After 120 h after inoculation, A. nomius had colonized the entire cadaver. The cadaver exhibited a mycoses appearance (). Extensive mycelial networking and sporulation were observed on the head region () and abdomen (). Weakly sclerotized intersegmental regions were colonized by the mycelium, which was also extruded from the surface of the cadaver (). The conidia of A. nomius were carried on vesicles () and conidiophores (), and conidiophores were observed throughout the cadaver.
Figure 9. Scanning electron representative microscopy images of the 3rd instar larvae of O. furnacalis infected with A. nomius(120 h post-infection). (a) head capsule with abundant conidia and mycelial growth. (b) Extensive growth of mycelia in the abdomen. (c and d) full view of a larva showing complete mycelial networking.
Extracellular enzyme assay and its relationship with A. nomius virulence
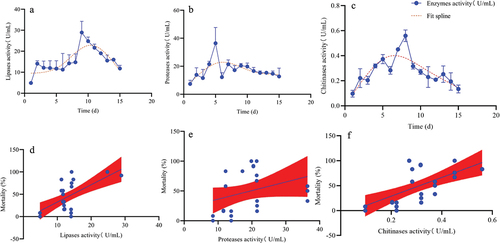
The lipase, protease, and chitinase activities of A. nomius gradually increased, peaked, and decreased slowly over the culture duration (). The maximum lipase activity (28.92 U/mL) occurred on day 9 (), while those of protease (21.42 U/mL) and chitinase (0.56 U/mL) were observed on days 5 ( (), respectively. Furthermore, minimum lipase (4.87 U/mL), protease (8.49 U/mL), and chitinase (0.10 U/mL) activities were observed on day 1 (). A strong correlation between lipase activity and the mortality rate of the 3rd instar larvae of O. furnacalis was noted (F = 21.14, P = 0.0001, R2 = 0.8451), and a good correlation among protease and chitinase activity with mortality (F = 11.81, P = 0.03, R2 = 0.5008, F = 18.48, P = 0.05, R2 = 0.4122) was observed.
Figure 10. Activities of extracellular enzymes and their correlation with the virulence of A. nomius. (a) lipase activity, (b) protease activity, (c) chitinase activity, (d) relationship between the mortality rate of the 3rd instar larvae of O. furnacalis and lipase activity, (e) relationship between the mortality rate of the 3rd instar larvae of O. furnacalis and protease activity, (f) relationship between the mortality rate of 3rd instar larvae of O. furnacalis and chitinase activity.
Discussion
Although there are several commercial mycoinsecticides, it is important to investigate the use of native EPF because native isolates ensure virulence, are environmentally adapted, and provide the least non-targeted effects when used on a field scale [Citation13]. Numerous studies have been conducted on the virulence of EPF; however, these studies have focused predominantly on certain life stages of an insect species [Citation41–44], although the potential of EPF to infect any life stage of an insect has been confirmed [Citation44]. The pathogenicity of EPF depends on the insect species, larval stage, and EPF strain owing to differences in the physical structure, chemical composition, and structure of the microbial community of the cuticle of each insect species [Citation42,Citation44,Citation45]. As the response to insect EPF stress differs throughout their life stages [Citation44], it is necessary to explore the mechanisms of action of indigenous and virulent EPF strains against different insect life stages to develop effective mycoinsecticides [Citation46]. Thus, this study was designed to assess the efficacy of A. nomius against O. furnacalis at different life stages to develop an efficient fungal biopesticide to combat this maize pest in China.
The key features of a highly pathogenic EPF strain are high mortality and mycosis and low LC50 and LT50 values [Citation2,Citation39,Citation47]. We compared the virulence of the A. nomius ACB1041 strain in O. furnacalis at different life stages and revealed that A. nomius had a significant ovicidal response in O. furnacalis eggs. Our results are consistent with those previous studies reporting that M. anisopliae and B. bassiana isolates significantly induced the mortality of Spodoptera frugiperda [Citation30,Citation35,Citation44] and Phthorimaea operculella eggs [Citation48]. In our study, for the larval stage, A. nomius provided a dose-dependent effect against O. furnacalis, and higher mortality and mycosis and lower LC50 and LT50 values were recorded in the younger larvae of O. furnacalis (1st, 2nd, and 3rd instar larvae); in contrast, comparatively lower mortality and mycosis and higher LC50 and LT50 values were observed in older larvae (4th and 5th instar larval), pupae, and adults of O. furnacalis. These results, for the first time, indicate that early instar larvae (1st, 2nd, and 3rd instar larvae) of O. furnacalis are highly susceptible to A. nomius; however, 3rd instar larvae were more highly susceptible to A. nomius, which may have been attributed to differences in the physical structure and chemical composition of cuticles of O. furnacalis at different life stages, as well as its nutrition [Citation29,Citation39,Citation44–46]. Notably, some adults that emerged from pupae infected with A. nomius were malformed, and the frequency of malformation was dose-dependent and increased with the spore concentration dose. This result is consistent with a previous study reporting a similar effect when I. fumosorosea was applied to Spodoptera littoralis (Boisd.) and Cydalima perspectalis [Citation56].
