ABSTRACT
The presence of significant liver inflammation is an important indication for antiviral treatment in patients with chronic hepatitis B (CHB) in the indeterminate phase. We aimed to establish a non-invasive nomogram to predict significant liver inflammation in these patients. A total of 195 CHB patients in the indeterminate phase were randomly split into training and validation sets. The least absolute shrinkage and selection operator and logistic regression were applied to identify risk factors and establish a predictive model. A calibration curve, decision curve analysis (DCA), and receiver operating characteristic (ROC) curve were applied to assess the performance of the nomogram. The median age was 42.0 y and 59.5% of the patients were male. Alkaline phosphatase, γ-glutamyl transpeptidase, and prothrombin time were independent predictors for significant liver inflammation and selected to establish the AGP-nomogram. The calibration plot demonstrated that the predicted results matched the actual values. The DCA showed a high net benefit when the threshold probability was 25-83% in the training set and 31-100% in the validation set. The areas under ROC curves of AGP-nomogram in predicting significant inflammation were significantly higher than ALT in the training set (0.744 vs. 0.642, P = 0.049) and validation set (0.766 vs. 0.660, P = 0.047). The ability of AGP-nomogram in predicting advanced inflammation was also superior to ALT. The AGP-nomogram can accurately identify significant inflammation in CHB patients in the indeterminate phase, and its application may reduce the need for liver biopsy and help identify candidates for antiviral treatment.
Abbreviations: AASLD: American Association for the Study of Liver Diseases; ALB: albumin; ALP: alkaline phosphatase; ALT: alanine aminotransferase; APRI: aspartate aminotransferase-to-platelet ratio index; AST: aspartate aminotransferase; AUROC: area under the receiver operating characteristic curve; CHB: chronic hepatitis B; CI: confidence interval; DCA: decision curve analysis; FIB-4: fibrosis index based on the four factors; GLB: globulin; GGT: γ-glutamyl transpeptidase; HBcAb: hepatitis B core antibody; HBeAg: hepatitis B e antigen; HBsAg: hepatitis B surface antigen; HBV: hepatitis B virus; HCC: hepatocellular carcinoma; HIV: human immunodeficiency virus; INR: international-normalized ratio; IQR: interquartile range; LASSO: least absolute shrinkage and selection operator; LB: liver biopsy; LR: Likelihood ratio; NAFLD: non-alcoholic fatty liver disease; NPV: negative predictive value; PLT: platelets; PPV: positive predictive value; PT: prothrombin time; ROC: receiver operating characteristic; TB: total bilirubin; TE: transient elastography; ULN: upper limit of normal.
Introduction
Hepatitis B virus (HBV) infection has always been a serious health threat worldwide, with approximately 296 million patients chronically infected and 820,000 people died from HBV-related diseases, particularly liver cirrhosis and hepatocellular carcinoma (HCC) in 2019 [Citation1]. Given the interaction between HBV and host immune response, the natural history of chronic hepatitis B (CHB) includes immune-tolerant CHB, inactive CHB, immune-active CHB with hepatitis B e antigen (HBeAg) positive and negative [Citation2]. The immune-active CHB patients with high HBV DNA, elevated alanine aminotransferase (ALT), and significant liver inflammation or fibrosis regardless of HBeAg status need to receive antiviral therapy, while regular monitoring is recommended for immune-tolerant and inactive CHB [Citation3]. However, a substantial proportion of patients with CHB do not meet any criteria of the above immune status and fall into an uncertain phenotype which is known as indeterminate phase or grey zone, while the severity of liver injury and the necessity for antiviral treatment have not been elucidated clearly [Citation4,Citation5].