This study showed that A. nomius isolates could infect O. furnacalis throughout its various life stages, indicating its potential use as a biological control for these pests. Although the pathogenicity of the Aspergillus genus to infect insects has been reported previously [Citation2,Citation15,Citation17,Citation39,Citation49], the insecticidal activity of some Aspergillus species may be attributed to various secondary metabolites it contains [Citation28]. In detail, Kaur et al. [Citation50] detected the presence of various phenolic compounds, including gallic acid, caffeic acid, quercetin and kaempferol, by UHPLC in A. flavus extract. Thus, the mycoses individuals in this study may represent secondary infections of dead animals killed by secondary metabolites or toxins. At present, whether the strain ACB1041 produces mycotoxins remains unclear; thus, further studies are required to clarify this. In addition, including mortality data from ingested conidia would be useful; therefore, exploring this topic in the future for reflecting a likely transmission route in the field was also necessary. Moreover, because the 3rd instar larvae of O. furnacalis showed the highest susceptibility to A. nomius, we selected them for subsequent experiments investigating the mode of action of A. nomius using SEM analysis. Therefore, more studies are required to explore the mechanisms underlying the highest pathogenicity of A. nomius against the 3rd instar larvae of O. furnacalis.
Entomopathogenic fungi attachment and germination to the host epidermis is the first and most critical step in the infection process [Citation24]. In our present study, extensive conidia of A. nomius adhered to surfaces containing protrusions, the thoracic foot, the head capsule, and the setal alveolus of the 3rd instar larvae of O. furnacalis. Furthermore, A. nomius produced infection structures, including appressoria and penetration pegs, and hyphae grew during the initial phases of infection. Successful germination of fungal conidia in the host cuticle is necessary for infection [Citation51]; these results indicated that a high conidial attachment to the larvae cuticle had occurred. These structures are important for the direct penetration of EPF into the host’s integument [Citation52]. In addition, the key stages of host EPF infection include adhesion, germination, penetration, colonization of the hemocoel, and conidiogenesis [Citation51]. This study preliminarily observed the infection symptoms, germination, attachment, penetration, and conidial sporulation of A. nomius. However, future research should be conducted to demonstrate the mechanism by which the fungus penetration and colonization of the hemocoel kill O. furnacalis and characterize the physiological interactions between fungi and insects as well as the comparative study of the similarities and differences between the 3rd instar larvae infected by A. nomius and other stages of O. furnacalis.
The conidia of several EPF do not attach to the head capsule of lepidopterans larvae because it is both sclerous and smooth [Citation24]. In this study, the conidia and hyphae of A. nomius were evident around the head capsule of the 3rd instar larvae of O. furnacalis, and they produced infection structures when the germ tube contacted a hard surface, which may explain the higher virulence of A. nomius against the 3rd instar larvae of O. furnacalis. The cuticles of some insects contain antifungal compounds that repress germination or the further development of EPF spores, ultimately leading to infection failure [Citation39,Citation53]. This phenomenon could occur in the later life-cycle stages of O. furnacalis. Nevertheless, further research is required to explore the mechanisms underlying the lower pathogenicity of A. nomius in the later life-cycle stages of O. furnacalis.