Hepatic pathology plays an essential role in the assessment of liver injury and determining the optimal timing of antiviral treatment, especially for patients in the indeterminate phase [Citation3]. However, as the gold standard method to assess liver injury, liver biopsy (LB) is hard to conduct routinely in clinical settings because of its invasive, high cost, sampling errors and adverse risks [Citation6]. Thus, several non-invasive measures for assessing liver fibrosis were developed as alternatives to LB, including aspartate transaminase-to-platelet ratio (APRI), fibrosis index based on the four factors (FIB-4) and transient elastography (TE) [Citation7,Citation8]. These measures have been validated with relatively high accuracy in distinguishing liver fibrosis [Citation9,Citation10]. However, few non-invasive indicators are available to reflect liver histological inflammation in CHB patients. ALT is the most widely used indicator to assess liver inflammation and determine the timing of antiviral therapy [Citation2,Citation6]. Nevertheless, several reports have shown that many CHB patients without elevated ALT levels had significant liver inflammation, suggesting that ALT levels were not always consistent with the severity of liver inflammation [Citation11,Citation12]. Moreover, the ideal cut-off values of ALT remain controversial [Citation13]. This study aimed to establish and validate a non-invasive nomogram to predict significant liver inflammation in CHB patients with indeterminate phase.
Materials and methods
Patients
We retrospectively included 1,980 consecutive CHB patients who underwent LB at four hospitals (Nanjing Drum Tower Hospital, Huai’an No. 4 People’s Hospital, Fifth People’s Hospital of Wuxi, and The Affiliated Infectious Diseases Hospital of Soochow University) in China from April 2004 to January 2022 (Figure S1). All patients were positive for hepatitis B surface antigen (HBsAg) over 6 months. The following exclusion criteria were used: (1) coexisting with non-alcoholic fatty liver disease (NAFLD), alcohol abuse, HCC, autoimmune liver disease, other viral hepatitis infection, syphilis, human immunodeficiency virus infection, or hereditary liver diseases; (2) insufficient clinical data. This study was approved by the ethics committees of Nanjing Drum Tower Hospital (approval number: 2008022), and the protocol was conducted in accordance with the Declaration of Helsinki. A waiver of informed consent was granted by the ethics committees due to a retrospective design.
Definition
According to the American Association for the Study of Liver Diseases (AASLD) 2018 hepatitis B guidance, the natural history of CHB was classified as follows: immune-active CHB was characterized by elevated ALT, with HBV DNA > 20,000 IU/mL in HBeAg-positive patients and HBV DNA > 2,000 IU/mL in HBeAg-negative patients, respectively. Immune-tolerant CHB was defined as HBeAg-positive, HBV DNA > 1 million IU/mL and normal ALT levels. Inactive CHB refers to normal ALT levels, absence of HBeAg, and HBV DNA < 2,000 IU/mL. The upper limit of normal (ULN) of ALT was 35 U/L for men or 25 U/L for women [Citation2]. Patients who cannot be classified into any of the above immune phases were classified as indeterminate phase.
Liver biopsy and clinical data
In this retrospective study, LB was performed to determine the severity of liver inflammation and fibrosis to assist the decision on starting antiviral treatment. LB was performed under ultrasound guidance by using suction biopsy needles. Liver tissues were processed with 10% formalin and embedded in paraffin. Two experienced pathologists evaluated liver inflammation based on the Scheuer scoring system and clarified it into five grades: G0 (no inflammation); G1 (inflammatory but no liver damage); G2 (focal necrosis or acidophil bodies); G3 (severe focal cell damage); and G4 (fusion necrosis) [Citation14]. G ≥2 and G ≥3 were defined as significant inflammation and advanced inflammation, respectively. We retrospectively reviewed individual medical records and collected demographic data and laboratory results within 2 weeks of LB, including age, sex, blood routine test, coagulation function, liver function, and HBV serological markers.
Statistical analyses
Data were analyzed in R version 4.2.0 (http://www.R-project.org) and SPSS version 26.0 (IBM Corp., Armonk, NY, USA). First, we randomly assigned patients to the training and validation sets in a 3:2 ratio. In the training set, we used the least absolute shrinkage and selection operator (LASSO) regression to screen independent risk factors for significant liver inflammation. Afterward, variables obtained from the LASSO regression were included in the logistic regression. Based on these results, a nomogram was established by the RMS package in R. The accuracy of the nomogram was verified by decision curve analysis (DCA), calibration curve, and receiver operating characteristic (ROC) curve. Constant values were presented as median (interquartile ranges, IQR) and categorical variables as frequency (percentage). Student’s t-test, Mann-Whitney U tests or Chi-square test were used for comparison between the two sets. P < 0.05 with two sides indicated statistical significance.