The insecticidal mechanism underlying the pathogenicity of EPF relates to the presence of cuticle-degrading enzymes and extracellular enzymes, which are the virulence factors of many EPFs [Citation22,Citation54]. Our results revealed differences in the activities of extracellular enzymes with an extension in the incubation time. The highest proteases, chitinase, and lipase activity were recorded on days 5, 8, and 9, respectively. The higher protease activity indicated that A. nomius is capable of protein digestion in the initial stages of infection, and protease activity may then ensure the success of chitinase and lipase to feasibly penetrate the O. furnacalis cuticle. A similar observation was reported, in which proteases were produced during invasion before chitinases, which are important in cuticle infiltration [Citation22]. Furthermore, in this study, A. nomius showed a high lipase activity, and lipases may be important for integument lipids in EPF development. Similar results have been reported in B. bassiana that infects Chilo suppressalis Walker [Citation13]. In this study, the activities of proteases, chitinases and lipase were highly correlated with mortality, which is consistent with the results of previous studies reporting that EPF with the highest enzymatic activity resulted in higher mortality rates [Citation53,Citation54], and that EPF with higher protease and chitinase activities resulted in lower LC50 and LT50 values [Citation22]. Currently, the expression genes of various extracellular enzymes of the A. nomius ACB1041 strain against ingests are unclear. Therefore, it is necessary not only to find the expression genes of various enzymes through genetic technology by the biotechnology method but also to express these genes in large quantities and finally achieve the effect of increasing the enzyme activity and enhancing the pathogen virulence [Citation55,Citation56]. Only after the above issues are clarified can we further develop this new resource and promote the application of biological pesticides.
In conclusion, investigating EPF virulence in different life stages of insect species may help determine the most appropriate insect life stage that can be targeted when using EPF as an effective biological control agent. In this study, the virulence of the A. nomius ACB1041 strain against the eggs, 1st‒5th instar larvae, pupae, and adults of O. furnacalis was compared, and the results showed that the insecticidal ability of the A. nomius ACB1041 strain was higher against younger larvae of O. furnacalis than against older larvae, pupae, and adults. Our results also showed that the A. nomius ACB1041 strain has a promising biological control effect against O. furnacalis, highlighting its potential for development as a new biological control agent.
Acknowledgements
This work was supported by the Natural Science Foundation of Xinjiang Uygur Autonomous Region under Grant 2022D01B35 and the Open Fund of the Key Laboratory of Integrated Pest Management of Crops in the Northwest Desert Oasis of the Ministry of Agriculture under Grant KFJJ202205.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data Availability statement
The data used to support the findings of this study are available from the corresponding author upon request.
Additional information
Funding
References
- Chen K, Chen J, Tang T, et al. Characterization and functional analysis of a relish gene from the Asian corn borer, Ostrinia furnacalis (Guenée). Arch Insect Biochem Physiol. 2021;108(2):e21841. doi: 10.1002/arch.21841
- Wang X, Ding X, Fu K, et al. Molecular identification and efficacy of entomopathogenic fungi isolates against larvae of the Asian corn borer Ostrinia furnacalis (Lepidoptera: Crambidae) in Xinjiang, China. J Appl Microbiol. 2022;133(5):2979–17. doi: 10.1111/jam.15749
- Bažok R, Pejić I, Čačija M, et al. Weather conditions and maturity group impacts on the infestation of first generation European corn borers in maize hybrids in Croatia. Plants (Basel). 2020;9(10):1387. doi: 10.3390/plants9101387
- Meihls LN, Kaur H, Jander G. Natural variation in maize defense against insect herbivores. Cold Spring Harb Symp Quant Biol. 2012;77:269–283. doi: 10.1101/sqb.2012.77.014662
- Ou D, Zhang LH, Guo CF, et al. Identification of a new Cordyceps javanica fungus isolate and its toxicity evaluation against Asian citrus psyllid. Microbiologyopen. 2019;8(6):e00760. doi: 10.1002/mbo3.760
- Chen J, Lai Y, Wang L, et al. CRISPR/Cas9-mediated efficient genome editing via blastospore-based transformation in entomopathogenic fungus Beauveria bassiana. Sci Rep. 2017;7(1):45763. doi: 10.1038/srep45763
- Duan X, He K, Wang Z, et al. Parasitoids and entomopathogens in overwintering larvae of Ostrinia furnacalis in China. Chin J Biol Control. 2014;30:823.
- Haihong W, Sheng L, Shuaiyu W, et al. Research and development of wettable powder of Beauveria bassiana and its control and application to Frankliniella occidentalis. Chin J Biol Control. 2021;36:858.
- Xu WJ, Sui L, Gao P, et al. Study and application of wettable powder of Beauveria bassiana to control corn borer. Chin J Biol Control. 2021;36:862.