Results
Patient characteristics
Of 1,980 CHB patients who underwent LB, 1,785 were excluded based on exclusion criteria, and 195 CHB patients in the indeterminate phase were included eventually (Figure S1). The median age was 42.0 y and 116 (59.5%) cases were male. The proportion of HBeAg-positive patients was 17.4%. The median HBsAg and HBV DNA levels were 2.8 log10 IU/mL and 3.4 log10 IU/mL, respectively. One hundred and three (52.8%) patients had G ≥2 liver inflammation and 26 (13.3%) had G ≥3 liver inflammation. The patients were randomly divided into a training set (n = 116) and a validation set (n = 79). No significant differences in demographic, clinical, and histological characteristics were observed between the two sets ().
Table 1. Characteristics of patients with chronic hepatitis B in the indeterminate phase.
Comparison of clinical features of patients with and without significant liver inflammation in the training set
We further compared the clinical features of CHB patients in the indeterminate phase with and without significant liver inflammation in the training set, as shown in Table S1. Patients with significant inflammation had higher levels of ALT, aspartate aminotransferase (AST), alkaline phosphatase (ALP), γ-glutamyl transpeptidase (GGT), prothrombin time (PT), interquartile range (INR), hepatitis B core antibody (HBcAb), and HBeAg positive rate while lower levels of albumin (ALB). The age, sex, HBsAg, and HBV DNA levels were comparable between patients with and without significant inflammation.
Development of a nomogram for predicting significant liver inflammation
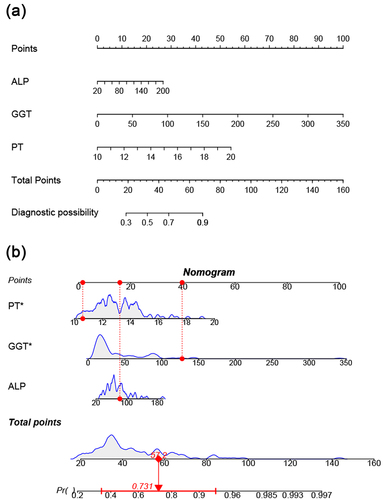
LASSO regression was conducted to select the parameters for assessing significant inflammation. Three predictor variables (ALP, GGT, and PT) positively associated with significant liver inflammation were eventually identified from 17 candidate variables (). These factors were included in multivariate logistic regression to build the predictive model presented as an AGP-nomogram (). We can calculate the total points on a vertical line from each variable to the point axis and obtain the risk probability of significant inflammation in CHB patients in the indeterminate phase. For example, a patient with PT of 10.6 s, GGT of 127.6 U/L and ALP of 82.3 U/L has a 73.1% probability of having significant liver inflammation ().
Figure 1. Predictor selection using LASSO regression analysis. (a) three predictors were selected by deriving the optimal λ. (b) the optimal penalty coefficient λ of LASSO regression was determined by 10-fold cross-validation and minimum criterion.
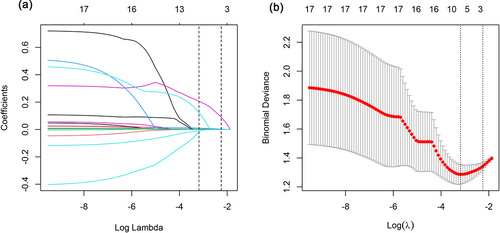
Figure 2. A nomogram for the prediction of significant liver inflammation in patients with chronic hepatitis B in the indeterminate phase (a). Draw a vertical line from the corresponding axis of each variable to the point axis. Calculate the points of all risk factors to determine the probability of significant inflammation. (b) a dynamic nomogram is used as an example.
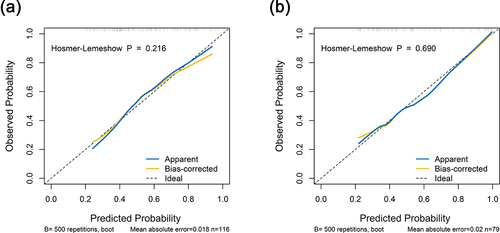
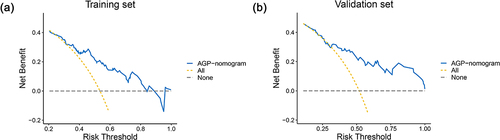
As shown in , the AGP-nomogram was calibrated by the Hosmer–Lemeshow test and the 500 times bootstrap resampling calibration plot, indicating that the nomogram predicted and the actually observed probability of significant inflammation had good consistency (P = 0.216 in the training set and P = 0.690 in the validation set). In addition, DCA revealed that the threshold probability ranges were 25–83% in the training set and 31–100% in the validation set for the nomogram’s ability to distinguish significant liver inflammation in CHB patients in the indeterminate phase ().
Comparison of predictive accuracy between the nomogram and ALT
The AGP-nomogram scores increased with the aggravation of liver inflammation (Figure S2). Correlation analysis also suggested that there was a significantly positive association between the nomogram scores and liver inflammation grades in CHB patients in the indeterminate phase both in the training set (r = 0.398, P < 0.001) and validation set (r = 0.457, P < 0.001). We further compared the performance of the AGP-nomogram and ALT in predicting liver inflammation (, Figure S3). In the training set, the AUROCs of AGP-nomogram were 0.744 (95% CI: 0.656–0.836) and 0.844 (95% CI: 0.729–0.958) in predicting significant inflammation and advanced inflammation, with optimal cut-off values of 29.665 and 50.698, respectively. By contrast, the AUROCs of AGP-nomogram were higher than ALT both in predicting significant inflammation (AUC: 0.642, 95% CI: 0.544–0.745, P = 0.049) and advanced inflammation (AUC: 0.663, 95% CI: 0.521–0.806, P = 0.025). Similarly, in the validation set, the AGP-nomogram also had higher AUROCs than ALT in predicting significant inflammation (0.766 vs. 0.660, P = 0.047) and advanced inflammation (0.925 vs. 0.744, P = 0.007).
Table 2. Diagnostic performances of AGP-nomogram and ALT in the training set and validation set.
Discussion
Accurate assessment of liver inflammation is crucial for making therapeutic decisions for CHB patients [Citation6]. Notably, significant liver inflammation was presented in approximately 52.8% of CHB patients in the indeterminate phase of our study, which suggested that it is necessary to distinguish these patients with significant inflammation and initiate antiviral therapy. Therefore, we established and validated a non-invasive nomogram based on three routine parameters (ALP, GGT, and PT). The AGP-nomogram showed better predicting accuracy for liver inflammation severity than ALT.
The immune status of CHB is dynamic and usually divided into four stages according to HBeAg, ALT, and HBV DNA levels. Identification of immune stages is conducive to prognostic prediction and therapeutic decision [Citation2]. However, approximately a third of patients do not meet any well-known immune status in the CHB population and fall into the indeterminate phase [Citation5,Citation15]. The severity of liver injury and the necessity for antiviral treatment in patients with indeterminate phase remains not fully clear [Citation16].
This study showed that more than half of patients with indeterminate phase had significant liver inflammation and the proportion was as high as 82.4% in HBeAg-positive patients. In previous study, we found that 72.7% (176/242) of patients in the indeterminate phase had significant liver histological diseases (G ≥ 2 and/or S ≥ 2). The proportion of significant liver histological diseases in patients with indeterminate phase was lower than that in the immune-active phase, while higher than in the immune-tolerant phase and inactive phase [Citation17]. Regarding the long-term prognosis, Bonacci et al. reported that only 6.3% of CHB patients with indeterminate phase transitioned to the immune-active state and no patients developed cirrhosis or severe liver fibrosis during follow-up [Citation18]. On the contrary, some investigators confirmed that patients with indeterminate phase had a higher risk of HCC. Daniel et al. found that the 10-y cumulative HCC incidence of patients persistently in the indeterminate phase was significantly higher than those remaining inactive (4.6% vs. 0.49%) [Citation19].