- Amatuzzi RF, Poitevin CG, Poltronieri AS, et al. Susceptibility of Duponchelia fovealis Zeller (Lepidoptera: Crambidae) to soil-borne entomopathogenic fungi. Insects. 2018;9(2):70. doi: 10.3390/insects9020070
- Deng J, Xu W, Lv G, et al. Associated bacteria of a pine sawyer beetle confer resistance to entomopathogenic fungi via fungal growth inhibition. Environ Microbiome. 2022;17(1):47. doi: 10.1186/s40793-022-00443-z
- Nussenbaum AL, Lecuona RE. Selection of Beauveria bassiana sensu lato and Metarhizium anisopliae sensu lato isolates as microbial control agents against the boll weevil (Anthonomus grandis) in Argentina. J Invertebr Pathol. 2012;110(1):1–7. doi: 10.1016/j.jip.2012.01.010
- Shahriari M, Zibaee A, Khodaparast SA, et al. Screening and virulence of the entomopathogenic fungi associated with Chilo suppressalis Walker. Walker J Fungi (Basel). 2021;7(1):34. doi: 10.3390/jof7010034
- Sayed AMM, Dunlap CA. Virulence of some entomopathogenic fungi isolates of Beauveria bassiana (Hypocreales: Cordycipitaceae) and Metarhizium anisopliae (Hypocreales: Clavicipitaceae) to Aulacaspis tubercularis (Hemiptera: Diaspididae) and Icerya seychellarum (Hemiptera: Monophlebidae) on mango crop. J Econ Entomol. 2019;112(6):2584–2596. doi: 10.1093/jee/toz187
- Lu Q, Wang P, Ali A, et al. Molecular identification and virulence of four strains of entomopathogenic fungi against the whitefly, Bemisia tabaci (Hemiptera: Aleyrodidae). J Econ Entomol. 2022;115(3):731–738. doi: 10.1093/jee/toac031
- Zhao X, Situ G, He K, et al. Functional analysis of eight chitinase genes in rice stem borer and their potential application in pest control. Insect Mol Biol. 2018;27(6):835–846. doi: 10.1111/imb.12525
- Chu Y, Liu Y, Shen D, et al. Serine proteases SP1 and SP13 mediate the melanization response of Asian corn borer, Ostrinia furnacalis, against entomopathogenic fungus Beauveria bassiana. J Invertebr Pathol. 2015;128:64–72. doi: 10.1016/j.jip.2015.02.010
- Qu S, Wang S. Interaction of entomopathogenic fungi with the host immune system. Dev Comp Immunol. 2018;83:96–103. doi: 10.1016/j.dci.2018.01.010
- Nahar P, Ghormade V, Deshpande MV. The extracellular constitutive production of chitin deacetylase in Metarhizium anisopliae: Possible edge to entomopathogenic fungi in the biological control of insect pests. J Invertebr Pathol. 2004;85(2):80–88. doi: 10.1016/j.jip.2003.11.006
- Bhadani RV, Gajera HP, Hirpara DG, et al. Characterization and bio-efficacy of entomopathogenic Beauveria associated with cuticle-degrading enzymes to restrain sucking pest Bemisia tabaci. Parasitol Res. 2022;121(7):2019–2031. doi: 10.1007/s00436-022-07557-w
- Lei Y, Hussain A, Guan Z, et al., 2021. Unraveling the mode of action of Cordyceps fumosorosea: Potential biocontrol agent against Plutella xylostella (Lepidoptera: Plutellidae). Insects 12, 179.
- Maqsoudi P, Ramzi S, Zibaee A, et al. Virulence comparison of two Iranian isolates of Beauveria bassiana Vuillemin against Pseudococcus viburni Signoret (Hemiptera: Pseudococcidae). Trends Entomol. 2018;14:63–70.