The current CHB guidelines recommended to monitor HBV DNA and ALT levels in CHB patients with indeterminate phase, and LB should be taken into consideration when necessary [Citation2]. Nevertheless, LB is challenging to apply in routine clinical practice due to its invasiveness and complexity [Citation6]. The ALT has always been recognized as a simple marker for reflecting hepatic inflammation activity [Citation20]. However, a previous study found that 72.1% of the patients with normal ALT had significant liver inflammation and 18.5% had advanced inflammation [Citation21]. Antiviral treatments should be recommended for CHB patients with detectable HBV DNA with the presence of significant liver inflammation [Citation22]. These findings suggested that the correlation of ALT with liver inflammation activity is not satisfactory in patients with CHB [Citation23]. Therefore, it is urgently needed to establish an accurate measure to assess the liver inflammation activity in CHB patients with indeterminate phase.
Although numerous of studies have established predictive models to assess liver inflammation, most of them used logistic regression analysis to identify relevant variables of liver inflammation [Citation24,Citation25]. Chen et al. analyzed the risk factors of significant liver inflammation by multivariate logistic regression, and the results showed that AST, PT, GGT, and HBcAb were predictors of significant liver inflammation [Citation26]. In another study, after adjusting for potential confounders, only GGT and PLT were independent risk factors of significant liver inflammation [Citation27]. However, multicollinearity and confounding factors should be considered in this process. Instead, before constructing the logistic regression model, we used LASSO regression analysis to screen the variables to avoid overfitting or poor fitting. We proposed the complex logistic regression formula in the form of a simple and intuitive nomogram. The DCA, calibration, and ROC were used to evaluate the accuracy of AGP-nomogram. The results showed that the model had excellent performance for predicting significant liver inflammation and advanced liver inflammation in both training set and validation set.
The AGP-nomogram contained three independent risk factors of significant liver inflammation, including ALP, GGT, and PT. GGT, a microsomal enzyme distributed in hepatic ductal and canalicular cells, may increase in several liver diseases, such as fatty liver disease, autoimmune liver disease, and drug-induced liver damage [Citation28]. Previous reports have revealed that GGT is associated with hepatic inflammation activity in CHB patients [Citation21]. ALP is also a marker of liver damage and distributes on the canalicular membrane of hepatocytes. PT indicates the activity of extrinsic coagulation system and may be prolonged in patients with significant hepatocellular dysfunction [Citation29].
Despite the AGP-nomogram being confirmed to be excellent in predicting liver inflammation, there were some limitations in our study. First, this is a retrospective and cross-sectional study, and the sample size is relatively small. Thus, the predicting value of AGP-nomogram needs to be validated in the future. Second, patients with indeterminate phase were identified based on the clinical data before biopsy, and the immune status of patients with indeterminate phase was likely temporary. Third, the AGP-nomogram was only validated in one internal set in the Asian population and needs to be validated in other ethnicities.
In conclusion, we established an AGP-nomogram that was accurate in predicting liver inflammation activity in CHB patients with indeterminate phase. The AGP-nomogram may be beneficial in reducing the need of LB and facilitating early identification and intervention in CHB patients with indeterminate phase with the presence of significant inflammation.
Authors’ contributions
All authors contributed to this study at different levels. All authors read and approved the final version. Study concept and design (Chao Wu, Rui Huang, Jie Li); acquisition of data (Jie Zhan, Jian Wang, Suling Jiang, Zhiyi Zhang, Jiacheng Liu, Yilin Liu, Ruifei Xue, Li Zhu, Juan Xia, Xiaomin Yan, Weimao Ding, Chuanwu Zhu, Yuanwang Qiu); statistical analysis and interpretation of data (Jie Zhan, Zhiyi Zhang, Suling Jiang); drafting of the manuscript (Jie Zhan, Jian Wang); critical revision of the manuscript for important intellectual content (Rui Huang, Chao Wu).
Supplemental Material
Download MS Word (482 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/21505594.2023.2268497.
Additional information
Funding
References
- Jeng W, Papatheodoridis G, AJL L, et al. Hepatitis B. Lancet. 2023;401(10381):1039–9. doi: 10.1016/s0140-6736(22)01468-4
- Terrault NA, Lok ASF, McMahon BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67:1560–1599. doi: 10.1002/hep.29800
- Lampertico P, Agarwal K, Berg T, et al. EASL 2017 clinical practice guidelines on the management of hepatitis B virus infection. Hepatol. 2017;67(2):370–398. doi: 10.1016/j.jhep.2017.03.021
- Hsu Y-N, Pan CQ, Abbasi A, et al. Clinical presentation and disease phases of chronic hepatitis B using conventional versus modified ALT criteria in Asian Americans. Dig Dis Sci. 2014;59:865–871. doi: 10.1007/s10620-014-3054-1
- Di Bisceglie AM, Lombardero M, Teckman J, et al. Determination of hepatitis B phenotype using biochemical and serological markers. J Viral Hepat. 2017;24:320–329. doi: 10.1111/jvh.12643
- Sarin SK, Kumar M, Lau GK, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10(1):1–98. doi: 10.1007/s12072-015-9675-4
- Degos F, Perez P, Roche B, et al. Diagnostic accuracy of FibroScan and comparison to liver fibrosis biomarkers in chronic viral hepatitis: a multicenter prospective study (the FIBROSTIC study). J Hepatol. 2010;53:1013–1021. doi: 10.1016/j.jhep.2010.05.035
- Alboraie M, Khairy M, Elsharkawy M, et al. Value of E gy- S core in diagnosis of significant, advanced hepatic fibrosis and cirrhosis compared to aspartate aminotransferase-to-platelet ratio index, FIB -4 and F orns’ index in chronic hepatitis C virus. Hepatol Res. 2015;45(5):560–570. doi: 10.1111/hepr.12385
- Xiao G, Yang J, Yan L. Comparison of Diagnostic accuracy of aspartate aminotransferase to platelet ratio index and fibrosis-4 index for detecting liver fibrosis in adult patients with chronic hepatitis B virus infection: a systemic review and meta-analysis. Hepatology. 2015;61:292–302. doi: 10.1002/hep.27382
- Lin C-L, Liu C-H, Wang C-C, et al. Serum biomarkers predictive of significant fibrosis and cirrhosis in chronic hepatitis B. J Clin Gastroenterol. 2015;49:705–713. doi: 10.1097/mcg.0000000000000250
- Duan M, Chi X, Xiao H, et al. High-normal alanine aminotransferase is an indicator for liver histopathology in HBeAg-negative chronic hepatitis B. Hepatol Int. 2021;15(2):318–327. doi: 10.1007/s12072-021-10153-2
- Liu J, Wang J, Yan X, et al. Presence of liver inflammation in Asian patients with chronic hepatitis B with normal ALT and detectable HBV DNA in absence of liver fibrosis. Hepatol Commun. 2022;6(4):855–866. doi: 10.1002/hep4.1859
- Wang H, Ru GQ, Yan R, et al. Histologic disease in Chinese chronic hepatitis B patients with low viral loads and persistently normal alanine aminotransferase levels. J Clin Gastroenterol. 2016;50:790–796. doi: 10.1097/mcg.0000000000000544