- Revathi N, Ravikumar G, Kalaiselvi M. Pathogenicity of three entomopathogenic fungi against helicoverpa armigera. J Plant Pathol Microbiol. 2011;2(04). doi: 10.4172/2157-7471.1000114
- Velavan V, Dhanapal R, Ramkumar G, et al. Characterization and evaluation of Metarhizium spp. (Metsch.) Sorokin isolates for their temperature tolerance. J Fungi (Basel). 2022;8(1):68. doi: 10.3390/jof8010068
- Mkiga AM, Mohamed SA, du Plessis H, et al. Metarhizium anisopliae and Beauveria bassiana: Pathogenicity, horizontal transmission, and their effects on reproductive potential of Thaumatotibia leucotreta (Lepidoptera: Tortricidae). J Econ Entomol. 2020;113(2):660–668. doi: 10.1093/jee/toz342
- Peng, G., Zhang S., Xia Y. Laboratory efficacy of insecticidal fungi for control of Spodoptera frugiperda. Chin. J. Biol. Control. 2019; 35 (5): 729-734. doi: 10.16409/j.cnki.2095-039x.2019.05.026
- Akutse KS, Kimemia JW, Ekesi S, et al. Ovicidal effects of entomopathogenic fungal isolates on the invasive fall armyworm Spodoptera frugiperda (Lepidoptera: Noctuidae). J Appl Entomol. 2019;143(6):626–634. doi: 10.1111/jen.12634
- Kaur M, Chadha P, Kaur S, et al. Aspergillus flavus induced oxidative stress and immunosuppressive activity in Spodoptera litura as well as safety for mammals. BMC Microbiol. 2021;21(1):180. doi: 10.1186/s12866-021-02249-4
- Yan J, Liu H, Idrees A, et al. First record of Aspergillus fijiensis as an entomopathogenic fungus against Asian citrus psyllid, Diaphorina citri Kuwayama (Hemiptera: Liviidae). J Fungi (Basel). 2022;8(11):1222. doi: 10.3390/jof8111222
- Zhou D, Wang Y, Liu B, et al. Asian corn borer artificial feeding research: I. A semi-artificial diet and improvement. Acta Phytophysiol Sin. 1980;2:113–122.
- Karthi S, Vaideki K, Shivakumar MS, et al. Effect of Aspergillus flavus on the mortality and activity of antioxidant enzymes of Spodoptera litura fab. (Lepidoptera: Noctuidae) larvae. Pestic Biochem Physiol. 2018;149:54–60. doi: 10.1016/j.pestbp.2018.05.009
- Amsalingam R, Gajjeraman P, Sam N, et al. Mechanism of fenpropathrin resistance in red spider mite, oligonychus coffeae (acarina: tetranychidae), infesting tea [Camellia sinensis L. (O. Kuntze)]. Appl Biochem Biotechnol. 2017;181(2):548–561. doi: 10.1007/s12010-016-2230-5
- Seye F, Faye O, Ndiaye M, et al. Pathogenicity of the fungus, Aspergillus clavatus, isolated from the locust, Oedaleus senegalensis, against larvae of the mosquitoes Aedes aegypti, anopheles gambiae and Culex quinquefasciatus. J Insect Sci. 2009;9(53):1–7. doi: 10.1673/031.009.5301
- Huffman J, Gerber R, Du L. Recent advancements in the biosynthetic mechanisms for polyketide-derived mycotoxins. Biopolymers. 2010;93(9):764–776. doi: 10.1002/bip.21483
- Guo J, Guo J, He K, et al. Physiological responses induced by Ostrinia furnacalis (lepidoptera: crambidae) feeding in maize and their effects on O. furnacalis performance. J Econ Entomol. 2017;110(2):739–747. doi: 10.1093/jee/tox060
- Wu T, Zhao Y, Wang Z, et al. β-1,3-glucan recognition protein 3 activates the prophenoloxidase system in response to bacterial infection in Ostrinia furnacalis guenée. Dev Comp Immunol. 2018;79(79):31–43. doi: 10.1016/j.dci.2017.10.004
- Zemek R, Konopická J, Ul Abdin Z. Low efficacy of Isaria fumosorosea against box tree moth cydalima perspectalis: are host plant phytochemicals involved in herbivore defence against fungal pathogens? J Fungi (Basel). 2020;6(4):342. doi: 10.3390/jof6040342
- Ntalli NG, Ferrari F, Giannakou I, et al. Synergistic and antagonistic interactions of terpenes against Meloidogyne incognita and the nematicidal activity of essential oils from seven plants indigenous to Greece. Pest Manag Sci. 2011;67(3):341–351. doi: 10.1002/ps.2070
- Lin W-J, Chiu M-C, Lin C-C, et al. Efficacy of entomopathogenic fungus Aspergillus nomius against dolichoderus thoracicus. BioControl. 2021;66(4):463–473. doi: 10.1007/s10526-021-10086-7