- Scheuer PJ. Classification of chronic viral hepatitis: a need for reassessment. J Hepatol. 1991;13:372–374. doi: 10.1016/0168-8278(91)90084-o
- Yao K, Liu J, Wang J, et al. Distribution and clinical characteristics of patients with chronic hepatitis B virus infection in the grey zone. J Viral Hepat. 2021;28:1025–1033. doi: 10.1111/jvh.13511
- Teng W, Chang T-T, Yang H-I, et al. Risk scores to predict HCC and the benefits of antiviral therapy for CHB patients in gray zone of treatment guidelines. Hepatol Int. 2021;15(6):1421–1430. doi: 10.1007/s12072-021-10263-x
- Wang J, Yan X, Zhu L, et al. Significant histological disease of patients with chronic hepatitis B virus infection in the grey zone. Aliment Pharmacol Ther. 2023;57:464–474. doi: 10.1111/apt.17272
- Bonacci M, Lens S, Mariño Z, et al. Anti-viral therapy can be delayed or avoided in a significant proportion of HBeAg-negative Caucasian patients in the grey zone. Aliment Pharmacol Ther. 2018;47:1397–1408. doi: 10.1111/apt.14613
- Huang DQ, Li X, Le MH, et al. Natural history and hepatocellular carcinoma risk in untreated chronic hepatitis B patients with indeterminate phase. Clin Gastroenterol Hepatol. doi: 10.1016/j.cgh.2021.01.019
- Gui HL, Wang H, Yang YH, et al. Significant histopathology in Chinese chronic hepatitis B patients with persistently high–normal alanine aminotransferase. J Viral Hepat. 2010;17(s1):44–50. doi: 10.1111/j.1365-2893.2010.01270.x
- Wang J, Xia J, Yan X, et al. The gamma-glutamyl transpeptidase to platelet ratio predicts liver inflammation in chronic hepatitis B with normal or mildly elevated alanine transaminase. Clin Res Hepatol Gastroenterol. 2020;44:913–922. doi: 10.1016/j.clinre.2020.01.011
- Chinese Society of Infectious Diseases CMA, Chinese Society of Hepatology CMA. The guidelines of prevention and treatment for chronic hepatitis B (2019 version). Zhonghua gan zang bing za zhi = Zhonghua ganzangbing zazhi =. Chinese J Hepat. 2019;27(12):938–961. doi: 10.3760/cma.j.issn.1007-3418.2019.12.007
- Chang X, Wang J, Chen Y, et al. A novel nomogram to predict evident histological liver injury in patients with HBeAg-positive chronic hepatitis B virus infection. EBioMedicine. 2021;67. doi: 10.1016/j.ebiom.2021.103389
- Li X, Xing Y, Zhou D, et al. A non-invasive model for predicting liver inflammation in chronic hepatitis B patients with normal serum alanine aminotransferase levels. Front Med (Lausanne). 2021;8:688091. doi: 10.3389/fmed.2021.688091
- Xie Y, Yi W, Zhang L, et al. Evaluation of a logistic regression model for predicting liver necroinflammation in hepatitis B e antigen-negative chronic hepatitis B patients with normal and minimally increased alanine aminotransferase levels. J Viral Hepat. 2019;26(S1):42–49. doi: 10.1111/jvh.13163
- Chen S, HJFim H. Clinical non-invasive model to predict liver inflammation in chronic hepatitis B with alanine aminotransferase ≤ 2 upper limit of normal. Front Med. 2021;8:661725. doi: 10.3389/fmed.2021.661725
- Wang J, Xia J, Zhang R, et al. A novel index using routine clinical parameters for predicting significant liver inflammation in chronic hepatitis B. J Viral Hepat. 2018;25:1151–1160. doi: 10.1111/jvh.12925
- Eminler AT, Irak K, Ayyildiz T, et al. The relation between liver histopathology and GGT levels in viral hepatitis: more important in hepatitis B. Turk J Gastroenterol. 2014;25:411–415. doi: 10.5152/tjg.2014.3693
- Kwo PY, Cohen SM, Lim JK. ACG clinical guideline: evaluation of abnormal liver chemistries. Am J Gastroenterol. 2017;112:18–35. doi: 10.1038/ajg.2016.517