- Xie Y, Liu W, Xue J, et al. Integument of soft scale insects and the invasion of the pathogenic fungus lecanicillium lecanii. Entomol Hell. 2017;19(2):66. doi: 10.12681/eh.11573
- Idrees A, Afzal A, Qadir ZA, et al. Bioassays of Beauveria bassiana isolates against the Fall armyworm, Spodoptera frugiperda. Spodoptera frugiperda J Fungi (Basel). 2022;8(7):717. doi: 10.3390/jof8070717
- Kim JC, Lee MR, Kim S, et al. Tenebrio molitor-mediated entomopathogenic fungal library construction for pest management. J Asia-Pac Entomol. 2018;21(1):196–204. doi: 10.1016/j.aspen.2017.11.018
- Pu S, Qin L, Chen M, et al. Host shift and host specificity analysis of Beauveria bassiana in Masson’s pine plantation based on SSR molecular marker. Mycosystema. 2013;32(04):698–709. doi: 10.13346/j.mycosystema.2013.04.004
- Sujithra M, Prathibha HV, Rajkumar M, et al. Entomopathogenic potential of simplicillium lanosoniveum native strain in suppressing invasive whitefly, aleurodicus rugioperculatus Martin (Hemiptera: aleyrodidae. Infesting Coconut J Fungi (Basel). 2021;7(11):964. doi: 10.3390/jof7110964
- Ni NT, Wu SS, Liao KM, et al. Evaluation of the potential entomopathogenic fungi purpureocillium lilacinum and fusarium verticillioides for biological control of forcipomyia taiwana (Shiraki). J Fungi (Basel). 2022;8(8):861. doi: 10.3390/jof8080861
- Khorrami F, Mehrkhou F, Mahmoudian M, et al. Pathogenicity of three different entomopathogenic fungi, Metarhizium anisopliae IRAN 2252, nomuraea rileyi IRAN 1020C and Paecilomyces tenuipes IRAN 1026C against the potato tuber moth, Phthorimaea operculella Zeller (Lepidoptera: Gelechiidae). Potato Res. 2018;61(4):297–308. doi: 10.1007/s11540-018-9378-z
- Bahar MH, Backhouse D, Gregg PC, et al. Efficacy of a Cladosporium sp. fungus against helicoverpa armigera (Lepidoptera: Noctuidae), other insect pests and beneficial insects of cotton. Biocontrol Sci Technol. 2011;21(12):1387–1397. doi: 10.1080/09583157.2011.622036
- Cafarchia C, Immediato D, Iatta R, et al. Native strains of Beauveria bassiana for the control of Rhipicephalus sanguineus sensu lato. Parasites Vectors. 2015;8(1):80. doi: 10.1186/s13071-015-0693-9
- Hassan AEM, Dillon RJ, Charnley AK. Influence of accelerated germination of conidia on the pathogenicity of Metarhizium anisopliae for Manduca sexta. J Invertebr Pathol. 1989;54(2):277–279. doi: 10.1016/0022-2011(89)90040-2
- Kaur M, Chadha P, Kaur S, et al. Schizophyllum commune induced genotoxic and cytotoxic effects in Spodoptera litura. Sci Rep. 2018;8(1):4693. doi: 10.1038/s41598-018-22919-0
- Butt TM, Coates CJ, Dubovskiy IM, et al. Entomopathogenic fungi: new insights into host-pathogen interactions. Adv Genet. 2016;94:307–364.
- Xiong Q, Xie Y, Zhu Y, et al. Morphological and ultrastructural characterization of Carposina sasakii larvae (Lepidoptera: Carposinidae) infected by Beauveria bassiana (Ascomycota: Hypocreales: clavicipitaceae). Micron. 2013;44:303–311. doi: 10.1016/j.micron.2012.08.002
- Ramzi S, Zibaee A. Biochemical properties of different entomopathogenic fungi and their virulence against Chilo suppressalis (Lepidoptera: Crambidae) larvae. Biocontrol Sci Technol. 2014;24(5):597–610. doi: 10.1080/09583157.2014.883360
- Lu Z, Zhu P, Gurr GM, et al. Rice pest management by ecological engineering: a pioneering attempt in China. In: Heong KL, J Cheng MM Escalada, editors. Rice planthoppers. Netherlands, Dordrecht: Springer Netherlands; 2015. pp. 161–178. doi: 10.1007/978-94-017-9535-7_8
- Fang W, Leng B, Xiao Y, et al. Cloning of Beauveria bassiana chitinase gene Bbchit1 and its application to improve fungal strain virulence. Appl Environ Microbiol. 2005;71(1):363–370. doi: 10.1128/AEM.71.1.363-370.2005
- Lin Y, Huang J, Akutse KS. Whitefly-induced tomato volatiles enhance the virulence of Lecanicillium lecanii. J Invertebr Pathol. 2021;183:107623. doi: 10.1016/j.jip.2021.107623